Abstract.
Strongyloidiasis is an intestinal parasitic infection becoming increasingly important outside endemic areas, not only because of the high prevalence found in migrant populations, but also because immunosuppressed patients may suffer a potentially fatal disseminated disease. The aim of these guidelines is to provide evidence-based guidance for screening and treatment of strongyloidiasis in non-endemic areas. A panel of experts focused on three main clinical questions (who should be screened and how, how to treat), and reviewed pertinent literature available in international databases of medical literature and in documents released by relevant organizations/societies. A consensus of the experts’ opinion was sought when specific issues were not covered by evidence. In particular, six systematic reviews were retrieved and constituted the main support for this work. The evidence and consensus gathered led to recommendations addressing various aspects of the main questions. Grading of evidence and strength of recommendation were attributed to assess the quality of supporting evidence. The screening of individuals at risk of the infection should be performed before they develop any clinical complication. Moreover, in immunosuppressed patients, the screening should be mandatory. The screening is based on a simple and widely accessible technology and there is now a universally accepted treatment with a high efficacy rate. Therefore, the screening could be implemented as part of a screening program for migrants although further cost-effectiveness studies are required to better evaluate this strategy from a public health point of view.
INTRODUCTION
Strongyloidiasis is a parasitic disease widely distributed in tropical and subtropical regions,1 with over 350 million people estimated to be infected worldwide.2 Migrant populations living in European countries present a high risk of having strongyloidiasis,3,4 and it has been reported that the prevalence in immigrants may range from 2% to 46%,5 but few studies have assessed the burden and risk factors of imported strongyloidiasis.3,6
The infection has three peculiar characteristics that are of importance from the clinical and public health point of view. First, more than half of infected subjects are asymptomatic or have mild, not specific complains,6 and eosinophilia is often the only finding.4 Therefore, they are usually unaware that they might harbor an infection.7 Second, Strongyloides stercoralis has the ability to replicate indefinitely inside the host (autoinfective cycle) without any further exposure to an infected site, thus causing a lifelong infection if left untreated.8,9 Third, immunosuppressed patients can develop the hyperinfection syndrome or the disseminated disease, which has a fatality rate of 60–70%.10 The most frequent trigger of this complication is a chronic therapy with steroids, but solid organ or bone marrow transplant recipients, patients with malignancies, or those under therapy with immunosuppressive drugs are also at risk.11 Human T-cell lymphotropic virus 1 (HTLV-1) is also a risk factor for severe disease and treatment failure.12,13
The rationale for a screening of S. stercoralis in non-endemic countries is based on the high estimated prevalence of the infection among migrants, the availability of a sensitive method for detection, and the potential to prevent fatal complications through early case detection. Currently, a few societies/organizations recommend screening for S. stercoralis in specific fields, such as solid organ transplantation,14 since it has been recognized that strongyloidiasis can be acquired from an infected donor.15–17
Different screening strategies include universal screening (when all individuals in a certain category are tested)18 and case finding (when only a well-defined group with risk factors are candidates for screening).19
OBJECTIVES
These guidelines are aimed to provide evidence-based guidance and, when not available, consensus opinion from a group of experts to address the screening and treatment of strongyloidiasis in non-endemic areas.
The following definitions were used in these guidelines:
Individuals with high risk of exposure to S. stercoralis: immigrants coming from endemic areas (Africa, Latin America, Asia, and Oceania), adopted children who have been living for at least 1 year in highly endemic area, expatriates 1) undertaking long trips (more than 1 year) to endemic countries and 2) with exposure to rural areas.
Individuals with intermediate to low risk of exposure to S. stercoralis: short-term (less than 1 year) travelers to highly endemic areas; elderly patients living in countries where transmission was occurring in the past, which include Northern Italy20 and the Spanish Region of Valencia.21
Immunosuppressed: patients in chronic treatment with corticosteroids, chemotherapy, immunosuppressant and immunomodulator agents, transplant recipients, patients with acquired immune deficiency syndrome or HTLV-1 infection or any immunosuppression condition.
Candidates for immunosuppression: candidates for immunosuppressant therapies (see above in definition number 3), candidates for solid or bone-marrow transplant. Patients with well-controlled human immunodeficiency virus infection should be managed like non-immunosuppressed individuals.
Disseminated strongyloidiasis: severe infection with presence of parasites outside the classical life cycle (i.e., in organs other than the skin, gastrointestinal tract, lungs).
