Abstract.
Intestinal infection caused by Cryptosporidium is a major contributor to diarrhea morbidity and mortality in young children around the world. Current treatments for children suffering from cryptosporidiosis are suboptimal. Lactoferrin is a glycoprotein found in breast milk. It has showed bacteriostatic and antimicrobial activity in the intestine. However, the effects of lactoferrin on the intestinal parasite Cryptosporidium have not been reported. In this study, we investigated the anticryptosporidial activity of human lactoferrin on different stages of Cryptosporidium. Physiologic concentrations of lactoferrin killed Cryptosporidium parvum sporozoites, but had no significant effect on oocysts viability or parasite intracellular development. Since sporozoites are essential for the infection process, our data reinforce the importance of breastfeeding and point to the potential of lactoferrin as a novel therapeutic agent for cryptosporidiosis.
Cryptosporidium species are major causes of diarrhea morbidity in children from resource-limited countries and an important cause of mortality in children below 24 months of age.1,2 Cryptosporidium is also a cause of persistent diarrhea and malnutrition.3 A large number of therapeutic agents have been evaluated for their anticryptosporidial activity, but only nitazoxanide, which has limited effectiveness in malnourished children, has been approved by the Food and Drug Administration.4,5 Therefore, the development of novel treatments for cryptosporidiosis is an important priority for child health.6,7 Lactoferrin is a multifunctional glycoprotein found in breast milk. Previous studies have demonstrated that this molecule has bacteriostatic, antimicrobial, and antiinflammatory activity.8–10 Lactoferrin chelates iron which is necessary for essential enzymes in microorganisms. Lactoferrin interacts with molecular and cellular components of both host and pathogens leading to the activation of immune responses.11 Lactoferrin has antimicrobial activity against enteric parasites and can cause ultrastructural damage to Giardia lamblia and binds the trophozoites of Entamoeba histolytica.12,13 Interestingly breast milk has been used to prevent and reduce infection by Cryptosporidium.14,15 In the current study, we assessed the effects of exposure of Cryptosporidium to physiological concentrations of lactoferrin.
We investigated the effect of lactoferrin (TATUA Dairy Company, New Zealand) on viability of Cryptosporidium oocysts by measuring the excystation rate. For these experiments, 1 × 106 oocysts (Iowa strain, University at Arizona) were exposed to lactoferrin by incubating the parasites for 4 hours in complete media (Roswell Park Memorial Institute-1640, 10% fetal bovine serum, and antibiotic–antimycotic solution) containing the following concentrations of lactoferrin: 0, 1, 2.5, 5, and 10 mg/mL, the experiment was conducted at 4°C to prevent spontaneous excystation. After incubation, the oocysts were concentrated by centrifugation (5 minutes at 500 × g), and washed (three times). To induce excystation, the oocysts were resuspended in acidic water (25 μL) and then incubated for 10 minutes on ice. After incubation, 250 μL of excystation media (complete media with 0.8% sodium taurocholate) was added to oocysts and incubated 1 hour at 37°C. Excystation rate was evaluated by microscopy counting the number of sporozoites as described before.14 We did not observe any effect of lactoferrin on excystation rate in treated oocyst compared with untreated samples (data not shown). These findings suggest that lactoferrin does not penetrate oocyst shell therefore it cannot affect sporozoites within oocyst. This result was expected since Cryptosporidium oocysts are quite hardy to the external environment.
