Abstract
Patient: Male, 49
Final Diagnosis: Pulmonary tumor thrombotic microangiopathy
Symptoms: Cough • dyspnea • fatigue • lightheadedness
Medication: —
Clinical Procedure: Trans-bronchial biopsy • right heart catheterization
Specialty: Pulmonology
Objective:
Rare disease
Background:
Pulmonary arterial hypertension (PAH) results from proliferative vasculopathy involving all layers of the blood vessel. Similar findings may be present in pulmonary hypertension (PH) associated with microscopic tumor embolism, which are thought to be related to the phenomenon of pulmonary tumor thrombotic microangiopathy (PTTM). PTTM is associated with the activation of the coagulation system at the surface of the tumor emboli, resulting in stenosis or occlusion of the vessel.
Case Report:
A 49-year-old man with stage IV gastro-esophageal junction adenocarcinoma presented with complaints of cough and shortness of breath. These symptoms coincided with the initiation of trastuzumab with a new experimental medication with receptor tyrosine kinase blocking activity. A trans-thoracic echocardiogram demonstrated severely increased right ventricle (RV) cavity size with severely decreased RV systolic function. A computed tomography angiography was negative for pulmonary embolism but demonstrated new bilateral pulmonary infiltrates. Bronchoalveolar lavage ruled out an infectious etiology. Trans-bronchial biopsies (TBBx) showed arteriole obliteration by smooth muscle proliferation suggestive of pulmonary vasculopathy. The right heart catheterization (RHC) confirmed severe pulmonary hypertension. Unfortunately, shortly after the RHC, the patient developed pulseless electrical activity cardiac arrest and died. Autopsy results were similar to those of the TBBx, except for diffuse dissemination of tumor cells in the lymphatic channels and small pulmonary vessels, confirming a diagnosis of PTTM.
Conclusions:
We highlight the limitations of trans-bronchial biopsies in evaluating PTTM. The final diagnosis of PTTM was not made until the autopsy was done.
MeSH Keywords: Adenocarcinoma; Biopsy; Bronchoalveolar Lavage; Cardiac Catheterization; Death, Sudden; Pulmonary Embolism
Background
Pulmonary arterial hypertension (PAH) results from proliferative vasculopathy caused by pulmonary vascular remodeling which involves all layers of the blood vessel. Pathologic findings include intimal hyperplasia and fibrosis, medial hypertrophy, and thrombi of the small pulmonary arteries and arterioles with occasional plexiform lesion formation [1,2]. Similar pathologic changes may be present in pulmonary hypertension (PH) associated with microscopic tumor embolism, which is thought to be related to the phenomenon of pulmonary tumor thrombotic microangiopathy (PTTM). Pulmonary tumor embolism is described as direct and entire occlusion of the pulmonary vessel by tumor emboli. In PTTM, tumor emboli do not occlude affected vessels; however, the microscopic tumor cell emboli induce the activation of the coagulation system at the surface of the tumor emboli, with fibrocellular intimal proliferation, resulting in stenosis and occlusion of the vessel [3]. PTTM manifests clinically as subacute respiratory failure with pulmonary hypertension and progressive right ventricular failure due to increased right ventricular afterload, and sudden death [4,5].
We present a case of PTTM developing in a patient with gastro-esophageal (GE) junction adenocarcinoma who was also treated with trastuzumab and a new experimental medication that is a human monoclonal antibody blocking the activity of ErbB3 receptor tyrosine kinase (RTK). This case was challenging in view of the rapid progression of right heart failure and a trans-bronchial biopsy suggesting an alternative diagnosis. We highlight the limitations of trans-bronchial biopsies in evaluating PTTM. The final diagnosis of PTTM was not made until the autopsy was done.
Case Report
A 49-year-old man with stage IV human epidermal growth factor receptor (HER2) positive gastro-esophageal (GE) junction adenocarcinoma presented to the hospital with complaints of cough, shortness of breath, lightheadedness, and increasing fatigue. Two years earlier, when his malignancy was diagnosed, he was initially started on FOLFIRINOX (fluorouracil, leucovorin, oxaliplatin, and irinotecan) with later transition to the single agent trastuzumab (anti-HER2 monoclonal antibody). FOLFOX was added to trastuzumab due to the signs of disease progression on the PET scan. However, restaging scans demonstrated progression of his malignancy; therefore, he was included in a phase 1 clinical trial of a new experimental medication with receptor tyrosine kinase (RTK) blocking activity. He had completed 2 cycles of this medication by the time of his admission to the hospital.
Upon admission, he was found to have hypoxemic respiratory failure. He had mildly elevated troponin T and troponin I at 0.03 ng/ml (normal range: <0.01 ng/mL) and 0.25 ng/ml (normal range: 0.00–0.08 ng/mL), respectively. His EKG showed new T wave inversions in precordial leads, suggestive of anterolateral ischemia. There was also a change of axis from the left axis deviation to the normal axis. A trans-thoracic echocardiogram (TTE) demonstrated severely increased right ventricle (RV) cavity size with severely decreased RV systolic function. McConnell’s sign (RV dysfunction with characteristic sparing of the apex) was noted. RV systolic pressure was estimated at 70 mmHg. Based on these results, a computed tomography angiography was obtained, which was negative for pulmonary embolism but demonstrated multiple nodules in both lungs, with surrounding ground-glass opacities and evidence of tree-in-bud pattern (Figure 1).
