Plants utilize conserved and developmentally essential activation of Rab5 GTPases for organizing endomembrane trafficking required for pre- and postinvasive innate immunity
Abstract
Plant innate immunity can effectively prevent the proliferation of filamentous pathogens. Papilla formation at the site of attack is essential for preinvasive immunity; in postinvasive immunity, the encasement of pathogen structures inside host cells can hamper disease. Whereas papillae are highly dependent on transcytosis of premade material, little is known about encasement formation. Here, we show that endosome-associated VPS9a, the conserved guanine-nucleotide exchange factor activating Rab5 GTPases, is required for both pre- and postinvasive immunity against a nonadapted powdery mildew fungus (Blumeria graminis f. sp hordei) in Arabidopsis thaliana. Surprisingly, VPS9a acts in addition to two previously well-described innate immunity components and thus represents an additional step in the regulation of how plants resist pathogens. We found VPS9a to be important for delivering membrane material to the encasement and VPS9a also plays a predominant role in postinvasive immunity. GTP-bound Rab5 GTPases accumulate in the encasement, but not the papillae, suggesting that two independent pathways form these defense structures. VPS9a also mediates defense to an adapted powdery mildew fungus, thus regulating a durable type of defense that works in both host and nonhost resistance. We propose that VPS9a plays a conserved role in organizing cellular endomembrane trafficking, required for delivery of defense components in response to powdery mildew fungi.
INTRODUCTION
Plants rely on innate immunity to defend themselves against viruses, bacteria, oomycetes, and fungi (Jones and Dangl, 2006; Dangl et al., 2013). Two functionally independent Arabidopsis thaliana pathways contribute to preinvasive immunity against the nonadapted powdery mildew fungus Blumeria graminis f. sp hordei (Bgh). One pathway, defined by the 1 β-glucoside hydrolase, PEN2, mediates synthesis and export of antimicrobial metabolites (Lipka et al., 2005; Stein et al., 2006; Clay et al., 2009; Bednarek et al., 2009). Here, the endoplasmic reticulum-resident P450 monooxygenase, CYP81F2, is thought to synthesize glucosinolate derivatives that are activated by PEN2 and finally secreted by the ATP binding cassette transporter protein, PEN3, thereby presenting potentially toxic compounds to the pathogen (Stein et al., 2006; Fuchs et al., 2016). Interestingly, loss of PEN2 and PEN3 also results in a substantial reduction of the hypersensitive response (HR) after recognition of the Pseudomonas syringae effectors, AvrRpm1 and AvrRps4, indicating that the role of the PEN2 pathway extends beyond that of preinvasive immunity (Johansson et al., 2014). In concert with PEN2, another pathway, defined by the plasma membrane (PM) syntaxin, PEN1, delivers building material for the papilla, which is a dome-shaped cell wall apposition formed in the outer cell wall of epidermal cells at the site of attack (Collins et al., 2003; Hückelhoven and Panstruga, 2011). The PEN1 pathway also includes the other membrane trafficking components, VAMP721/722 and GNOM, the loss of which also results in the phenotypes of the pen1 mutant, i.e., reduced preinvasive immunity and delayed papilla formation (Kwon et al., 2008; Nielsen et al., 2012). While PEN1 is required for this type of preinvasive immunity, it is not strictly required for delivery of papilla material to the attack site, only the timing of it. This suggests that to obtain preinvasive immunity, the papilla should form during the early phase of penetration. The PEN1 pathway is thought to act independently of the PEN2 pathway, as the pen1 pen2 double mutant showed elevated penetration frequencies in comparison to the respective single null mutants (Lipka et al., 2005). Moreover, whereas PEN2 mediates both pre- and postinvasive immunity, PEN1 predominantly acts in preinvasive immunity (Wen et al., 2011; Johansson et al., 2014).
Syntaxins, together with other interacting SNARE proteins, facilitate the fusion of two opposing membranes, thus allowing for membrane trafficking of cargos to specific destinations within the cell (Lipka et al., 2007). The PEN1 pathway relies on transcytosis, where preformed papilla material at the PM is transported to the attack site and secreted into the apoplast. Interestingly, this PM material includes PEN1 itself, visualized as GFP-PEN1. This process is dependent on GNOM (Nielsen et al., 2012), which is a transcytosis-associated ARF GTPase guanine-nucleotide exchange factor (GEF; Geldner et al., 2003). While we envisage GNOM to act on the PM, initiating the endocytosis event, PEN1 likely acts at two sites. First, it is believed to act at the trans-Golgi network (TGN) receiving the endocytic vesicles internalized at the PM, and second, at the PM where it receives the papilla material from the TGN, directed to the site of attack (Nielsen and Thordal-Christensen, 2013). The recycling of papilla material helps to explain how plants are able to mount a fast response to the invading pathogen and maintain cell integrity.
Based on the observation that the papillary accumulation of the membrane-embedded GFP-PEN1 occurs outside of the PM, as well as on several electron microscopy studies, it has been suggested that delivery of papilla material involves secretion of exosomes by fusing multivesicular bodies (MVBs) with the PM (An et al., 2006; Meyer et al., 2009; Nielsen et al., 2012; Nielsen and Thordal-Christensen, 2013). Yet, due to the topology of GFP-PEN1, the SNARE domain pointing toward the lumen of the exosomes, the papillary accumulation of GFP-PEN1 is considered to be biological irrelevant and instead could be the result of ineffective sorting at the TGN during pathogen attack. Plant exosomes are speculated to contain small peptides and RNAs that could hamper fungal growth (Rutter and Innes, 2017). In all eukaryotes, proper MVB maturation and fusion to target membranes is highly dependent on a small family of Rab5 GTPases (Carney et al., 2006). In mammals, yeast, and plants, VPS9a-like GEFs are key activators of the Rab5 GTPases that regulate the transition of early endosomes (EEs) into MVBs that ultimately deliver cargo to the vacuole/lysosome. Interestingly, introduction of Q→L mutant versions of Rab5 GTPases, which remain GTP-locked due to their disrupted GTPase enzyme activity, stimulates homotypic fusions of the outer MVB membrane (Stenmark et al., 1994). Rab5QL at the same time prevents fusion of MVBs with their target membrane (Jia et al., 2013). In plants, the TGN is believed to function as the EE (Dettmer et al., 2006), and plant Rab5 GTPases are suggested to be recruited to the TGN to promote MVB maturation, as it has been described for EE in mammals and yeast (Singh et al., 2014). Arabidopsis has two functionally redundant conventional Rab5 GTPases, ARA7 and RHA1, and a plant-specific Rab5 GTPase, ARA6. Hitherto, only the latter has been suggested to mediate a fusion of MVBs with the PM (Ebine et al., 2011). In addition, ARA6-labeled endosomes were found to accumulate near the site of Bgh attack, suggestive of a direct role in delivering exosomes to the forming papilla (Nielsen et al., 2012).
