Abstract.
The declining efficacy of chloroquine in the early 2000s in Ghana led to its replacement with artesunate/amodiaquine (AS/AQ) combination as first-line drug for treating uncomplicated malaria in 2005. Since then efficacy studies have been ongoing in the country to provide continuous data on the efficacy of AS/AQ and other alternative antimalarials (artemether/lumefantrine and dihyroartemisinin/piperaquine combinations) introduced in 2008. In vivo AS/AQ efficacy studies were conducted between June and October 2014 among children aged 6 months to 14 years, in two sentinel sites representing the forest and coastal zones of the country. The 2009 World Health Organization protocol for monitoring antimalarial drug efficacy was used in these studies. The studies showed an overall cumulative polymerase chain reaction-corrected day 28 cure rate of 97.2% (95% confidence interval [CI]: 93.6–99.1): 97.7% (95% CI: 92.0–99.7) within the forest zone and 96.7% (95% CI: 90.7–99.3) within the coastal zone (P = 0.686). Prevalence of fever declined from 100% to < 4% after first day of treatment in both ecological zones. All children in the coastal zone had cleared parasites by day 2. Three children (3.2%) in the forest zone were parasitemic on day 2, whereas one child was parasitemic on day 3. Gametocytemia was absent in both zones after day 14, and mean hemoglobin concentration significantly increased from 10.3 g/dL (95% CI: 10.1–10.5) on day 0 to 11.8 g/dL (95% CI: 11.6–12.0) on day 28. We conclude that AS/AQ combination remains efficacious in the treatment of uncomplicated malaria in Ghana.
INTRODUCTION
Malaria remains a major public health problem in the world affecting mainly low-and middle-income countries. It is estimated that 149–303 million malaria cases and 236,000–635,000 malaria deaths occurred in the world in 2015. The World Health Organization (WHO) African Region is estimated to bear about 90% of all the malaria deaths in 2015.1 In Ghana, malaria accounted for 30.9% of all outpatient clinic visits, 27.9% of all admissions and 7.2% of all deaths in 2014.2 The national malaria prevalence among children aged 6–59 months is estimated to be 27%.3
Malaria interventions in Ghana include case management based on prompt diagnosis and adequate treatment; use of long-lasting insecticide treated nets; intermittent preventive treatment in pregnancy; indoor residual spraying; and recently, seasonal malaria chemoprevention.4 Case management has been a major focus of malaria control in Ghana for decades. In 2005, artesunate-amodiaquine (AS/AQ) combination was introduced in Ghana to replace chloroquine as first-line drug for treating uncomplicated malaria based on evidence of declining efficacy of chloroquine.5,6 In the same year, 10 sentinel sites were established by the Noguchi Memorial Institute for Medical Research (NMIMR), University of Ghana, in collaboration with the National Malaria Control Program to continuously monitor the efficacy of the newly introduced artemisinin-based combination therapy (ACT). In 2008, artemether/lumefantrine and dihydroartemisinin/piperaquine combinations were added as alternative first-line antimalarials for patients who were unable to tolerate AS/AQ.6 Antimalarial drug resistance in Ghana has been studied using in vivo, in vitro, and molecular approaches over the years.6–14 This paper reports in vivo efficacy of AS/AQ for treatment of uncomplicated malaria in children aged 6 months to 14 years in two sentinel sites located in the forest and coastal zones of Ghana.
MATERIALS AND METHODS
Study sites.
The study was conducted in two of the 10 sentinel sites established in 2005 to monitor antimalarial drug efficacy in Ghana. The two sites were Begoro District Hospital and Ewim Polyclinic. The Begoro District Hospital is located in the Fanteakwa District within the forest zone of Ghana. The district has an estimated population of 108,614, and has an annual rainfall of 1,500–2,000 mm with double maxima rainfall in June and October each year. Malaria transmission in the district is perennial and intense.15,16 The Ewim Polyclinic is located in the Cape Coast Metropolis within the coastal zone of Ghana. The metropolis has an estimated population of 169,894, and has an annual rainfall of 750–1,000 mm with double maxima rainfall in June and December. Malaria transmission in the metropolis is perennial.6
Patient enrolment, treatment, and follow-up.
