Abstract.
Spotted fever group (SFG) rickettsioses are endemic in Tennessee, with ∼2,500 cases reported during 2000–2012. Because of this substantial burden of disease, we performed a three-part evaluation of Tennessee's routine surveillance for SFG rickettsioses cases and deaths to assess the system's effectiveness. Tennessee Department of Health (TDH) SFG rickettsioses surveillance records were matched to three patient series: 1) patients with positive serologic specimens from a commercial reference laboratory during 2010–2011, 2) tertiary medical center patients with positive serologic tests during 2007–2013, and 3) patients identified from death certificates issued during 1995–2014 with SFG rickettsiosis–related causes of death. Chart reviews were performed and patients were classified according to the Council of State and Territorial Epidemiologists' case definition. Of 254 SFG Rickettsia–positive serologic specimens from the reference laboratory, 129 (51%) met the case definition for confirmed or probable cases of rickettsial disease after chart review. The sensitivity of the TDH surveillance system to detect cases was 45%. Of the 98 confirmed or probable cases identified from the medical center, the sensitivity of the TDH surveillance system to detect cases was 34%. Of 27 patients identified by death certificates, 12 (44%) were classified as confirmed or probable cases; four (33%) were reported to TDH, but none were correctly identified as deceased. Cases of SFG rickettsioses were underreported and fatalities not correctly identified. Efforts are needed to improve SFG rickettsiosis surveillance in Tennessee.
INTRODUCTION
The spotted fever group (SFG) rickettsioses are tick-borne diseases caused by gram-negative, intracellular bacteria of the genus Rickettsia.1 SFG rickettsioses are nationally notifiable conditions and are reportable in Tennessee.2,3 During 2000–2012, approximately 25,000 cases were reported in the United States, with approximately 2,500 cases in Tennessee. The incidence rate in Tennessee is 52.8 cases per million person-years, approximately six times the national rate of 8.9 cases per million person-years.3,4
The most common and clinically severe SFG rickettsiosis is Rocky Mountain spotted fever (RMSF), caused by Rickettsia rickettsii. Case fatality rates were approximately 70% during the preantibiotic era, but according to data from the Centers for Disease Control and Prevention, have declined to < 1% during more recent years.1,3,5 However, this low rate is likely substantially confounded by cases caused by rickettsial species other than R. rickettsii; as demonstrated in Arizona, where a high proportion of SFG rickettsiosis cases are caused by R. rickettsii and the case fatality rate is 10%. Case fatality rates are also higher among American Indians and children, and fatality rates without treatment can be as high as 25%.3,5,6 Tetracyclines (e.g., doxycycline) are the primary choice for therapy, and morbidity and mortality are decreased when treatment is started within the first 5 days of symptoms; however, delayed treatment increases the risk for death.7,8 Multiple other Rickettsia species are found in the United States, including Rickettsia parkeri, Rickettsia amblyommii, and Rickettsia sp. 364D, which can cause milder, but clinically similar syndromes.3 In Tennessee specifically, studies have demonstrated the presence of R. amblyommii, Rickettsia montanensis, and R. parkeri in addition to R. rickettsii.9 Available serologic assays for R. rickettsii can cross-react with these other Rickettsia species, making both infections with different species, and subclinical exposures, difficult to distinguish.8 Because of these clinical and laboratory similarities, and a high probability that the existing passive surveillance system for RMSF was already capturing clinical cases caused by other Rickettsia species, the Council for State and Territorial Epidemiologists (CSTE) changed the national public health case definition to include all SFG rickettsioses beginning in 2010.10