Here, we show, in contrast to previous speculations, that ARA6 is not required for preinvasive immunity. Instead, impaired activation of conventional Rab5 GTPase, ARA7, by the common GEF, VPS9a, led to loss of both pre- and postinvasive immunity to Bgh. We follow up by showing that the preinvasive immunity mediated by VPS9a works in addition to PEN1 and PEN2. Moreover, VPS9a is required for proper delivery of membrane material to the encasement that mediates postinvasive immunity. Unlike GFP-PEN1, GTP-locked ARA6 and ARA7 were found to accumulate only in the encasement and not in the papilla, suggesting that two independent pathways deliver membrane material to these defense structures. Based on our findings, we speculate that VPS9a mainly mediates delivery of membrane material to the encasement and to a lesser degree to the papilla. Surprisingly, VPS9a also provides pre- and postinvasive immunity against an adapted powdery mildew fungus. In case of preinvasive immunity, this was unexpected, as this has been suggested overcome by this fungus. We propose that plants have co-opted the highly conserved and developmentally essential activation of Rab5 GTPases also to organize endomembrane trafficking needed for innate immunity.
RESULTS
Activation of a Conventional Rab5 GTPase Mediates Preinvasive Immunity
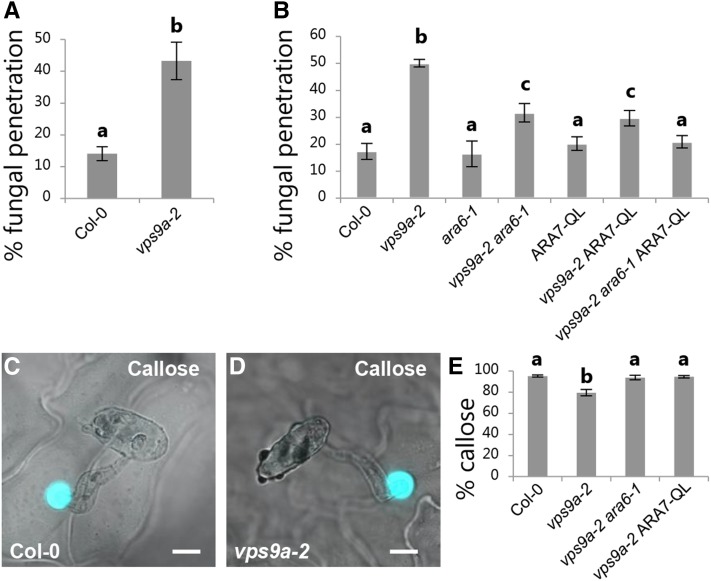
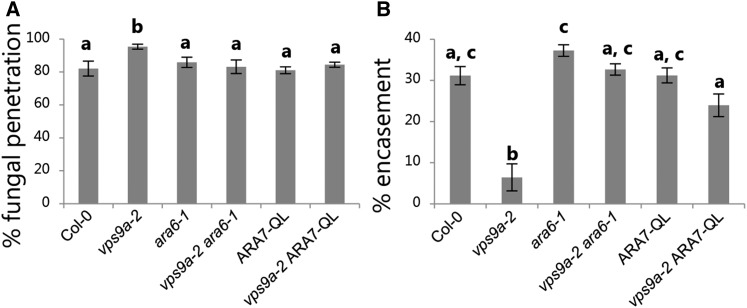
To elucidate the suggested role of Rab5 GTPases in delivering exosomes to the papillary matrix, we first analyzed the role of VPS9a, which is the common GEF for all three Rab5 GTPases (Goh et al., 2007). While VPS9a serves as an optimal target for determining the involvement of the Rab5 GTPases in plant innate immunity, a complete loss of VPS9a function results in seedling lethality. Therefore, we made use of the weak mutant allele, vps9a-2, which results in an intermediate phenotype with distorted vacuolar transport, polarity defects, and incomplete cell plate formation (Goh et al., 2007; Inoue et al., 2013). Nonetheless, vps9a-2 plants are viable and their immunity can be scored. When challenged with Bgh, the vps9a-2 mutant had a clear increase in penetration events (Figure 1A). In addition, we analyzed plants expressing the constitutively active mutant Rab5 GTPases, ARA6QL or ARA7QL, which lack GTPase enzymatic activities. Previous investigations have shown that overexpression of GTP-locked ARA7 effectively prevents fusion of MVBs with the tonoplast and results in enlarged endosomes (Jia et al., 2013). Yet in contrast to vps9a-2, neither expression of ARA6QL nor ARA7QL affected the penetration frequency (Supplemental Figure 1A). Previous reports have shown that the phenotypes of vps9a-2 can be suppressed either by removing the substrate, ARA6, which likely focuses the remaining activity of VPS9a-2 on activation of ARA7/RHA1, or they can be suppressed by introducing ARA7QL (Goh et al., 2007; Ebine et al., 2011). However, when analyzing such rescued vps9a-2 lines (i.e., vps9a-2 ara6-1 and vps9a-2 ARA7QL), it became clear that both lines had intermediate phenotypes both in terms of development and preinvasive immunity. Therefore, ara6-1 and ARA7QL were combined in the vps9a-2 mutant. In comparison to the single rescued lines, vps9a-2 ara6-1 and vps9a-2 ARA7QL, the double rescued vps9a-2 ara6-1 ARA7QL grew considerably larger and the preinvasive immunity was fully rescued (Figure 1B; Supplemental Figure 1B). This result confirms the phenotype of vps9a-2 and that activation of the conventional Rab5 GTPase, ARA7, is important for preinvasive immunity against Bgh. Surprisingly, however, there seems to be no dependency on their GTPase enzymatic activity and transport to the vacuole. From inspections of nonpenetrated attack sites at 24 h after inoculation (hai), we could not distinguish the quality of the papillae in Col-0 and vps9a-2 in terms of their content of callose (Figures 1C and 1D; Supplemental Figure 1C). We also didn’t detect a delay in the papilla formation in response to Bgh (Supplemental Figure 1D), which has otherwise been described for plants hampered in the PEN1 pathway, including VAMP721/722 and GNOM, for preinvasive immunity (Assaad et al., 2004; Kwon et al., 2008; Nielsen et al., 2012). However, we found consistently that the frequency of papillae at nonpenetrated sites of attack was slightly lower in vps9a-2 at 24 hai (Supplemental Figure 1D). Introducing either ara6-1 or ARA7QL alone was sufficient for fully rescuing the papilla frequency as observed by accumulation of callose (Figure 1E).
Figure 1.
VPS9a Is Required for Preinvasive Immunity.
(A) and (B) Frequency of penetration by Bgh in leaves of 5-week-old plants at 48 hai.
(C) and (D) Focal accumulation of callose in response to Bgh attack at 24 hai. Bars = 10 μm.
(E) Papilla frequency in response to Bgh attack at 24 hai as observed by accumulation of callose.
(A), (B), and (E) All values are mean ± sd (n = 4 leaves per genotype). Differences are significant at P ≤ 0.001 estimated using logistic regression.