The target population was children aged between 6 months and 14 years. Any child within the targeted age-group with a history of fever suggestive of malaria was screened for the presence of malaria parasitemia. The inclusion criteria were as follows: mono infection with Plasmodium falciparum; parasitemia of 1,000–250,000/μL blood; hemoglobin level of 5 g/dL or higher; ability of child to swallow oral medication; ability and willingness of parent/guardian to comply with study protocol and study visit schedule; absence of severe malaria; and parental informed consent for all children and child assent for children aged 12 years and above.
Children meeting all inclusion criteria were given a nonfixed formulation of AS/AQ combination (Camosunate®; Danadams, Accra, Ghana) by body weight (Table 1). All treatments were given by direct observation by the study nurse. After each treatment, a child was observed for 30 minutes to ascertain retention of medication. Children who vomit within the 30-minute observation period were given a repeated dose of AS/AQ. Children with repeated vomiting were given rectal artesunate (10 mg/kg body weight) or intravenous artesunate (2.4 mg/kg body weight) with supportive treatment until they were able to swallow oral medication as per national treatment guidelines, and excluded from the study.4
Table 1.
Treatment doses for artesunate/amodiaquine combination (Camosunate®)
| Weight of child (kg) | Artesunate/amodiaquine base | Total dose | Day | ||
|---|---|---|---|---|---|
| 0 | 1 | 2 | |||
| ≥ 4.5 to < 9 | 25 mg/75 mg | 3 | 1 | 1 | 1 |
| ≥ 9 to < 18 | 50 mg/150 mg | 3 | 1 | 1 | 1 |
| ≥ 18 to < 36 | 100 mg/300 mg | 3 | 1 | 1 | 1 |
| ≥ 36 | 100 mg/300 mg | 6 | 2 | 2 | 2 |
The follow-up schedule covered days 1, 2, 3, 7, 14, 21, and 28 with day of commencement of treatment counted as day 0. Clinical assessment was done on each clinic visit by the study physician, and findings recorded on a case record form (CRF). Parasitological assessment was done by microscopy on days 2, 3, 7, 14, 21, 28, and any day a study child was brought to the clinic with fever. Prepared thick and thin smears were stained with 3% Giemsa for 30–45 minutes, and asexual parasites counted per 200 white blood cells using a hand tally counter. Presence of gametocytes was also noted. Each slide was read by two independent microscopists for quality assurance purposes. Parasitemia levels were expressed per µL blood assuming white blood cell count of 8,000 per µL blood. Discordant slides (in terms of presence/absence of sexual or asexual parasites; species diagnosis; and day 0 count meeting the inclusion criterion of 1,000–250,000/μL blood) were read by a third senior microscopist. When a result of the third microscopist agreed with that of the first microscopist, the result of the first microscopist was considered final, and when a result of the third microscopist agreed with that of the second microscopist, the result of the second microscopist was considered final. Reinfection was distinguished from recrudescence by polymerase chain reaction (PCR) genotyping using merozoite surface protein 2 (MSP2). DNA extraction was by the Chelex method.17 As per WHO protocol, each sample was genotyped by amplifying the polymorphic regions of MSP2 by nested PCR assay. Secondary nested PCR was performed using MSP2-specific primers: FC 27 and 3D7.18 All samples were classified as reinfection when pretreatment and posttreatment alleles had different band sizes. Hemoglobin levels were assessed on days 0 and 28.
Data analysis.
A minimum sample size of 88 was computed for each ecological zone in this study, which was a one-arm prospective evaluation of clinical and parasitological responses to treatment of uncomplicated malaria. The sample size was based on an estimated PCR-corrected AS/AQ failure rate of 5% per zone at 95% confidence level, 5% precision, and 20% loss to follow-up. Data on completed CRFs were captured by two independent officers using EpiData 3.1 (The Epidata Association, Odense, Denmark), and corrections made until validation reports showed no errors. The validated data were exported to IBM SPSS Statistics 21 (IBM Corporation, Armonk, NY) for analysis. Per protocol analysis was used to assess treatment outcomes on days 14 and 28 based on WHO criteria. Early treatment failure (ETF) was defined as danger signs or severe malaria on days 1, 2, or 3 in the presence of parasitemia; parasitemia on day 2 higher than on day 0, irrespective of axillary temperature; parasitemia on day 3 with axillary temperature ≥ 37.5°C or parasitemia on day 3 ≥ 25% of count on day 0. Late clinical failure (LCF) was defined as danger signs or severe malaria in the presence of parasitemia on any day between days 4 and 28 in patients who did not previously meet any of the criteria of ETF or presence of parasitemia on any day between days 4 and 28 with axillary temperature ≥ 37.5°C in patients who did not previously meet any of the criteria of ETF. Late parasitological failure (LPF) was defined as presence of parasitemia on any day between days 7 and 28 and axillary temperature < 37.5°C in patients who did not previously meet any of the criteria of ETF or LCF. Adequate clinical and parasitological response (ACPR) or cure was defined as absence of parasitemia on day 28, irrespective of axillary temperature, in patients who did not previously meet any of the criteria for ETF, LCF, or LPF.19 Kaplan–Meier survival curve was used to describe the cumulative incidence of treatment success over the 28-day follow-up period.
