Abstract.
Soil-transmitted helminths, or parasitic intestinal worms, are among the most prevalent and geographically widespread parasitic infections in the world. Accurate diagnosis and quantification of helminth infection are critical for informing and assessing deworming interventions. The Kato–Katz thick smear technique, the most widely used laboratory method to quantitatively assess infection prevalence and infection intensity of helminths, has often been compared with other methods. Only a few small-scale studies, however, have considered ways to improve its diagnostic sensitivity. This study, conducted among 4,985 school-age children in an area of rural China with moderate prevalence of helminth infection, examines the effect on diagnostic sensitivity of the Kato–Katz technique when two fecal samples collected over consecutive days are examined and compared with a single sample. A secondary aim was to consider cost-effectiveness by calculating an estimate of the marginal costs of obtaining an additional fecal sample. Our findings show that analysis of an additional fecal sample led to increases of 23%, 26%, and 100% for Ascaris lumbricoides, Trichuris trichiura, and hookworm prevalence, respectively. The cost of collecting a second fecal sample for our study population was approximately USD4.60 per fecal sample. Overall, the findings suggest that investing 31% more capital in fecal sample collection prevents an underestimation of prevalence by about 21%, and hence improves the diagnostic sensitivity of the Kato–Katz method. Especially in areas with light-intensity infections of soil-transmitted helminths and limited public health resources, more accurate epidemiological surveillance using multiple fecal samples will critically inform decisions regarding infection control and prevention.
INTRODUCTION
Soil-transmitted helminth (STH) infections are among the most prevalent and geographically widespread parasitic infections in the world. According to the World Health Organization (WHO), around 24% of the world population is currently infected.1 In developing countries, approximately one billion people are infected with at least one of the four common species of STHs: roundworm, Ascaris lumbricoides; whipworm, Trichuris trichiura; and the hookworms, Necator americanus and Ancylostoma duodenale.2 Furthermore, STH infection has significant health costs, with symptoms ranging between weakness, blood loss, and anemia to cognitive and physical impairment.1
Detection of helminth infection is critical for epidemiologic surveillance and treatment plans. Accuracy is especially important because public health officials rely on infection statistics to determine where and when to implement a deworming intervention. Epidemiological assessment of helminth infection usually examines two components: prevalence, the proportion of infected persons in the population at a given time, as well as the intensity of infection, the number of eggs per gram (EPG) of feces. Parasitological diagnosis of infection is performed with microscopic techniques that detect helminth eggs in fecal samples. The Kato–Katz thick smear technique, recommended by WHO, is the most widely used laboratory method to quantitatively assess both infection prevalence and infection intensity of STHs.3 The Kato–Katz method involves microscopic examination of a fecal sample to count the number and species of eggs that are present.4
The technique is particularly suited for diagnosis of helminths in endemic countries because the protocol is low cost, simple to perform, and easy to reproduce.3 Various studies have compared the Kato–Katz technique to other methods and confirmed its high sensitivity for diagnosis of STHs, although the degree of sensitivity varies among the different species of helminths.5–11 A meta-analysis of diagnostic tests for STH infections assessed the sensitivity of the Kato–Katz technique and found that the estimated sensitivity of the test ranged from 63.0% for hookworm, to 64.6% for A. lumbricoides, to 84.8% for T. trichiura.12 Another analysis by a different research team found that sensitivity of the Kato–Katz technique was 65.2% for hookworm, 96.9% for A. lumbricoides, and 91.4% for T. trichiura.13
A key critique of the Kato–Katz technique is that it may be less sensitive in a setting of lower intensity STH infections.14,15 One reason for this reduced sensitivity points to the cyclical egg-laying nature of helminths, which results in daily fluctuation in the quantity of eggs excreted in the feces.16,17 For a female A. lumbricoides, the daily egg production may vary between 73,000 and 227,000 eggs on six consecutive days.18 For T. trichiura and hookworm, however, a much smaller number of helminth eggs are excreted over days and may escape detection in the small quantity of feces examined with the Kato–Katz smear.14 Thus, there is a high probability of false negative test results (diminished sensitivity) with the diagnostic method when only one fecal sample is obtained.
