Abstract.
Mucosal leishmaniasis (ML) is observed only in about 3% of patients with American tegumentary leishmaniasis (ATL) but has a high potential for destructive, disfiguring, and disabling sequelae. Prior reports of clinical and epidemiologic features of ML are limited by small numbers of cases. In this study, we evaluated changes in the demographic features and clinical presentation of ML in an endemic area of Leishmania braziliensis transmission over a period of 20 years. The charts of 327 patients with ML diagnosed between 1995 and 2014 were reviewed. The majority of patients (67%) were male. Age ranged from 8 months to 103 years, with a median age of 38.5 years (interquartile range: 22–58 years). The greatest number of patients was between 19 and 39 years (31%). Over the study period, there was an increase in patients with ML more than 60 years of age, an increase in ML with concomitant cutaneous lesions, a decrease in the period of time between the documentation of cutaneous lesions and the diagnosis of mucosal disease, and an increase in the frequency of patients presenting with stage I and V of ML. Moreover, there was a positive correlation between severity of mucosal disease and both age and the period of time between cutaneous lesion and mucosal disease. Response to therapy of ML remained similar over a period of 20 years. Despite the improvement in medical care during the study period, the prevalence of ML did not change and severe disease continues to be a major challenge for the management of these patients.
INTRODUCTION
The World Health Organization (WHO) estimates that there are 900,000 to 1.3 million new cases of leishmaniasis annually throughout the world, with approximately 200,000–400,000 of the visceral form and 700,000 to 1.2 million of the tegumentary form.1,2
Tegumentary leishmaniasis (TL) affects the skin and mucous membranes and may present as different clinical forms: cutaneous, mucosal, disseminated, and diffuse. Cutaneous leishmaniasis (CL) is the most common presentation of the disease, and mucosal leishmaniasis (ML) occurs in approximately 3–5% of cases of CL caused by Leishmania braziliensis. Classic ML occurs secondary to cutaneous lesions. In a minority of cases, however, the ML is primary, without prior or concomitant history of skin lesions.2,3 Additionally, in a study with 23 cases of ML caused by Leishmania panamensis, in Colombia mucosal lesions occurred concomitantly with cutaneous ulcers.4 ML affects mainly the nasal mucosa (90% of cases), followed by the pharyngeal mucosa, larynx, and mouth.5 However, the comparative frequency of nasal, pharyngeal, laryngeal, buccal, and labial involvement varies in different studies. Since the nasal mucosa is the most affected site in most of these works, it can vary from 70% to 90%.6–9 And other, even rarer sites of mucosal involvement are the genital organs.10
TL is a worldwide public health problem, with an estimated 350 million people living in endemic regions in 88 countries on 4 different continents (Asia, Africa, Europe, and America).1,2 It is considered by the WHO to be one of the six major neglected infectious diseases,2 based on not only the high incidence but also the potential for destructive, disfiguring, and disabling sequelae.
In Brazil, where the incidence is likely underestimated, the cumulative number of cases of American tegumentary leishmaniasis (ATL) from 1980 to 1990 was 154,000 and from 1985 to 1999 it was 388,000.1,11 The number of cases, however, is increasing because of both ongoing transmission in old foci and outbreaks attributable to an expanding agricultural frontier and encroachment on the outskirts of cities.9
There is a paucity of published data on the clinical and epidemiological profiles of ML. Most of the studies related to the epidemiology of ML present a limited number of cases and do not demonstrate the severity of mucosal disease. Estimate of the frequency of ML varies from 0.4% in southern Brazil to 20% in Bolivia.11 In a recent retrospective study by García Bustos, there was a significant increase in the number of ML cases (35%) between 2000 and 2014 in northwestern Argentina.12 Herein, we report the clinical and epidemiological profiles of ML in an endemic area of L. braziliensis over a period of 20 years.
METHODS
Patients and diagnosis.
