Abstract
Objective
Whether sex differences contribute to the heterogeneity of mild traumatic brain injury (mTBI) and repeated mTBI (RmTBI) outcomes in adolescents is unknown. Therefore, this study examined changes in, and differences between, male and female rats following single mTBI and RmTBI.
Methods
Rats were given a single mTBI, RmTBI (i.e., 3x), or sham injuries. Injuries were administered using a lateral impact model that mimics forces common in human mTBI. After the final injury, rats underwent extensive behavioral testing to examine cognition, motor function, and anxiety‐ and depressive‐like behavior. Postmortem analyses investigated gene expression and structural changes in the brain.
Results
Many of the outcomes exhibited a sex‐dependent response to RmTBI. While all rats given RmTBI had deficits in balance, motor coordination, locomotion, and anxiety‐like behavior, only male rats given RmTBI had short‐term working memory deficits, whereas only females given RmTBI had increased depressive‐like behavior. Volumetric and diffusion weighted MRI analyses found that while RmTBI‐induced atrophy of the prefrontal cortex was greater in female rats, only the male rats exhibited worse white matter integrity in the corpus callosum following RmTBI. Sex‐dependent changes in brain expression of mRNA for glial fibrillary acidic protein, myelin basic protein, and tau protein were also observed following injury.
Interpretation
These findings suggest that in adolescent mTBI, sex matters; and future studies incorporating both male and females are warranted to provide a greater understanding of injury prognosis and better inform clinical practice.
Introduction
Traumatic brain injury is one of the leading causes of death and disability with mild traumatic brain injury (mTBI) comprising the greatest proportion of this endemic.1 For children and adolescents, mTBI resulting from sports‐related events and accidental falls, are the most common form of neurological insult, accounting for approximately 80–90% of all brain injuries.2 Although many of these children recover without incident, a significant portion, especially those exposed to repeated mTBIs (RmTBI), go on to suffer from lingering symptoms (i.e., postconcussion syndrome; PCS).3 Despite extensive and ongoing research, clinicians still struggle with PCS prognosis, with no reliable process for distinguishing between those that will develop PCS and those that will not. Maturational brain changes that typify childhood and adolescent development,4, 5, 6 along with significant sex differences in neurological organization and function,7, 8, 9 likely contribute to the heterogeneity in injury outcomes and some of the difficulty associated with determining prognosis. To date, a majority of the animal and human studies that examine mTBI and RmTBI have focused on adult males,10 creating a void in the literature with respect to the female brain and the role of maturation.
As males and females often report different PCS symptoms,11, 12 understanding whether or not underlying structural or epigenetic changes following mTBI and RmTBI contribute to these heterogeneous outcomes is an important undertaking. Owing to sex differences in brain maturation,7 an injury at a given age has the potential to affect the developmental trajectory of males and females in distinct ways. Further complicating this picture, the impact of repeated injuries would in theory, also be affected by sex‐dependent variations in brain maturation and the structural organization that existed prior to the initial injury.13 Given the inherent difficulty that studying these phenomena in a medical setting presents, animal models provide a practical platform for initial assessments and investigations that may inform clinical practice and provide a greater understanding of outcome heterogeneity and injury prognosis.
Utilizing an innovative modeling platform to induce mTBIs that mimicked the acceleration/deceleration and rotational forces often observed in sports‐related concussion,14, 15 this study aimed to examine the behavioral, gene expression, and structural changes associated with mTBI and RmTBI in adolescent rats of both sexes. Behavioral changes were assessed with a battery of tests previously demonstrated to evaluate symptomology consistent with PCS, including acute loss of consciousness (LOC), deficits in balance and motor coordination, anxiety, short‐term working memory, and depressive‐like behaviors.16 Changes in corpus callosum (CC) and prefrontal cortex (PFC) expression of glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), neurofilament light (NFL), and tau protein (Tau) were examined. Structural changes in the CC and PFC were assessed using volumetric and diffusion weighted magnetic resonance imaging (MRI).
Materials and Methods
Animals and mTBI Induction
Each of the experiments was carried out in accordance with the Canadian Council of Animal Care and approved by the University of Calgary Conjoint Facilities Research Ethics board. All 85 (44 Male, 41 Female) animals were in‐house bred and maintained on a 12 h:12 h light/dark cycle, with ad libitum access to food and water. Animals were weaned at postnatal day 21 (P21) and housed in same‐sex groups of four. Animals were randomly assigned to either: single mTBI; RmTBI, consisting of three mTBIs; and sham controls. Sample size calculations were based on our previous behavioral and MRI studies (α = 0.05, power = 80%, effect size ≥ 20%).17, 18, 19 The mTBI(s) were induced with the lateral impact device as described elsewhere.14, 20 Briefly, animals were lightly anesthetized (~30 sec, until nonresponsive to a toe pinch) and placed chest down with the left side of their head facing the impactor. Using a pneumatic barrel, a 100 g weight was accelerated at 5.48 m/sec (±0.14) toward a small ‘helmet’ on the rat's head, producing an impact at 20.29 G, which propelled the rat into a 180° rotation. Following the mTBI or sham injury, the rat received a topical administration of lidocaine to the head. The lateral impact device induces lateral acceleration/deceleration and rotational forces that produce an injury that mimics mTBI.15 Animals in the single mTBI group (15 Male, 14 Female) received an mTBI at P30 and were briefly anesthetized on P34 and P38, animals in the RmTBI group received three mTBIs at P30, P34, and P38 (15 Male, 14 Female), while animals in the sham injury group were briefly anesthetized at P30, P34 and P38, but did not experience the mTBI (14 Male, 13 Female). There were no cases of mortality resulting from the mTBI, and no rats were excluded from the study. Following each sham injury or mTBI(s), the time each rat took to right itself in the recovery cage was recorded as the time‐to‐right. The age range selected for the RmTBIs in rodents represents late‐childhood/early adolescence, while the behavioral outcomes span the adolescent period.21, 22 This time period is particularly important for investigation given the large degree of brain maturation occurring in this time period, specifically in white matter development and functional connectivity, of brain regions like the CC and PFC.23, 24, 25, 26, 27
Behavioral testing
Researchers blinded to the experimental conditions performed all behavioral testing.
Beam walking
Animals were tested in the beam‐walking paradigm on P31 and P39 (i.e., 24 h after the first injury and 24 h after the third injury). The rats were required to traverse a tapered beam (165 cm long), moving from wide to narrow, ending at the rat's home‐cage. The tapered beam was suspended 1 m in the air, but was equipped with ‘safety ledges’ (2 cm wide) that caught the rat's foot if it slipped while walking. Rats were given a single trial to learn the task, and four additional videotaped trials that were scored for the number of hind‐leg foot slips.
Open field
Forty‐eight hours following the third injury (i.e., postinjury day 2; PID2), animals were tested in the open field arena as a measure of overt locomotor activity and exploratory behavior. Rats were placed in the center of the open field (diameter 135 cm) and were permitted to explore the environment for 10 min. An overhead camera was used to track the rat's movement (distance travelled and speed of travel).