Secondary outcomes analyzed were fever clearance and parasite clearance using proportions (with 95% confidence interval [CI]) of children febrile or with temperature ≥ 37.5°C or parasitemic during the first week of follow-up. Pre- and posttreatment hemoglobin levels were assessed using days 0 and 28 levels. All proportions were compared using χ2 and Fisher’s exact tests. Student’s t test was used to compare normally distributed continuous data, whereas skewed distributions such as parasite densities were log transformed before using the normal approximation to perform the t test. Differences with P < 0.05 were considered significant.
Ethical considerations.
This study protocol was reviewed and approved by the Institutional Review Board of the NMIMR, University of Ghana. Written informed consent was obtained from parents or guardians of all children before enrolment. An additional written assent was obtained from all children aged 12 years and above. Parents or guardians and children giving assent were informed of the objectives, methods, anticipated benefits, and potential risks of the study. They were also informed of their right to withdraw from the study without any penalty as well as the confidentiality of the data collected.
RESULTS
Patient characteristics.
Of the total 301 children (119 in the forest zone and 182 in the coastal zone) screened between June and October 2014, 191 aged 6 months to 14 years met study inclusion criteria and were enrolled: 95 in the forest zone and 96 in the coastal zone. For both ecological zones, male to female ratio of patients enrolled was 1:1. Mean age (years) of patients was 5.4 (±3.1) in the forest zone and 5.7 (±3.7) in the coastal zone (P = 0.544). The proportion of patients aged between 5 and 9 years was significantly higher in the forest zone compared with the coastal zone (46.3%; 95% CI: 36.1–56.8 versus 29.2%; 95% CI: 20.6–39.5; P < 0.022) (Table 2). Mean axillary temperature (°C) was significantly higher among patients in the coastal zone compared with the forest zone (38.7 ± 1.2 versus 37.8 ± 1.2; P = 0.000), whereas geometric mean parasite count was 50,754/μL blood in the forest zone and 37,781/μL in the coastal zone. Gametocytemia was absent in both ecological zones. There were no significant differences in hemoglobin concentrations between the two zones (Table 2). No patient studied had a history of previous intake of an antimalarial.
Table 2.
Demographic, clinical, parasitological, and hematological characteristics at baseline by ecological zone
| Characteristic | Total (N = 191) | Ecological zone | P value | |
|---|---|---|---|---|
| Forest N = 95 | Coastal N = 96 | |||
| Male/female | 96/95 | 47/48 | 49/47 | 0.942 |
| Mean age in years (SD) | 5.5 (3.4) | 5.4 (3.1) | 5.7 (3.7) | 0.544 |
| Age group (years) | ||||
| < 5 | 90 (47.1%) | 39 (41.1%) | 51 (53.1%) | 0.129 |
| 5–9 | 72 (37.7%) | 44 (46.3%) | 28 (29.2%) | 0.022 |
| 10–14 | 29 (15.2) | 12 (12.6%) | 17 (17.7%) | 0.434 |
| Axillary temperature in °C | ||||
| Mean temperature (SD) | 38.2 (1.3) | 37.8 (1.2) | 38.7 (1.2) | 0.000 |
| Range (min, max) | 35.5, 40.7 | 35.5, 40.5 | 36.0, 40.7 | |
| Parasitemia/µL | ||||
| Geometric mean | 43,756 | 50,754 | 37,781 | 0.015 |
| Range (minimum, maximum) | (1,757, 248,618) | (1,757, 248,618) | (2,640, 242,880) | |
| Hemoglobin level in g/dL | ||||
| Mean (sd) | 10.3 (1.6) | 10.2 (1.7) | 10.4 (1.6) | 0.403 |
| Range (minimum, maximum) | (6.5, 14.0) | (6.5, 14.3) | (7.2, 14.0) | |
SD = standard deviation.