This is a retrospective study aimed at evaluating changes in the prevalence and clinical presentation of ML in an area of L. braziliensis transmission over a 20-year period. A total of 15,770 patients with diagnosis of ATL were evaluated at the Corte de Pedra Health Clinic, located in southeastern state of Bahia, Brazil, from 1995 to 2014. This Health Clinic is a reference center for the diagnosis and treatment of ATL. All patients were evaluated by a physician and patients with suspicious mucosal lesions were evaluated by an otolaryngologist. ML was diagnosed based on the presence of a typical ML lesion associated with a positive L. braziliensis polymerase chain reaction (PCR), parasite isolation, or histopathologic findings of ML with a positive intradermal skin test with leishmania antigen (LST). The leishmania antigen used for the intradermal skin test was obtained from an isolate of L. braziliensis and was prepared and used as previously described.13 The indurated area was measured after 48 hours, and a reaction was considered to be positive if the measurement was 5 mm or greater in diameter.
All medical records of 327 patients who had diagnoses of ML were reviewed. Two otolaryngologists reviewed all data from the charts and confirmed the diagnosis of ML. The inclusion criterion was an adequate description of an ML lesion (defined as the presence of nodular or granular lesions, ulcers, infiltration, or perforation at nasal cavity, pharynx, oral cavity, and/or larynx). Exclusion criteria were charts without a clear description of the mucosal disease and patients with disseminated leishmaniasis. Disseminated leishmaniasis was defined by the presence of 10 or more acneiform, papular, and ulcerated lesions on at least two parts of the body.14 In this particular clinical form of ATL, mucosal lesions were observed in up to 40% of the patients and mucosal damage was usually contiguous to a skin lesion. These patients were a distinct subset of ML patients regarding their clinical manifestations and immunologic features15. Information about human immunodeficiency virus (HIV) coinfection was recorded. The prevalence of HIV in the endemic area was very low and since 2005 the serology for HIV was performed routinely in the clinic. This study was approved by the Institutional Review Board of the Federal University of Bahia.
Clinical evaluation and diagnosis of leishmaniasis.
As part of standard care, the otolaryngologist examined the anterior and posterior nose, nasopharynx, and oropharynx. In patients suspected of having more widespread lesions, endoscopies of the nose, pharynx, and larynx were performed with rigid endoscope of 0°angle and 4 mm in diameter or 70° angle and 8 mm in diameter. Patients with ML were classified in stages ranging from I to V according to severity of nasal disease as previously described.16 Stage I is characterized by nodular lesions without ulcerations typically along the cartilaginous septum, nasal floor, and lateral wall. In Stage II, patients had fine granular lesions with superficial ulcerations. Stage III is characterized by a deep ulcer with a more intense granulation and mucosal infiltration. In Stage IV, necrosis of cartilage in the anterior septum with septum perforation is observed. Stage V is characterized by involvement of the nasal pyramid with changes of facial architecture as a consequence of severe tissue destruction. The diagnosis of CL was based on evidence of leishmania infection by parasite isolation or histopathological findings or documentation of DNA for L. braziliensis by PCR or on the presence of a typical skin ulcer associated with a positive intradermal LST. A typical scar was also considered as previous CL. The locations of cutaneous lesions and previous treatment of CL were recorded. Time of CL means the time between presentation of CL and development of ML.
Patients with CL were treated with a 20-day course of intravenous pentavalent antimony (Glucantime®, Rhodia Laboratories, Paris, France) at a dose of 20 mg/kg per day. Patients diagnosed with ML were treated with a 30-day course of pentavalent antimony at a dose of 20 mg/kg per day. Some patients received antimony at the same dose plus pentoxifylline 400 mg three times a day for 30 days. Cure was defined as complete reepithelization of the mucosal tissue and no evidence of inflammatory activity in day 90 after initiation of therapy. Therapeutic failure was defined as presence of active lesions at day 90.
Statistical analysis.
Descriptive statistics for the study population were reported. Continuous variables were expressed as medians (interquartile range [IQR]). Nonparametric tests were used, as the samples did not follow a Gaussian distribution. Years were grouped into two decades or into four categories of 5-year periods. Variables were compared using Mann–Whitney U test, Fisher’s exact test, Pearson’s χ2 test, Mantel–Haenszel trend test, or Spearman correlation as appropriate. All tests were two-tailed and results were considered significant when P < 0.05. Analyses were conducted using Prism (GraphPad Software Inc., San Diego, CA) and SPSS® software, version 20 (IBM Inc., Armonk, NY).
