Abstract.
Visceral leishmaniasis (VL) is a chronic parasitic disease associated with suppressed T cell responses. Although parasites reside intracellularly in macrophages during chronic VL, neutrophils are the first host cell to infiltrate the infection site and phagocytose the parasite. Subsets of neutrophils with unusual characteristics have been documented in human VL, but whether the total neutrophil population is aberrant during disease is not known. Therefore, we examined phenotypic characteristics of unfractionated polymorphonuclear leukocyte (neutrophils) from subjects with active VL, and compared these with neutrophils from healthy controls or subjects who have been treated for VL. The data showed decreased mRNA and diminished amounts of the neutrophil chemoattractant CXCL8 (interleukin [IL]-8), increased IL-10 mRNA and protein, and elevated transcripts encoding arginase-1, which is involved in suppressing T cell responses. Neutrophils from VL subjects showed enhanced capacity to phagocytose Leishmania spp. promastigotes. The results suggest that neutrophils may contribute to immunosuppression in subjects with active VL.
Visceral leishmaniasis (VL) is a potentially fatal vector-borne parasitic disease prevalent in populations afflicted by poverty and malnutrition. The regions of highest incidence include India, Bangladesh, Ethiopia, Sudan, South Sudan, and Brazil.1,2
Neutrophils have been implicated as first responders at the site of infection in murine models of both cutaneous and VL, possibly serving as conduits for transferring parasites to macrophages where they survive long term.3,4 Known for their ability to phagocytose and kill microbes, recent data emphasize additional roles for neutrophils as immune system modulators that interact either directly or through cytokines with other hematopoietic elements.6 In the case of VL, it is hypothesized that a low-density subset of circulating neutrophils contributes to suppression of T cell responses due to their high arginase content.7 Our prior studies of Indian VL and parallel studies of Brazilians with cutaneous leishmaniasis have shown expansion of a subset of polymorphonuclear leukocyte (neutrophils) (PMNs) that is partially activated and lower density than conventional PMNs, presumably due to loss of granule contents.8
Adaptive immune responses are ineffective at clearing Leishmania spp. parasites during acute VL.9 We hypothesized that the dysfunctional systemic immune responses would correlate with an altered functional state of neutrophils. Although unusual subsets of neutrophils are reported in VL, it is not clear whether all or the majority of neutrophils are impaired in subjects with leishmaniasis. Thus the purpose of this study was to examine phenotypic characteristics of unfractionated PMNs from subjects with active VL, and compare these with neutrophils from healthy controls or subjects who have been treated for VL.
Human subjects recruited into this study included inpatients with VL at the Kala-Azar Medical Research Center in Muzaffarpur, Bihar, India. A diagnosis of VL was made by suggestive symptoms, positive serologic test for rK39,10 and in most cases detection of amastigotes in a splenic aspirate. A total of 37 patients, all of whom were human immunodeficiency virus (HIV)-negative and more than 6 years of age, were included. No serious complications or deaths occurred in these patients. Additional data from the charts of 572 VL patients were reviewed. Endemic control subjects (ECs) were healthy household members of patients (N = 16). Study protocols were approved by Institutional Review Boards (IRBs) at Banaras Hindu University, the University of Iowa, and the National Institutes of Health. The Banaras Hindu University IRB is registered with the NIH. All subjects and/or guardians of children under age 18 provided written informed consent, and children ages 12–17 signed an assent form.
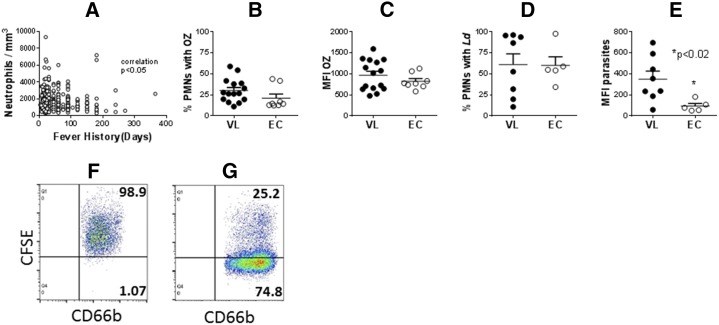
Neutropenia, a well-documented feature of VL,11 was confirmed in subjects admitted to KAMRC. Plotting against duration of fever according to the patient’s history, the degree of neutropenia was significantly correlated with the duration of fever (Figure 1A).