VPS9a Mediates Preinvasive Immunity Distinct from PEN1 and PEN2
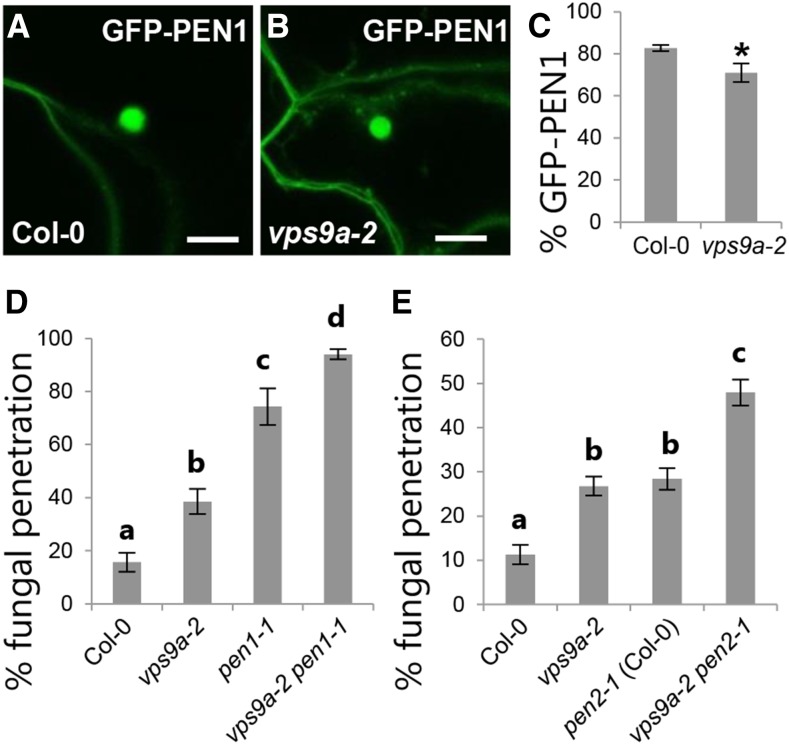
Previously we have shown that ARA6 accumulates near attack sites, while ARA7 localization is unaffected during pathogen invasion (Nielsen et al., 2012). As loss of functional ARA6 did not affect the preinvasive immunity, we speculated whether ARA7 could functionally replace ARA6 in preinvasive immunity. Yet, localization of ARA7 was not altered in plants lacking ARA6 (Supplemental Figure 2). Combined, these observations suggest that the accumulation of ARA6 at attack sites does not reflect a direct involvement in preinvasive immunity as previously thought. Also, as vps9a-2 is affected in transport from the TGN to the vacuole (Goh et al., 2007; Ebine et al., 2011), we speculated that preinvasive immunity is dependent on vacuolar transport. Previous studies found an intimate genetic interaction between the MVB/tonoplast localized SYP22 and the Rab5 GTPases (Ebine et al., 2011). Therefore, we tested the syp22-4 mutant for loss of preinvasive immunity. As described for vps9a-2, loss of SYP22 results in pleiotropic phenotypes (Ohtomo et al., 2005). Yet, we could not detect any change in the penetration frequency (Supplemental Figure 3A). This suggested that the loss of preinvasive immunity in vps9a-2 could be separated from transport to the vacuole. To further characterize the involvement of VPS9a in preinvasive immunity, we wanted to study its relationship with the delivery of exosomes carrying GFP-PEN1. Unexpectedly, accumulation of GFP-PEN1 in the papilla was indistinguishable from the wild type with only a slight reduction of papilla frequency at 24 hai, comparable to that found for callose (Figures 2A to 2C; Supplemental Figures 3B and 3C). The fact that vps9a-2 only is affected in the frequency of papilla formation, and not their quality, contrasts with plants lacking PEN1, VAMP721/722, or GNOM and suggested to us that VPS9a mediates preinvasive immunity independently of the PEN1 pathway. We tested this further by combining vps9a-2 with pen1-1 and found a significant increase in penetration level compared with each of the single mutants (Figure 2D). Moreover, combining vps9a-2 with pen2-1, which affects the second preinvasive immunity pathway, also elevated the penetration level significantly above the levels in the respective single mutants (Figure 2E). As pen1-1 and pen2-1 are both true null alleles of their respective pathways, the additive effects with vps9a-2 shows that VPS9a likely mediates a distinct preinvasive immunity that works in addition to both PEN1 and PEN2.
Figure 2.
VPS9a Makes an Independent Contribution to Immunity.
(A) and (B) Focal accumulation of GFP-PEN1 in response to Bgh attack at 24 hai. Bars = 10 μm.
(C) Papilla frequency in response to Bgh attack at 24 hai as observed by accumulation of GFP-PEN1.
(D) and (E) Frequency of penetration by Bgh in leaves of 5-week-old plants at 48 hai.
(C) to (E) All values are mean ± sd (n = 4 leaves per genotype). Differences are significant at P ≤ 0.001 estimated using logistic regression.
Similar to VPS9a, GNOM plays an important role in mediating endomembrane trafficking needed for preinvasive immunity (Nielsen et al., 2012). Yet, introduction of the transheterozygote mutant gnomB4049/emb30-1 in pen1-1 and pen2-1 only elevated penetration levels in the pen2-1 background (Geldner et al., 2004; Supplemental Figure 4). This confirms genetically that GNOM works on the PEN1 pathway, as previously suggested by pharmacological data (Nielsen and Thordal-Christensen, 2013), and underlines that VPS9a acts independently of PEN1 in preinvasive immunity.
Postinvasive Encasement-Based Immunity Is Compromised in vps9a-2
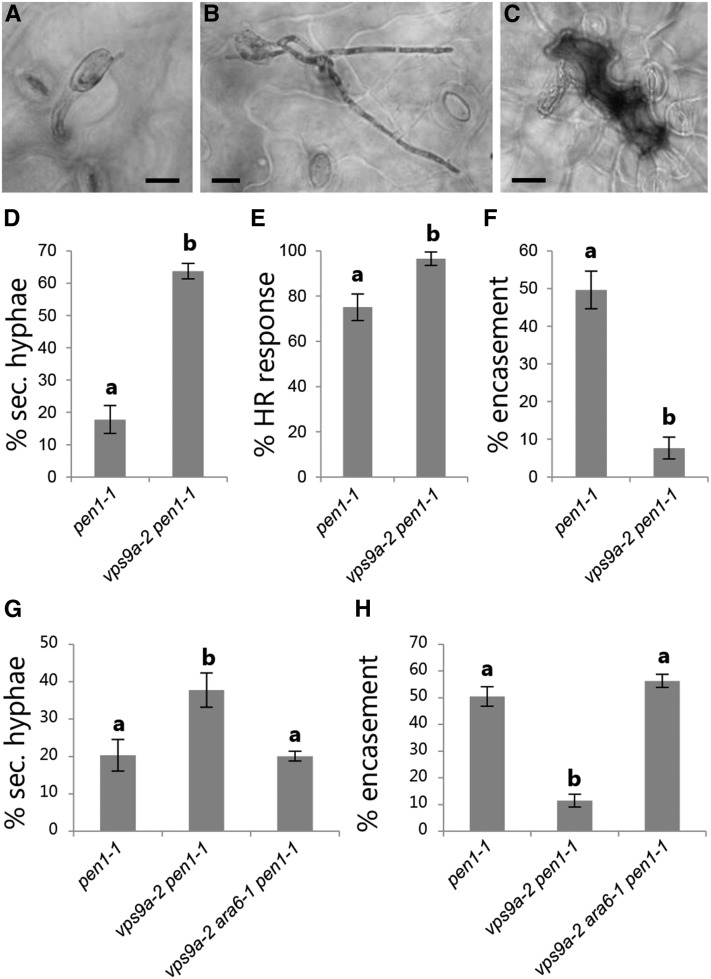
Upon successful penetration, the powdery mildew fungus establishes a fungal feeding structure, named the haustorium, by which it acquires nutrients from the host cell (Hückelhoven and Panstruga, 2011). This enables the fungus to produce secondary hyphae, which will seek to establish additional haustoria and eventually produce the next generation of conidia. From our analysis of the vps9a-2 mutant, we initially noticed a remarkably high frequency of Bgh conidia with secondary hyphal growth compared with the wild type, suggesting an involvement of VPS9a in postinvasive immunity as well as in preinvasive immunity (Figures 3A and 3B). To increase the number of successful penetrations and thereby improve the statistical analysis, we scored the frequency of secondary hyphal growth in the pen1-1 mutant background, since this is only mildly affected in postinvasive immunity (Wen et al., 2011; Johansson et al., 2014). In support of our initial observations, the vps9a-2 mutation caused a significant increase in the frequency of secondary hyphal growth in the pen1-1 background, showing that VPS9a activity mediates postinvasive immunity (Figure 3D).