RESULTS
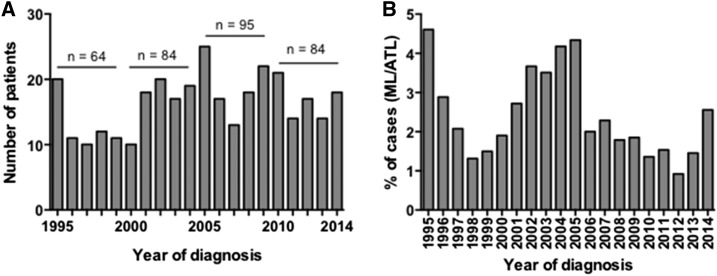
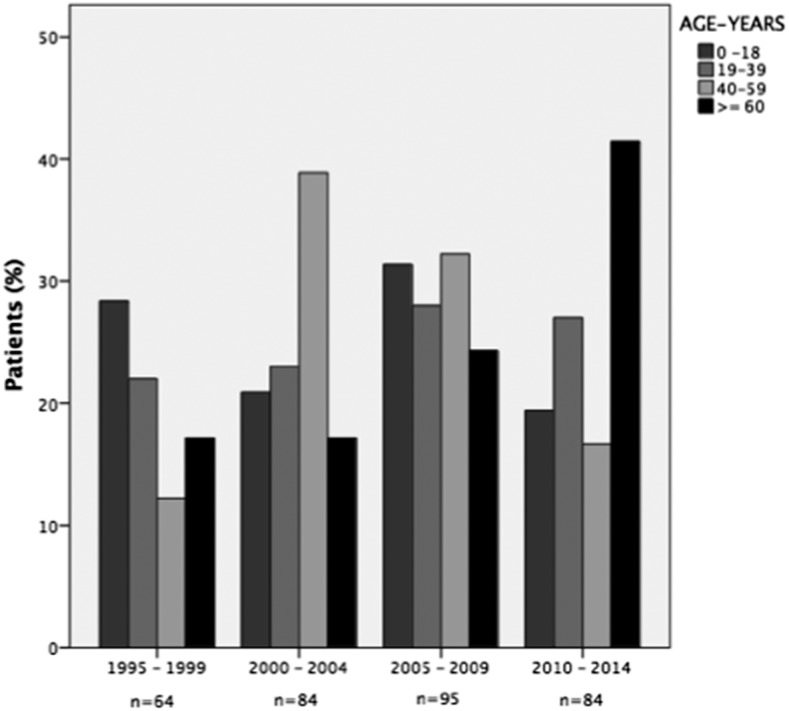
Figure 1A depicts the annual number of ML cases from 1995 to 2014, which totaled 327. There appeared to be some periodicity with peak numbers of cases centering around the years 1995, 2005, 2009, and 2010. The percentages of ML relative to total cases of ATL are shown in Figure 1B. The majority of patients (67%) were male. Age ranged from 8 months to 103 years, with a median age of 38.5 (IQR: 22–58 years). The greatest number of patients was between 19 and 39 years (N = 100 [31%]). Over the 20-year period of the study, the proportion of older patients (greater than 60 years) increased from 19% (12 patients) between 1995 and 1998 to 35% (29 patients) between 2010 and 2014 as demonstrated in Figure 2 (P = 0.02; Mantel–Haenszel trend test). Over time from 2000, the proportion of cases 40–59 years of age is inversely related to the proportion of cases 60 years or older (Figure 2).
Figure 1.
Annual number of patients diagnosed with mucosal leishmaniasis (ML) (A) and percentage of mucosal leishmaniasis from American tegumentary leishmaniasis (ATL) total cases (B) at the Corte de Pedra Health Clinic from 1995 to 2014.
Figure 2.
Age distributions of patients with mucosal leishmaniasis by time period.