Figure 1.
Neutrophil abundance and phagocytic capacity. (A) Peripheral blood neutrophil counts from subjects with visceral leishmaniasis (VL) were plotted against the number of days of fever according to the patient history. Data were analyzed with Spearman nonparametric correlation. (B–E) polymorphonuclear leukocyte (neutrophils) (PMNs) were isolated from peripheral blood of subjects with acute VL or from endemic controls (EC). PMNs were incubated with either FITC-labeled opsonized zymosan (OZ) or opsonized Leishmania donovani promastigotes for 30 minutes. The percentage of CD66b + cells with fluorescence indicating associated OZ (B) or parasites (D), or the mean fluorescence index of FITC-OZ (C) or carboxyfluorescein succinimidyl ester-parasites (E) were quantified by flow cytometry. N = 24 VL and 8 EC subjects (B and C) or 8 VL and 5 EC subjects (D and E). Statistical analyses were performed with unpaired t test. (F) and (G) show representative dot plots of CD66b neutrophils with CFSE-labeled intracellular L. donovani from (F) a subject with VL or (G) an endemic control.
The ability of PMNs to phagocytose fluorescent particles was quantified by flow cytometry. Leishmania promastigotes were stained with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) and used to assess phagocytosis as in our prior publication.12 Neutrophils were isolated on a Ficoll density gradient after erythrocyte sedimentation with Dextran.13 We previously reported that dextran eliminates the low-density neutrophil population,14 so the population under study included mostly normal density neutrophils depleted of the low-density subset. Neutrophils isolated by this procedure contained 86.2% ± 4.5% neutrophils, assessed by flow cytometry (N = 4). PMNs were incubated with fluorescein isothiocyante (FITC)-labeled opsonized zymosan (OZ; Invitrogen Cat: Z2841) or CFSE-labeled Leishmania donovani promastigotes under conditions favoring phagocytosis (37°C, 5% CO2, 30 minutes), at a 2:1 or 5:1 particle:PMN ratio, respectively. Neutrophils were stained with BD Anti Human CD66b (BD Biosciences, Billerica, MA, Cat. No. 555724) and analyzed on a FACS Calibur flow cytometry (Model 4CS, BD Biosciences).
Fluorescence associated with CD66b+ PMNs was quantified both as the percentage of PMNs with green fluorescence, which correlates with the percentage of neutrophils that were infected (Figures 1B and D), and the mean fluorescence index of the PMN population (Figures 1C and E), which reflects the relative burden of infection.12 Phagocytosis by PMNs from VL patients or ECs was compared.
The difference between the proportion of PMNs that phagocytosed OZ in PMNs from VL subjects versus ECs did not reach statistical significance (Figure 1B), although there was a trend toward higher phagocytosis in VL subject PMNs. The two outliers in the EC group accounted for the lack of significance at the alpha = 0.05 level. The percentage of neutrophils that phagocytosed parasites was not different between VL subjects and ECs (Figure 1D), but the parasite burden was significantly higher in PMNs from VL patients compared with ECs (Figure 1E), suggesting a difference in the phagocytic capacity. Representative plots of neutrophils with intracellular parasites from a subject with VL or an endemic control are shown in panels F and G of Figure 1, respectively. Phagocytosis of Leishmania spp. by macrophages is facilitated via macrophage surface receptors, and differences in receptors used have been implicated in differential uptake in prior studies.15,16 One possible explanation for the enhanced uptake of promastigotes but not OZ by VL subject PMNs would be a difference in receptor-mediated phagocytosis.
Activated neutrophils produce an array of cytokines and chemokines.5 Despite studies documenting inflammatory cytokines produced by T cells and macrophages from individuals with VL,17 there is a paucity of information on proteins produced by PMNs. Therefore, we purified PMNs from VL subjects or EC by positive selection with biotinylated CD66abce Microbeads using a Magnetic LS column (Miltenyi Biotec, Aubern, CA). Although this method does not expose neutrophils to dextran and yields both low- and high-density neutrophils, the method was preferred because it isolates > 97% pure neutrophils, minimizing contaminating monocyte transcripts. Cells were suspended in 500 μL of RNA Later (Qiagen, Germantown, MD) and total RNA was isolated using the RNeasy minikit and Qiashredder homogenizers (Qiagen). cDNA was synthesized using high-capacity cDNA Archive kit with random primers and Multiscribe TM MuLV reverse transcriptase (Applied Biosystems, Carlsbad, CA). Differential gene expression was quantified using an ABI Prism 7900HT real-time PCR system (Applied Biosystems), with primers listed in Table 1. mRNA quantities were calculated with the △△CT method, with β2 microglobulin mRNA as an endogenous control. △Ct values of patients were compared with the mean △Ct of the same transcript in EC cells. Results are shown as the fold change (2−△△CT) between VL subjects and EC subjects.