Strongyloides hyperinfection: increase in the number of larvae in the stools and/or sputum along with clinical manifestations limited to the respiratory and gastrointestinal systems, and peritoneum.
METHODS
Panel composition.
We convened a panel of six experts, all of them specialists in migrant health and imported diseases, with a particular experience in strongyloidiasis.
The panel addressed the following three clinical questions:
Who should be screened?
How to screen strongyloidiasis?
How to treat strongyloidiasis?
Literature review and analysis.
Panel members thoroughly reviewed the literature pertinent to each of the question using Pubmed/Medline and Cochrane library.
They particularly evaluated the results of four recent systematic reviews (SRs) about strongyloidiasis published by the COHEMI project. All these SRs had been undertaken by five members of the panel. The COHEMI project comprehensively reviewed different aspects of strongyloidiasis and the final results were four SRs published in peer-reviewed journals3,7,10,22 and another study that evaluated the accuracy of five different serological assays for the screening, diagnosis, and follow-up of S. stercoralis infection.23,24
Moreover, other SRs on strongyloidiasis have been additionally included for the guidelines development. For this purpose, panel members thoroughly reviewed the literature pertinent to each of the question using PubMed/Medline, Embase, CINAHL, Cochrane CENTRAL, as well as grey literature for other relevant documents as well as published guidelines and reports on screening for strongyloidiasis in relevant organizations (e.g., European Centre for Disease Prevention and Control, World Health Organization [WHO]) databases.
Process overview.
In creating the guidelines, the panel applied the same principles as the Agency for Healthcare Research and Quality.25
This included the available evidence based on the SRs and the grading of the recommendations. The panel members reviewed each recommendation, their strengths, and the quality of evidence. Discrepancies were discussed and resolved, to achieve a consensus for each recommendation. The strength assigned to a recommendation reflects the panel’s confidence that the benefits of following the recommendation are likely to outweigh potential harms.
Grading of evidence.
Ia: SR or meta-analysis of randomized controlled trials (RCTs).
Ib: at least one RCT.
IIa: at least one well-designed controlled study without randomization.
IIb: at least one well-designed quasi-experimental study, such as a cohort study.
III: well-designed nonexperimental descriptive studies, such as comparative studies, correlation studies, case-control studies, and case series.
IV: expert committee reports, opinions, and/or clinical experience of respected authorities.
Grading of recommendations.
A: based on hierarchy I evidence.
B: based on hierarchy II evidence or extrapolated from hierarchy I evidence.
C: based on hierarchy II evidence or extrapolated from hierarchy I or II evidence.
D: directly based on hierarchy IV evidence or extrapolated from hierarchy I, II, or III evidence.
RESULTS
Six SRs have been finally included in Table 1.
Table 1.
Systematic reviews finally included
| Title | Author | Year | Topic on Strongyloides | Reference |
|---|---|---|---|---|
| Imported strongyloidiasis: epidemiology, presentations, and treatment | Buonfrate and others | 2012 | Prevalence | 26 |
| Prevalence of strongyloidiasis in Latin America: a systematic review of the literature | Buonfrate and others | 2015 | Prevalence | 7 |
| Strongyloides stercoralis: global distribution and risk factors. | Schär and others | 2013 | Prevalence | 5 |
| The laboratory diagnosis and follow-up of strongyloidiasis: a systematic review | Requena-Méndez and others | 2013 | Diagnosis | 22 |
| Severe strongyloidiasis: a systematic review of case reports | Buonfrate and others | 2013 | Clinical presentations | 10 |
| Ivermectin vs. albendazole or thiabendazole for S. stercoralis infection | Henriquez-Camacho and others | 2016 | Treatment | 53 |
Who should be screened?
First, epidemiological data are important to identify patients at risk of exposure to S. stercoralis. However, there is limited evidence in the literature providing prevalence data of strongyloidiasis. In one SR about imported strongyloidiasis, prevalence ranged from 0.4 to 46%, which varied depending on the diagnostic technique used and the targeted population (migrant and/or refugees).26 Another SR suggests that S. stercoralis affects between 10% and 40% of the population in most tropical and subtropical countries5; this study also estimates high infection rates in refugees and migrants living in non-endemic areas, reaching prevalences up to 75%.5 However, infection rates varied substantially depending on the refugees’ country of origin and the studies analyzed suggest that the infection may be underreported, especially in sub-Saharan Africa and southeast Asia.5
Second, we should differentiate between 1) patients with high risk of exposure to S. stercoralis and 2) patients with intermediate–low risk of exposure, as defined previously.