Table 1 summarizes the demographic and clinical features of the patients stratified by decade. There was no difference in gender distribution by time period. The median size of the LST was greater in the first decade (P = 0.02; Mann–Whitney U test). There was no association between LST and stage of disease or location of lesions (data not shown). The presence of a concomitant cutaneous lesion or a scar suggestive of past leishmaniasis lesion was detected in 257 (79%) of the 327 patients. The presence of concomitant cutaneous and mucosal lesions increased from 19% in the first decade to 30% between 2005 and 2014 (P < 0.03; Fisher’s exact test). The history of previous cutaneous disease with scar was more frequent in the first decade (P = 0.007; Fisher’s exact test). Of patients with concomitant cutaneous and mucosal lesions, 22 patients (81%) had cutaneous lesions above the waist between 1995 and 2004 (P = 0.008; Fisher’s exact test) and 36 patients (71%) had cutaneous lesions above the waist in the second decade (P = 0.02; Fisher’s exact test) (data not shown). The median number of cutaneous lesions was 1 (IQR: 1–2) in the first decade and 2 (1–2) in the second decade (P < 0.01; Mann–Whitney U test). The median time between the onset or detection of cutaneous lesions and the diagnosis of ML was 6 years (IQR: 0.7–20) between 1995 and 2004, whereas this was 1.5 years (IQR: 0.1–15) between 2005 and 2014 (P = 0.02; Mann–Whitney U test).
Table 1.
Demographic and clinical features of mucosal leishmaniasis over a 20-year period
| 1995–2004 (N = 148) | 2005–2014 (N = 179) | P | |
|---|---|---|---|
| Age (years): median (IQR) | 37.5 (21–52) | 36.5 (20–59) | 0.2* |
| Gender: M (%) | 101 (68%) | 119 (66.5%) | 0.8† |
| LST (mm): median (IQR) | 20 (15–25) | 17.5 (13–22) | 0.02* |
| Duration of ML (days): median (IQR; n) | 180 (60–818; 86) | 90 (30–240; 72) | 0.009* |
| Previous CL‡ | 95 (65%) | 88 (49%) | 0.007† |
| Concomitant CL† | 28 (19%) | 54 (30%) | 0.03† |
| No CL | 29 (19%) | 41 (23%) | 0.5† |
| Number of CL lesions: median (IQR; n) | 1 (1–2; 117) | 2 (1–2; 125) | 0.0001* |
| Localization of CL | |||
| Above the waist (%; n) | 69 (59%; 116) | 73 (58%; 125) | 0.8† |
| Below the waist (%; n) | 47 (41%; 116) | 52 (58%; 125) | 0.8† |
| Face (%, n) | 30 (26%; 116) | 37 (30%; 125) | 0.5† |
| Time of CL (years): median (IQR; n) | 6 (0.7–20; 99) | 1.5 (0.1–15; 125) | 0.02* |
CL = cutaneous lesion; IQR = interquartile range; M = male.
Mann–Whitney U test.
Fisher’s exact test.
Individuals could be counted in both groups.
ML in the absence of a previous or concomitant cutaneous lesions was observed in 21% of the patients, and there was no change in the frequency of these cases over the period of the study. There was no difference between demographic and clinical features of patients with ML without cutaneous lesions or ML with active disease or scar of CL (data not shown). Only three patients had HIV coinfection.
Table 2 summarizes the locations of mucosa lesions. Nasal cavity was the most common site affected followed by pharynx, oral cavity, and larynx. The majority of the patients had only nasal involvement. In those who had mucosal lesions in two sites, the disease was most common in the nose and pharynx, and in patients who had involvement of three sites, the nasal cavity, pharynx, and oral cavity were the most frequently affected sites. There was no significant difference in mucosal lesion location observed over time.
Table 2.