Table 1.
Primers used for qPCR. SYBR green was incorporated into qPCR reactions with the primers listed below, using cDNA prepared from PMN samples as described in the text
| Transcript | Forward primer | Reverse primer |
|---|---|---|
| ARG-1 | 5′-AAGCAGACCAGCCTTTCTCA-3′ | 5′-GCCAAGTCCAGAACCATAGG-3′ |
| CTLA4 | 5′-TGGAGATGCATACTCACACACA-3′ | 5′-TTCATCCCTGTCTTCTGCAA-3′ |
| CXCL8 | 5′-CTGGCCGTGGCTCTCTTG-3′ | 5′-CCTTGGCAAAACTGCACCTT-3′ |
| IL1-β | 5′-ACGAATCTCCGACCACCACT-3′ | 5′-CCATGGCCACAACAACTGAC-3′ |
| IL10 | 5′-GGTGATGCCCCAAGCTGA-3′ | 5′-TCCCCCAGGGAGTTCACA-3′ |
| IL12p40 | 5′-CGGTCATCTGCCGCAAA-3′ | 5′-CAAGATGAGCTATAGTAGCGGTCCT-3′ |
| IFN-γ | 5′-CCAACGCAAAGCAATACATGA-3′ | 5′-CGCTTCCCTGTTTTAGCTGC-3′ |
| β-Defensin | 5′-GCAGTGGAGGGCAATGTCTC-3′ | 5′-TTCCCTCTGTAACAGGTGCCTT-3′ |
| β2-MG | 5′-CTCCGTGGCCTTAGCTGTG-3′ | 5′-TTTGGAGTACGCTGGATAGCCT-3′ |
| CXCL9 | 5′-TGCAAGGAACCCCAGTAGTGA-3′ | 5′-GGTGGATAGTCCCTTGGTTGG-3′ |
| CXCL10 | 5′-TGAAATTATTCCTGCAAGCCAA-3′ | 5′-CAGACATCTCTTCTCCCCTTCTTT-3′ |
| CXCR1 | 5′-AGG GGC CAC ACC AAC CTT CTG-3′ | 5′-AGT GCC TGC CTC AAT GTC TCC A-3′ |
| CCL4/MIP-1 | 5′-CTGCTCTCCAGCGCTCTCA-3′ | 5′-GTAAGAAAAGCAGCAGGCGG-3′ |
| CCL5/RANTES | 5′-GACACCACACCCTGCTGCT-3′ | 5′-TACTCCTTGATGTGGGCACG-3′ |
IFN = interferon; IL = interleukin; PMN = polymorphonuclear leukocyte (neutrophil); qPCR = quantitative polymerase chain reaction.
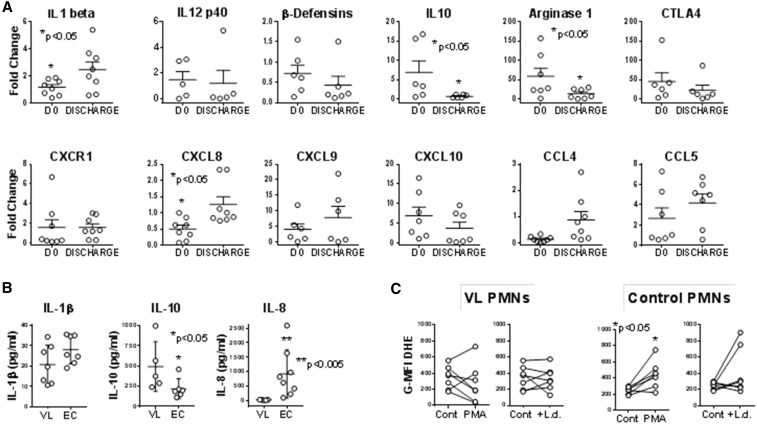
Comparisons of PMN transcript abundance in subjects with VL during acute disease and after treatment, both shown as a fold-change compared with endemic control PMNs, are shown in Figure 2. Three transcripts encoding proteins associated with enhanced inflammatory responses, interleukin (IL)-β and CCL4, were expressed at lower levels during acute disease but increased significantly after recovery. IL-1β promotes production of CXCL8 (IL-8), which in turn is a chemoattractant for neutrophils to sites of infection or injury; thus the coincident decreases in IL-1β and CXCL8 transcripts are logical.18
Figure 2.