Elevated plus maze
Rats were tested in the EPM 72 h following the third injury (i.e., postinjury day 3; PID3). The EPM is a well‐established behavioral test for anxiety in rodents.28 The EPM was elevated 55 cm above ground and contained two open arms and two closed arms. Rats were tested for five‐minute sessions in a brightly lit room, and the amount of time spent in the center of the platform and the amount of time in the open arms was recorded.
Novel context mismatch
Rats were tested in the NCM paradigm from postinjury days 6–9 (PID6‐9). The NCM task is a measure of short‐term working memory that is dependent upon PFC function. In the learning phase of the test (PID6‐8) the rat learns two distinct contexts: A and B. They are placed in context A for 5 min and then moved to context B for 5 min before being place back into their home cage. Context A is comprised of an opaque circular enclosure with two identical objects inside, while context B is a clear rectangular box with a different pair of identical objects inside. The fourth day is used as the probe day, in which the animal is placed in context A for 5 min, then context B for 5 min, followed by their home cage for 5 min. Lastly, they are placed in the probe context C for 5 min, which is comprised of either box A or B, as well as one object from each box. This context C is videotaped and time spent with the novel and familiar object is measured.29
Forced swim paradigm
On postinjury day 12 (PID12), rats were tested in a FS task to measure depressive‐like behavior. A cylindrical tank (diameter: 30 cm; height: 60 cm) was filled with water (~25°C) to a level that did not allow the rat's tail to touch the bottom of the tank. Each testing session lasted seven minutes and the amount of time each rat spent immobile was recorded.
RT‐qPCR analysis
Once behavioral testing was complete (i.e., P52), all of the rats were euthanized. Half of the animals were used for gene expression analysis; rats (n = 6/group) were anaesthetized, weighed, and rapidly decapitated. Brain tissue from the CC and PFC was removed and flash frozen on dry ice. RNA was extracted from the CC and PFC using the RNA/DNA Mini kit according to the manufacturer's protocols (Qiagen, Germany). Quantitative real time PCR (qRT‐PCR) was performed on cDNA synthesized from the CC and PFC RNA. The expression of four genes were investigated: GFAP, which has been used as an estimate of astrocyte activation and is considered a characteristic marker of brain injury;30 MBP, a myelin protein essential for normal myelination and axonal signal conduction31 which may be vulnerable to proteolysis following TBI;32 NFL, exclusive to neurons and involved in maintaining neuronal shape and function.33, 34; and Tau, a highly soluble microtubule‐associated protein that is an established biomarker of axonal injury after mTBI.35 Primer sequences and cycling parameters for GFAP, MBP, NFL, and Tau can be found in detail in prior studies carried out in this laboratory.28 Ten nanograms of cDNA with 0.5 μM of each of the forward and reverse primers and 1x SYBR Green FastMix with Rox was used for qRT‐PCR analysis on the CFX Connect‐Real‐Time PCR Detection system (BioRad, Hercules, CA). Each sample was tested in duplicate and PCR efficiency was between 91.5 and 105.8%.
MRI
The other half of the animals (n = 5–7/group), were deeply anesthetized with sodium pentobarbital and transcardially perfused with phosphate‐buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Whole brains were embedded in 2–3% agar for scanning with a 4.7 T Bruker scanner (Bruker™, Ettlingen, Germany Biospin®, USA). T2*‐weighted images were acquired with a multiple gradient echo sequence with the following imaging parameters: repetition time (TR) = 8 sec; number of echoes = 12; echo time (TE) = 15, 22.5…97.5 msec; matrix size = 144 × 128; number of slices = 74; resolution = 160 × 160 × 160 μm3; and number of excitations (NEX) = 2. Diffusion weighted imaging (DWI) was performed using a 2D echo planar imaging sequence with eight segments and the following imaging parameters: TR = 10 sec; TE = 35 msec; matrix size = 96 × 96; number of slices = 38; and resolution = 300 × 300 × 300 μm3. Diffusion weighting was performed in 81 directions with duration (δ) = 6 msec, gradient separation (Δ) = 17 msec and b‐value = 5000 s/mm2. Two volumes without diffusion‐weighting (b 0) were also acquired.
T2*‐weighted image analysis was performed as described previously.19 Individual echoes were averaged and template images were generated for each cohort using Advanced Normalization Tools (ANTs, http://stnava.github.io/ANTs/). The resulting templates were then combined to create a study‐specific template. The ipsilateral and contralateral CC and PFC were delineated on the study‐specific template, transformed into subject space, and the total volumes calculated for each.
DWI preprocessing was performed using the MRtrix software package (www.mrtrix.org) as described previously.19, 36 Images were corrected for spatial intensity in homogeneity, normalized, upsampled by a factor of two and the fiber orientation distribution (FOD) estimated for each voxel. As in the T2*‐weighted image analysis described above, a study‐specific FOD template was constructed from FOD template images generated for each cohort.
Tractograms were generated for each rat, registered to the study‐specific FOD template, and three track‐weighted images were then generated using properties of the tractogram streamlines: track density imaging (TDI), which maps the total number of streamlines passing through each voxel; average pathlength mapping (APM), which maps the mean length of each streamline traversing the voxel; and mean curvature, which maps the mean curvature of each streamline traversing the voxel.19 Diffusion tensor metrics including fractional anisotropy (FA), apparent diffusion coefficient (ADC), radial diffusivity (RD) and axial diffusivity (AD) were also generated for each subject and transformed into the study‐specific template space using MRtrix. The mean FA image was calculated and the same four regions of interest (ROIs) including the ipsilateral and contralateral CC and PFC were outlined for analysis of each of the diffusion metrics.
Statistical analyses
All outcomes were analyzed with SPSS 24.0 software (IBM Corp, Armonk, NY). Time‐to‐right and beam walking data was analyzed by mixed design analysis of variance (ANOVA) with injury and sex as between subject factors and time as a within subject factor. All other data was analyzed by two‐way ANOVA, with injury and sex as the between‐subjects factors. Bonferroni post hoc comparisons were carried out when appropriate. Statistical significance was set as P ≤ 0.05.
Results
mTBI validation and behavioral outcomes
At the time of injury induction, there were no significant differences in body weight between males and females (Males; 136.44 ± 11.13 g, Females; 117.00 ± 9.23 g). The induction of the mTBIs was confirmed with the time‐to‐right and acute measure of balance and motor coordination in the beam walking task. Sex differences in behavioral performance were identified in the beam‐walking task, NCM, and FS paradigm following RmTBI.
Time‐to‐right
A mixed design ANOVA demonstrated a significant effect of time (F 1,80 = 9.50, P < 0.01), and a significant time by injury effect (F 2,30 = 5.24, P < 0.01). Post hoc analyses indicated that following the induction of the first injury at P30, animals in both the single mTBI and RmTBI group exhibited significant increases in the time‐to‐right when compared to sham animals (Fig. 1A). Following induction of the third injury for the RmTBI group at P38 (both the sham and single mTBI animals were anesthetized but did not experience an injury), animals in the RmTBI group exhibited a significant increase in time‐to‐right that was not present in the single mTBI or sham group. The mTBI group also had a longer righting reflex on P30 (i.e., after an mTBI) compared to P38 (i.e., after a sham injury; P < 0.05). There was no difference within the RmTBI group on P30 (i.e., after the first mTBI) compared to P38 (i.e., after the third mTBI).
Figure 1.