Primary outcomes.
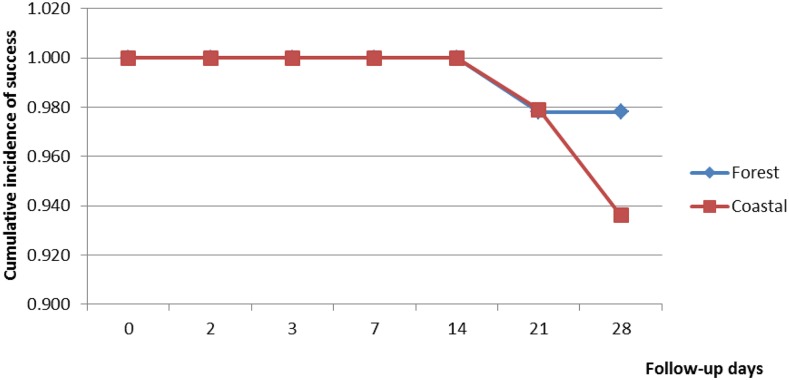
Of the 191 children enrolled 186 were assessed on day 14 (92 in the forest zone and 94 in the coastal zone) and 182 assessed on day 28 (88 in the forest zone and 94 in the coastal zone). A cumulative total of nine children were therefore not assessed on day 28. Of these nine children, four did not complete their treatment; one had Plasmodium malariae infection on day 3; and four were lost to follow-up. There was no treatment failure observed on day 14 in the two ecological zones. Out of the evaluable patients assessed on day 28, 97.7% (95% CI: 91.3–99.6) in the forest zone and 93.6% (95% CI: 86.1–97.4) in the coastal zone showed PCR-uncorrected ACPRs (P = 0.325) (Table 3). PCR-uncorrected treatment failure in the forest zone occurred only among children aged 5–9 years: 2.4% (95% CI: 0.1–14.4) for LCF and 2.4% (95% CI: 0.1–14.4) for LPF. Age-specific analysis of PCR-uncorrected treatment failures in the coastal zone showed treatment failure among children under 5 years (10%; 95% CI: 3.7–22.6) and children aged 5–9 years (3.7%; 95% CI: 0.2–20.9) (P = 0.590) (Table 3). There was no treatment failure among children aged 10–14 years in the two ecological zones. For both ecological zones, PCR-uncorrected Kaplan–Meier survival analysis showed a cumulative treatment success incidence of 1.000 until day 21, when incidence declined to 0.978 (95% CI: 0.913–0.994) in the forest zone and 0.979 (95% CI: 0.918–0.996) in the coastal zone (P = 0.652). Cumulative incidence of treatment success remained 0.978 in the forest zone from days 21 to 28, whereas it declined to 0.936 (95% CI: 0.863–0.971) in the coastal zone (P = 0.314) (Figure 1). PCR-corrected analyses showed an overall cumulative cure rate of 97.2% (95% CI: 93.6–99.1): 97.7% (95% CI: 92.0–99.7) within the forest zone and 96.7% (95% CI: 90.7–99.3) within the coastal zone (P = 0.686). The 3.3% PCR-corrected treatment failure in the coastal zone was among children under 5 years, whereas the 2.3% failure in the forest zone was among children aged 5–9 years.
Table 3.