Neutrophil transcripts, protein, and oxidation. (A) Transcripts. Neutrophils were separated from blood of subjects with visceral leishmaniasis (VL) either prior to VL or after treatment (Tx) or from endemic control (EC) subjects using the Miltenyi CD66abce microbead kit. cDNA was generated and used to quantify transcripts using SYBR green with the primers listed in Table 1. Data show fold change in polymorphonuclear leukocyte (neutrophils) (PMNs) from VL subjects pre- or posttreatment relative to expression of the same gene in PMNs from controls (EC). All CT values were normalized to the abundance of β2 microglobulin as an endogenous constitutively expressed reference. Statistical analyses were done by paired t test. N = 7 VL and seven EC subjects. (B) Proteins secreted by neutrophils were detected by enzyme-linked immunosorbent assay (ELISA). Neutrophils from subjects with VL or ECs were incubated in culture for 6 hours, supernatants were collected and analyzed by ELISA. The data show the amounts of interleukin (IL)-1β, IL-10, or IL-8 protein in supernatants. (C) Oxidation of dihydroethidium (DHE) was documented in neutrophils exposed to stimuli. Isolated PMNs from subjects with acute VL prior to treatment, or from EC subjects were incubated with buffer (Cont), 5 μg/mL phorbol myristate acetate (PMA), or 5:1 opsonized Leishmania donovani promastigotes (+L.d.) for 15 minutes, after which 5 μM DHE (Molecular Probes) was added. DHE oxidation was quantified in CD66b + PMNs by flow cytometry, and plotted as the geometric mean fluorescence index of oxidized DHE fluorescence. N = 7 VL and 7 EC subjects.
Among the transcripts encoding proteins associated with immunosuppression, significantly more arginase-1 and IL-10 mRNAs were detected in PMNs from VL subjects before versus after treatment (Figure 2E). Transcripts encoding microbicidal proteins myeloperoxidase (MPO) or beta defensins did not change. The major source of arginase in peripheral blood cells is PMNs, and arginase has been implicated as a suppressor of T cell responses in PMNs of subjects with VL.7,19 Thus this increase could be associated with the suppressed adaptive cellular responses characteristic of human VL.17,20
The functional capacity of PMNs isolated after dextran sedimentation of erythrocytes and Ficoll purification was examined. Neutrophils were further purified by positive selection on CD66abc microbeads, exposed to 5:1 promastigotes or buffer for 6 hours. Supernatants were collected and concentrations of IL-1β, IL-10, and IL-8 (CXCL-8) were measured by enzyme-linked immunosorbent assay (Figure 2B). The amount of IL-10 was significantly higher, and IL-8 was significantly lower in supernatants of neutrophils from subjects with VL compared with ECs.
The ability of these neutrophils to generate oxidants was estimated by incubation in dihydroethidium, and detected by flow cytometry. Dihydroethidium fluoresces blue in its reduced state. The compound can be oxidized intracellularly yielding a red fluorescent dye that intercalates into cellular DNA, a response that occurs in the presence of superoxide (.O2−). Dihydroethidium can also participate in competing reactions with heme proteins complicating interpretations, but dihydroethidium (DHE) oxidation in response to a single stimulus such a phagocytosis or PMA can reflect oxidant production.21
Shown as the mean fluorescence index in CD66b + PMNs from VL subjects or ECs, the data showed that exposure to 5 μg/mL phorbol myristate acetate (PMA) or L. donovani (Figure 2C) revealed no significant differences between DHE oxidation by VL neutrophils after either PMA or L. donovani exposure compared with unstimulated PMNs. However, control neutrophils from healthy subjects were able to oxidize significantly more DHE in the presence of PMA. There were no significant differences in DHE oxidation by neutrophils from VL subjects, either after phagocytosis or PMA exposure. The basal level of DHE oxidation seems higher in VL compared with control neutrophils, tempting one to question whether the basal redox state was different between VL and control subject neutrophils. However this could also be explained by different levels of MPO protein in VL subject PMNs, precluding drawing any conclusions.