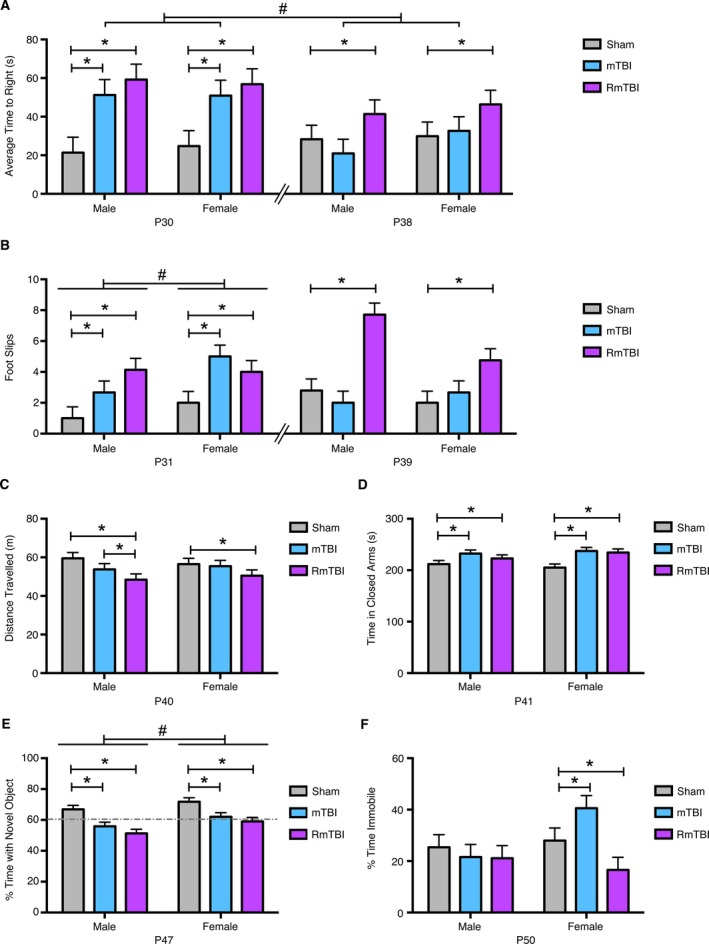
Behavioral performance following the mTBI(s). (A) At P30 animals in the single mTBI group and the repetitive mTBI (RmTBI) group were administered injuries, with both groups taking significantly longer to wake from anesthetic than the sham control group (*P < 0.01). At P38, the RmTBI group experienced their third injury while the sham and mTBI group were only anesthetized. At P38, sham and mTBI animals were indistinguishable, but RmTBI took significantly longer to wake (*P < 0.01). The mTBI group also had a longer righting reflex on P30 (i.e., after an mTBI) compared to P38 (i.e., after a sham injury; # P < 0.05). (B) At P31, animals in the mTBI and RmTBI group were significantly impaired (*P < 0.05), with females also displaying more slips than males on the beam task (# P < 0.01) while at P39, only animals in the RmTBI group displayed performance impairment on the beam task (*P < 0.05). (C) Males exhibited a linear reduction in locomotor behavior in the open field following mTBI and RmTBI whereas females only displayed locomotor reductions following RmTBI (*P < 0.01). (D) Injured animals spent longer in the closed arm of the EPM irrespective of sex or injury paradigm (*P < 0.01). (E) In the NCM, performance was impaired following both mTBI and RmTBI (*P < 0.01), but males exhibited a significantly greater reduction in performance than their female counterparts (# P < 0.01). (F) Time immobile in the FS was altered in females only, whereby mTBI increased immobility time, but RmTBI significantly decreased it (*P < 0.05). Bar graphs show mean + SEM.
Beam walking
Hind‐leg foot slips on the beam walking task was measured on P31 and P39 (i.e., 24 h following the first and third injuries). The mixed design ANOVA demonstrated a significant time by sex effect (F 2,30 = 22.18, P = 0.05), and a significant time by injury effect (F 2,30 = 21.54, P < 0.05). Post hoc analyses indicated that animals in the mTBI and RmTBI groups exhibited significantly more hind‐leg foot slips than the sham animals at P31 (P < 0.05), with females also exhibiting significantly more impairment than males (P < 0.01; Fig. 1B). On P39, the RmTBI group exhibited significantly more hind‐leg foot slips than the sham and mTBI animals (P < 0.05). There were also nonsignificant trends suggesting that the RmTBI group had more slips on P39 (i.e., after the third mTBI) compared to P31 (i.e., after the first mTBI; P = 0.078), and that the mTBI group had more slips on P31 (i.e., after an mTBI) compared to P39 (i.e., after a sham injury; P = 0.061).
Open field
The average distance travelled for animals in the open field was measured on PID2 (i.e., P40). The two‐way ANOVA showed a main effect of injury (F 2,80 = 3.14, P < 0.05), with post hoc analyses identifying significant differences between the sham and RmTBI group, and sham and mTBI for males, but only between sham and RmTBI for the females (Fig. 1C).
Elevated plus maze
On PID3 (i.e., P41) animals were tested for anxiety like behaviors in the EPM (Fig. 1D). The two‐way ANOVA demonstrated a main effect of injury (F 2,80 = 5.21, P < 0.01), with post‐hoc analyses indicating that mTBI and RmTBI groups exhibited increased anxiety, spending significantly longer in the closed arms of the EPM than the sham groups. This finding was further validated by time spent in the open arms; the two‐way ANOVA demonstrated a main effect of injury (F2, 80 = 3.97, P = 0.03; data not shown). There were no effects of injury for males or females with respect to time spent in the center of the EPM (P's > 0.05; data not shown).
Novel context mismatch
When tested in the NCM on PID9 (i.e., P47) both males and females demonstrated impairment in working memory following the mTBI and RmTBI, however post hoc analyses revealed that the impairment was significantly more severe for males (Fig. 1E). The two‐way ANOVA demonstrated a main effect of injury (F 2,80 = 11.29, P < 0.01) and of sex (F 1,84 = 7.25, P < 0.01).
Force swim paradigm
All animals were tested in the FS paradigm on PID12 (i.e., P50) and it was found that only females were significantly affected by the mTBI and RmTBI, with a single injury increasing the percentage of time spent immobile but the RmTBI associated with a decrease in the time spent immobile (Fig. 1F). The two‐way ANOVA demonstrated a significant sex by injury interaction (F 2,80 = 3.82, P < 0.05).
Epigenetic changes in gene expression
Examination of the qRT‐PCR results demonstrated that changes in expression of GFAP, NFL, and Tau were more pronounced in RmTBI animals as compared to the sham and mTBI groups. Sex‐differences in GFAP and Tau expression were identified in the CC for each TBI group, but just for GFAP in the PFC.