The public health case definition is complex, involving both clinical and laboratory criteria.10,11 A clinically compatible case requires a history of fever and ≥ 1 of the following clinical or laboratory findings: rash, eschar, headache, myalgia, anemia, thrombocytopenia, or any hepatic transaminase elevation. Next, cases can either be classified as laboratory confirmed or laboratory supportive. Laboratory-confirmed cases require either paired serum samples (acute and convalescent) documenting a ≥ 4-fold change in IgG-specific antibody titer by indirect immunofluorescence assay in paired specimens (collected 2–4 weeks apart), detection of SFG DNA by polymerase chain reaction (PCR), isolation of SFG Rickettsia in cell culture, or positive immunohistochemistry result on a tissue specimen. In contrast, laboratory-supportive cases require only a single positive serologic result (recommended cutoff ≥ 1:64) of elevated IgG- or IgM-specific antibody.11 On the basis of these different clinical and laboratory criteria, cases can be classified as confirmed, probable, suspect, or not a case; only confirmed and probable cases are included in state and national incidence statistics.10
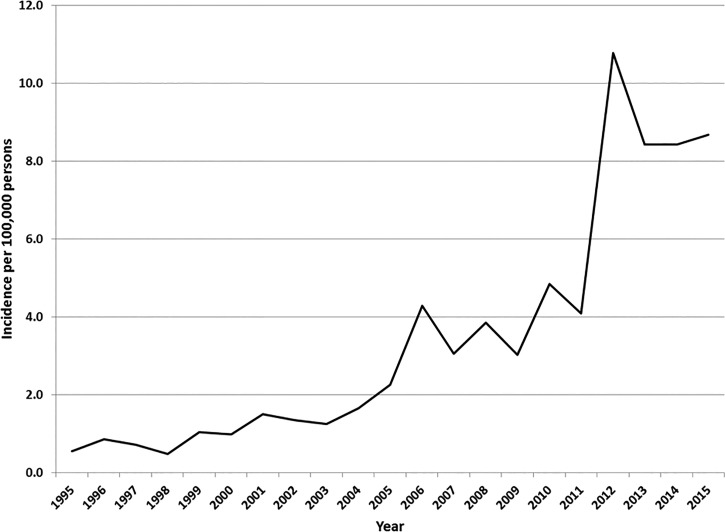
The incidence of infections from SFG rickettsioses in Tennessee has increased from 0.6/100,000 persons in 1995 to 8.7/100,000 persons in 2015 (Figure 1). During 2012–2015, a mean of 2,494 (range: 2,382–2,726) reports to the health department occurred annually; approximately 25% were classified as confirmed or probable, and the remainder were classified as suspect or not a case. Because of Tennessee's substantial burden of disease from the SFG rickettsioses and rising incidence rates during the last two decades, we performed a three-part evaluation of Tennessee's routine surveillance for SFG rickettsioses cases and deaths to assess the system's effectiveness.
Figure 1.
Incidence of reported spotted fever group rickettsioses infections—Tennessee, 1995–2015.
MATERIALS AND METHODS
Surveillance system.
Possible cases of SFG rickettsiosis are reported to the Tennessee Department of Health (TDH) either through standard methods (submission of the Notifiable Disease Report Form [PH-1600] by a laboratory or healthcare provider through standard mail or fax) or through electronic laboratory reporting.12,13 Local health department epidemiologists then complete an investigation to definitively classify cases according to the national case definition. Investigation methods vary between regional health departments, but can include calling the patient to obtain a more detailed description of symptoms, formally requesting medical records from the healthcare provider, sending a form with specific questions pertaining to the case definition to the provider for completion, or other techniques. The information collected is then entered into the TDH surveillance system (the National Electronic Disease Surveillance System Base System).14
Evaluation methods.
We performed a three-part evaluation of Tennessee's routine surveillance for SFG rickettsioses cases and deaths to assess the system's effectiveness. TDH SFG rickettsioses surveillance system records were matched to three patient series: 1) patients with positive serologic specimens from a commercial reference laboratory during 2010–2011, 2) tertiary medical center patients with positive serologic tests during 2007–2013, and 3) patients identified from death certificates issued during 1995–2014 with SFG rickettsiosis–related causes of death. Cases identified from these patient series were classified according to the criteria in the national (CSTE) public health case definition, not by any clinical definitions or diagnoses in the medical record.
In a previously completed study, all SFG Rickettsia–positive serologic specimens from a commercial reference laboratory during 2010–2011 underwent confirmatory testing at the TDH laboratory.9 The corresponding outpatient medical records were reviewed to classify cases by the national case definition. Cases classified as confirmed or probable were then matched to TDH surveillance records. Cases were matched by first name, last name, and date of birth. The case classification assigned in the TDH surveillance system was compared with that determined by medical record review.