Per protocol PCR-uncorrected treatment outcomes for evaluable patients in two ecological zones in Ghana
| Day of assessment | Treatment outcome | Patient age-group (forest zone) | Patient age-group (coastal zone) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| < 5 years | 5–9 years | 10–14 years | Total | < 5 years | 5–9 years | 10–14 years | Total | ||
| Day 14 | N = 39 | N = 41 | N = 12 | N = 92 | N = 50 | N = 27 | N = 17 | N = 94 | |
| ETF (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| LCF (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| LPF (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| ACPR (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Day 28 | N = 36 | N = 41 | N = 11 | N = 88 | N = 50 | N = 27 | N = 17 | N = 94 | |
| ETF (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| LCF (%) | 0 | 2.4 (0.1–14.4)* | 0 | 1.1 (0.1–7.0)* | 0 | 0 | 0 | 0 | |
| LPF (%) | 0 | 2.4 (0.1–14.4)* | 0 | 1.1 (0.1–7.0)* | 10 (3.7–22.6)* | 3.7 (0.2–20.9)* | 0 | 6.4 (2.6–13.9)* | |
| ACPR (%) | 100 | 95.1 (82.2–99.2)* | 100 | 97.7 (91.3–99.6)* | 90 (77.4–96.3)* | 96.3 (79.1–99.8)* | 100 | 93.6 (86.1–97.4)* | |
ACPR = adequate clinical and parasitological response; ETF = early treatment failure; LCF = late clinical failure; LPF = late parasitological failure.
95% confidence interval.
Figure 1.
Polymerase chain reaction-uncorrected Kaplan–Meier survival curve for children treated with artesunate–amodiaquine combination in two ecological zones of Ghana. This figure appears in color at www.ajtmh.org.
Secondary outcomes.
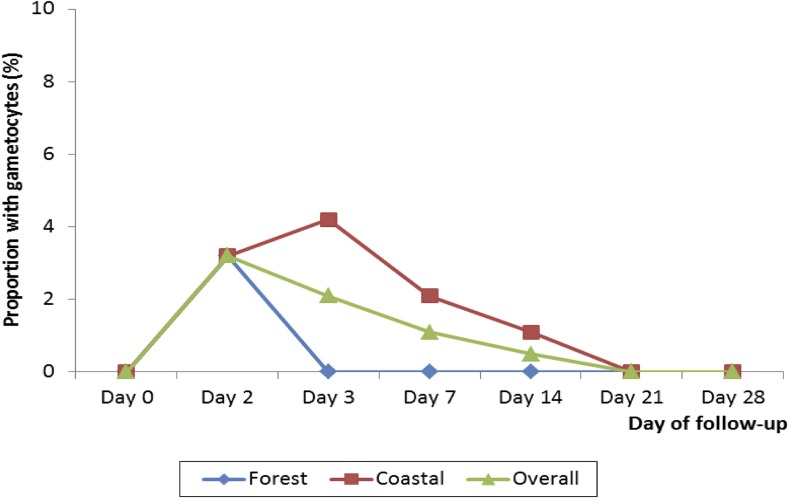
Assessment of prevalence of fever during the first week of follow-up showed that only one child (1.1%) in the forest zone and three children (3.1%) in the coastal zone were febrile after first day of treatment. In the forest zone, no child was febrile on days 3 and 7. In the coastal zone, only two children (2.1%) and one child (1.1%) were febrile on days 3 and 7, respectively (Table 4). Regarding presence of parasites during the first week of follow-up, no child in the coastal zone was parasitemic on days 2, 3, and 7. In the forest zone, three children (3.2%) were parasitemic on day 2, whereas one child (1.1%) was parasitemic on day 3 (Table 4). Two of the three children who were parasitemic on day 2 were aparasitemic during the remaining follow-up period, whereas the third child was aparasitemic from days 3 to 14 but became parasitemic on day 21, and was confirmed as a case of P. falciparum recrudescence. The child with parasitemia on day 3, aged 45 months, had a monoinfection with P. malariae, and was subsequently withdrawn from the study. For both ecological zones, gametocytemia was absent on day 0 but present on day 2 at a rate of 3.2%. Gametocytemia was absent from days 3 to 28 in the forest zone; declined to 1.1% on day 14 in the coastal zone; and absent on days 21 and 28 in the coastal zone (Figure 2). For both ecological zones, mean hemoglobin concentration significantly increased after treatment. Mean hemoglobin in the forest zone increased from 10.2 g/dL (±1.7) on day 0 to 12.1 g/dL (±1.2) on day 28 (P < 0.001) (mean difference = 1.9 (95% CI: 1.5–2.3), and from 10.4 g/dL (±1.6) on day 0 to 11.1 g/dL (±1.0) on day 28 (P = 0.026) (mean difference = 0.70 (95% CI: 0.1–1.3) in the coastal zone. No serious adverse event was observed or reported during the first week of follow-up. The only adverse event observed/reported was vomiting. A total of 10 out of 191 patients vomited during the first week of follow-up: seven out of 95 in the forest zone and three out of 96 in the coastal zone (P = 0.321). All three patients who vomited in the coastal zone were aged less than 5 years. In the forest zone, four out of seven patients who vomited were aged less than 5 years; two were aged 5–9 years; and one was aged 10–14 years.