Therefore, in theory, obtaining multiple fecal samples can increase the sensitivity of the Kato–Katz technique. Indeed, a few studies have documented an increase in the detection of helminths by obtaining multiple fecal samples collected over several consecutive days.13–15,19,20 These studies, however, had relatively small samples, with sample sizes ranging between 101 and 342.14,15,19 The two largest existing studies reached sample sizes of N = 1,05520 and N = 2,14913 individuals. Further, while some of the studies used up to five fecal samples,15 none of them provided a cost-benefit analysis. Without cost analysis, we know little about the marginal monetary costs of adding samples compared with the marginal gains in sensitivity of each extra sample.
In this study, we address the two gaps in the current literature. In particular, we consider a large sample of 6,720 school-age children in rural Guizhou Province, where prevalence of STH infection is endemic.21 For the 4,895 children who provided two stool samples, we compare the prevalence obtained by the Kato–Katz thick smear technique when examining two fecal samples collected over consecutive days versus a single fecal sample.
Furthermore, we offer insight into the cost-effectiveness of multiple fecal sampling. Despite the size of the study, we brought in necessary structures that were not already in place. These structures included hired local health practitioners for fecal sample collection and transportation for the fecal samples, as well as payment for laboratory workers. By setting in place such logistics from the ground up, and monitoring their costs, we are able to estimate the cost-effectiveness of obtaining an additional sample in a real-world helminth surveillance scenario.
METHODS
Study population.
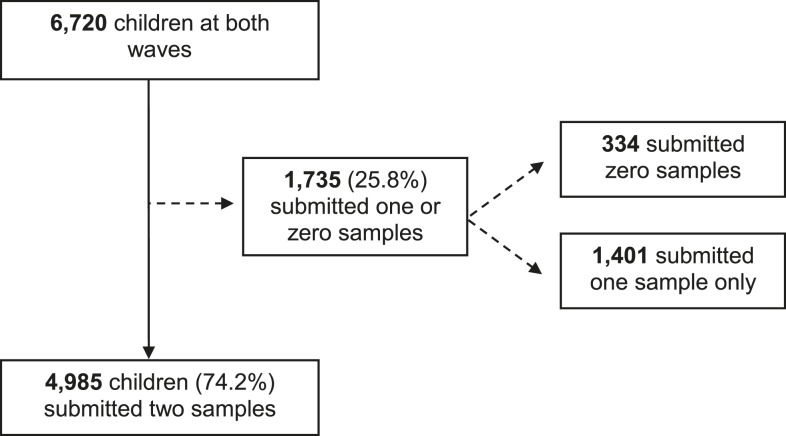
We conducted a cross-sectional study among schoolchildren in rural Guizhou Province in southwest China, where previous epidemiological surveys found an endemic prevalence of STH infection.21,22 The study had two waves, in May 2013 and April 2014. Over the two waves, we asked 6,720 schoolchildren from 112 townships to participate. Out of this sample, 4,985 provided two fecal samples (Figure 1).
Figure 1.
Flow chart of study participants and compliance with the fecal sampling procedure. Reasons that children did not provide a second stool sample include: The child did not have a bowel movement on one of the sample collection days; the child was absent from school on one of the sample collection days; and the child accidently misplaced their stool sample container. Of the 4,985 children that submitted two samples, 2,764 were included in both waves of data collection, whereas the remainder (2,221) were in only one wave.
Field and laboratory procedures.
Fecal sampling and parasitological testing occurred once during each wave. All children participating in the study were informed about the sampling process, and were asked to submit two fecal samples over two consecutive days. The study team visited the schools once per day to collect the samples, which were stored in a temperature-controlled cooler until delivery to the laboratory. Members of the study team transported the fecal samples in a temperature-controlled cooler to the laboratory of the county branch of the Centers for Disease Control and Prevention (CDC). All fecal samples were tested on the same day they were collected.
The two fecal samples for each child were processed and analyzed microscopically using the Kato–Katz thick-smear technique4 for four types of STH infections: A. lumbricoides (Ascaris), Trichuris trichura (Trichuris), and hookworm, (which include both Ancylostoma duodenale and Necator americanus). From each sample, two smears were taken: one smear was tested the same day on-site, and the second was treated using a formaldehyde preservation technique and sent for quality control to the headquarters of the National Institute for Parasitic Diseases in Shanghai.