Figure 3.
VPS9a Is Required for Postinvasive Immunity.
(A) and (B) Conidia of Bgh without (A) and with (B) secondary hyphal growth.
(C) Pavement cell with HR. Bars = 20 μm.
(D) and (G) Frequency of Bgh conidia with secondary hyphae following penetration at 48 hai.
(E) Frequency of HR upon penetration by Bgh at 72 hai.
(F) and (H) Frequency of fully encased Bgh haustoria at 48 hai.
(D) to (H) Scoring of leaves of 5-week-old plants. All values are mean ± sd (n = 4 leaves per genotype). Differences are significant at P ≤ 0.001 estimated using logistic regression.
The final option by which a penetrated pavement cell can prevent a powdery mildew fungus from proliferating is by undergoing HR. This is a form of programmed cell death that prevents obligate biotrophs, such as powdery mildew fungi, from taking up nutrients from the host cell (Figure 3C). Less HR in penetrated cells would, according to previous findings, allow more conidia to produce secondary hyphae (Lipka et al., 2005). However, the frequency of HR in response to penetration by Bgh was in fact higher in vps9a-2 and therefore could not explain the increased growth of secondary hyphae (Figure 3E). Instead, we found that encasements formed around haustoria were often incomplete, not fully enclosing the developing haustoria (Figure 3F). The encasement is thought to mediate postinvasive immunity by secluding the invading fungus from the host cell and preventing nutrient uptake. Therefore, a delayed or incomplete encasement response could explain the observed increase of secondary fungal growth in vps9a-2. Also, the hampered ability to fully seclude the haustoria from the host cell could explain the elevation in HR frequency observed at 3 dai, as this may provide better access for fungal effectors to the host cytosol. As described for the rescue of the papilla frequency, we found that introducing either ara6-1 or ARA7QL was sufficient for fully rescuing the secondary hyphal growth as well as the encasement response of vps9a-2 (Figures 3G and 3H; Supplemental Figure 5). Thus, the loss of pre- and postinvasive immunity phenotypes as well as the developmental phenotypes of vps9a-2 appeared to be directly correlated to the activation of ARA7.
Deposition of Membrane Material in the Encasement Is Impaired in vps9a-2 Mutants
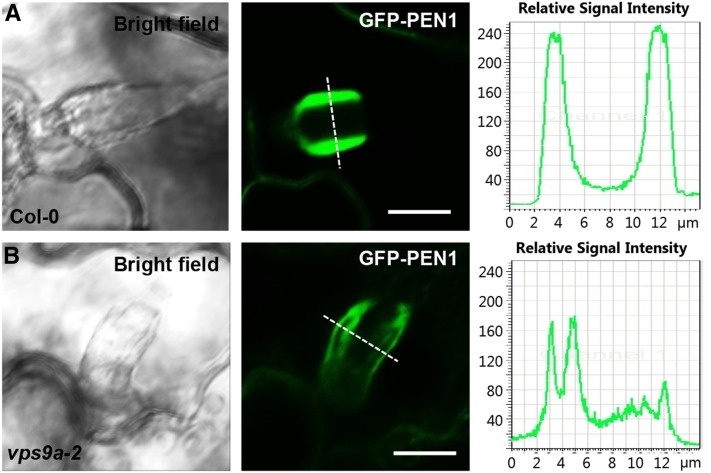
Encasements have been observed in diverse plant species in response to a range of haustoria-forming pathogens. Yet, little is known as to how this structure is formed (Kemen and Jones, 2012; Caillaud et al., 2014). Callose, fluorescent versions of PEN1 and its interacting partner SNAP33 accumulate strongly in papillae and encasements, thereby serving as valid markers for the formation of these structures (Meyer et al., 2009). As mentioned above, we observed reduced frequencies of papillae with both callose and GFP-PEN1 in vps9a-2. Yet, we were unable to detect a difference in papilla quality. In contrast, the encasement accumulation of these components was different in Col-0 and vps9a-2. While GFP-PEN1 in 20 out of 23 observed encasements uniformly labels the interior matrix of the encasement in Col-0 (Figure 4A), it was found to deposit in lower amounts and aberrantly in the encasement of vps9a-2 as only 8 out of 30 encasements showed normal labeling (Figure 4B; Supplemental Figures 6A to 6C). To support our observations of irregular GFP-PEN1 localization in the encasements of vps9a-2, we introduced the lipophilic membrane dye FM4-64, which was previously shown to colocalize with GFP-PEN1 in the encasement (Meyer et al., 2009). Yet, encasements in vps9a-2 were clearly less accessible to the dye than those in the wild type. Often, this led to distinct localizations of the two signals (data not shown). Therefore, FM4-64 could not be used to validate the irregular localization GFP-PEN1. In contrast to this, we found that the accumulation of callose in the encasement was unchanged in the vps9a-2 mutant (Supplemental Figure 7). Combined, we take these observations as evidence that VPS9a is required for correct delivery of membrane material including GFP-PEN1 to the matrix of the encasement.
Figure 4.
VPS9a Is Required for Correct Formation of the Encasement.
Encasements forming around Bgh haustoria accumulating GFP-PEN1 48 hai of leaves of 5-week-old plants of Col-0 (A) and vps9a-2 (B). Intensity profile was measured at the dotted lines. Bars = 10 μm.
GTP-Locked Rab5 GTPases Marks the Encasement Matrix
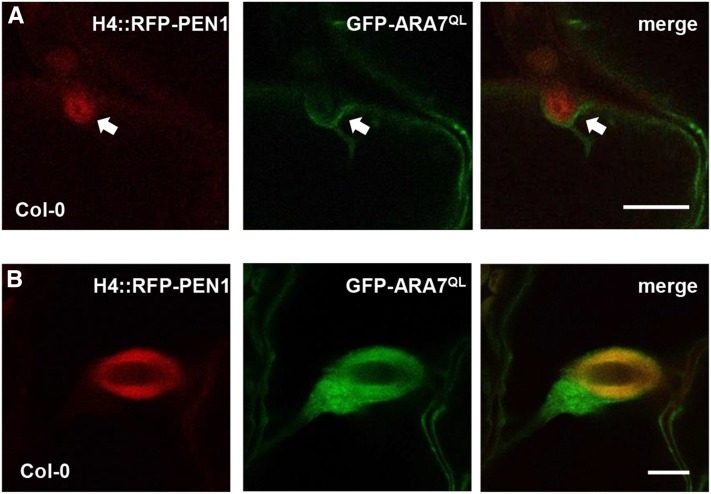
Having identified a requirement for a Rab5 GEF in encasement formation, we monitored the localization of ARA6-GFP and GFP-ARA7 in relation to this. However, in accordance with a recent observation (Inada et al., 2016), we could only find a weak increase in the GFP signal near the encasement as compared with the unencased part of the forming haustoria (Supplemental Figures 8A and 8B). We also included the GTP-locked, QL versions of both GTPases in this study. Here, we could detect clear signals from both ARA6QL-GFP and GFP-ARA7QL inside the encasement, but only weak signals from the same markers inside the papilla (Figures 5A and 5B; Supplemental Figures 8C and 8D). To our knowledge, ARA6QL and ARA7QL are the first reported markers that can distinguish these defense structures, underlining that they differ in composition, although similar in appearance. In line with the result that ARA7QL did not affect penetration resistance in the wild type (Figure 1B), this GTP-locked GTPase neither affected RFP-PEN1 accumulation in papillae nor in encasements when this syntaxin was expressed from the S-phase specific histone H4 promoter (Reichardt et al., 2011; Figures 5A and 5B). As these studies are made in mature epidermal cells, in which only previously expressed RFP-PEN1 is found, this result shows that recycled membrane material is delivered to both structures. Yet, this is not prevented by coexpression of a Rab5 GTPase with blocked enzymatic activity.