Table 4.
Prevalence of fever and parasitemia during first week of follow-up
| Day | Forest zone | Coastal zone | Overall | |||
|---|---|---|---|---|---|---|
| Fever (%) | Parasitemia (%) | Fever (%) | Parasitemia (%) | Fever (%) | Parasitemia (%) | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1 | 1.1 (0.1–6.7)* | ND | 3.1 (0.9–9.5)* | ND | 2.1 (0.7–5.6)* | ND |
| 2 | 1.1 (0.1–6.7)* | 3.2 (0.8–9.8)* | 3.2 (0.7–9.4)* | 0 | 2.1 (0.7–5.7)* | 1.6 (0.4–5.0)* |
| 3 | 0 | 1.1 (0.1–6.7)* | 2.1 (0.4–8.2)* | 0 | 1.1 (0.2–4.3)* | 0.5 (0.0–3.3)* |
| 7 | 0 | 0 | 1.1 (0.7–6.7)* | 0 | 0.5 (0.0–2.4)* | 0 |
ND = not done.
95% confidence interval.
Figure 2.
Prevalence of gametocytemia in two ecological zones in Ghana. This figure appears in color at www.ajtmh.org.
DISCUSSIONS
Following the introduction of AS/AQ combination as first-line treatment of uncomplicated malaria in 2005 in Ghana, the provision of continuous data on its therapeutic efficacy has become important in building the confidence of the National Malarial Control Program regarding the adequacy of current treatment recommendations or the need for a revision. It was in this light that efficacy of AS/AQ was monitored between June and October 2014 in two sentinel sites representing the forest and coastal zones of the country. The main strength of the study is the strict use of directly observed therapy for all treatments given, and this allows for the proper interpretation of treatment outcomes.
This study has shown that AS/AQ remains efficacious in the two ecological zones studied. Day 28 cure rates for the forest and coastal zones were 97.7% (95% CI: 92.0–99.7) and 96.7% (95% CI: 90.7–99.3), respectively. The more than 90% cure rates after AS/AQ treatment compares well with findings from other studies conducted in Ghana after the introduction of AS/AQ in 2005.10,20,21 Studies in other parts of Africa have also shown cure rates greater than 90%.22–25 There was no treatment failure observed during the first 2 weeks of follow-up. Late treatment failure occurred on days 21 and 28 in the two ecological zones. This finding supports other studies showing that most episodes of AS/AQ treatment failure occur after day 14.10,20,26,27 Treatment failure was prevalent among children aged 5–9 years in the forest zone, and among children under 5 years and 5–9 years in the coastal zone. There was, however, no significant difference between the two age-groups in the coastal zone (P > 0.05).
The global deployment of artemisinin-based combination drugs in the early 2000s was based on the high efficacy of artemisinin derivatives in rapid clearance of malaria symptoms and parasites, among other reasons.28–31 This study has shown that AS/AQ continues to rapidly clear fever in patients suffering from malaria in Ghana. Prevalence of fever was less than 4% after first day of treatment in the two ecological zones studied. Regarding parasite clearance, all children studied in the coastal zone had cleared all parasites by day 2. Only three (3.2%; 95% CI: 0.8, 9.8) children in the forest zone were parasitemic on day 2 and one child was parasitemic on day 3. These findings support the continuous efficacy of AS/AQ, and to a large extent ACTs, in rapidly clearing malaria parasites in Ghana. Gametocytemia, which was present in both zones on day 2 (3.2%), was absent in the forest zone from days 3 to 28. Prevalence in the coastal zone was just about 1% on day 14, and absent on days 21 and 28. These findings show that artemisinin derivatives remain efficacious in reducing gametocyte carriage.
For both ecological zones, treatment yielded significant improvement in mean hemoglobin concentration after treatment with AS/AQ: mean difference between days 0 and 28 was 1.9 (95% CI: 1.5–2.3) in the forest zone and 0.70 (95% CI: 0.1–1.3) in the coastal zone. This finding compares well with several studies, and suggests that effective malaria treatment using ACT is significant in the prevention of anemia in malaria endemic areas.7,10,32–34
In conclusion, AS/AQ remains significantly relevant in malaria control efforts in Ghana, as it continues to yield positive clinical and parasitological outcomes among children under 15 years.