As part of a previous study performed at a tertiary medical center, all patients during 2007–2013 with a positive SFG rickettsiosis serology (IgM or IgG) were identified.15 The case classification for each patient was determined through medical record review of clinical symptoms and laboratory results from the tertiary medical center. Case classifications were assigned using the national public health case definition. For the current study, cases classified as confirmed or probable were then matched to TDH surveillance records. Cases were matched by first name, last name, and laboratory specimen collection date. The case classification assigned in the TDH surveillance system was compared with that determined by medical record review.
As the third component of the evaluation, death certificates from the Tennessee Office of Vital Records with any SFG rickettsiosis–related diagnostic codes as the primary or underlying cause of death (International Classification of Diseases, ninth revision [ICD-9] and International Classification of Diseases, tenth revision [ICD-10]) during 1995–2015 were obtained. These years were selected due to the availability of electronic TDH surveillance records for the corresponding years. Complete death certificates, autopsy records, and medical records were reviewed, when available, to classify cases according to the national public health case definition. Cases identified from death certificates were then matched to TDH surveillance records by first and last name and confirmed with date of birth and event date.
All three parts of this evaluation were separately reviewed. The study conducted at the tertiary medical center was determined to pose minimal risk to study participants and was approved by the center's Institutional Review Board. The other two portions of the evaluation were deemed public health practice and did not meet criteria for human subjects research.
RESULTS
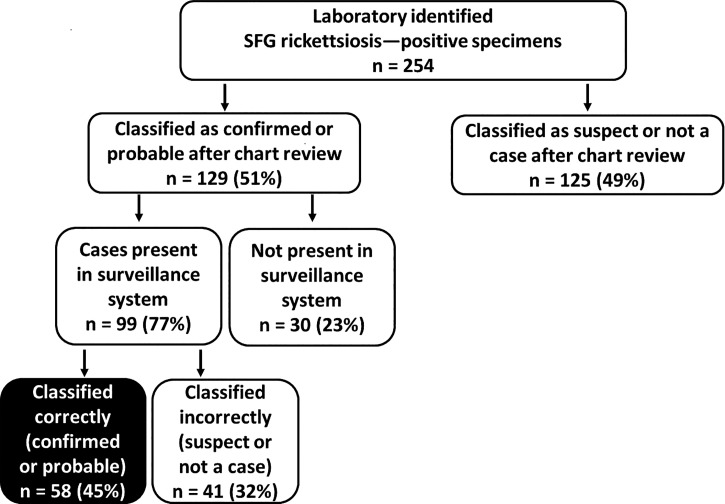
The reference laboratory identified 254 SFG Rickettsia–positive serologic specimens from Tennessee residents during 2010–2011 (Figure 2). Of these, 129 (51%) were classified as confirmed or probable cases after chart review. Ninety-nine (77%) were present in the TDH surveillance system and 30 (23%) were not present. Of those present in the TDH surveillance system, 58 (59%) were correctly classified in TDH records as confirmed or probable, and 41 (41%) were incorrectly classified as suspect or not a case. Therefore, the sensitivity of the TDH surveillance system to detect cases was 45% (58/129).
Figure 2.
Proportion of spotted fever group rickettsiosis cases identified from a reference laboratory that were correctly classified in the Tennessee Department of Health surveillance system, 2010–2011.