Figure 5.
GTP Locked ARA7 Accumulates in Encasements, Not Papillae.
Localization of H4::RFP-PEN1 and GFP-ARA7QL at papilla ([A], arrow) and encasement (B) in response to Bgh attack 48 hai of leaves of 5-week-old plants. Bars = 10 μm.
Activation of ARA7 Mediates Pre- and Postinvasive Immunity to an Adapted Powdery Mildew Fungus
Finding that VPS9a plays a vital role in immunity against the nonadapted Bgh, we decided also to study the immunity to the adapted powdery mildew fungus, Golovinomyces orontii (Go), for which Arabidopsis is host. Unexpectedly, we found that penetration levels were higher in vps9a-2 (Figure 6A). This was unlike in pen1-1 and pen2-1, where Go’s penetration frequency was unaffected (Lipka et al., 2005). Interestingly, the penetration levels in vps9a-2, rescued by ara6-1 or ARA7QL, were indistinguishable from the wild type (Figure 6A). As for Bgh, haustoria from Go became encased in wild-type plants, although this occurred later and at a lower frequency. This Go-induced encasement was clearly affected in vps9a-2 and fully restored by either ara6-1 or ARA7QL (Figure 6B). Combined, this shows that while the adapted pathogen is able to evade or suppress the preinvasive immunity mediated by PEN1 and PEN2, it is unable to completely suppress pre- and postinvasive immunity mediated by VPS9a. As vps9a-2 displayed a higher penetration rate and reduced encasement formation, we expected vps9a-2 to be more susceptible to Go. Yet, we could not detect any difference in susceptibility, at the macroscopic level or quantitatively (Supplemental Figures 9A and 9B). Moreover, vps9a-2 did not positively affect hyphal growth rates of Go (Supplemental Figure 9C). Therefore, while loss of VPS9a activity leads to an enhanced penetration frequency and a hampered encasement response, this does not result in an improvement of the overall fungal fitness. This would indicate that encasements are not efficient in restricting proliferation of the adapted powdery mildew fungus. We speculate that Go partially suppresses or evades the encasement response and thereby obtains full capacity to generate secondary haustoria to sustain fungal growth, despite the encasement still being visible.
Figure 6.
VPS9a Mediates Immunity to an Adapted Powdery Mildew.
(A) Frequency of penetration by Go in leaves of 5-week-old plants at 48 hai.
(B) Frequency of fully encased Go haustoria at 4 d after inoculation (dai).
All values are mean ± sd (n = 4 leaves per genotype). Differences are significant at P ≤ 0.001 estimated using logistic regression.
DISCUSSION
From previous analyses of mutants and from pharmacological studies, it is well established that PEN1 and PEN2 define two distinct preinvasive fungal immunity pathways (Lipka et al., 2005; Stein et al., 2006; Clay et al., 2009; Bednarek et al., 2009; Collins et al., 2003; Kwon et al., 2008; Hückelhoven and Panstruga, 2011; Nielsen et al., 2012; Underwood and Somerville, 2013). While the PEN2 pathway is found to mediate secretion of glucosinolates at the site of attack, the PEN1 pathway is thought to mediate resistance by delivering papilla material to this site. Finding that GFP-PEN1 is secreted into the matrix of papillae and encasements was taken as a sign of involvement of exosomes by mechanisms similar to what has been described for mammals and yeast (Meyer et al., 2009; Nielsen et al., 2012; Nielsen and Thordal-Christensen, 2013). The activity of Rab5 GTPases has been shown to be vital for the maturation and fusion of the MVBs that secrete exosomes into the apoplast in mammals (Baietti et al., 2012). Based on previous findings in Arabidopsis, the plant-specific Rab5 GTPase ARA6 was suggested to have a similar role in the preinvasive immunity against Bgh (Ebine et al., 2011; Nielsen et al., 2012). Yet, we found in the present study no absolute requirement for ARA6 in this form of defense. Moreover, plants expressing GTP-locked ARA6 or ARA7 were also not affected in preinvasive immunity against Bgh. As introducing a GTP-locked Rab5 GTPase exerts a dominant-negative effect on exosome secretion in mammals, our findings could question whether the plant exosomes secreted during preinvasive immunity has a similar origin. Yet, as little is known on the formation of exosomes in plants, we felt that further inquiries of PEN1 secretion were required to elucidate the molecular machinery behind and to establish the functional role of exosomes in plant innate immunity.