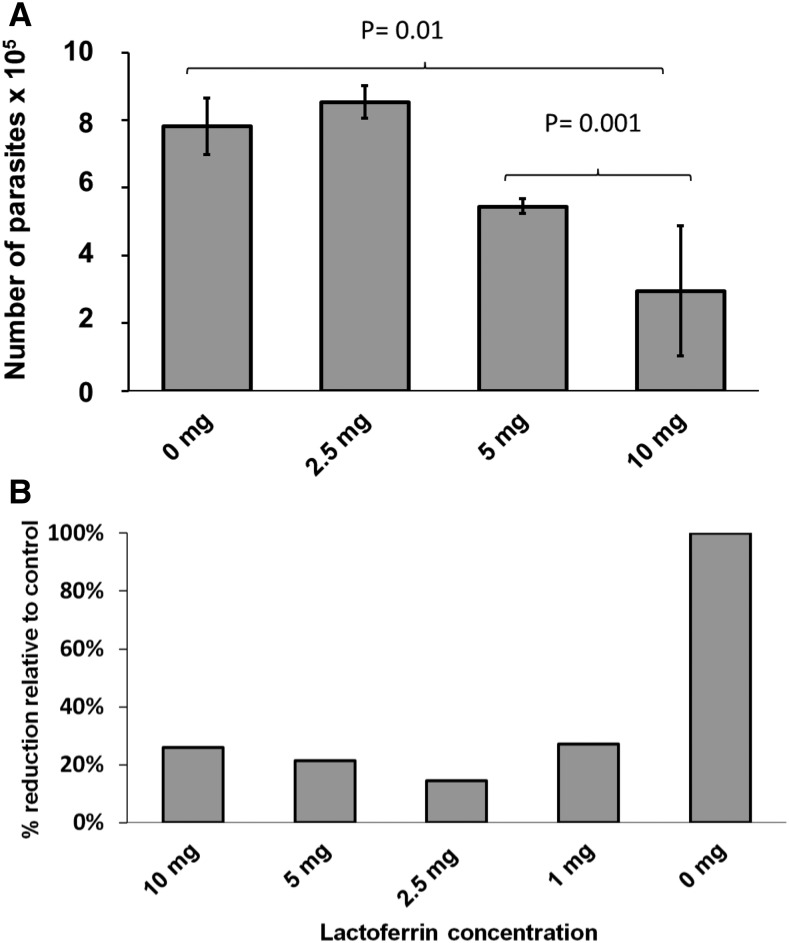
The effect of lactoferrin on sporozoites was evaluated measuring the infectivity and viability of fresh sporozoites. Oocysts were treated with acid water and then excystation media as described earlier. Sporozoites were separated by filtration with 3 µm pore-sized mesh and then exposed to lactoferrin. For these experiments, 1 × 106 sporozoites were incubated at 37°C for 2 hours with 500 µL of complete media containing lactoferrin at concentrations of 0, 1, 2.5, 5, or 10 mg/mL. After exposure, the sporozoites were washed by centrifugation (5 minutes × 500 g) and the pellet was resuspended in 500 µL of complete media and then the parasites were used to infect HCT-8 cells cultured in 24 well plates as described before.16 After 24 hours of infection, media was removed and the cells were washed three times with phosphate-buffered saline (PBS). The cells were harvested and RNA extracted and infection quantitated by SYBR green (Quanta Biosciences, Gaithersburg, MD) RT-PCR.17 The results showed that sporozoites exposed to low concentrations of lactoferrin were able infect HCT-8 cells. However, sporozoites treated with ≥ 5 mg/mL lactoferrin had significantly reduced numbers of parasites present after 24 hours of infection (Figure 1A). We observed that lactoferrin at a concentration of 10 mg/mL reduced parasite burden by approximately 60% in comparison to untreated sporozoites (Figure 1A). We have hypothesized that this reduction of parasites observed is caused by decreased viability of sporozoites and not by blocking proliferation after infection. To test this hypothesis we analyzed the viability of sporozoites treated with lactoferrin by fluorescent microscopy using a vital dye. For these experiments, 1 × 106 oocysts underwent excystation and resulting sporozoites were incubated with either complete media alone or with 10 mg/mL lactoferrin at 37°C for 2 hours. The sporozoites were then pelleted by centrifugation 500 × g for 5 minutes. An aliquot was resuspended in complete media and stained with carboxyfluorescein succinimidyl ester (CellTrace™ CFSE, Waltham, MA) as described before.16 Ten microliters of the stained samples were placed in microscopy slides and counterstained with 4’,6-diamidino-2-phenylindole (DAPI). Fluorescent microscopy showed fluorescence in all of the untreated parasites (Figure 2A) indicating that these parasite were metabolically active and alive. Nuclear staining (DAPI) also was detected in all untreated parasites (Figure 2B). In the treated samples, we observed the sporozoites by bright field microscopy (Figure 2E), but no staining was observed with either vital dye (Figure 2D and Figure 2F) or nuclear staining DAPI (Figure 2C). Since in natural conditions sporozoites may take less than 2 hours to enter host cells (after excystation), we evaluate the effect of lactoferrin on sporozoites during excystation-invasion process. We suspended fresh oocysts to excystation media supplement with or without lactoferrin (Sigma Aldrich L4040, St. Louis, MO) and added them directly to host cells for 2 hours. The cells were then washed to remove free parasites and we quantitated intracellular infection by SYBR green RT-PCR as described before. The results confirmed that when sporozoites were exposed to lactoferrin during excystation infectivity decreased (Figure 1B).