GFAP
Two‐way ANOVA revealed a main effect of injury (F 2,18 = 11.75, P < 0.01), and sex (F 1,23 = 28.58, P < 0.01), on CC GFAP expression with post hoc analyses identifying significantly increased expression in male mTBI and female RmTBI groups when compared to their sham‐injured counterparts (Fig. 2A). The sex by injury interaction did not reach significance (F 2,18 = 2.82, P = 0.08). GFAP expression in the PFC was significantly elevated in both sexes following RmTBI (Fig. 2B). The two‐way ANOVA demonstrated a main effect of injury (F 2,18 = 20.03, P < 0.01), sex (F 1,23 = 15.71, P < 0.01), and a significant interaction (F 2,18 = 9.85, P < 0.01). Post hoc analyses revealed significantly elevated GFAP expression in both males and females following RmTBI, and while mTBI also increased GFAP expression in female rats, it was significantly decreased following mTBI in males.
Figure 2.
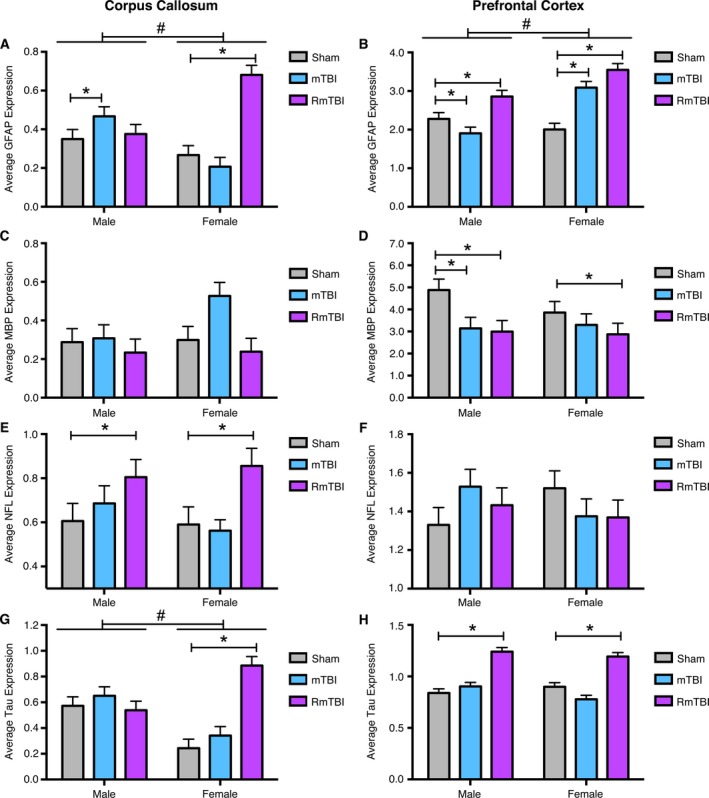
Changes in gene expression in the corpus callosum (CC) and prefrontal cortex (PFC) for GFAP, MBP, NFL, and Tau. (A) Significant sex differences were identified in GFAP expression in the CC (# P < 0.01) with male mTBI and female repetitive mTBI (RmTBI) groups having significantly elevated GFAP expression compared to their sham counterparts (*P < 0.05). (B) There were also sex differences in GFAP expression in the PFC (# P < 0.01). Both males and females exhibited significantly elevated GFAP expression following RmTBI, but while mTBI also increased GFAP expression in female rats, it was significantly decreased following mTBI in males (*P < 0.05). (C) MBP was unaltered in the CC of males and females. (D) In the PFC,MBP expression was reduced in males following mTBI and RmTBI, and in females following RmTBI (*P < 0.05). (E) NFL was elevated in the CC of both sexes following RmTBI (*P < 0.05), but was not altered in the (F) PFC. (G) Finally, in addition to elevated Tau expression in the CC of females following RmTBI (*P < 0.05), sex differences were identified across all injury groups for this brain region (# P < 0.05). (H) Tau expression was also significantly elevated in the PFC of both males and females following RmTBI (*P < 0.05) but not in response to mTBI. Bar graphs show mean + SEM.
MBP
The two‐way ANOVA of MBP expression in the CC failed to identify significant main effects, or sex by injury interaction (Fig. 2C). MBP expression was reduced in the PFC of males following mTBI and RmTBI, and in females following RmTBI (Fig. 2D). The two‐way ANOVA demonstrated a main effect of injury (F 2,18 = 3.34, P < 0.05).
NFL
The two‐way ANOVA demonstrated a main effect of injury (F 2,18 = 4.71, P < 0.05) on NFL expression in the CC (Fig. 2E). Post hoc analyses revealed significantly elevated NFL expression in both sexes following RmTBI. There were no main effects, nor significant interaction, of NFL in the PFC (Fig. 2F).
Tau
The two‐way ANOVA demonstrated a main effect of injury (F 2,18 = 8.31, P < 0.01), and sex (F 1,23 = 26.93, P < 0.01), and post hoc analyses revealed significantly elevated Tau expression in the female RmTBI group (Fig. 2G). In the PFC, two‐way ANOVA demonstrated a main effect of injury (F 2,18 = 13.19, P < 0.01) and post hoc analyses revealed significantly elevated Tau expression in the PFC of both sexes following RmTBI (Fig. 2H).
MRI
Volumetrics
As shown in Figure 3, an ROI analysis that included the ipsilateral and contralateral PFC and CC was completed to examine for evidence of brain atrophy. Two‐way ANOVA identified a significant interaction between injury and sex in the ipsilateral PFC (F 2,30 = 4.511, P < 0.05; Fig. 3B). Post hoc analyses indicated that the female RmTBI group had significantly reduced volume compared to the female sham group, the female mTBI group, and the male RmTBI group, whereas the male RmTBI group did not significantly differ from either of the other male groups. Two‐way ANOVA also identified a significant effect of sex in the contralateral CC (F 1,30 = 5.986, P < 0.05), with males having larger volumes (Fig. 3F).
Figure 3.
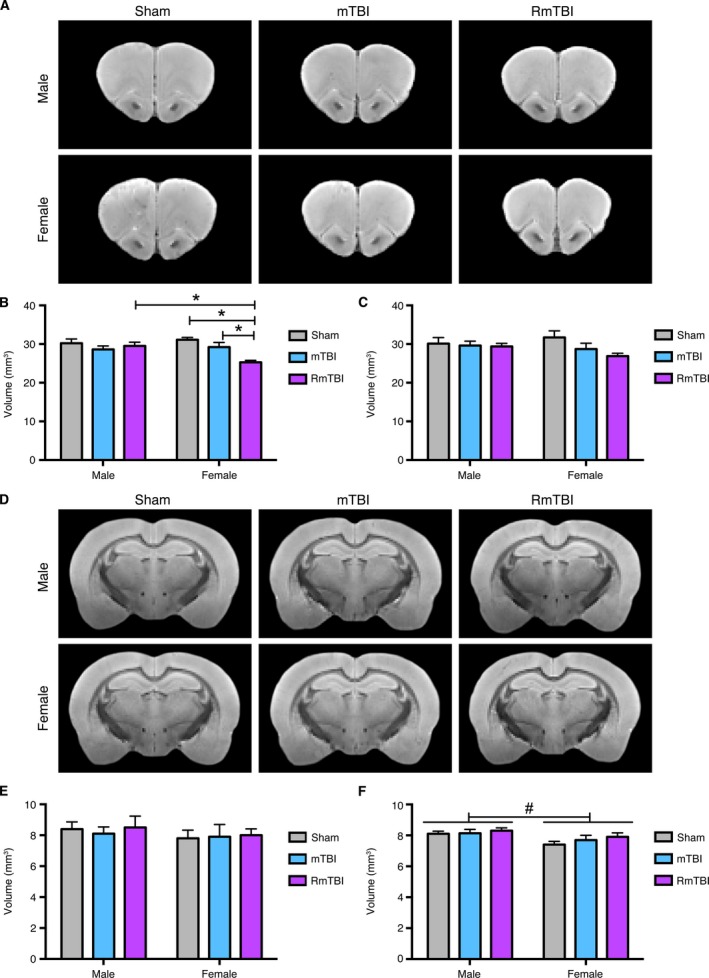
Volumetric MRI analyses of the prefrontal cortex (PFC) and corpus callosum (CC). Template T2*‐weighted structural images showing the (A) PFC and (D) CC in male and female rats given sham, single, or repetitive mTBIs (RmTBI). (B) Female RmTBI rats had reduced ipsilateral PFC volume compared to the female sham and single mTBI groups, as well as the male RmTBI group (*P < 0.05), whereas the male RmTBI group did not significantly differ from either of the other male groups. There were no statistically significant findings in the contralateral (C) PFC or (E) ipsilateral CC. (F) There was a significant effect of sex in the contralateral CC, with males having larger volumes (# P < 0.05). Bar graphs show mean + SEM.