Anatomic location of mucosal lesions
| N = 326 | From 1995 to 2004 | From 2005 to 2014 | P* | |
|---|---|---|---|---|
| Affected sites | ||||
| Nasal cavity | 318 (98%) | 144 (98%) | 174 (97%) | 0.7 |
| Pharynx | 36 (11%) | 21 (14%) | 15 (8%) | 0.1 |
| Oral cavity | 18 (6%) | 6 (4%) | 12 (7%) | 0.3 |
| Larynx | 5 (1.5%) | 3 (2%) | 2 (1%) | 0.6 |
| One site affected | ||||
| Nasal cavity | 279 (86%) | 124 (84%) | 155 (87%) | 0.6 |
| Oral cavity | 7 (2%) | 2 (2.4%) | 6 (3.4%) | 0.3 |
| Two sites affected | 29 (9%) | 15 (10%) | 14 (8%) | 0.5 |
| Three sites affected | 11 (3%) | 6 (4%) | 5 (3%) | 0.5 |
Fisher’s exact test.
ML patients with nasal mucosal disease were classified in stages ranging from I to V according to severity16 (Table 3).The majority of patients were classified as stages II and III. Between 2005 and 2014, patients with mild (stage I) and severe (stage V) disease increased when compared with the first decade (P = 0.018, Pearson’s χ2 test). A direct correlation was observed between the severity of ML (stage of nasal disease) and time between the cutaneous lesion and diagnosis of ML (r = 0.4, P < 0.01; Spearman correlation) and there was also a direct correlation between age and the stage of nasal disease (r = 0.4, P < 0.01; Spearman correlation). Longer duration of ML was associated with more advanced stages of the disease (r = 0.5, P < 0.001; Spearman correlation). The involvement of other anatomical sites (pharynx, oral cavity, and/or larynx) was associated with more severe form of nasal disease (clinical stages), although this finding was not statistically significant (P = 0.051; linear trend test).
Table 3.
Stage of nasal disease over two decades
| 1995–2004 (N = 140) | 2005–2014 (N = 172) | P | |
|---|---|---|---|
| Stage | 0.018* | ||
| I | 7 (5%) | 25 (14.5%) | |
| II | 45 (32%) | 55 (32%) | |
| III | 43 (31%) | 43 (25%) | |
| IV | 35 (25%) | 29 (17%) | |
| V | 10 (7%) | 20 (12%) |
Pearson’s χ2 test.
Of the 327 participants in the study, 175 had prior CL and 53 (30.2%) of them received standard treatment. The frequency of CL cases that received standard therapy was similar in the two periods (Table 4). When comparing severity of mucosal disease, previous treatment of CL did not affect disease severity (median clinical stage III in both groups, IQR: II–IV, P = 0.373), or duration of mucosal disease (median 90 days in both groups, IQR: 30–365 days, P = 0.423).
Table 4.
Treatment of patients with mucosal leishmaniasis
| 1995–2004 (N = 105) | 2005–2014 (N = 146) | P | |
|---|---|---|---|
| Treatment of ML | <0.001† | ||
| Pentavalent antimony 30 days | 98 (93%) | 110 (75%) | |
| Pentavalent antimony + pentoxifylline 30 days | 7 (7%) | 36 (25%) | |
| Time until healing (days), median (IQR) | 75 (60–120) | 90 (60–120) | 0.375* |
| Therapeutic failure | 28 (27%) | 44 (30%) | 0.672† |
| Relapse | 13 (9%) | 12 (7%) | 0.626† |
| Previous treatment of CL (%; n) | 29 (31%; 95) | 24 (27%; 88) | 0.148† |
CL = cutaneous leishmaniasis; IQR = interquartile range; ML = mucosal leishmaniasis.
Mann–Whitney U test.
Fisher’s exact test.
Data about ML treatment were reported in 251 patients (Table 4). Time until healing, therapeutic failure, and relapse rates were similar in both periods.