Acknowledgments:
We thank the staff and management of Ewim polyclinic in the central region of Ghana and the Begoro District Hospital in the Eastern region of Ghana for their support in the conduct of this study. We also acknowledge the technical support of Christiana Ownona and Messrs. Charles Attiogbe, Abdul Haruna, and Joseph Otchere. We appreciate the consent of parents/guardians of all children who participated in the study.
REFERENCES
- 1.World Health Organization , 2015. World Malaria Report 2015. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Ministry of Health , 2015. National Malaria Control Programme, 2014 Annual Report. Accra, Ghana: Ministry of Health. [Google Scholar]
- 3.Ghana Statistical Service (GSS), Ghana Health Service (GHS), and ICF International , 2015. 2014 Ghana Demographic and Health Survey (GDHS) Key Findings. Rockville, MD: GSS, GHS, and ICF International. [Google Scholar]
- 4.Ministry of Health , 2014. Guidelines for Case Management of Malaria in Ghana, 3rd edition Accra, Ghana: Ministry of Health. [Google Scholar]
- 5.Koram KA, 2003. Mapping Response of Plasmodium falciparum to Chloroquine and Other Antimalarial Drugs in Ghana. Project ID 980034. Final report submitted to MIM/TDR. Accra, Ghana: Noguchi Memorial Institute for Medical Research.
- 6.Abuaku B, Duah N, Quaye L, Quashie N, Koram KA, 2012. Therapeutic efficacy of artemether-lumefantrine combination in the treatment of uncomplicated malaria among children under five years of age in three ecological zones in Ghana. Malar J 11: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koram KA, Abuaku B, Duah N, Quashie N, 2005. Comparative efficacy of antimalarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in Ghana. Acta Trop 95: 194–203. [DOI] [PubMed] [Google Scholar]
- 8.Duah NO, Wilson MD, Ghansah A, Abuaku B, Edoh D, Quashie NB, Koram KA, 2007. Mutations in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance genes, and treatment outcomes in Ghanaian children with uncomplicated malaria. J Trop Pediatr 53: 27–31. [DOI] [PubMed] [Google Scholar]
- 9.Quashie NB, Duah NO, Abuaku B, Koram KA, 2007. The in-vitro susceptibilities of Ghanaian Plasmodium falciparum to antimalarial drugs. Ann Trop Med Parasitol 101: 391–398. [DOI] [PubMed] [Google Scholar]
- 10.Koram KA, Quaye L, Abuaku B, 2008. Efficacy of amodiaquine/artesunate combination therapy for uncomplicated malaria in children under five years in Ghana. Ghana Med J 42: 55–60. [PMC free article] [PubMed] [Google Scholar]
- 11.Duah NO, Abuaku B, Quashie N, Kronmann K, Koram K, 2012. Surveillance of molecular markers of Plasmodium falciparum resistance to sulphadoxine-pyrimethamine resistance 5 years after the change of malaria treatment policy in Ghana. Am J Trop Med Hyg 87: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duah NO, et al. , 2013. Increased pfmdr1 gene copy number and the decline in pfcrt and pfmdr1 resistance alleles in Ghanaian Plasmodium falciparum isolates after change of anti-malarial drug treatment policy. Malar J 12: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quashie NB, et al. , 2013. A SYBR Green 1-based in-vitro test of susceptibility of Ghanaian Plasmodium falciparum clinical isolates to a panel of antimalarial drugs. Malar J 12: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abuaku B, Quaye L, Quashie N, Quashie N, Koram KA, 2014. Managing antimalarial drug resistance in Ghana: the importance of surveillance. Koram KA, Ahorlu CSK, Wilson MD, Yeboah-Manu D, and Bosompem KM, eds. Towards Effective Disease Control in Ghana: Research and Policy Implications, Vol. 1. Accra, Ghana: University of Ghana Reader Series.
- 15.Ghana Statistical Service , 2012. 2010 Population and Housing Census. Summary Report of Final Results. Accra, Ghana: Ghana Statistical Service. [Google Scholar]
- 16.Ministry of Food and Agriculture , 2012. Fanteakwa District. Available at: http//www.mofa.gov.gh/site/?page_id=1512. Accessed August 30, 2015.