Figure 1.
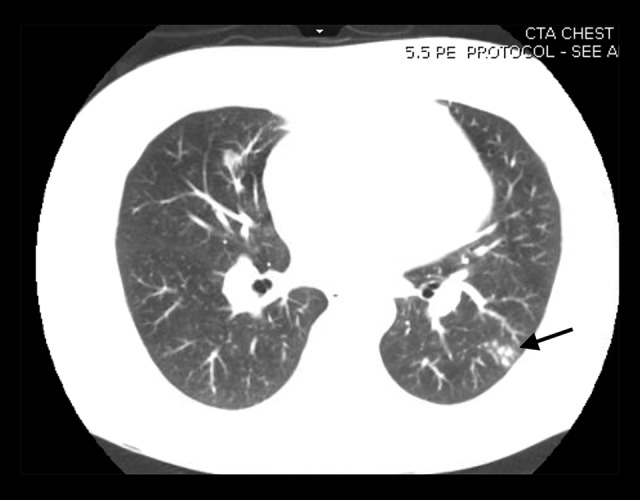
Chest CT image with nodular opacities in the left lower lobe (arrow).
Due to concern for possible fungal or atypical bacterial infection, the patient underwent bronchoscopy with bronchoalveolar lavage (BAL). BAL stains and cultures ruled out an infectious etiology. After prolong discussion with the family, who opted to proceed with the trans-bronchial biopsies due to concerns for possible drug-related lung injury rather than further advancement of malignancy, the procedure was performed with enormous caution and vigilance due to the newly diagnosed pulmonary hypertension, which in general is considered a contradiction for the procedure. The patient was also empirically started on treatment with prednisone 1 mg/kg daily due to concern for possible drug-related lung injury. Trans-bronchial biopsies showed arteriole obliteration by smooth muscle proliferation, consistent with pulmonary vasculopathy (Figure 2A) and suggestive of pulmonary arterial hypertension (PAH) [1,2].
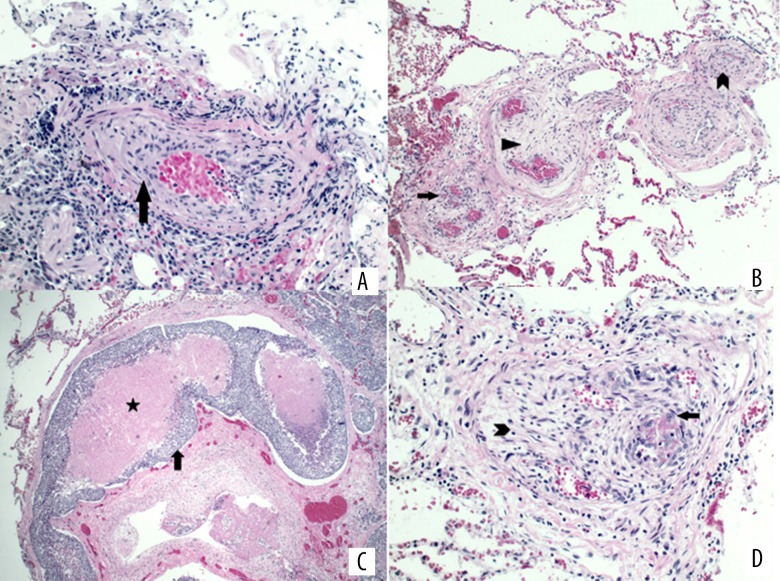
Figure 2.
(A) Pathology of the trans-bronchial biopsy shows arteriole obliteration by smooth muscle proliferation (arrow), consistent with pulmonary vasculopathy. (B) Postmortem lung pathology shows arteriolar smooth muscle proliferation (arrowhead) and fibrosis (triangle) with recanalization of thromboemboli (arrow). Recanalization features multiple small vascular channels containing red blood cells embedded in the fibrotic tissue. (C) Representative pathology of the postmortem lung outlines a distended lymphatic channel filled by tumor thromboembolus (arrow, clumps of blue cells) with necrotic center (star, pink area). (D) Pathology of postmortem lung shows arteriolar myofibroblastic proliferation (arrowhead) in small pulmonary vessels. Also noted are tumor cells (arrow, blue cells with enlarged nuclei) forming thrombi in recanalized lymphatic channels.
PAH was previously reported in patients receiving dasatinib, which can be reversed following discontinuation of the therapy [6]. Due to the RTK-blocking activity of the experimental medication, it was argued at the time that it may have played a role in the pathogenesis of this patient’s condition, in a similar way that dasatinib causes pulmonary vasculopathy. Given the elevated estimated (by echo) RV pressure with hemodynamic stability at the time, the patient was planned for consideration of initiation of infused prostanoid analogues therapy and thus required right heart catheterization (RHC). The RHC confirmed severe pulmonary hypertension with mean pulmonary artery pressure of 70 mmHg and a pulmonary vascular resistance of 20 Wood units. A vasodilatory challenge test was not done because of his low cardiac index (1.25 liters/minute/m2). Cytologic examination of blood aspirated from the pulmonary artery catheter (not in a wedged inflated balloon position) showed presence of circulating tumor cells.