Diffusion MRI
An ROI analysis that included the ipsilateral and contralateral CC and PFC was also completed using TWI (i.e., curvature, APM, and TDI) and DTI (i.e., FA, ADC, AD, and RD) measures. Two‐way ANOVA identified a significant interaction between injury and sex in the ipsilateral CC (F 2,30 = 3.805, P < 0.05) on the measure of curvature (Fig. 4B). Post‐hoc analyses found that the male RmTBI group had significantly reduced curvature compared to the male sham‐injured, male mTBI, and female RmTBI groups, whereas there were no significant differences among the female groups. Two‐way ANOVA also found a significant effect of sex in the contralateral CC on the measures of curvature (F 1,30 = 5.655, P < 0.05; Fig 4C) and APM (F 1,30 = 5.598, P < 0.05), with males having significantly lower curvature and APM values than females. There was also a significant effect of injury in the contralateral CC on the measures of AD (F 2,30 = 3.758, P < 0.05; Fig. 4D) and ADC (F 2,30 = 3.510, P < 0.05; Fig. 4E), with the RmTBI group having significantly lower AD and ADC values than the sham‐injured group. With regards to the ipsilateral PFC, two‐way ANOVA found a significant effect of injury on the measure of FA (F 2,30 = 6.517, P < 0.005) with the RmTBI rats having significantly reduced FA values compared to both the sham and mTBI rats.
Figure 4.
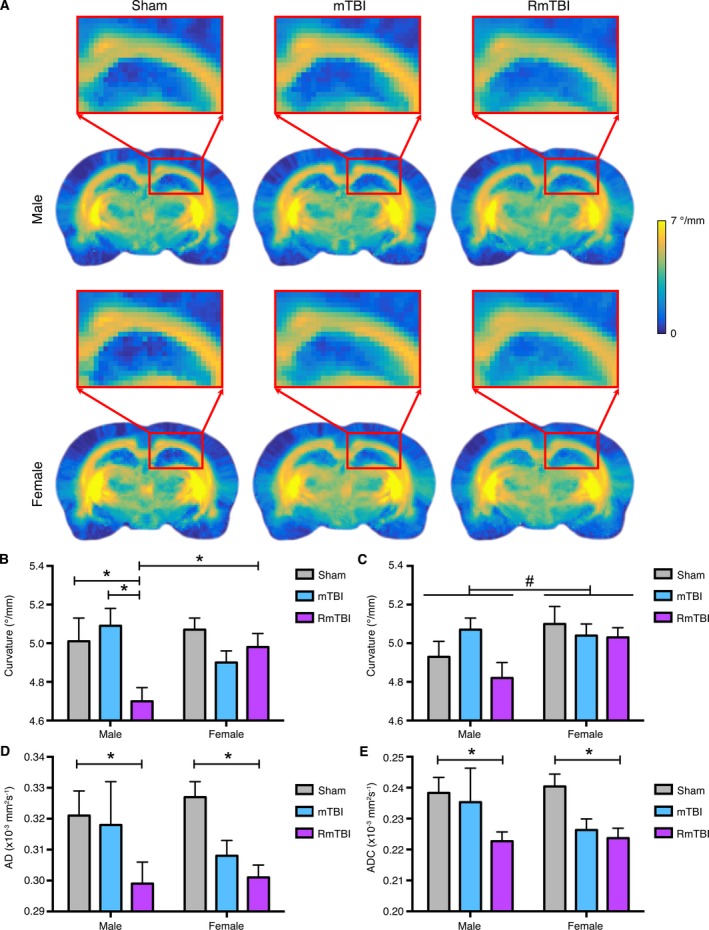
Diffusion weighted MRI analysis of the prefrontal cortex (PFC) and corpus callosum (CC). (A) Template curvature images showing the CC in male and female rats given sham, single, or repetitive mTBIs (RmTBI). (B) Male RmTBI rats had significantly reduced curvature in the ipsilateral CC compared to the male sham and single mTBI groups, as well as the female RmTBI group (*P < 0.05), whereas there were no significant differences among the female groups. (C) There was also a significant effect of sex in the contralateral CC on the measure of curvature, with males having significantly lower curvature than females (#P < 0.05). RmTBI rats were found to have significantly lower (D) AD and (E) ADC values than the sham‐injured group (* P < 0.05). Bar graphs show mean + SEM.
Discussion
Adolescence is characterized by significant brain maturation and pubertal differentiation,23, 24, 25, 26, 27, 37, 38, 39, 40, 41 suggesting that the pathophysiological response to mTBI and RmTBI might differ from adults, the most commonly studied population. There is also a gap in the literature with respect to how the female brain responds to mTBI. Given that it is often difficult to study these cohorts in clinical settings, animal models offer a significant advantage.42, 43 This study provided a comprehensive examination of the outcomes associated with exposure to mild brain injuries during adolescence, for both male and female rodents. We demonstrate that RmTBI during adolescence induced pronounced changes in behavioral, gene expression, and MRI outcomes. Of particular interest, many of the behavioral, gene expression and MRI findings exhibited a sex‐dependent response to the injuries.
The effects of mTBIs in adolescents
There is mounting evidence that RmTBIs can result in cumulative and lasting effects in the adult brain. However, few studies have investigated the effects of repetitive mild brain injury in adolescents. Here we found that repeated mTBIs in adolescent rats induced a range of behavioral abnormalities that included cognitive deficits, motor deficits, anxiety‐like behavior, and depression when compared to sham controls. Furthermore, although the design of this study limits the interpretation of the differences between the single and RmTBI groups (i.e., different recovery times before the onset of behavioral testing – discussed further below), there was a trend in the beam testing data indicating that the RmTBI rats had more slips and falls 24 h after the third mTBI compared to 24 h after the first mTBI, suggesting a cumulative effect with the repeated injuries. Although there is some preliminary human evidence that adolescents may experience worse mTBI outcomes than young adults (see Semple et al., 2015),44 many of the changes in the current study are similar to those observed in adult rats given repeated mTBI. For example, adult male rats administered repeated mild fluid percussion injuries had cumulative and persisting cognitive deficits, motor abnormalities, anxiety‐like behavior, and depression.45, 46 Although we are unaware of any studies that have directly compared the effects of repeated mTBIs at different ages in rats, it should be noted that age‐specific changes do occur after more severe TBI. Specifically, a recent study that administered a diffuse TBI (i.e., moderate midline fluid percussion) to both adolescent and adult rats, found that rats given a TBI during adolescence had motor and cognitive deficits, whereas TBI in adulthood resulted in increased anxiety‐like behavior.47 Future studies might use a similar study design to comprehensively examine how age affects behavioral outcomes after repeated mTBI.