- 17.World Health Organization , 2008. Methods and Techniques for Clinical Ttrials on Antimalarial Drug Efficacy: Genotyping to Identify Parasite Populations. Geneva, Switzerland: WHO. [Google Scholar]
- 18.Wooden J, Kyes S, Sibley CH, 1993. PCR and strain identification in Plasmodium falciparum. Parasitol Today 9: 303–305. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization , 2009. Methods for Surveillance of Antimalarial Drug Efficacy. Geneva, Switzerland: WHO. [Google Scholar]
- 20.Adjei GO, Kurtzhals JA, Rodrigues OP, Alifrangis M, Hoegberg LC, Kitcher ED, Badoe EV, Lamptey R, Goka BQ, 2008. Amodiaquine-artesunate vs artemether-lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow-up. Malar J 7: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owusu-Agyei S, Asante KP, Owusu R, Adjuik M, Amenga-Etego S, Dosoo DK, Gyapong J, Greenwood B, Chandramohan D, 2008. An open label, randomized trial of artesunate+amodiaquine, artesunate+chlorproguanil-dapsone and artemether-lumefantrine for the treatment of uncomplicated malaria. PLoS One 3: e2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yavo W, Konaté A, Kassi FK, Djohan V, Angora EK, Kiki-Barro PC, Vanga-Bosson H, Menan EIH, 2015. Efficacy and safety of artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sentinel sites across Côte d’Ivoire. Malar Res Treat 2015: 878132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paczkowski M, Mwandama D, Marthey D, Luka M, Makuta G, Sande J, Ali D, Troell P, Mathanga DP, Gutman J, 2016. In vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine for uncomplicated Plasmodium falciparum malaria in Malawi, 2014. Malar J 15: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndounga M, Mayengue PI, Casimiro PN, Koukouikila-Koussounda F, Bitemo M, Matondo BD, Diakou LAN, Basco LK, Ntoumi F, 2015. Artesunate-amodiaquine versus artemether-lumefantrine for the treatment of acute uncomplicated malaria in Congolese children under 10 years old living in a suburban area: a randomized study. Malar J 14: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nji AM, et al. , 2015. Randomized non-inferiority and safety trial of dihydroartemisinin-piperaquine and artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroonian children. Malar J 14: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schramm B, et al. , 2013. Efficacy of artesunate-amodiaquine and artemether-lumefantrine fixed-dose combinations for the treatment of uncomplicated Plasmodium falciparum malaria among children aged six to 59 months in Nimba County, Liberia: an open-label randomized non-inferiority trial. Malar J 12: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeka A, Lameyre V, Afizi K, Frederick M, Lukwago R, Kamya MR, Talisuna AO, 2014. Efficacy and safety of fixed-dose artesunate-amodiaquine vs. artemether-lumefantirne for repeated treatment of uncomplicated malaria in Ugandan children. PLoS One 9: e113311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization , 2001. Antimalarial Drug Combination Therapy. Report of a WHO Technical Consultation, 4–5 April 2001. Geneva, Switzerland: WHO. [Google Scholar]
- 29.Mutabingwa TK, 2005. Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop 95: 305–315. [DOI] [PubMed] [Google Scholar]
- 30.Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, McGready R, ter Kuile F, Looareesuwan S, White NJ, 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356: 297–302. [DOI] [PubMed] [Google Scholar]
- 31.Adjuik M, et al. , 2004. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363: 9–17. [DOI] [PubMed] [Google Scholar]
- 32.Mårtensson A, Strömberg J, Sisowath C, Msellem MI, Gil JP, Montgomery SM, Olliaro P, Ali AS, Björkman A, 2005. Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis 41: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 33.Sagara I, Piarroux R, Djimde A, Giorgi R, Kayentao K, Doumbo OK, Gaudart J, 2014. Delayed anemia assessment in patients treated with oral artemisinin derivatives for uncomplicated malaria: a pooled analysis of clinical trials data from Mali. Malar J 13: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oguche S, et al. , 2014. Efficacy of artemisinin-based combination treatments of uncomplicated falciparum malaria in under-five-year-old Nigerian children. Am J Trop Med Hyg 91: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]