DISCUSSION
ML has been predominantly associated with infections caused by L. braziliensis, but it is also observed in patients infected by Leishmania amazonensis, Leishmania guyanensis, L. panamensis, and Leishmania peruviania.11,17,18 Diffuse cutaneous leishmaniasis, disseminated leishmaniasis, and ML are the more severe forms of ATL. The severity of mucosal disease is important not only because of the risk of facial disfiguration but also because of the potential rare complication of airway obstruction leading to asphyxia and death.19 Herein, we report the clinical epidemiology of ML in 327 patients diagnosed over a two decade period in an endemic region of northeastern Brazil. We found that the frequency of ML did not change over 20 years, but there was an increase in patients with ML more than 60 years of age, an increase in ML with concomitant cutaneous lesions, a decrease in the period between the documentation of cutaneous lesions and the diagnosis of mucosal disease, and an increase in the proportion of patients presenting with stages I and V of the ML. Moreover analyzing the total number of cases, we found a positive correlation between severity of mucosal disease and both age and the period of time between cutaneous lesion and ML. The absence of previous therapy for CL was not associated with severity of ML and the response to therapy of ML was similar over the period of time.
Fluctuations in the number of cases of both visceral leishmaniasis and CL have been reported to be associated with climate events, viral infections, and earthquakes.20–23 In the same endemic area where this study was performed, an increased incidence of ATL was observed with a periodicity of approximately 10 years.9 Regarding mucosal disease, we also observed a peak in the number of patients in the 10th year and the number of patients seen was higher in the second decade. As the majority of the patients who have mucosal disease have or had CL concomitantly or preceding the mucosal involvement, it is expected that the fluctuations in the number of cases with CL affected the periodicity of ML cases.
ATL is a disease predominantly of male adults. Physicians who have worked for up to 30 years in the Health Post of Corte de Pedra, however, have observed an increase in the number of children and women with ATL. Although we did not find an increase in children or women with mucosal disease, over the study period, there was an increase in the proportion of patients age 60 years and older. We know that infected sandflies can be documented inside of houses and in peridomiciliary areas,24 which might increase the risk of exposure among children, women, and older adults who are more likely to spend time in these areas compared with young adult men who tend to do daytime agricultural work. The lack of an increase in the number of ML cases in children and women, however, makes it unlikely that the observed trend in older adults is related to a change in the transmission pattern. It is possible that an increase in the number of cases with CL over the study period may have contributed to the more frequent appearance of mucosal disease in the older age group. Alternatively, an impairment in the immune response may account for this finding. Recently, we showed that elderly patients with CL produce more interleukin 10, had more frequently a history of a previous episode of CL, and a higher frequency of mucosal disease than young controls.25
Although the frequency of patients with history of CL decreased over time, there was an increase in the proportion of patients with concomitant cutaneous and mucosal lesions. Since we have systematically performed nose, mouth, and through examination in all patients with cutaneous lesions above the waist in the last 15 years of the study period, it is unlikely that the increase in mucocutaneous lesions is due to a detection bias. We have observed that L. braziliensis is polymorphic in this endemic area and that there is an association between genetic profiles of the parasites and clinical forms of the disease.26 This may explain the increase in cases of patients with cutaneous and mucosal lesions. One important finding of this study was the high rate of patients with ML without history of CL. Clinically, we found no difference between patients with mucosal disease with or without history of cutaneous lesions. We do not believe that individuals who had a subclinical L. braziliensis may develop ML later. In contrast to ML patients who have an exaggerated inflammatory response, subjects with subclinical L. braziliensis infection have a downmodulated immune response.27 It is likely that difference among parasites strains may explain the tropism for the nasal mucosa in patients who had ML without previous CL.
It is well known that up to 90% of ML cases have nasal involvement. In the last 15 years, we began to stratify nasal lesions into stages, a classification that was recently validated.16 Herein we observed an increase in the frequency of patients with both stage I and stage V nasal lesions over the study period. Although the increase in the frequency of patients with initial and mild lesions was probably due to the active search for mucosal disease in the last 15 years of the study period, the increase in number of patients with stage V was unexpected. Although we found a positive correlation between age and the stage of nasal disease, we also found an increase in the total number of patients with ML more than 60 years of age in the last 10 years of the study period. Future studies should be performed to evaluate the influence of age on the severity of ML.