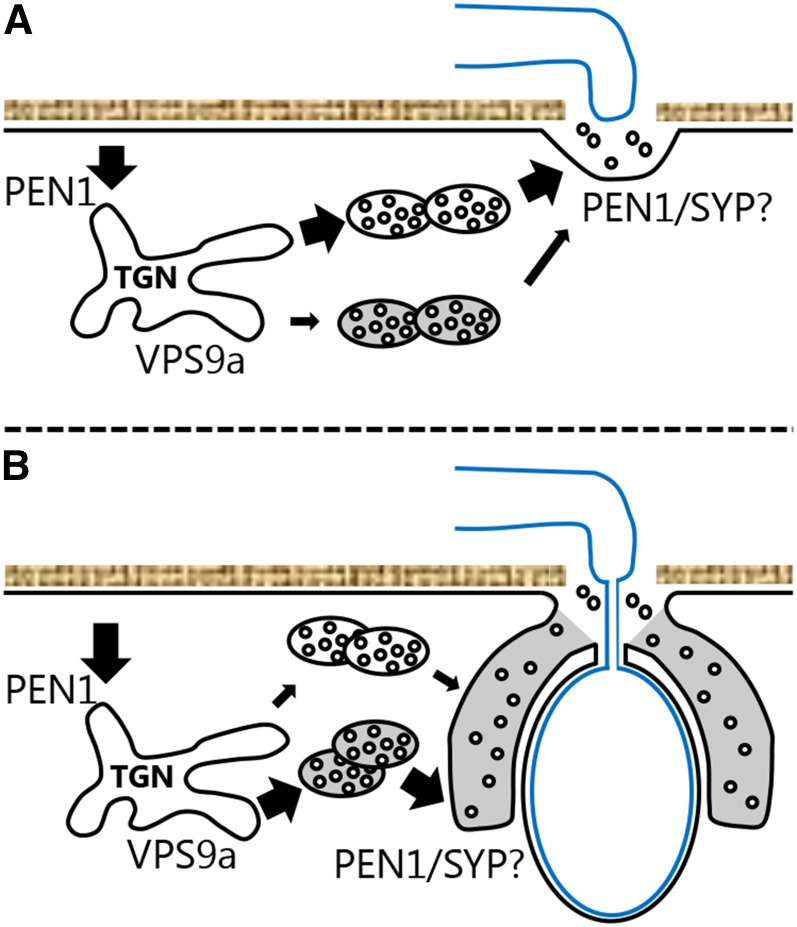
In this work, we have shown that VPS9a is crucial for both pre- and postinvasive immunity against powdery mildew fungi. Moreover, this previously undescribed immunity mediated by VPS9a works in addition to the known immunity components, PEN1 and PEN2. However, we cannot exclude the possibility that VPS9a mediates a membrane trafficking step, shared by both PEN1 and PEN2. We failed to correlate the loss of preinvasive immunity in vps9a-2 with altered papilla quality. However, in context of postinvasive immunity, vps9a has a clear mislocalization of GFP-PEN1 in the encasement forming around the invading fungus. This occurs with more secondary hyphal growth of the nonadapted fungus. Finding that both ARA6QL and ARA7QL accumulate strongly in the encasement, but only weakly in the papilla, indicates that the vesicles and/or endosomes directed to the papilla and the encasement are different. As GTP-locked Rab GTPases are not released from their target membrane (Stenmark, 2009), the membrane accumulating in the encasement can be traced back to the point at which ARA6 and ARA7 attach to the membrane. As for MVBs, Rab5 GTPases are essential for autophagosome formation. These double membrane structures could mediate the secretion of membrane material to the encasement as an alternative pathway (Ao et al., 2014). However, from previous investigations, loss of autophagy leads to increased powdery mildew resistance, rather than susceptibility, which is the opposite effect of the vps9a-2 mutant (Wang et al., 2011). We therefore consider it unlikely that autophagosomes are directly involved in the formation of the encasement. Instead, since ARA7 recently has been suggested recruited to a subdomain of the TGN (Singh et al., 2014), we find that this compartment is the most likely candidate for supplying the encasement with membrane material. Interestingly, we found that the GTP-locked, but not the wild-type versions, of ARA6 and ARA7 are transported into the encasement. We speculate that these QL versions cannot escape the outer membrane of the MVB during its maturation and consequently end up on the intraluminal vesicles, which eventually become exosomes in the encasement. That ARA6QL and ARA7QL readily accumulate in the encasement and not in the papilla underlines that the membranous materials destined for the matrices in the two defense structures are dependent on two unrelated membrane trafficking pathways. Nonetheless, finding that recycled RFP-PEN1 accumulates in both structures shows that the two trafficking pathways receive membrane material from the PM and at least partially overlap. Therefore, we envisage that separation of the membranous material occurs at the TGN, which in plants acts as a major sorting station for both secretory, recycling, and vacuolar membrane trafficking (Figure 7; Richter et al., 2014).
Figure 7.
Membrane Trafficking during Powdery Mildew Attack.
At the TGN, PEN1 plays a predominant role in mediating the membrane transport needed for preinvasive resistance (A), whereas membrane transport dependent on VPS9a ensures correct delivery of material to the encasement, which mediates postinvasive immunity (B). In both scenarios, PEN1 and one or more complementary syntaxin mediate the fusion of MVBs with the plasma membrane.
Based on our observations, we suggest that membrane trafficking to the papilla is mainly delivered by the PEN1 pathway, where recycled material from the PM is directed to the site of attack (Figure 7A). Here, PEN1 initially works at the TGN receiving papilla material from the PM and subsequently at the site of attack fusing MVBs with the PM. This is supported by our previous findings that papilla formation, including GFP-PEN1 accumulation, is severely hampered in the absence of functional GNOM, which acts on the PEN1 pathway (Nielsen et al., 2012; Supplemental Figure 4). It should be noted that although GNOM is considered to work on the recycling endosome (Geldner et al., 2003), there is as yet no direct evidence showing which membrane GNOM acts on in preinvasive immunity. As opposed to the PEN1 pathway, VPS9a plays only a minor role in directing membrane material to the papilla, which would explain why the formation of papillae appears to be almost normal in vps9a-2 and why the effect on preinvasive immunity is relatively weak. In contrast to the papilla, we envisage that the membrane trafficking to the encasement to a larger degree depends on VPS9a (Figure 7B). This is supported by the finding that loss of PEN1 only causes a slight reduction in encasement formation in response to penetration by the poorly adapted sow thistle powdery mildew fungus (Wen et al., 2011). At the same time, this suggests that delivery of material for the encasement does not require PEN1, but likely one or more other PM syntaxin. We envisage that the encasement matrix is filled by a combination of callose and membrane material. As callose deposition seems unaffected in the vps9a-2 mutant, the hampered delivery of membrane material is not able to keep up with the speed at which callose accumulates or is delivered unevenly to the growing rim of the encasement. This would explain why encasements in vps9a-2 forms at a slower rate than in the wild type and results in an aberrant PEN1 accumulation. As our data clearly show that VPS9a-mediated immunity is dependent on GTP-bound ARA7, and not ARA6, we propose that the exchange of GDP with GTP in ARA7 by VPS9a plays a major role in organizing the endosomal membrane trafficking at the TGN and that this is needed for proper plant innate immunity. As mentioned above, ARA7 is thought to be functionally redundant to the second conventional Rab5 GTPase, RHA1. We speculate that this redundancy extends to also cover innate immunity. Interestingly, recent reports point to a clear difference in the composition of ARA6- and ARA7-positive endosomes (Heard et al., 2015) and that plant Rab5 GEFs seem to have evolved a specific domain that regulates ARA6 only (Sunada et al., 2016). Combined, these findings underline a likely difference in ARA6 and ARA7 functionality that we find extends to innate immunity. Previously, we have suggested that PEN1 acts at the TGN in preinvasive immunity (Nielsen and Thordal-Christensen, 2013). Thus, the plant TGN may play an important role in directing membrane trafficking during disease resistance. Yet, it is at this stage difficult to visualize how VPS9a and PEN1 control separate contributions to membrane trafficking toward the site of attack.
In contrast to what has been described for mammals and yeast, in plants, the TGN has taken over the function of the EE in directing material to the vacuole and recycling of PM proteins (Dettmer et al., 2006). Thus, the plant TGN serves as a major sorting station for secretory, vacuolar, and endocytic membrane trafficking (Richter et al., 2014). At the TGN, GTP activation of the conserved Rab5 GTPases, ARA7/RHA1, is crucial for initiation of MVB maturation that ultimately delivers cargo to the vacuole. In addition, activated ARA7/RHA1 is also crucial for establishing cell polarity as defined by the localization of the auxin efflux carriers, PIN1 and PIN2 (Goh et al., 2007; Inoue et al., 2013). Yet, whereas vacuolar trafficking requires Rab5 GTPase enzymatic activity to complete the maturation of MVBs and fusion with the vacuole (Jia et al., 2013), there appears to be no such requirement for PIN localization, as plants expressing GTP-locked versions of ARA6 or ARA7 display normal cell polarity (Goh et al., 2007). Similarly, while activation of the conventional Rab5 GTPase, ARA7, is important for execution of innate immunity, we found that its GTPase enzymatic activity is not required. This indicates that VPS9a-dependent cell polarity and immunity is organized at the TGN and does not appear to involve the downstream events of MVB formation and fusion. However, it remains enigmatic how the GTP-locked ARA7 can contribute to the formation of the encasement-localized exosomes.