A child was considered positive for STH infection if at least one of his/her fecal samples tested positive for one or more species of STHs. Among fecal samples that tested positive for STH, fecal egg counts were measured on a per-species basis, by calculating the geometric mean number of EPG in each fecal sample.
Statistical analysis.
Statistical analyses were performed using STATA 12.0 (STATA Corp., College Station, TX). P values below 0.05 were considered to be statistically significant. The statistical significance of differences in outcomes by subgroup populations was assessed using the t test function in STATA.
Ethical approval.
This study received ethical approval from the Stanford University Institutional Review Board (Protocol ID 25027), and from the Sichuan University Ethical Review Board (Protocol ID 2013005-02). All participating children gave oral assent prior to data collection, and the children’s legal guardians gave written consent for their children’s involvement in the study.
RESULTS
Study population and procedure compliance.
Figure 1 details the study participation and procedure compliance of the 6,720 children enrolled in the study. Overall, the two-sample fecal collection procedure had a compliance rate of 74.2%. Only the subgroup of 4,985 children who submitted two fecal samples over consecutive days were included in the analysis. Table 1 shows background characteristics of the final study population. Supplemental Appendix Table 1 presents t test results of differences in background characteristics by compliance with the fecal sampling procedure. Except for a few characteristics, there were no significant differences between children who submitted one versus two fecal samples.
Table 1.
Background characteristics of the study population (N = 4,985)
| Characteristic | Mean |
|---|---|
| Individual characteristics | |
| Age, years (mean) | 11.11 |
| Female (%) | 46.42 |
| Boarding at school (%) | 30.07 |
| Dong ethnic minority (%) | 45.72 |
| Miao ethnic minority (%) | 36.25 |
| Shui ethnic minority (%) | 3.71 |
| Zhuang ethnic minority (%) | 2.09 |
| Household characteristics | |
| Number of siblings | 1.24 |
| Pieces of durable assets | 8.19 |
| Parents are migrant workers (%) | 30.73 |
| Mother attended secondary school (%) | 6.97 |
| Father attended secondary school (%) | 11.89 |
| Sanitation and hygiene | |
| Washes hands before eating (%) | 86.63 |
| Washes hands after using toilet (%) | 89.23 |
| Drinks boiled water only (%) | 10.66 |
| Wears shoes while playing outside (%) | 35.41 |
| House has dirt floor (%) | 13.69 |
| House has dirt-based latrine (%) | 17.92 |
| Family uses feces as fertilizer (%) | 68.05 |
Infection prevalence and intensity.
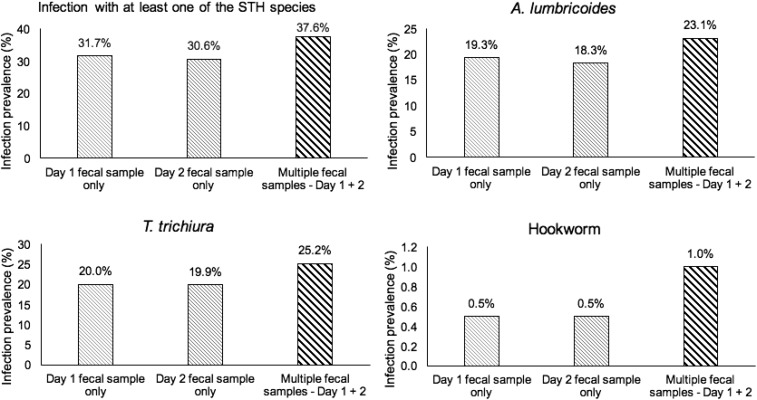
Table 2 presents findings on the calculated per-species prevalence and infection intensities from examination of a single fecal sample compared with two fecal samples. Prevalence of infection with any of the three main types of STHs was found to be 31.7% (95% confidence interval [CI] = 30.4–33.0) using the first samples only, and 30.6% (95% CI = 29.4–31.9) using the second samples only.
Table 2.