Unfortunately, shortly after the RHC, the patient developed pulseless electrical activity cardiac arrest and died after resuscitation efforts were unsuccessful. Autopsy results confirmed dilated cardiomyopathy of the right ventricle, and postmortem lung pathologies showed arteriolar smooth muscle proliferation and fibrosis with recanalization of thromboemboli (Figure 2B), and the precise thickness of the RV was measured at 8 mm. These findings are largely similar to those of the trans-bronchial biopsies, except for diffuse dissemination of tumor cells in the lymphatic vessels (Figure 2C) and small pulmonary vessels (Figure 2D), consistent with PTTM [3–5].
Discussion
PTTM is a very rare complication of metastatic cancer. Unfortunately, it is difficult to identify clinically and is predominantly diagnosed postmortem [7]. Evidence of tree-in-bud pattern on the chest CT was very evocative of PTTM in the setting of PH [8], but infectious processes were also high on the differential in this immunocompromised patient undergoing chemotherapy. In this case, trans-bronchial biopsies were suggestive of PAH without any evidence of lymphangitic tumor spread. Moreover, the patient had a history of being treated with RTK-blocking medications. Tyrosine kinase inhibitors (TKI) are known for their inhibition of platelet-derived growth factor (PDGF), which is known to be implicated in the development of PAH [3,4]. The trans-bronchial biopsies could not rule out this possibility (drug-associated pulmonary vascular disease), which turned out (postmortem) to not be the case.
Taking into consideration all the differentials at the time, we performed cytologic examination of blood aspirated from the pulmonary artery catheter, which confirmed the presence of circulating tumor cells. Unfortunately, due to the rapidly progressive nature of the disease, the patient died right after the RHC. Even if he had been properly diagnosed, this is almost uniformly fatal with a background of advanced malignancy that does not respond to first-line therapies. Recent reports give us hope that with the appropriate clinical suspicion, the outcomes may be less dismal in a select group of patients [9]. The data on the effective treatment of pulmonary tumor emboli is insufficient but it is generally directed at the primary tumor. Different proposed therapies, such as combination chemotherapy with dexamethasone, warfarin, and aspirin, and pulmonary vasodilators, do not improve the prognosis under such circumstances, but may improve patient symptoms [4,5,7].
In this perplexing and unfortunate case, PTTM was missed on the trans-bronchial biopsy but confirmed on autopsy. Diagnosing PTTM on trans-bronchial biopsies would not have changed the inevitable outcome of this case, as the patient rapidly deteriorated and there are no known effective therapies, especially in the setting of such an advanced aggressive malignancy.
Conclusions
It is important to consider PTTM as a differential diagnosis for rapidly progressive respiratory failure in patients with metastatic tumors. Antemortem diagnosis of pulmonary tumor embolism may be difficult to establish, and we show that even trans-bronchial biopsies may be misleading.
References:
- 1.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 3.von Herbay A, Illes A, Waldherr R, Otto HF. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587–92. doi: 10.1002/1097-0142(19900801)66:3<587::aid-cncr2820660330>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Price LC, Wells AU, Wort SJ. Pulmonary tumour thrombotic microangiopathy. Curr Opin Pulm Med. 2016;22:421–28. doi: 10.1097/MCP.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 5.Kuwabara H, Yoshida S, Takasu T, et al. Pulmonary tumor thrombotic microangiopathy caused by gastric cancer. Pulmonary tumor thrombotic microangiopathy caused by gastric cancer. Ann Thorac Med. 2012;7:168–69. doi: 10.4103/1817-1737.98853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumitrescu D, Seck C, ten Freyhaus H, et al. Fully reversible pulmonary arterial hypertension associated with dasatinib treatment for chronic myeloid leukaemia. Eur Respir J. 2011;38:218–20. doi: 10.1183/09031936.00154210. [DOI] [PubMed] [Google Scholar]
- 7.Patrignani A, Purcaro A, Calcagnoli F, et al. Pulmonary tumor thrombotic microangiopathy: The challenge of the antemortem diagnosis. J Cardiovasc Med (Hagerstown) 2014;15:828–33. doi: 10.2459/JCM.0b013e328354e473. [DOI] [PubMed] [Google Scholar]
- 8.Franquet T, Giménez A, Prats R, et al. Thrombotic microangiopathy of pulmonary tumors: a vascular cause of tree-in-bud pattern on CT. Am J Roentgenol. 2002;179:897–99. doi: 10.2214/ajr.179.4.1790897. [DOI] [PubMed] [Google Scholar]
- 9.Merad M, Savale L, Antoun S, Vincent F. Is there hope of improving the prognosis of pulmonary tumour thrombotic microangiopathy? Eur Respir J. 2016;47:688–90. doi: 10.1183/13993003.01276-2015. [DOI] [PubMed] [Google Scholar]