Figure 1.
Effects of lactoferrin on sporozoites and oocsyst/sporozoites during Cryptosporidium parvum infection. (A) Sporozoites were incubated (or not) with lactoferrin and then used to infect HCT-8 cells, grey bars indicate the average of three experiments, P values were calculated from a t test, error bars indicates standard deviation. (B) Oocyst were added to HCT-8 cells and incubated with excystation media containing (or not) lactoferrin, the bars represent the median of 2 independent experiments conducted by duplicate.
Figure 2.
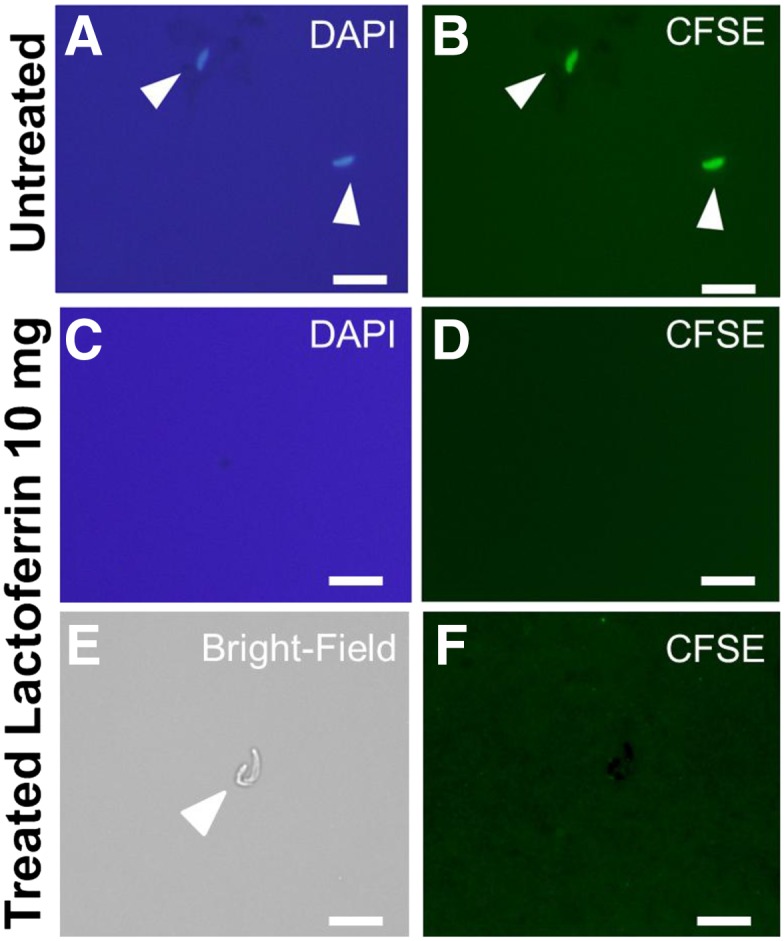
Fluorescent microscopy (×100) after exposure to lactoferrin. Samples were incubated with complete media alone (A and B) or with complete media supplemented with lactoferrin at 10 mg/mL (C, D, E, and F). White arrows point sporozoites, line scale = 5 µm.
To confirm the hypothesis that lactoferrin affects only sporozoites and has no effect on intracellular stages we studied lactoferrin treatment postinfection. Of oocysts, 1 × 106 underwent excystation and were added to HCT-8 cells cultured in 24 well plates. After 2 hours, the cells were washed with PBS and then complete media containing lactoferrin at concentrations of 0, 1, 2.5, 5, or 10 mg/mL was added to the cells and incubated for 24 hours at 37°C (5% CO2). The infected cells were harvested and the parasite burden analyzed by SYBR green RT-PCR. The results showed that lactoferrin did not change the number of parasites when added after infection and had no effects on intracellular stages (data not shown).
To test if lactoferrin reduce infection by blocking host molecules that are essential for invasion process, then we exposed HCT-8 cells to complete media containing lactoferrin at concentrations of 0, 1, 2.5, 5, or 10 mg/mL concentrations for 2 hours at 37°C. After exposure, cells were washed with complete media and infected with 1 × 106 excystated parasites for 24 hours. We did not observe difference between control and treated groups.