Observed infection prevalence and fecal egg counts from examination of a single fecal sample and multiple fecal samples
| Single fecal sample | Multiple fecal samples | ||
|---|---|---|---|
| Day 1 only; 95% CI | Day 2 only; 95% CI | Day 1 + 2; 95% CI | |
| Infection prevalence (%) | |||
| Ascaris lumbricoides | 19.3 (18.2–20.4) | 18.3 (17.2–19.4) | 23.1 (21.9–24.3) |
| Trichuris trichiura | 20.0 (18.9 –21.1) | 19.9 (18.8–21.0) | 25.2 (24.0–26.4) |
| Hookworm | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | 1.0 (0.7–1.2) |
| At least one of the STH species | 31.7 (30.4–33.0) | 30.6 (29.4–31.9) | 37.6 (36.3–39.0) |
| Fecal egg count among infected (EPG) | |||
| A. lumbricoides | 765.9 (623.75–907.96) | 780.12 (616.35–943.89) | |
| T. trichiura | 60.68 (48.25–73.11) | 51.61 (41.87–61.35) | |
| Hookworm | 2.83 (−3.40–9.07) | 1.50 (−2.05–5.05) | |
CI = confidence interval; EPG = eggs per gram; STH = soil-transmitted helminth.
Figure 2 displays the increases in the infection prevalence that are observed on examination of two fecal samples. Multiple fecal sampling resulted in increases in the determined infection prevalence for all helminth species. The observed prevalence of infection with any of the three main types of STHs was 37.6% (95% CI = 36.3–39.0) with the examination of two fecal samples, which was an 18.6% increase (P < 0.001) from the prevalence obtained if only the first day’s samples were tested, and a 22.9% increase (P < 0.001) from the prevalence if only the second day’s samples were tested. The average of the two increments is almost 21%.
Figure 2.
Infection prevalence (%) of soil-transmitted helminths in relation to number of fecal samples examined.
Supplemental Appendix Table 2 presents findings on the calculated per-species prevalence and infection intensities from examination of a single smear slide compared with two slides. Prevalence of infection with any of the three main types of STHs was found to be 32.0% (95% CI = 29.0–34.9) using the first slide only, and 34.8% (95% CI = 31.8–37.8) using the second slide only. Multiple slides resulted in increases in the determined infection prevalence for all helminth species. The observed prevalence of infection with any of the three main types of STHs was 40.2% (95% CI = 37.2–43.3) with the examination of two slides, which was a 33.1% increase (P < 0.001) from the prevalence obtained if only the first slide was tested, and a 15.5% increase (P < 0.001) from the prevalence if only the second slide was tested. The average of the two increments is almost 24%.
Cost of examining an additional fecal sample.
Table 3 presents a breakdown of cost estimates for collecting fecal samples for 6,720 children, 4,985 of whom provided the two samples. The fixed costs of the epidemiologic surveillance involved setting up the fecal collection sites in schools, setting up the laboratory at the county CDC, and training of field collectors and laboratory scientists. These costs totaled USD10.23 (62.40 yuan, at an exchange rate of 6.1 Chinese yuan to USD1 at the time of the survey) per student. The variable costs, on the other hand, included sample collection and laboratory costs, which depended on the number of samples. For one fecal sample, the variable costs were USD4.60 (28 yuan).
Table 3.
Cost breakdown for obtaining a second fecal sample for helminth infection surveillance among 4,985 schoolchildren at 118 schools in 112 townships
| Expenditure items | Cost in USD* | Cost in RMB yuan | Description |
|---|---|---|---|
| Part I. Fixed costs per student (total number of students 6,720, as planned) | |||
| A. Costs of establishing set-up in schools | |||
| Set-up in school with guidance from CDC and township health workers | 2.07 | 12.6 | 360 yuan payment/school/time × 2 times × 118 schools. Of the 360 yuan, 100 yuan for the health worker from CDC, 100 yuan for the health worker from the township, 100 yuan for the school principle, 60 yuan for the teacher |
| Obtaining consent from students and their guardians | 0.69 | 4.2 | 60 yuan payment/ teacher/school/time × two teachers/school/time × 118 schools × two times |
| Training teachers to collect and store samples | 1.38 | 8.4 | 120 yuan payment/ teacher/school/time × two teachers/school/time × 118 schools × two times |
| B. Laboratory costs at county CDC laboratory | |||
| Laboratory set-up and equipment (i.e., microscopes, temperature-controlled coolers) | 4.99 | 30.4 | 9,300 Yuan rent/laboratory/time × two times × 11 laboratories. Of the 11 laboratories, four of the seven bigger sample counties each has two laboratories, while the reminder three counties each has one laboratory |
| Laboratory cleaning | 0.54 | 3.3 | 1,000 yuan/laboratory/time × two times × 11 laboratories |
| C. Training of field and laboratory workers | |||