Moreover, the risk of developing a severe disease is not the same in all patients harboring the infection. Most infected subjects will never incur in the complicated form throughout their life,8 whereas immunocompromised patients are at risk of developing a severe, life-threatening disease.10
Therefore, when considering the screening for S. stercoralis, we should differentiate two clinical situations.
Immunocompetent patients.
The economic benefits of soil-transmitted infections screening in asymptomatic immunocompetent individuals, both in cost per hospitalization averted and disability-adjusted life years, have been evaluated through cost-effectiveness studies conducted in the United States.27,28
The results of these economic analyses showed that universal screening and presumptive antiparasitic treatment were more cost-effective strategies to control soil-transmitted helminths in immigrants entering United States, compared with a “watchful waiting” strategy.27 However, these studies did not consider serology as a screening method, nor new data about the efficacy of ivermectin for the treatment of strongyloidiasis.29
Testing for S. stercoralis has been suggested only for patients with eosinophilia (> 500 eosinophils-per-microliter of blood) returning from the tropics.30 Eosinophilia is a frequent (48–78%) finding in patients with strongyloidiasis,31–33 but clearly, its absence does not exclude the infection.22 It is a too weak predictor of strongyloidiasis in migrants.22,34,35
Hence, strongyloidiasis should be ruled out in any individual at risk of the infection and with eosinophilia as part of the differential diagnosis of eosinophilia. However, a two-step screening strategy (blood count and serological test if eosinophilia is present) is not recommended considering 1) the need of two accesses of the patient to the laboratory and 2) the insufficient sensitivity of eosinophilia.
Recommendation.
“Immunocompetent patients who present high risk of exposure to S. stercoralis infection should be routinely screened for strongyloidiasis.”
Grading of evidence: III
Grading of recommendations: D
Immunosuppressed patients/candidates for immunosuppression (see definitions 3 and 4).
People exposed to immunosuppressant conditions should be particularly targeted due to the increased risk of developing severe disease that has a high mortality rate.10,36 A study that evaluated the risk factors for developing strongyloidiasis hyperinfection concluded that all patients with severe disease were immunocompromised.37 As it has already been mentioned, a wide variety of predisposing factors has been described: hematologic malignancies, transplantation, immunosuppressant drugs. Steroids remain the most frequent risk factor for developing severe disease, which has been reported even during short steroid courses.37,38 It is difficult to quantify the risk of developing hyperinfection or disseminated disease in case of immunosuppression and also the amount of risk of complication involved in each particular type of immunosuppression is unknown. To sum up, immunosuppression poses the patients at risk of developing the severe disease; it has been recommended to screen the patients for S. stercoralis before administering immunosuppressant therapy, as well as before transplantation or other immunosuppressant conditions.10
Finally, and considering the high efficacy and tolerability of ivermectin, it might be probably worth treating high-risk patients preemptively in case an appropriate test (stool culture or serology) is not available.10
Recommendation.
“Immunosuppressed patients and candidates for immunosuppression should be routinely screened for strongyloidiasis if they have high or intermediate risk of exposure to S. stercoralis.
If an appropriate diagnostic test is not available, specific treatment with ivermectin should be preemptively provided.”
Grading of evidence: Ia
Grading of recommendations: B
How to screen?
The diagnosis of S. stercoralis infection is hampered by the low sensitivity of fecal-based tests and the suboptimal specificity of most serological test.22
Direct methods (parasitological-based methods).
A single stool examination fails to detect S. stercoralis larvae in up to 70% of cases. Repeated examinations of stool specimens improve the chances of finding parasites; in some studies, diagnostic sensitivity increases to 50% with three stool examinations.39,40
A recent meta-analysis on the evaluation of conventional parasitological methods found the highest sensitivity (89%) for agar plate culture, followed by the Baermann technique (72%), formol-ether concentration technique (FECT) (48%), and direct wet smear (21%).41 In most of the diagnostic studies on strongyloidiasis, the reference standard used was based on fecal methods.22 However, the sensitivity of any fecal-based reference standard may be suboptimal, especially in chronic infections where larval output is often very low.
Indirect methods (serology).