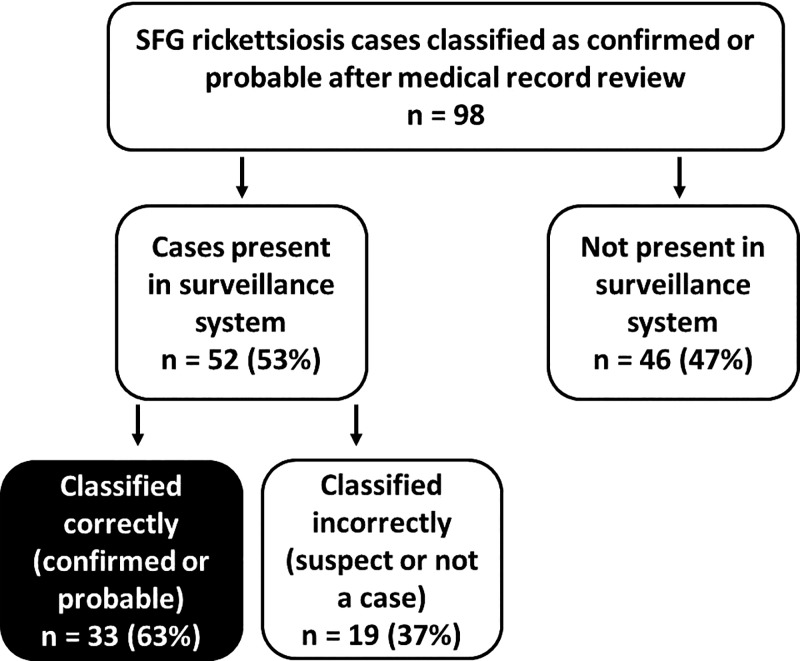
Ninety-eight cases that met the national public health case definition for a confirmed or probable SFG rickettsiosis case were identified from tertiary medical center data during 2007–2013 (Figure 3). Of these, 52 (53%) were present in the TDH surveillance system and 46 (47%) were not present. Thirty-three (63%) of those present in TDH records were correctly classified as confirmed or probable, and 19 (37%) were incorrectly classified as suspect or not a case. The sensitivity of the TDH surveillance system to detect cases was 34% (33/98). Of the 98 cases identified, 82 (84%) had only a single titer drawn (i.e., no convalescent serum was tested), 29 (30%) had only a positive IgM result, and 34 (35%) had either an IgM or IgG result that was equal to 1:64 (none were < 1:64 as this is not considered a positive result).
Figure 3.
Proportion of spotted fever group rickettsiosis cases identified from a tertiary medical center that were correctly classified in the Tennessee Department of Health surveillance system, 2007–2013.
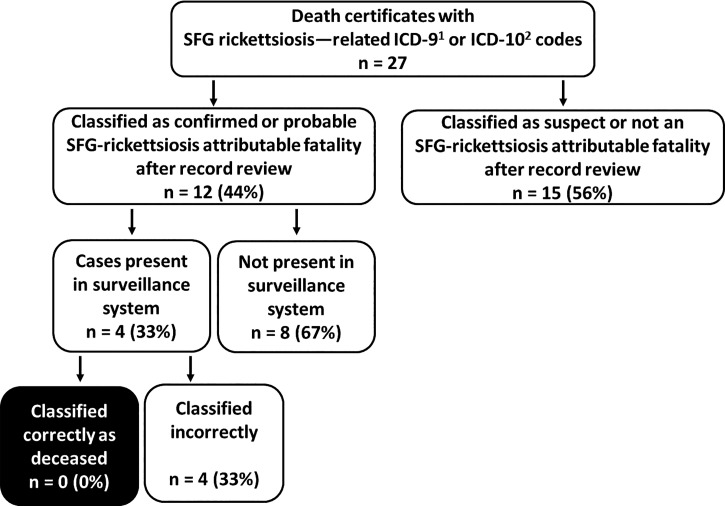
Vital records data during 1995–2015 identified 27 death certificates with an SFG rickettsiosis–related ICD-9 or ICD-10 diagnosis code as either the primary or underlying cause of death. Death certificates, medical records, or autopsy reports were reviewed on all 27 deaths. After this additional review, 12 (44%) were classified as a confirmed or probable SFG rickettsiosis–attributable fatality (Figure 4). Of these, four (33%) had been identified as cases in the TDH surveillance system, but none of the patients were correctly identified as deceased. The sensitivity of the TDH surveillance system to detect SFG rickettsiosis–attributable fatalities was 0%.
Figure 4.
Proportion of spotted fever group (SFG) rickettsiosis-attributable deaths identified from vital records data that were correctly classified in the Tennessee Department of Health (TDH) surveillance system. 1) International Classification of Disease, Ninth Revision; 2) International Classification of Disease, Tenth Revision.
DISCUSSION
Tennessee has among the highest reported incidence rates of SFG rickettsioses in the United States, and rates have been increasing during the last two decades. Despite this, our analysis reveals a low sensitivity of the public health surveillance system to correctly classify cases, and that cases and fatalities from SFG rickettsioses are frequently misclassified or not reported. In addition, fatalities are inadequately documented, indicating that these increased rates are even more substantial than have been identified by the existing surveillance system. Finally, a substantial proportion of SFG rickettsiosis reports to public health are incorrectly classified after further investigation by local public health officials.