MRI analyses also indicated that repeated mTBIs negatively affect adolescent rats. While many of these findings were sex‐dependent and are discussed further in the next section, DTI metrics revealed a significant effect of injury in the contralateral CC with RmTBI rats having significantly lower AD and ADC than shams regardless of sex. Reduced ADC has been observed in the CC of adolescents with mTBI,48 and in university students including concussed male49 and female50 athletes. In the latter study, and consistent with our results here, concussed athletes also had reduced AD in the CC. There was also a significant effect of injury in the PFC with RmTBI rats having significantly reduced FA compared to sham and mTBI rats. This is consistent with results in clinical51 and preclinical52 studies of mTBI.
This study also found that repeated mTBI resulted in significantly elevated GFAP and Tau expression in the PFC, reduced MBP expression in the PFC, and increased expression of NFL in the CC, in both males and females following RmTBI. Of note, previous studies have investigated the abovementioned markers in TBI patients and found evidence for increases in NFL, 53, 54 GFAP,55 and Tau 56 concentrations, as well as decreased MBP,57 in cerebral spinal fluid and/or serum. While serum and CSF were not analyzed in this study, RmTBI did affect expression of these markers in brain tissue in a similar manner. GFAP, MBP, NFL, and Tau have all been implicated in functional deficits after TBI and neurodegeneration,30, 33, 57, 58, 59, 60, 61, 62, 63, and therefore may be involved in the behavioral and MRI results observed in this study. For example, Budde et al., investigated DTI changes following blast TBI in rats and found reduced FA in the medial PFC at both four and 30 days postinjury, with rats exposed to high‐blast exhibiting a wider pattern of reduced FA compared to those exposed to low‐blast.52 Histology revealed greater astrocyte hypertrophy evident within the ipsilateral cortex following injury. As discussed above, gliosis, a characteristic marker of brain injury,30 is also consistent with the observed increase in GFAP expression in the PFC following RmTBI seen here. Although research demonstrating causal relationships between changes in these markers and outcomes following adolescent mTBI is in its infancy and requires further exploration, this study provides a preliminary overview of how brain injuries in adolescence may influence their expression.
Nature of sex differences after mTBIs
Consistent with epidemiological findings, postinjury symptomology differed between males and females.12, 64 Males given mTBIs exhibited greater impairment on the cognitive task (i.e., NCM), whereas females given mTBIs exhibited increased impairments on the forced swim task. Previous studies in our laboratories have also found evidence for sex‐dependent outcomes in rodent models of TBI.65, 66 For example, there are sex‐dependent social deficits in juvenile mice given a mild TBI.66 Another study found that juvenile males and females did not differ on a range of sensorimotor outcomes, which is consistent with our findings of motor‐related deficits in both male and female rats given RmTBI.67 Clinical studies of sex differences following mTBI in high school and collegiate students are currently conflicted; some have reported females are more likely to be cognitively impaired and experience more subjective and objective symptoms,11 while others have reported no differences in the number of symptoms, but sex differences in the type of symptoms experienced.64 Given the significant degree of brain maturation occurring during this critical period of development, sex‐dependent variations, and the differences in symptomology associated with single versus repetitive mTBI, it is not surprising that clinical studies have failed to produce reliable results regarding symptom presentation. For these reasons, extensive animal studies involving males and females that span childhood, adolescence, and early adulthood may be the most efficacious way to resolve the gaps that currently exist in the clinical literature.
Similar to the behavioral measures, examination of mRNA expression also exhibited sex‐dependent responses after RmTBI, with females exhibiting increased expression of GFAP in the CC and PFC, as well as increased Tau in the CC. The finding of increased GFAP expression in female rats given RmTBI suggests that they may have had elevated astrogliosis and inflammation relative to the male rats.30, 68 There is some evidence that biological sex can alter astrogliosis and inflammation after different forms of brain insult, including stroke and more severe TBI.69 However, these studies were undertaken in adults, and sex hormones are thought to play a central role in these differences.68, 69, 70 As such, future studies are needed to examine how sex influences astrogliosis and inflammation in the developing brain. Although a female‐specific increase in levels of Tau mRNA was also found in the CC after RmTBI, sex differences in baseline levels of Tau were also identified in the CC, demonstrating premorbid differences in Tau expression that may be associated with variations in injury response.
Further evidence of a sex‐dependent response to RmTBI was seen in MRI outcomes. Female rats had reduced ipsilateral PFC volume following RmTBI when compared to female sham and mTBI rats, while there were no differences among the male groups. Interestingly, while the gray matter of the PFC appeared to be adversely affected in female rats following RmTBI, male rats given RmTBI had worse white matter integrity in the CC. Specifically, analysis of DWI metrics revealed that male RmTBI rats had significantly reduced mean curvature in the ipsilateral CC than male sham, male mTBI, and female RmTBI rats, while there were no differences between the female groups. These findings are consistent with previous studies reporting reduced mean curvature in the CC following lateral fluid percussion injury in male rats.19, 36 Taken together, these findings suggest a sex‐dependent response in adolescent mTBI and RmTBI, and future treatment strategies may need to consider the sex of the individual.
Limitations and future studies
There are some limitations that should be considered when interpreting the outcomes of this study. When comparing the effects of single versus repeated mTBI in the developing brain it is not possible to control for both the age at the initial mTBI as well as the post‐injury recovery time of testing using one single mTBI group. Here we administered the initial injury to both the single and repeated mTBI groups at P30 because the developmental timing of the initial injury might influence outcome. The limitation of this design is that the single mTBI group has had a longer recovery time than the RmTBI group. Because of this limitation, it is not possible to determine whether differences between the single and RmTBI groups are due to the number of injuries or the recovery time. Future studies could include an additional single mTBI group that is administered an mTBI at P38 to address this issue. This study was also limited that it only examined acute and sub‐acute injury outcomes. Although it is appropriate to investigate early changes in gene expression and how they relate to structural and behavioral changes following injury, there is some evidence and growing societal concern surrounding the long‐term and neurodegenerative effects of mild brain injuries. Therefore, future studies examining chronic postinjury consequences would be of significant interest. Moreover, while this study examined mRNA changes related to pathophysiological markers of brain injury, additional studies will need to examine biologically meaningful markers such as BDNF. It should also be noted that this study employed ex vivo MRI, which allows for accurate measurements but reduces the translational value of the findings that in vivo MRI would have afforded. However, the MRI scan time for this study was only 2 h (i.e., feasible for in vivo scans), and the MRI sequences and parameters measured are similar to those that we have previously applied in in vivo studies that identified significant volumetric changes after TBI.59, 60, 71 Another potential limitation is the lack of histological evidence to corroborate our MRI findings. Although previous studies have validated MRI measures against histology,72, 73, 74 this study would have been strengthened by complementary histological studies. Lastly, while it is unlikely that the rats reached sexual maturity during the course of this study, future investigations may want to examine the effects of estrous cycle on postinjury recovery.