The presence of lesions in the pharynx and/or larynx in association with nasal involvement is a sign of severe disease. Severe nasal disease, however, may not be associated with pharyngeal and laryngeal involvement likely representing different facets of disease severity. Of note, there has been greater use of newer and more efficacious therapies for cutaneous and ML over the past 20 years, including amphotericin B, miltefosine, combination of antimony and pentoxifylline, and antimony with granulocyte/macrophage colony-stimulating factor. Despite increase in use of these new therapies, which are more effective than antimony, and decrease in the healing time of mucosal disease,28 the frequency of leishmaniasis involving the pharynx and larynx did not change in the study period. There was also an increase in the frequency of cases with more severe nasal involvement. It is possible that severity of mucosal disease may be matched to the genotypic characteristics of the parasite26 or the presence of leishmania RNA virus.29,30 Therefore, studies should be designed to evaluate both genotypic differences between isolates from cases of severe mucosal disease compared with those from patients with stage II or III mucosal disease, and differences in the presence of leishmania RNA virus.
ML is usually observed in less than 5% of ATL cases, but due to severity of the disease, this is an important clinical form of ATL. This study showed that although some data are consistent with the published literature, there were changes in the clinical and epidemiological profile of ML over a 20-year period, with an increase in late-stage nasal lesions, as well an increase in the number of affected elderly. This reinforces the concept that ML should not be underestimated.
Acknowledgments:
We thank the health-care professionals who provided outstanding medical care at the Corte de Pedra field site. We also thank Cristiano Franco for secretarial assistance.
REFERENCES
- 1.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M; WHO Leishmaniasis Control Team, 2012. Leishmaniasis worldwide and global estimates of its incidence. PloS One 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, 2010.. Control of Leishmaniasis: Report of Who Expert Committee. Geneva, Switzerland: World Health Organization, 1–15. [Google Scholar]
- 3.Boaventura VS, Café V, Costa JML, Oliveira F, Bafica ALB, Rosato AB, Freitas LAR, Brodskyn CI, Barral MN, Barral AMP, 2006. Short report: concomitant early mucosal and cutaneous leishmaniasis in brazil. Am J Trop Med Hyg 75: 267–269. [PubMed] [Google Scholar]
- 4.Osorio LE, Castillo CM, Ochoa MT, 1998. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg 59: 49–52. [DOI] [PubMed] [Google Scholar]
- 5.Lessa MM, Lessa HA, Oliveira A, Castro TWN, Scherifer A, Machado P, 2007. Mucosal leishmaniasis: epidemiological and clinical aspects. Rev Bras Otorrinolaringol 73: 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsden PD, Llanos-Cuentas A, Lago EL, Cuba-Cuba AC, Barreto AC, Costa JM, Jones TC, 1984. Human mucocutaneous leishmaniasis in Três Braços, Bahia, Brazil. An area of Leishmania braziliensis braziliensis transmission. III. Mucosal disease: presentation and initial evolution. Rev Soc Bras Med Trop 17: 179–186. [Google Scholar]
- 7.Falqueto A, Sessa PA, Ferreira AL, Vieira VP, Santos CB, Varejão JBM, Cupolillo E, Porrozzi R, Carvallho-Paes LE, Grimaldi G, Jr, 2003. Epidemiological and clinical features of Leishmania (Viannia) braziliensis American cutaneous and mucocutaneous leishmaniasis in the State of Espirito Santo, Brazil. Mem Inst Oswaldo Cruz 98: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 8.Jones TC, et al. , 1987. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis 156: 73–83. [DOI] [PubMed] [Google Scholar]
- 9.Jirmanus L, Glesby MJ, Guimarães LH, Lago E, Rosa ME, Machado PR, Carvalho EM, 2012. Epidemiological and clinical changes in American tegumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20-year period. Am J Trop Med Hyg 86: 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osório RC,, et al. , 2009. Tegumentary leishmaniasis caused by Leishmania Viannia braziliensis in genital organs. Gaz Méd Bahia 79 (Suppl 3): 91–94. [Google Scholar]
- 11.Ministério da Saúde do Brasil, 2007. Manual da Vigilância da Leishmaniose Tegumentar Americana, 2a edição. Brasília (DF): Editora do Ministério da Saúde.