| Training of field collectors and laboratory scientists | 0.55 | 3.4 | 11,370 yuan/training/time × 1 training/time × two times. Of the 11,370 yuan, Airfare: 2,600 yuan/round trip Shanghai CDC to Guiyang/training × two trainers; Room and board: 600 yuan/day/trainer × three days/training × two trainers; laboratory rent 2,970/training |
| Fixed costs subtotal per student | 10.23 | 62.4 | |
| Part II. Variable costs (per fecal sample) | |||
| A. Fecal sample collection | |||
| Payment for the labor of field collectors and laboratory scientists | 0.82 | 5.00 | 80 yuan/person/day × 52 persons/time × 6 days/ × two times |
| Transportation costs | 1.48 | 9.00 | 680 yuan/van/day/team/time × 11 teams/time × 6 days/× two times |
| Room and board for collectors and testers | 1.64 | 10.0 | 160 yuan/person/day × 52 persons/time × 6 days/× two times |
| B. Laboratory costs | |||
| Materials used for the testing of a fecal sample (i.e., microscope slides) | 0.66 | 4.0 | |
| Variable Costs Subtotal (per fecal sample) | 4.60 | 28.0 | |
| TOTAL COST of the first fecal sample* | 14.83 | 90.4 | |
| TOTAL COST of the two fecal samples† | 19.43 | 118.4 | |
| MARGINAL COST of the second fecal sample | 4.60 | 28.0 | |
CDC = Centers for Disease Control and Prevention; RMB = Renminbi.
At the exchange rate of 1 Dollar = 6.1 RMB Yuan at the time of the survey.
Fixed costs + variable costs.
Fixed costs + (2 × variable costs).
The cost of obtaining the first fecal sample was calculated as the sum of the fixed costs and the variable costs for one sample, which together totaled USD14.83 (90.40 yuan). To add one additional fecal sample, the total expenditure increased by an amount equal to the variable costs per sample. More precisely, USD4.60 (28 yuan) was the cost of obtaining the additional sample and increasing the sensitivity by 21%.
DISCUSSION
The aim of this study—conducted in a helminth-endemic region of rural Guizhou, China—was to compare the diagnostic sensitivity for the multiple fecal sampling method versus the single sampling one when using the Kato–Katz technique to detect STH infection. A one-sample examination for 4,985 children resulted in a prevalence (the percentage of children who were infected with at least one of the helminth species) of 31.7% on day 1 and 30.6% on day 2. The cumulative prevalence, however, revealed by the two-sample method was 37.6%. This 21% average increase in the true positive rate, and the subsequent improvement of the Kato–Katz diagnostic sensitivity, we conclude, represent the value of examining an additional fecal sample.
The observed infection prevalence also increased on a per-species basis: increases of 23%, 26%, and 100% occurred for A. lumbricoides, T. trichiura, and hookworm infection, respectively, when data from two fecal samples were considered versus one on either day. This biologic variability in egg output means that unless multiple fecal samples are taken across consecutive days, a single sample may miss the diagnosis of helminth infections in over one-fifth of the population.
Our study fills in a gap in the current literature with two strengths. The first is the large sample size (N = 4,985). Our results precisely and substantially corroborate the findings of these studies, which noted increases in infection prevalence on examining multiple fecal sampling.
Importantly, this study accounts for the feasibility and cost-effectiveness of the surveillance methodology. Previous studies have considered the use of three14,19 or even five15 fecal samples. Theoretically, added samples should improve the sensitivity of the technique. Practically, as we demonstrate and observe, it would be very costly to attain these last increments of improved sensitivity. As shown by the cost breakdown, doubling the number of fecal samples, though beneficial, increased the costs by 31%. Using more samples would add even more expenses to an already resource-intensive undertaking. Areas with persistently high rates of helminth infection are generally poor and have limited access to public health resources, which means that surveillance programs will have to bring in the needed resources such as laboratory equipment and workers. Further, in areas where the population in question is geographically spread-out, additional costs may be incurred. Our study population, for instance, was spread-out over 112 rural townships in seven counties in Guizhou, China. This geographic expanse led to additional costs including worker compensation, transportation, and local guide fees. Using more fecal samples would further increase the costs and are unlikely to produce significant gains in sensitivity. As our results show, investing an additional USD4.60 for one additional fecal sample was sufficient to generate substantial improvements in diagnostic sensitivity.