Serological methods are the most sensitive available diagnostic tools. There are several serologic tests that demonstrated better sensitivity compared with stool methods.42–49 However, false-negative results occur, especially in acute infections50 and in immunosuppressed patients22,33,51,52 and false-positive results can occur because of other helminthic infection, especially nematodes.23
A diagnostic accuracy trial has evaluated five different serological tests for S. stercoralis, including the two commercially available Bordier-ELISA (enzyme-linked immunosorbent assay) and IVD-ELISA.23 The two latter tests showed a high sensitivity and specificity: 91.2% and 99.1% for IVD-ELISA, 89.5% and 98.3% for Bordier-ELISA.
Recommendation.
“Screening should be performed with a highly sensitive serological test. If not available, improved fecal techniques could also be used (Baermann or agar plate culture).”
Grading of evidence: Ia
Grading of recommendations: B
Recommendation.
“In immunosuppressed patients, a combination of serological and parasitological methods (see above in the definition section) is mandatory, and screening should be performed before the immunosuppression if possible, first to avoid the risk of severe disease and second because serology is less sensitive once immunosuppression has already been established.”
Grading of evidence: III
Grading of recommendations: D
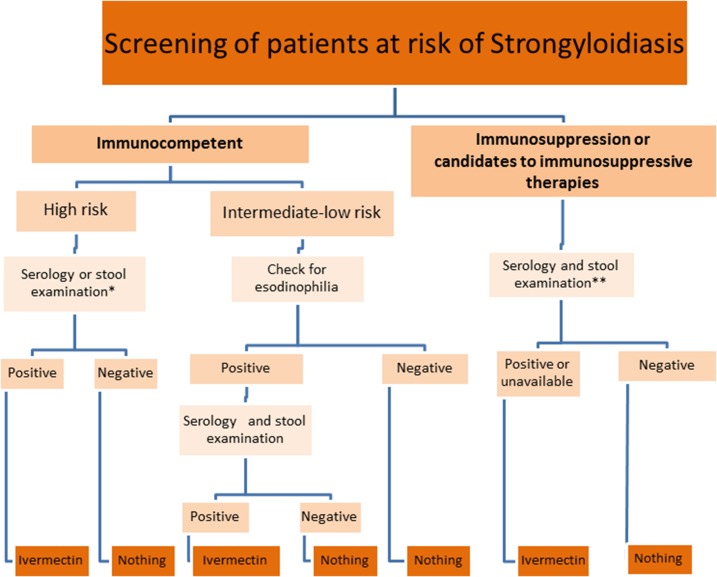
COHEMI recommendations for screening are resumed in Figure 1.
Figure 1.
Algorithm of screening for strongyloidiasis. *Serology is preferable but if not available, improved faecal techniques could also be used (Baerman or APC). **When serology or more sensitive stool techniques (Baermann or stool culture) is not available, consider empiric treatment with ivermectin. This figure appears in color at www.ajtmh.org.
How to treat?
A recent Cochrane SR has reported a higher cure rate of strongyloidiasis with ivermectin compared with albendazole and a better tolerance. Similar cure rates were observed when ivermectin was compared with thiabendazole but more adverse events were reported with the second drug.53
Most trials were relatively small, with less than 100 patients per arm. All trials but one exclusively relied on fecal diagnostic methods for the assessment of cure.
The main findings of the trials are summarized in Table 2 (that includes also trials not considered in the Cochrane review). The number needed to treat was also calculated for each trial.
Table 2.