- 12.García Bustos MF,, et al. , 2015. Clinical and epidemiological features of leishmaniasis in northwestern-Argentina through a retrospective analysis of recent cases. Acta Trop 154: 125–132. [DOI] [PubMed] [Google Scholar]
- 13.Reed SG, Badaró R, Masur H, Carvalho EM, Lorenco R, Lisboa A, Teixeira R, Johnson WD, Jr, Jones TC, 1986. Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg 35: 79–85. [DOI] [PubMed] [Google Scholar]
- 14.Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, Mobashery N, Johnson WD, Jr, Carvalho EM, 2002. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis 186: 1829–1834. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho EM, Barral A, Costa JM, Bittencourt A, Marsden P, 1994. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop 56: 315–325. [DOI] [PubMed] [Google Scholar]
- 16.Lessa HA, Lessa MM, Guimarães Lima CM, Arruda S, Machado PR, Carvalho EM, 2012. A proposed new clinical staging system for patients with mucosal leishmaniasis. Trans R Soc Trop Med Hyg 106: 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silveira FT, Lainson R, Brito AC, Oliveira MRF, Paes MG, Souza AAA, Silva BM, 1997. Leishmaniose tegumentar Americana. In: Leão RNQ. Doenças Infecciosas e Parasitárias: Enfoque Amazônico. Belém: Editora CEJUP. [Google Scholar]
- 18.Osorio LE, Castillo CM, Ochoa MT, 1998. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg 59: 49–52. [DOI] [PubMed] [Google Scholar]
- 19.Marsden PD, 1994. Mucosal leishmaniasis due to Leishmania (Viannia) braziliensis L(V)b in Três Braços, Bahia-Brazil. Rev Soc Bras Med Trop 27: 93–101. [DOI] [PubMed] [Google Scholar]
- 20.Franke CR, Ziller M, Staubach C, Latif M, 2002. Impact of the El Niño/Southern Oscillation on visceral leishmaniasis, Brazil. Emerg Infect Dis 8: 914–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebêlo JM, 2008. El Niño episodes and temporal distribution of kala azar on São Luís island, Maranhão State, Brazil. Cad Saude Publica 24: 1713–1714. [DOI] [PubMed] [Google Scholar]
- 22.Lewnard JA, Jirmanus L, Júnior NN, Machado PR, Glesby MJ, Ko AI, Carvalho EM, Schriefer A, Weinberger DM, 2014. Forecasting temporal dynamics of cutaneous leishmaniasis in northeast Brazil. PLOS Negl Trop Dis 8: e3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schriefer A,, et al. , 2004. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American tegumentary leishmaniasis. Infect Immun 72: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho AM, Amorim CF, Barbosa JLS, Lago AS, Carvalho EM, 2015. Age modifies the immunologic response and clinical presentation of American tegumentary leishmaniasis. Am J Trop Med Hyg 92: 1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machado PRL, Lessa H, Lessa M, Guimarães LH, Bang H, Ho JL, Carvalho EM, 2007. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis 44: 788–793. [DOI] [PubMed] [Google Scholar]
- 26.Queiroz A, Souza R, Heline C, Cardoso M, Guimarães LH, Machado PRL, Carvalho EM, Riley LW, Wilson ME, Shriefer A, 2012. Association between an emerging disseminated form of Leishmaniasis and Leishmania (Viannia) braziliensis strain polymorphisms. J Clin Microbiol 50: 4028–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muniz AC, Bacellar O, Lago EL, Carvalho AM, Carneiro PP, Guimarães LH, Rocha PN, Carvalho LP, Glesby M, Carvalho EM, 2016. Immunologic markers of protection in Leishmania (Viannia) braziliensis infection: a 5-year cohort study. J Infect Dis 214: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ives A, , et al. , 2011. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 331: 775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantanhêde LM, da Silva Júnior CF, Ito MM, Felipin KP, Nicolete R, Salcedo JM, Porrozzi R, Cupolillo E, Ferreira R de G, 2015. Further evidence of an association between the presence of Leishmania RNA virus 1 and the mucosal manifestations in tegumentary leishmaniasis patients. PLoS Negl Trop Dis 15: e0004079. [DOI] [PMC free article] [PubMed] [Google Scholar]