The policy implications of our study are considerable. An improvement in sensitivity of 21% could be enough to move a population from one WHO treatment category to another, which has the potential to dramatically change the suggested intervention for said population (from either no mass treatment to annual treatment, or from annual treatment to biannual treatment). For populations with relatively higher prevalence of T. trichiura and hookworm, the improvement in sensitivity could be even higher.
A limitation of our study was that the majority of fecal samples from the children were not produced on-site at the time of collection, meaning that there could have been a delay of up to a few hours between children delivering their samples and reaching refrigeration facilities at the school or village clinic. One study of helminth diagnosis in different conditions observed that a time delay of more than 3 hours between fecal collection and laboratory testing led to nearly a 50% decrease in sensitivity for hookworm detection using the Kato–Katz technique.23 Our study team made an effort to transport the refrigerated samples as quickly as possible from the field to the laboratory; however, it is possible that time delays between fecal production (by the children) and parasitological testing led to some degradation of hookworm eggs. This may have contributed to the relatively low hookworm prevalence identified in our population. Although the Kato–Katz technique has high sensitivity for diagnosis of A. lumbricoides and T. trichiura, the sensitivity for detection of hookworm eggs is significantly lower.12,13 Thus, future studies examining the improvement in diagnostic sensitivity with multiple fecal samples may achieve greater accuracy in assessing hookworm detection if another diagnostic method, such as the Koga agar plate technique,24 is used in combination with the Kato–Katz technique.14
One limitation to our cost calculations relates to the potentially larger sample size involved in a conducting a formal surveillance survey. Some of the fixed costs that we list (such as the laboratory fees) may be truly fixed and might not change significantly regardless of sample size. If, during a scaling up process, the fixed costs rise at a lower rate than the variable costs, the marginal cost would be proportionately higher than what we observe in our study, potentially leading to a different cost-benefit assessment. That said, our calculations are based on a population size of nearly 5,000 students. This is more than sufficient for a typical surveillance program, so we do not anticipate significant increases in sample size that would affect the calculations reported here.
Diagnostic sensitivity is critical for accurate epidemiological surveillance, particularly in areas with light-intensity infection of STHs. Public health officials in endemic countries rely on infection prevalence from the field to make decisions regarding whether a control program is needed, and how frequently to treat and prevent infection. The WHO, for instance, recommends different treatment strategies for different prevalence.25 We find that examining a single fecal sample to determine infection prevalence insufficiently measures infections for all three types of STHs. Such an underestimation could lead to inadequate or inappropriate treatment program strategies, which would make reduction efforts inadequate.
We conclude that in areas with light-intensity infections of STHs, epidemiological surveillance can be substantially improved by obtaining two fecal samples on consecutive days for improved diagnostic sensitivity to determine infection prevalence.
Supplementary Material
Acknowledgments:
We are grateful to the thousands of public health officials and practitioners, school principals, teachers, village clinicians, and students who have helped us throughout this project.
Note: Supplemental appendix tables appear at www.ajtmh.org.
REFERENCES
- 1.World Health Organization, 2017. Soil-Transmitted Helminth Infections Geneva, Switzerland: World Health Organization. http://www.who.int/mediacentre/factsheets/fs366/en/. Accessed July 20, 2016.
- 2.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J, 2008. Helminth infections: the great neglected tropical diseases. J Clin Invest 118: 1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization, 1994. Bench Aids for the Diagnosis of Intestinal Parasites Planches pour le diagnostic des parasites intestinaux http://www.who.int/iris/handle/10665/37323. Accessed July 19, 2016.