Interestingly, both PIN proteins and encasements, in response to fungal invasion, are thought to have evolved early in the history of land plants (Krings et al., 2007; Bennett, 2015). Therefore, it is tempting to speculate that the first land plants co-opted the existing VPS9a-based molecular machinery to facilitate these two major innovations in plant evolution.
METHODS
Plant and Fungal Growth
All experiments were performed using Arabidopsis thaliana ecotype Col-0 plants, which were grown at 21°C, with 8 h of light at 125 μE s−1 m−2 per day. The barley powdery mildew fungus, Bgh (Blumeria graminis f. sp hordei, isolate C15), was propagated on barley (Hordeum vulgare), and the Arabidopsis powdery mildew fungus, Go (Golovinomyces orontii), was propagated on Arabidopsis Col-0 eds1-2. All plant lines have previously been reported (Collins et al., 2003; Geldner et al., 2004; Lipka et al., 2005; Ohtomo et al., 2005; Goh et al., 2007; Ebine et al., 2011; Reichardt et al., 2011). For scoring of penetration success, leaf material was trypan blue stained at 48 h after Bgh or Go inoculation (except for scoring of HR, which was assessed at 72 hai). For each leaf, a minimum of 50 penetration attempts were counted. These were determined by the presence of a fungal appressorium using light microscopy. Penetration was determined by the presence of a fungal haustorium. Callose staining was made using 0.01% aniline blue in 1 M glycine (NaOH, pH 9.5) and visualized by UV epifluorescence. All experiments were repeated a least twice with similar results. Data from the studies described above represent discrete variables, since they record whether or not a certain event had taken place (e.g., whether or not a conidia caused penetration). Consequently, these data were analyzed by logistic regression, assuming a binomial distribution (corrected for overdispersion when present) (Collett, 1991). Hypotheses were rejected at P < 0.001. All data were analyzed by PC-SAS (release 9.4; SAS Institute).
Quantification of Powdery Mildew Fungal Growth by qPCR
Quantification of fungal material was done as previously described (Weßling and Panstruga, 2012) with the following alterations. Seven-week-old plants were used for inoculation with Go. At 5 d after inoculation, four leaf discs (∼0.63 cm2) from four individual plants of each genotype were harvested for DNA extraction using primer combinations R189/R192 and R193/194 for the qPCR.
Fluorescent Protein and Callose Detection Using Confocal Microscopy
Samples were examined using a 63× water immersion lens mounted on a Leica CLSM TCS SP5 confocal microscope. For detection and localization of the fluorophores and stains, GFP was excited at 488 nm and detected between 500 and 520 nm. RFP was excited at 543 nm and detected between 575 and 595 nm. Aniline blue-stained callose was excited at 405 nm and detected between 450 and 495 nm. Imaging data were collected at the Center for Advanced Bioimaging Denmark, University of Copenhagen. Projections of serial confocal sections, image overlays, and contrast enhancement were performed using image processing software (GIMP 2).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: VPS9a (At3g19770), ARA7 (At4g19640), ARA6 (At3g54840), PEN1, (At3g11820), PEN2 (At2g44490), and GNOM (At1g13980).
Supplemental Data
Supplemental Figure 1. GTP-locked Rab5 does not affect preinvasive immunity.
Supplemental Figure 2. Loss of ARA6 does not induce accumulation of ARA7 at Bgh attack sites.
Supplemental Figure 3. SYP22 is not required for preinvasive immunity.
Supplemental Figure 4. GNOM works on the PEN1 pathway in preinvasive immunity.
Supplemental Figure 5. Compromised encasement formation in vps9a-2 is rescued by ARA7QL.
Supplemental Figure 6. Accumulation of GFP-PEN1 in the encasement is altered in vps9a-2.
Supplemental Figure 7. Accumulation of callose is unaffected in vps9a-2.
Supplemental Figure 8. Only GTP-locked Rab5 accumulates in the encasement.
Supplemental Figure 9. vps9a-2 does not display enhanced susceptibility to Go.
Acknowledgments
We thank Takashi Ueda for providing mutant and transgenic lines. This project was supported by grants from the Carlsberg Foundation (2011_01_0789), the Villum Foundation (VKR023502), the Danish Strategic Research Council (10-093504), and the Deutsche Forschungsgemeinschaft (Ju 79/18-1).
AUTHOR CONTRIBUTIONS
M.E.N., G.J., and H.T.-C. designed experiments. M.E.N. performed all experiments and analyses. M.E.N. and H.T.-C. wrote the article.
Glossary
- HR
hypersensitive response
- PM
plasma membrane
- GEF
guanine-nucleotide exchange factor
- TGN
trans-Golgi network
- EE
early endosome
- MVB
multivesicular body
- hai
hours after inoculation
Footnotes
Articles can be viewed without a subscription.
References
- An Q., Hückelhoven R., Kogel K.H., van Bel A.J. (2006). Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell. Microbiol. 8: 1009–1019. [DOI] [PubMed] [Google Scholar]
- Ao X., Zou L., Wu Y. (2014). Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 21: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad F.F., Qiu J.L., Youngs H., Ehrhardt D., Zimmerli L., Kalde M., Wanner G., Peck S.C., Edwards H., Ramonell K., Somerville C.R., Thordal-Christensen H. (2004). The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol. Biol. Cell 15: 5118–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., Zimmermann P., David G. (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14: 677–685. [DOI] [PubMed] [Google Scholar]
- Bednarek P., Pislewska-Bednarek M., Svatos A., Schneider B., Doubsky J., Mansurova M., Humphry M., Consonni C., Panstruga R., Sanchez-Vallet A., Molina A., Schulze-Lefert P. (2009). A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106. [DOI] [PubMed] [Google Scholar]
- Bennett T. (2015). PIN proteins and the evolution of plant development. Trends Plant Sci. 20: 498–507. [DOI] [PubMed] [Google Scholar]
- Caillaud M.C., Wirthmueller L., Sklenar J., Findlay K., Piquerez S.J., Jones A.M., Robatzek S., Jones J.D., Faulkner C. (2014). The plasmodesmal protein PDLP1 localises to haustoria-associated membranes during downy mildew infection and regulates callose deposition. PLoS Pathog. 10: e1004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D.S., Davies B.A., Horazdovsky B.F. (2006). Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol. 16: 27–35. [DOI] [PubMed] [Google Scholar]
- Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. (2009). Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett D. (1991). Modelling Binary Data. (London: Chapman & Hall; ). [Google Scholar]
- Collins N.C., Thordal-Christensen H., Lipka V., Bau S., Kombrink E., Qiu J.L., Hückelhoven R., Stein M., Freialdenhoven A., Somerville S.C., Schulze-Lefert P. (2003). SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425: 973–977. [DOI] [PubMed] [Google Scholar]