Conclusion
This study demonstrated that repetitive mTBI during adolescence induces changes in behavior, gene expression, and brain structure after single and repetitive mTBI in adolescent rats of both sexes. Of particular interest here, many of the findings exhibited a sex‐dependent response to injury within the CC and PFC. Male rats given RmTBI exhibited deficits in short‐term working memory, balance and motor coordination, and locomotion, while females given RmTBI had increased depressive‐like behavior and deficits in locomotion. Volumetric MRI analyses found that atrophy of the ipsilateral PFC after RmTBIs was only present in female rats, while advanced diffusion MRI analysis found that only the male rats exhibited decreased white matter integrity in the ipsilateral CC following RmTBI. Sex‐dependent changes in GFAP, MBP, and Tau, were also observed following injury, and may provide mechanistic insight into the behavioral and structural changes. Altogether these findings suggest that in adolescent mTBI, sex matters, and future studies incorporating both male and female cohorts are warranted to provide a greater understanding of injury prognosis and better inform clinical practice.
Author Contributions
RM and SRS conceptualized and designed the study. DKW, RM and SRS acquired and analyzed data. DKW, RM, SRS, and TOB interpreted results and prepared the manuscript.
Conflicts of Interest
Nothing to report.
Acknowledgements
The authors thank the Alberta Children's Hospital Research Foundation, the Integrated Concussion Research Program and the National Health and Medical Research Council for funding. We acknowledge the animal MRI facility at the Florey Institute of Neuroscience and Mental Health, a node of the National Imaging Facility.
Funding Statement
This work was funded by Alberta Children's Hospital Research Foundation grant ; Integrated Concussion Research Program grant ; National Health and Medical Research Council grant .
References
- 1. Gerbeding JL, Binder S. Report to congress on mild traumatic brain injury in the United States: steps to preventing a serious public health problem. Atlanta (GA): Centers for Disease Control and Prevention, 2003. [Google Scholar]
- 2. Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002‐2006. Atlanta GA: Centers for Disease Control and Prevention, 2010. [Google Scholar]
- 3. Rose SC, Weber KD, Collen JB, Heyer GL. The diagnosis and management of concussion in children and adolescents. Pediatr Neurol 2015;53:108–118. [DOI] [PubMed] [Google Scholar]
- 4. Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci 2006;29:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sowell ER, Thompson PM, Leonard CM, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 2004;24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004;101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex 2001;11:552–557. [DOI] [PubMed] [Google Scholar]
- 8. Gur RC, Turetsky BI, Matsui M, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci 1999;19:4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCarthy MM, Auger AP, Bale TL, et al. The epigenetics of sex differences in the brain. J Neurosci 2009;29:12815–12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shultz SR, McDonald SJ, Haar CV, et al. The potential for animal models to provide insight into mild traumatic brain injury: translational challenges and strategies. Neurosci Biobehav Rev 2016;76:396–414. [DOI] [PubMed] [Google Scholar]
- 11. Broshek DK, Kaushik T, Freeman JR, et al. Sex differences in outcome following sports‐related concussion. J Neurosurg 2005;102:856–863. [DOI] [PubMed] [Google Scholar]
- 12. Covassin T, Schatz P, Swanik CB. Sex differences in neuropsychological function and post‐concussion symptoms of concussed collegiate athletes. Neurosurgery 2007;61:345–350. [DOI] [PubMed] [Google Scholar]
- 13. Cloots RJH, Gervaise HMT, van Dommelen JAW, Geers MGD. Biomechanics of traumatic brain injury: influences of the morphologic heterogeneities of the cerebral cortex. Ann Biomed Eng 2008;36:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viano DC, Hamberger A, Bolouri H, Säljö A. Concussion in professional football: animal model of brain injury–part 15. Neurosurgery 2009;64:1162–1173. [DOI] [PubMed] [Google Scholar]
- 15. Viano DC, Casson IR, Pellman EJ. Concussion in professional football: biomechanics of the struck player–part 14. Neurosurgery 2007;61:313–327. [DOI] [PubMed] [Google Scholar]
- 16. Mychasiuk R, Farran A, Esser MJ. Assessment of an experimental rodent model of pediatric mild traumatic brain injury. J Neurotrauma 2014;31:749–757. [DOI] [PubMed] [Google Scholar]
- 17. Sharma P, Wright DK, Johnston LA, et al. Differences in white matter structure between seizure prone (FAST) and seizure resistant (SLOW) rat strains. Neurobiol Dis 2017;104:33–40. [DOI] [PubMed] [Google Scholar]
- 18. Hehar H, Mychasiuk R. The use of telomere length as a predictive biomarker for injury prognosis in juvenile rats following a concussion/mild traumatic brain injury. Neurobiol Dis 2016;87:11–18. [DOI] [PubMed] [Google Scholar]
- 19. Wright DK, Trezise J, Kamnaksh A, et al. Behavioral, blood, and magnetic resonance imaging biomarkers of experimental mild traumatic brain injury. Sci Rep 2016;6:28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mychasiuk R, Hehar H, Candy S, et al. The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J Neurosci Methods 2016;257:168–178. [DOI] [PubMed] [Google Scholar]
- 21. Kolb B, Mychasiuk R, Muhammad A, et al. Experience and the developing prefrontal cortex. Proc Natl Acad Sci 2012;109:17186–17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mychasiuk R, Metz GAS. Epigenetic and gene expression changes in the adolescent brain: What have we learned from animal models? Neurosci Biobehav Rev 2016;70:189–197. [DOI] [PubMed] [Google Scholar]
- 23. Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol 2000;54:241–257. [DOI] [PubMed] [Google Scholar]
- 24. Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 1999;2:861–863. [DOI] [PubMed] [Google Scholar]
- 25. Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health 2008;42:335–343. [DOI] [PubMed] [Google Scholar]
- 26. Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 2006;30:718–729. [DOI] [PubMed] [Google Scholar]
- 27. Paus T, Zijdenbos A, Worsley K, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science 1999;283:1908–1911. [DOI] [PubMed] [Google Scholar]
- 28. Mychasiuk R, Hehar H, Ma I, Esser MJ. Dietary intake alters behavioral recovery and gene expression profiles in the brain of juvenile rats that have experienced a concussion. Front Behav Neurosci 2015;9:e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spanswick S, Sutherland R. Object/context‐specific memory deficits associated with loss of hippocampal granule cells after adrenalectomy in rats. Learn Mem 2010;17:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamphuis W, Mamber C, Moeton M, et al. GFAP Isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer Disease. PLoS ONE 2012;7:e42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci 2006;63:1945–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu MC, Akle V, Zheng W, et al. Extensive degradation of myelin basic protein isoforms by calpain following traumatic brain injury. J Neurochem 2006;98:700–712. [DOI] [PubMed] [Google Scholar]