Summary of published trials of strongyloidiasis treatment
| Author | Drug(s), dose | Diagnostic methods | Cured (%) | NTT | P value | Reference |
|---|---|---|---|---|---|---|
| Pene and others | Placebo | Harada-Mori | 0/31 (0) | 2.08 | NS | 54 |
| Albendazole 400 mg/day × 3 days | 12/25 (48) | |||||
| Singthong and others | Albendazole 800 mg bid for 3 days repeated after 1 week | Agar plate culture (APC) | 51/57 (87.9) | 64.8 | NS | 55 |
| Albendazole 800 mg bid for 5 days repeated after 1 week | 51/58 (89.5) | |||||
| Datry and others | Albendazole 400 mg/day × 3 days | Fecal smear, Kato, FECT/Baermann | 9/24 (38) | 2.2 | < 0.01 | 56 |
| Ivermectin 150–200 µg/kg single dose | 24/29 (83) | |||||
| Marti and others | Albendazole 400 mg/day × 3 days | Baermann method/Kato-Katz | 67/149 (45) | 2.6 | < 0.01 | 57 |
| Ivermectin 200 µg/kg single dose | 126/152 (83) | |||||
| Toma and others | Albendazole 800 mg bid for 3 days | Harada-Mori APC | 65/84 (77.4) | 1.35 (iv vs. pyr) | < 0.01 | 58 |
| Ivermectin 6 mg single dose | 65/67 (97.0) | 5.1 (iv vs. alb) | ||||
| Pyrvinium pamoate 5 mg/kg for 3 days | 14/60 (23.3) | – | ||||
| Nontasut and others | Albendazole 400 mg bid for 5 days | Kato-Katz culture, APC | 26/33 (78.8) | 5 | < 0.01 | 59 |
| Ivermectin 200 µg/kg single dose | 77/78 (98.7) | |||||
| Suputtamongkol | Albendazole 800 mg/day × 7 days | FECT | 8/21 (38.1) | 2.625 | 0.029 | 72 |
| Ivermectin 200 µg/kg single dose | 16/21 (76.2) | |||||
| Suputtamongkol and others | Ivermectin 200 µg/kg single dose | Fecal smear, FECT, APC | 30/31 (96.8) | 3 | NS < 0.01 | 60 |
| Ivermectin 200 µg/kg single dose for 2 days | 27/29 (93.1) | 3.6 | ||||
| Albendazole 800 mg/day × 7 days | 19/30 (63.3) | – | ||||
| Albendazole 400 mg bid for 5 days | Fecal smear, Harada-Mori, larva count (Stool and Sasa method) | 22/23 (95) | 23 | |||
| Thiabendazole 1 g bid for 5 days | 12/12 (100) | |||||
| Gann and others | Ivermectin 200 µg/kg single dose | Baermann | 16/16 (100) | 19 | NS | 62 |
| Ivermectin 200 µg/kg single dose for 2 days | 18/18 (100) | 19 | ||||
| Thiabendazole 25 mg/kg bid for 3 days | 18/19 (94.7) | – | ||||
| Adenusi and others | Ivermectin 200 µg/kg single dose | Baermann | 95/113 (84.1) | 18.4 | NS | 63 |
| Thiabendazole 25 mg/kg bid for 3 days | 81/103 (78.6) | |||||
| Bisoffi and others | Ivermectin 200 µg/kg single dose | APC, serology (IFAT) | 32/47 (68.1) | NS | 29 | |
| Thiabendazole 25 mg/kg bid for 3 days | 31/45 (68.9) | 124.411 |
NS = non-significant; NTT = number needed to treat.
Albendazole versus placebo.
A double-blind, placebo-controlled trial evaluated the efficacy of albendazole for several intestinal helminths, including S. stercoralis at the dose of 400 mg daily for 3 consecutive days, and showing a cure rate of 48%.54
Albendazole at high dosage.
An RCT comparing two different, high dosage schedules of albendazole showed an efficacy of 87.9% for albendazole (800 mg twice daily for 3 days) and 89.5% for albendazole (800 mg twice daily for 5 days) with no significant difference.55
Albendazole versus ivermectin.
Six RCTs were carried out from 1994 to 2011, on ivermectin single standard dose for 1 or 2 days, versus albendazole at different dose schedules, including high dosage. All invariably showed a superiority of ivermectin, with cure rates ranging from 83% to 100% for the latter, and from 38% to 79% for albendazole.56–60
Albendazole versus thiabendazole.
We retrieved a single RCT61 reporting a similar high cure rate for albendazole at high dose (800 mg daily for 5 consecutive days, with cure rate 95%) and thiabendazole (1 g twice daily for 5 days, with cure rate 100%). The sample size of this study was particularly small, with 35 patients enrolled overall and a short duration of follow-up (21 days).
Thiabendazole versus ivermectin.
Three RCTs compared the two drugs,62–64 all demonstrating equivalent efficacy and a much higher incidence of untoward effects for thiabendazole.
Recommendations.
“Chronic (uncomplicated) strongyloidiasis should be treated with ivermectin.”