- Dangl J.L., Horvath D.M., Staskawicz B.J. (2013). Pivoting the plant immune system from dissection to deployment. Science 341: 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebine K., et al. (2011). A membrane trafficking pathway regulated by the plant-specific RAB GTPase ARA6. Nat. Cell Biol. 13: 853–859. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Kopischke M., Klapprodt C., Hause G., Meyer A.J., Schwarzländer M., Fricker M.D., Lipka V. (2016). Immobilized subpopulations of leaf epidermal mitochondria mediate PENETRATION2-dependent pathogen entry control in Arabidopsis. Plant Cell 28: 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Anders N., Wolters H., Keicher J., Kornberger W., Muller P., Delbarre A., Ueda T., Nakano A., Jürgens G. (2003). The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230. [DOI] [PubMed] [Google Scholar]
- Geldner N., Richter S., Vieten A., Marquardt S., Torres-Ruiz R.A., Mayer U., Jürgens G. (2004). Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 131: 389–400. [DOI] [PubMed] [Google Scholar]
- Goh T., Uchida W., Arakawa S., Ito E., Dainobu T., Ebine K., Takeuchi M., Sato K., Ueda T., Nakano A. (2007). VPS9a, the common activator for two distinct types of Rab5 GTPases, is essential for the development of Arabidopsis thaliana. Plant Cell 19: 3504–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard W., Sklenář J., Tomé D.F., Robatzek S., Jones A.M. (2015). Identification of regulatory and cargo proteins of endosomal and secretory pathways in Arabidopsis thaliana by proteomic dissection. Mol. Cell. Proteomics 14: 1796–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückelhoven R., Panstruga R. (2011). Cell biology of the plant-powdery mildew interaction. Curr. Opin. Plant Biol. 14: 738–746. [DOI] [PubMed] [Google Scholar]
- Inada N., Betsuyaku S., Shimada T.L., Ebine K., Ito E., Kutsuna N., Hasezawa S., Takano Y., Fukuda H., Nakano A., Ueda T. (2016). Modulation of plant RAB GTPase-mediated membrane trafficking pathway at the interface between plants and obligate biotrophic pathogens. Plant Cell Physiol. 57: 1854–1864. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kondo Y., Naramoto S., Nakano A., Ueda T. (2013). RAB5 activation is required for multiple steps in Arabidopsis thaliana root development. Plant Cell Physiol. 54: 1648–1659. [DOI] [PubMed] [Google Scholar]
- Jia T., Gao C., Cui Y., Wang J., Ding Y., Cai Y., Ueda T., Nakano A., Jiang L. (2013). ARA7(Q69L) expression in transgenic Arabidopsis cells induces the formation of enlarged multivesicular bodies. J. Exp. Bot. 64: 2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson O.N., Fantozzi E., Fahlberg P., Nilsson A.K., Buhot N., Tör M., Andersson M.X. (2014). Role of the penetration-resistance genes PEN1, PEN2 and PEN3 in the hypersensitive response and race-specific resistance in Arabidopsis thaliana. Plant J. 79: 466–476. [DOI] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. (2006). The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Kemen E., Jones J.D.G. (2012). Obligate biotroph parasitism: can we link genomes to lifestyles? Trends Plant Sci. 17: 448–457. [DOI] [PubMed] [Google Scholar]
- Krings M., Taylor T.N., Hass H., Kerp H., Dotzler N., Hermsen E.J. (2007). Fungal endophytes in a 400-million-yr-old land plant: infection pathways, spatial distribution, and host responses. New Phytol. 174: 648–657. [DOI] [PubMed] [Google Scholar]
- Kwon C., et al. (2008). Co-option of a default secretory pathway for plant immune responses. Nature 451: 835–840. [DOI] [PubMed] [Google Scholar]
- Lipka V., et al. (2005). Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310: 1180–1183. [DOI] [PubMed] [Google Scholar]
- Lipka V., Kwon C., Panstruga R. (2007). SNARE-ware: the role of SNARE-domain proteins in plant biology. Annu. Rev. Cell Dev. Biol. 23: 147–174. [DOI] [PubMed] [Google Scholar]
- Meyer D., Pajonk S., Micali C., O’Connell R., Schulze-Lefert P. (2009). Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J. 57: 986–999. [DOI] [PubMed] [Google Scholar]
- Nielsen M.E., Feechan A., Böhlenius H., Ueda T., Thordal-Christensen H. (2012). Arabidopsis ARF-GTP exchange factor, GNOM, mediates transport required for innate immunity and focal accumulation of syntaxin PEN1. Proc. Natl. Acad. Sci. USA 109: 11443–11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.E., Thordal-Christensen H. (2013). Transcytosis shuts the door for an unwanted guest. Trends Plant Sci. 11: 611–616. [DOI] [PubMed] [Google Scholar]
- Ohtomo I., Ueda H., Shimada T., Nishiyama C., Komoto Y., Hara-Nishimura I., Takahashi T. (2005). Identification of an allele of VAM3/SYP22 that confers a semi-dwarf phenotype in Arabidopsis thaliana. Plant Cell Physiol. 46: 1358–1365. [DOI] [PubMed] [Google Scholar]
- Reichardt I., Slane D., El Kasmi F., Knöll C., Fuchs R., Mayer U., Lipka V., Jürgens G. (2011). Mechanisms of functional specificity among plasma-membrane syntaxins in Arabidopsis. Traffic 12: 1269–1280. [DOI] [PubMed] [Google Scholar]
- Richter S., Kientz M., Brumm S., Nielsen M.E., Park M., Gavidia R., Krause C., Voss U., Beckmann H., Mayer U., Stierhof Y.D., Jürgens G. (2014). Delivery of endocytosed proteins to the cell-division plane requires change of pathway from recycling to secretion. eLife 3: e02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter B.D., Innes R.W. (2017). Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 173: 728–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.K., Krüger F., Beckmann H., Brumm S., Vermeer J.E., Munnik T., Mayer U., Stierhof Y.D., Grefen C., Schumacher K., Jürgens G. (2014). Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr. Biol. 24: 1383–1389. [DOI] [PubMed] [Google Scholar]
- Stein M., Dittgen J., Sánchez-Rodríguez C., Hou B.H., Molina A., Schulze-Lefert P., Lipka V., Somerville S. (2006). Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18: 731–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Parton R.G., Steele-Mortimer O., Lütcke A., Gruenberg J., Zerial M. (1994). Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13: 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. (2009). Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10: 513–525. [DOI] [PubMed] [Google Scholar]
- Sunada M., Goh T., Ueda T., Nakano A. (2016). Functional analyses of the plant-specific C-terminal region of VPS9a: the activating factor for RAB5 in Arabidopsis thaliana. J. Plant Res. 129: 93–102. [DOI] [PubMed] [Google Scholar]
- Underwood W., Somerville S.C. (2013). Perception of conserved pathogen elicitors at the plasma membrane leads to relocalization of the Arabidopsis PEN3 transporter. Proc. Natl. Acad. Sci. USA 110: 12492–12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Nishimura M.T., Zhao T., Tang D. (2011). ATG2, an autophagy-related protein, negatively affects powdery mildew resistance and mildew-induced cell death in Arabidopsis. Plant J. 68: 74–87. [DOI] [PubMed] [Google Scholar]
- Wen Y., Wang W., Feng J., Luo M.C., Tsuda K., Katagiri F., Bauchan G., Xiao S. (2011). Identification and utilization of a sow thistle powdery mildew as a poorly adapted pathogen to dissect post-invasion non-host resistance mechanisms in Arabidopsis. J. Exp. Bot. 62: 2117–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weßling R., Panstruga R. (2012). Rapid quantification of plant-powdery mildew interactions by qPCR and conidiospore counts. Plant Methods 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]