- 33. Neselius S, Zetterberg H, Blennow K, et al. Increased CSF levels of phosphorylated neurofilament heavy protein following bout in amateur boxers. PLoS ONE 2013;8:e81249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Geel WJA, Rosengren LE, Verbeek MM. An enzyme immunoassay to quantify neurofilament light chain in cerebrospinal fluid. J Immunol Methods 2005;296:179–185. [DOI] [PubMed] [Google Scholar]
- 35. Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol 2013;9:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wright D, Johnston L, Kershaw J, et al. Changes in apparent fibre density and track‐weighted imaging metrics in white matter following experimental traumatic brain injury. J Neurotrauma 2017;. https://doi.org/10.1089/neu.2016.4730. [DOI] [PubMed] [Google Scholar]
- 37. Sowell ER, Thompson PM, Holmes CJ, et al. In vivo evidence for post‐adolescent brain maturation in frontal and striatal regions. Nat Neurosci 1999;2:859–861. [DOI] [PubMed] [Google Scholar]
- 38. Blakemore S‐J, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry 2006;47:296–312. [DOI] [PubMed] [Google Scholar]
- 39. Mayer C, Acosta‐Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha‐signaling in kisspeptin neurons. Proc Natl Acad Sci 2010;107:22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neurosci Biobehav Rev 2002;26:381–391. [DOI] [PubMed] [Google Scholar]
- 41. Rzeczkowska PA, Hou H, Wilson MD, Palmert MR. Epigenetics: a new player in the regulation of mammalian puberty. Neuroendocrinology 2014;99:139–155. [DOI] [PubMed] [Google Scholar]
- 42. O'Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: a critical evaluation. Pharmacol Ther 2011;130:106–113. [DOI] [PubMed] [Google Scholar]
- 43. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci 2013;14:128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Semple BD, Lee S, Sadjadi R, et al. Repetitive concussions in adolescent athletes ‐ translating clinical and experimental research into perspectives on rehabilitation strategies. Front Neurol 2015;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Webster KM, Wright DK, Sun M, et al. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long‐term outcomes in a rat model of repeated mild traumatic brain injury. J Neuroinflammation 2015;12:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shultz SR, Bao F, Omana V, et al. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long‐term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma 2012;29:281–294. [DOI] [PubMed] [Google Scholar]
- 47. Rowe RK, Ziebell JM, Harrison JL, et al. Aging with traumatic brain injury: effects of age at injury on behavioral outcome following diffuse brain injury in rats. Dev Neurosci 2016;38:195–205. [DOI] [PubMed] [Google Scholar]
- 48. Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008;70:948–955. [DOI] [PubMed] [Google Scholar]
- 49. Henry LC, Tremblay J, Tremblay S, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma 2011;28:2049–2059. [DOI] [PubMed] [Google Scholar]
- 50. Chamard E, Lefebvre G, Lassonde M, Théoret H. Long‐term abnormalities in the corpus callosum of female concussed athletes. J Neurotrauma 2016;33:1220–1226. [DOI] [PubMed] [Google Scholar]
- 51. Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion‐tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 2009;252:816–824. [DOI] [PubMed] [Google Scholar]
- 52. Budde MD, Shah A, McCrea M, et al. Primary blast traumatic brain injury in the rat: relating diffusion tensor imaging and behavior. Front Neurol 2013;4:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88:1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gatson JW, Barillas J, Hynan LS, et al. Detection of neurofilament‐H in serum as a diagnostic tool to predict injury severity in patients who have suffered mild traumatic brain injury. J Neurosurg 2014;121:1232–1238. [DOI] [PubMed] [Google Scholar]
- 55. Mondello S, Kobeissy F, Vestri A, et al. Serum concentrations of ubiquitin C‐terminal hydrolase‐L1 and glial fibrillary acidic protein after pediatric traumatic brain injury. Sci Rep 2016;6:28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shahim P, Tegner Y, Wilson DH, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol 2014;71:684–692. [DOI] [PubMed] [Google Scholar]
- 57. Su E, Bell MJ, Kochanek PM, et al. Increased CSF concentrations of myelin basic protein after TBI in infants and children: Absence of significant effect of therapeutic hypothermia. Neurocrit Care 2012;17:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Metting Z, Wilczak N, Rodiger LA, et al. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 2012;78:1428–1433. [DOI] [PubMed] [Google Scholar]
- 59. Tan XL, Wright DK, Liu S, et al. Sodium selenate, a protein phosphatase 2A activator, mitigates hyperphosphorylated tau and improves repeated mild traumatic brain injury outcomes. Neuropharmacol 2016;108:382–393. [DOI] [PubMed] [Google Scholar]
- 60. Shultz SR, Wright DK, Zheng P, et al. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 2015;138:1297–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gabbita SP, Scheff SW, Menard RM, et al. Cleaved‐Tau: a biomarker of neuronal damage after traumatic brain injury. J Neurotrauma 2005;22:83–94. [DOI] [PubMed] [Google Scholar]
- 62. McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68:709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Elder GA. Age‐related atrophy of motor axons in mice deficient in the mid‐sized neurofilament subunit. J Cell Biol 1999;146:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Frommer LJ, Gurka KK, Cross KM, et al. Sex differences in concussion symptoms of high school athletes. J Athl Train 2011;46:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Semple BD, Dixit S, Shultz SR, et al. Sex‐dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury. Behav Brain Res 2017;319:48–62. [DOI] [PubMed] [Google Scholar]
- 66. Mychasiuk R, Hehar H, Farran A, Esser MJ. Mean girls: sex differences in the effects of mild traumatic brain injury on the social dynamics of juvenile rat play behaviour. Behav Brain Res 2014;259:284–291. [DOI] [PubMed] [Google Scholar]
- 67. Russell KL, Kutchko KM, Fowler SC, et al. Sensorimotor behavioral tests for use in a juvenile rat model of traumatic brain injury: assessment of sex differences. J Neurosci Methods 2011;199:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Villapol S, Loane DJ, Burns MP. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 2017;65:1423–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chisholm NC, Sohrabji F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol Dis 2016;85:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cordeau P, Lalancette‐Hébert M, Weng YC, Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke 2008;39:935–942. [DOI] [PubMed] [Google Scholar]
- 71. Wright DK, Liu S, van der Poel C, et al. Traumatic brain injury results in cellular, structural and functional changes resembling motor neuron disease. Cereb Cortex 2016;13. doi:10.1093/cercor/bhw254. 1–13. [DOI] [PubMed] [Google Scholar]
- 72. Gyengesi E, Calabrese E, Sherrier MC, et al. Semi‐automated 3D segmentation of major tracts in the rat brain: comparing DTI with standard histological methods. Brain Struct Funct 2014;219:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kooy RF, Verhoye M, Lemmon V, van der Linden A. Brain studies of mouse models for neurogenetic disorders using in vivo magnetic resonance imaging (MRI). Eur J Hum Genet 2001;9:153–159. [DOI] [PubMed] [Google Scholar]
- 74. Wu Q‐Z, Yang Q, Cate HS, et al. MRI identification of the rostral‐caudal pattern of pathology within the corpus callosum in the cuprizone mouse model. J Magn Reson Imaging 2008;27:446–453. [DOI] [PubMed] [Google Scholar]