The data presented in this short report indicate that PMNs from subjects with VL do indeed have different characteristics from control subjects. Studies of transcript abundance and secreted cytokines suggest enhanced expression of the suppressive protein IL-10, and decreased IL-8 mRNA and protein. Transcripts encoding arginase-1 are augmented, and oxidation of dihydroethidium was not detected despite intact phagocytic capacity. The observed changes in gene expression reverse after successful treatment. Literature reports document unusual PMN subsets in VL patients.7,8 The data presented herein are consistent with a contribution of the neutrophil population in VL subjects to the immunosuppressive state during acute VL, that recovers after successful therapy.
Acknowledgments:
This work was supported in part by Tropical Medicine Research Center P50 AI-074321 from the NIH, and NIH grants R01 AI076233 and R01 AI045540 (MEW). Financial assistance by the Council of Scientific and Industrial Research is gratefully acknowledged.
REFERENCES
- 1.World Health Organization, 2016. Leishmaniasis Available at: http://www.who.int/topics/leishmaniasis/en/.
- 2.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters NC, Sacks DL, 2009. The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cell Microbiol 11: 1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charmoy M, Auderset F, Allenbach C, Tacchini-Cottier F, 2010. The prominent role of neutrophils during the initial phase of infection by Leishmania parasites. J Biomed Biotechnol 2010: 719361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mocsai A, 2013. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 210: 1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A, 2013. Neutrophils in innate and adaptive immunity. Semin Immunopathol 35: 377–394. [DOI] [PubMed] [Google Scholar]
- 7.Abebe T, et al., 2013. Arginase activity: a marker of disease status in patients with visceral leishmaniasis in ethiopia. PLoS Negl Trop Dis 7: e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Davis RE, Srivastva S, Nylen S, Sundar S, Wilson ME, 2016. A subset of neutrophils expressing markers of antigen-presenting cells in human visceral leishmaniasis. J Infect Dis 214: 1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta G, Oghumu S, Satoskar AR, 2013. Mechanisms of immune evasion in leishmaniasis. Adv Appl Microbiol 82: 155–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boelaert M, et al., 2008. Diagnostic tests for kala-azar: a multi-centre study of the freeze-dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Trans R Soc Trop Med Hyg 102: 32–40. [DOI] [PubMed] [Google Scholar]
- 11.Jeronimo SMB, Sousa AdQ, Pearson RD, 2006. Leishmaniasis. Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases. Philadelphia, PA: Elsiever, Inc., 1095–1113. [Google Scholar]
- 12.Chang HK, Thalhofer C, Duerkop BA, Mehling JS, Verma S, Gollob KJ, Almeida R, Wilson ME, 2007. Short term fluorescent labeling of a protozoan parasite to assess phagocytosis and oxidant generation. Infect Immun 75: 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalf JA, Gallin JI, Nauseef WM, Root RK, 1986. Laboratory Manual of Neutrophil Function. New York, NY: Raven Press. [Google Scholar]
- 14.Davis RE, Sharma S, Conceicao J, Carneiro P, Novais F, Scott P, Sundar S, Bacellar O, Carvalho EM, Wilson ME, 2017. Phenotypic and functional characteristics of HLA-DR+ neutrophils in Brazilians with cutaneous leishmaniasis. J Leukoc Biol 101: 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno N, Bratt CL, Rodriguez NE, Wilson ME, 2009. Differences in human macrophage receptor usage, lysosomal fusion kinetics and survival between logarithmic and metacyclic Leishmania infantum chagasi promastigotes. Cell Microbiol 11: 1827–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno N, Wilson ME, 2012. Receptor-Mediated Phagocytosis of Leishmania: implications for intracellular survival. Trends Parasitol 28: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar R, Nylen S, 2012. Immunobiology of visceral leishmaniasis. Front Immunol 3: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim GY, Lee JW, Ryu HC, Wei JD, Seong CM, Kim JH, 2010. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J Immunol 184: 3946–3954. [DOI] [PubMed] [Google Scholar]
- 19.Munder M, et al., 2006. Suppression of T-cell functions by human granulocyte arginase. Blood 108: 1627–1634. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho EM, Teixeira RS, Johnson JWD, 1981. Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun 33: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nauseef WM, 2014. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim Biophys Acta 1840: 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]