This study demonstrated that lactoferrin has antimicrobial activity against Cryptosporidium sporozoites either when treated as sporozoites or when lactoferrin was included in excystation buffer with oocysts. By contrast, lactoferrin had no effect on infection when exposure occurred only during the oocyst stage or intracellular stages. Interestingly, these experiments were conducted with a concentration, 10 mg/mL, equivalent to that found in colostrum. Considering Cryptosporidium’s importance as a diarrheal pathogen in children, our findings support the observations that breast milk reduces diarrhea in infants. This suggests that a continuous presence of breast milk in an infant’s diet may decrease or inhibit Cryptosporidium infection. Although the hardy oocysts and intracellular stages were resistant, the extracellular sporozoites were highly susceptible. We suspect that the extracellular merozoite stage (which spreads infection from cell to cell) would also be killed by lactoferrin. If so, it also suggests potential for lactoferrin as a lead compound to treat cryptosporidiosis. It has already been seen that the severity of diarrhea episodes in children have benefited from supplementation with lactoferrin, however, the pathogen-specific effects of this treatment have yet to be determined.16 Thus, further clinical studies are needed to confirm these findings in cases of cryptosporidiosis in children and other at-risk patients. If successful, lactoferrin may be a much-needed treatment option not only for children.
Acknowledgments:
JLP was supported by the Annual Medical Grant “Francisco Tejada and Semiramis Reátegui” from Universidad Peruana Cayetano Heredia.
REFERENCES
- 1.Sow SO, et al. , 2016. The burden of cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 10: e0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, et al. , 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3: e564–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korpe PS, et al. , 2016. Natural history of cryptosporidiosis in a longitudinal study of slum-dwelling Bangladeshi children: association with severe malnutrition. PLoS Negl Trop Dis 10: e0004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabada MM, White AC, Jr, 2010. Treatment of cryptosporidiosis: do we know what we think we know? Curr Opin Infect Dis 23: 494–499. [DOI] [PubMed] [Google Scholar]
- 5.Sparks H, Nair G, Castellanos-Gonzalez A, White AC, Jr, 2015. Treatment of Cryptosporidium: what we know, gaps, and the way forward. Curr Trop Med Rep 2: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoultz DA, de Hostos EL, Choy RK, 2016. Addressing cryptosporidium infection among young children in low-income settings: the crucial role of new and existing drugs for reducing morbidity and mortality. PLoS Negl Trop Dis 10: e0004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Checkley W, et al. , 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortner CA, Miller RD, Arnold RR, 1986. Bactericidal effect of lactoferrin on Legionella pneumophila. Infect Immun 51: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conneely OM, 2001. Antiinflammatory activities of lactoferrin. J Am Coll Nutr 20: 389S–395S; discussion 396S–397S. [DOI] [PubMed] [Google Scholar]
- 10.Aguila A, Herrera AG, Morrison D, Cosgrove B, Perojo A, Montesinos I, Perez J, Sierra G, Gemmell CG, Brock JH, 2001. Bacteriostatic activity of human lactoferrin against Staphylococcus aureus is a function of its iron-binding properties and is not influenced by antibiotic resistance. FEMS Immunol Med Microbiol 31: 145–152. [DOI] [PubMed] [Google Scholar]
- 11.Pierce A, Legrand D, Mazurier J, 2009. Lactoferrin: a multifunctional protein [in French] Med Sci (Paris) 25: 361–369. [DOI] [PubMed] [Google Scholar]
- 12.Turchany JM, McCaffery JM, Aley SB, Gillin FD, 1997. Ultrastructural effects of lactoferrin binding on Giardia lamblia trophozoites. J Eukaryot Microbiol 44: 68–72. [DOI] [PubMed] [Google Scholar]
- 13.Ochoa TJ, Cleary TG, 2009. Effect of lactoferrin on enteric pathogens. Biochimie 91: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaacs CE, 2005. Human milk inactivates pathogens individually, additively, and synergistically. J Nutr 135: 1286–1288. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Hafeez EH, Belal US, Abdellatif MZ, Naoi K, Norose K, 2013. Breast-feeding protects infantile diarrhea caused by intestinal protozoan infections. Korean J Parasitol 51: 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castellanos-Gonzalez A, Yancey LS, Wang HC, Pantenburg B, Liscum KR, Lewis DE, White AC, Jr, 2008. Cryptosporidium infection of human intestinal epithelial cells increases expression of osteoprotegerin: a novel mechanism for evasion of host defenses. J Infect Dis 197: 916–923. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos-Gonzalez A, et al. , 2013. A novel calcium dependent protein kinase inhibitor as a lead compound for treating cryptosporidiosis. J Infect Dis 208: 1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]