Multiple challenging aspects of the public health surveillance system exist, including difficulty in interpreting laboratory diagnostics for the SFG rickettsioses. In general, cases reported to public health are laboratory reports of a single elevated immunoglobulin (IgG or IgM) level above the ≥ 1:64 cutoff value. A single titer can represent either past infection or acute infection, and without a paired convalescent specimen differentiation is impossible. This is likely further confounded by an increasing seroprevalence with age among the general population. A prior study demonstrated a strong relationship between age and seroprevalence, and documented that 19.5% of Nashville and 9.0% of Memphis children (1–17 years of age) tested had a R. rickettsii titer > 1:64.16 Diagnostic methods other than serology (e.g., PCR, immunohistochemistry, or cell culture) require more elaborate or invasive testing and are typically only performed by reference laboratories. Additionally, a considerable amount of clinical information is required to classify cases according to the national public health case definition. Public health authorities can obtain this information through medical record requests, provider interviews, patient interviews, or other methods; however, all approaches are labor intensive, inefficient, and variably sensitive. This combination of laboratory diagnostics with limited sensitivity for acute infection, along with the requirement for considerable supplemental clinical information creates a substantial burden for public health authorities to accurately monitor cases of SFG rickettsiosis.
Approximately 75% of SFG Rickettsia reports to public health in Tennessee are ultimately not verified as confirmed or probable cases, which translates to a minimum of four case investigations by local public health authorities to identify one confirmed or probable case. However, our analysis also demonstrates that a substantial proportion of reported cases are misclassified by public health authorities. Approximately one-quarter of cases in our analysis were incorrectly classified as suspect or not a case when they should have been confirmed or probable cases.
More concerning are findings that none of the fatalities identified through vital records were identified as deceased in our surveillance system, and that only half of the fatalities identified had been reported to public health authorities at all. With a historic, untreated mortality rate of approximately 70%, it is important that public health authorities accurately identify patients who might have died of this preventable cause.6 Multiple factors likely contribute to this discrepancy, including challenges in diagnosing fatalities and the variable availability and quality of supplemental clinical information required for public health case classification. In addition, fatality information might not be readily available to public health investigators; or a definitive cause of death may never be determined or may be delayed until after completion of a postmortem examination with additional laboratory testing, likely after the public health investigation was completed. Postmortem testing information is not retroactively entered into the medical record, nor do vital records data interface with the public health surveillance system; therefore, definitive cause of death information is not brought to the attention of public health authorities. Lastly, since the majority of fatal cases of SFG rickettsiosis occur within 10 days of illness and antibodies are not detectable in 85% of patients during the first week, definitive diagnosis of fatal cases requires a high index of suspicion to direct postmortem testing.5,17
Despite challenges, the SFG rickettsiosis surveillance system in Tennessee is performing adequately for many of its intended purposes, including monitoring disease trends by person, place, and time to guide targeted prevention messages. On the basis of our evaluation, although we are capturing only approximately one-third of all confirmed or probable cases in Tennessee, trends are detectable and we described a steadily rising incidence during the last decade. However, the cause of this rising incidence is unclear. Although the national public health case definition was changed in 2010, it primarily acknowledged that infections caused by rickettsial species other than R. rickettsii were already being captured and counted as RMSF, and no major procedural change concerning how SFG rickettsiosis surveillance is conducted occurred, indicating the increase is not caused by a surveillance artifact. Greater awareness of tick-borne diseases among the public and health-care providers might contribute to increased testing and more reported cases. However, recent studies have reported a decrease in reported cases of RMSF and have proposed that up to one-third of reported SFG rickettsioses cases are because of infections from rickettsial species other than R. rickettsii, suggesting that this increased incidence may primarily be due to infections from non-RMSF species.18,19
Our analysis in Tennessee demonstrated limited sensitivity to identify fatalities, a substantial proportion of misclassified or missed cases, and a considerable volume of case reports that are ultimately not verified as confirmed or probable. Although no surveillance system will capture all cases, the SFG rickettsiosis surveillance system can be improved. In Tennessee, changes have been made to the surveillance system data entry form to clarify the case definition and simplify case classifications. Many regional health departments conduct educational activities for, and engage with, providers annually during the peak incidence of tick-borne disease in the state. In addition, public health authorities can explore improved methods of linking vital records data to surveillance data, increasing the probability of capturing SFG rickettsiosis–attributable fatalities. Finally, CSTE should consider convening a working group to further discuss status and strategies to improve SFG rickettsiosis surveillance in the United States, as demonstrated by some of these challenges in Tennessee. Improved surveillance will empower public health authorities with superior data for action.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Dantas-Torres F, 2007. Rocky Mountain spotted fever. Lancet Infect Dis 150: 480–488. [DOI] [PubMed] [Google Scholar]
- 2.Tennessee Department of Health , 2016. Reportable Diseases and Events Matrix. Available at: https://apps.health.tn.gov/ReportableDiseases/Common/Updated_ReportableDiseasesMatrix_160203.pdf. Accessed July 28, 2016.