- 4.Na K, Chaves A, Pellegrino JP, 1971. A simple device for quantitative stool thick-smear technique in Schistomiasis mansoni. ResearchGate 14: 397–400. [PubMed] [Google Scholar]
- 5.Yu JM, de Vlas SJ, Jiang QW, Gryseels B, 2007. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int 56: 45–49. [DOI] [PubMed] [Google Scholar]
- 6.Speich B, Knopp S, Mohammed KA, Khamis IS, Rinaldi L, Cringoli G, Rollinson D, Utzinger J, 2010. Comparative cost assessment of the Kato-Katz and FLOTAC techniques for soil-transmitted helminth diagnosis in epidemiological surveys. Parasit Vectors 3: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levecke B, et al. , 2011. A comparison of the sensitivity and fecal egg counts of the McMaster egg counting and Kato-Katz thick smear methods for soil-transmitted helminths. PLoS Negl Trop Dis 5: e1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glinz D, Silué KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, Rinaldi L, Cringoli G, N’Goran EK, Utzinger J, 2010. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis 4: e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knopp S, et al. , 2014. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg 90: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barda B, Cajal P, Villagran E, Cimino R, Juarez M, Krolewiecki A, Rinaldi L, Cringoli G, Burioni R, Albonico M, 2014. Mini-FLOTAC, Kato-Katz and McMaster: three methods, one goal; highlights from north Argentina. Parasit Vectors 7: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habtamu K, Degarege A, Ye-Ebiyo Y, Erko B, 2011. Comparison of the Kato-Katz and FLOTAC techniques for the diagnosis of soil-transmitted helminth infections. Parasitol Int 60: 398–402. [DOI] [PubMed] [Google Scholar]
- 12.Nikolay B, Brooker SJ, Pullan RL, 2014. Sensitivity of diagnostic tests for human soil-transmitted helminth infections: a meta-analysis in the absence of a true gold standard. Int J Parasitol 44: 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarafder MR, Carabin H, Joseph L, Balolong E, Olveda R, McGarvey ST, 2010. Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a “gold standard.” Int J Parasitol 40: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, Marti H, Utzinger J, 2008. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis 2: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booth M, Vounatsou P, N’goran EK, Tanner M, Utzinger J, 2003. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d’Ivoire. Parasitology 127: 525–531. [DOI] [PubMed] [Google Scholar]
- 16.Hall A, 1981. Quantitative variability of nematode egg counts in faeces: a study among rural Kenyans. Trans R Soc Trop Med Hyg 75: 682–687. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RM, Schad GA, 1985. Hookworm burdens and faecal egg counts: an analysis of the biological basis of variation. Trans R Soc Trop Med Hyg 79: 812–825. [DOI] [PubMed] [Google Scholar]
- 18.Bossche HV, Thienpont D, Janssens PG, 2012.. Chemotherapy of Gastrointestinal Helminths. Springer Berlin Heidelberg: Heidelberg, Germany. [Google Scholar]
- 19.Steinmann P, Du Z-W, Wang L-B, Wang X-Z, Jiang J-Y, Li L-H, Marti H, Zhou X-N, Utzinger J, 2008. Extensive multiparasitism in a village of Yunnan province, People’s Republic of China, revealed by a suite of diagnostic methods. Am J Trop Med Hyg 78: 760–769. [PubMed] [Google Scholar]
- 20.Lin D-D, et al. , 2008. Routine Kato–Katz technique underestimates the prevalence of Schistosoma japonicum: a case study in an endemic area of the People’s Republic of China. Parasitol Int 57: 281–286. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, et al. , 2015. Soil-transmitted helminths in Southwestern China: a cross-sectional study of links to cognitive ability, nutrition, and school performance among children. PLoS Negl Trop Dis 9: e0003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Zhang L, Luo R, Wang G, Chen Y, Medina A, Eggleston K, Rozelle S, Smith DS, 2012. Soil-transmitted helminth infections and correlated risk factors in preschool and school-aged children in rural southwest China. PLoS One 7: e45939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dacombe RJ, Crampin AC, Floyd S, Randall A, Ndhlovu R, Bickle Q, Fine PEM, 2007. Time delays between patient and laboratory selectively affect accuracy of helminth diagnosis. Trans R Soc Trop Med Hyg 101: 140–145. [DOI] [PubMed] [Google Scholar]
- 24.Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, Takatsuka N, Kita K, Ohtomo H, 1991. A modified agar plate method for detection of Strongyloides stercoralis. Am J Trop Med Hyg 45: 518–521. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization, 2016. Intestinal Worms Geneva, Switzerland: WHO. http://www.who.int/intestinal_worms/more/en/. Accessed July 20, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.