- 3.Drexler NA, Dahlgren FS, Heitman KN, Massung RF, Paddock CD, Behravesh CB, 2016. National surveillance of spotted fever group rickettsioses in the United States, 2008–2012. Am J Trop Med Hyg 94: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Openshaw JJ, Swerdlow DL, Krebs JW, Holman RC, Mandel E, Harvey A, Haberling D, Massung RF, McQuiston JH, 2010. Rocky Mountain spotted fever in the United States, 2000–2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg 83: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlgren FS, Holman RC, Paddock CK, Callinan LS, McQuiston JH, 2012. Fatal Rocky Mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg 86: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Childs JE, Paddock CD, 2002. Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? Am J Trop Med Hyg 66: 450–457. [DOI] [PubMed] [Google Scholar]
- 7.Regan JJ, et al., 2015. Risk factors for fatal outcome from Rocky Mountain spotted fever in highly endemic area: Arizona, 2002–2011. Clin Infect Dis 60: 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Scola B, Raoult D, 1997. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol 35: 2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delisle J, Mendell NL, Stull-Lane A, Bloch KC, Bouyer DH, Moncayo AC, 2016. Human infections by multiple spotted fever group rickettsiae in Tennessee. Am J Trop Med Hyg 94: 1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Council of State and Territorial Epidemiologists , 2009. Position Statement: Public Health Reporting and National Notification for Spotted Fever Rickettsiosis (including Rocky Mountain spotted fever). Available at: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/09-ID-16.pdf. Accessed August 17, 2016.
- 11.Centers for Disease Control and Prevention , 2010. Spotted Fever Rickettsiosis (Rickettsia spp.): 2010 Case Definition. Available at: https://wwwn.cdc.gov/nndss/conditions/spotted-fever-rickettsiosis/case-definition/2010/. Accessed August 31, 2016.
- 12.Tennessee Department of Health , 2015. Reportable Diseases. Available at: https://apps.health.tn.gov/ReportableDiseases/ReportingOverview.aspx. Accessed August 17, 2016.
- 13.Tennessee Department of Health Reportable Diseases and Events , 2012. PH-1600. Available at: https://tn.gov/assets/entities/health/attachments/ph-1600.pdf. Accessed August 17, 2016.
- 14.Centers for Disease Control and Prevention , 2015. National Notifiable Diseases Surveillance System (NNDSS). Available at: https://wwwn.cdc.gov/nndss/nedss.html. Accessed August 17, 2016.
- 15.Ivey KS, Pruthi S, Bloch KC, 2014. Neurologic manifestations of Rocky Mountain spotted fever in children and adults. Open Forum Infect Dis 1: S241. [Google Scholar]
- 16.Marshall GS, et al., 2003. Antibodies reactive to Rickettsia rickettsii among children living in the southeast and south central regions of the United States. Arch Pediatr Adolesc Med 157: 443–448. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention , 2010. Rocky Mountain Spotted Fever: Symptoms, Diagnosis, and Treatment. Available at: http://www.cdc.gov/rmsf/symptoms/.Accessed December 8, 2016.
- 18.Raoult D, Parola P, 2008. Rocky Mountain spotted fever in the USA: a benign disease or a common diagnostic error? Lancet Infect Dis 8: 587–589. [DOI] [PubMed] [Google Scholar]
- 19.Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, Behravesh CB, 2016. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg 94: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]