Grading of evidence: Ia
Grading of recommendations: A
At the moment, the recommended dosage is a stat dose of 200 µg/kg (as reported in the patient information leaflet), although some authors suggest that multiple doses might increase the efficacy.65 The WHO model drug formulary66 gives both options: 1 day versus 2 consecutive days, single dose. Two trials compared the two different regimens of ivermectin, the first one published in 199462 and with small numbers reported a cure rate of 100% with both schemes, whereas the second and more recent one60 reported a slightly higher cure rate (not statistically significant) for the single dose (97% versus 93%). A multicenter RCT is currently underway (including serology for assessment of cure), comparing single to multiple doses of ivermectin.67
Empiric treatment.
In case adequate laboratory facilities are not available, and the infection cannot be excluded, empiric treatment might be worth, in consideration of the good tolerability of the drug and the potential harm caused by a missed diagnosis.65 This is particularly advised for patients who are candidate to be immunosuppressed, such as, but not limited to, transplant recipients.68
Recommendation.
“Empiric treatment of patients at risk of immunosuppression, if past exposure cannot be excluded, is indicated without testing in case of lack of adequate diagnostic facilities (see the section ‘How to screen?’).”
Grading of evidence: IV
Grading of recommendations: D
Follow-up after treatment.
Evidence summary.
A posttreatment evaluation with parasitological methods does not reliably exclude the infection, as the sensitivity of these methods is low. Several studies have reported that the serologic titer usually tends to decrease after treatment,48,64,69–71 but uniform criteria to define cure have not been established.22,42 Recently, it has been shown that, for all of the five tests analyzed by a diagnostic study (three ELISA tests, one Luciferase Immunoprecipitation Systems (LIPS), and one indirect fluorescent antibody test [IFAT]), the optical density (OD)/luminescence/titer consistently showed a diminishing trend with time, tending to negativization, for the cases treated successfully, although the time required may be as long as 12 months or more.24 Failure to achieve a significant reduction in titer or OD (to 50% or less of the OD prior to treatment, or at least two IFAT dilutions) should be considered as a potential treatment failure, even if fecal-based tests are negative.
Recommendations.
“Posttreatment follow-up should be performed with the most sensitive technique available. Serology should be done at baseline and repeated after 6 and 12 months after treatment to monitor the decrease in OD/titer or negativization.”
Grading of evidence: IIb
Grading of evidence: C
DISCUSSION
There are several reasons that justify the screening in asymptomatic people.
In the first place, an early detection of the infection in individuals at risk, before they develop any clinical complication, is in itself a sufficient argument to propose a screening. Moreover, in immunosuppressed patients, the screening should be mandatory. Second, there is a drug, ivermectin, which is now the universally accepted treatment with a high efficacy rate and a low rate of adverse effects. Third, the screening is based on a simple and widely accessible technology, including commercially available tests that are highly sensitive. The screening could be implemented as part of a screening program for migrants, although further cost-effectiveness studies are required to better evaluate this strategy from a public health point of view.
REFERENCES
- 1.Greaves D, Coggle S, Pollard C, Aliyu SH, Moore EM, 2013. Strongyloides stercoralis infection. BMJ 347: f4610 Available at: http://www.ncbi.nlm.nih.gov/pubmed/23900531. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Muñoz J, Krolewiecki AJ, Gotuzzo E, Mena MA, Chiodini PL, Anselmi M, Moreira J, Albonico M, 2013. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 7: e2214 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3649953&tool=pmcentrez&rendertype=abstract. Accessed May 27, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buonfrate D, Angheben A, Gobbi F, Muñoz J, Requena-Mendez A, Gotuzzo E, Mena MA, Bisoffi Z, 2012. Imported strongyloidiasis: epidemiology, presentations, and treatment. Curr Infect Dis Rep 14: 256–262. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22322601. Accessed June 25, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Montes M, Sawhney C, Barros N, 2010. Strongyloides stercoralis: there but not seen. Curr Opin Infect Dis 23: 500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P, 2013. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7: e2288. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3708837&tool=pmcentrez&rendertype=abstract. Accessed July 18, 2013. [DOI] [PMC free article] [PubMed]
- 6.Gonzalez A, Gallo M, Valls ME, Munoz J, Puyol L, Pinazo MJ, Mas J, Gascon J, 2010. Clinical and epidemiological features of 33 imported Strongyloides stercoralis infections. Trans R Soc Trop Med Hyg 104: 613–616. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20637483. Accessed August 3, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Buonfrate D, Mena MA, Angheben A, Requena-Mendez A, Muñoz J, Gobbi F, Albonico M, Gotuzzo E, Bisoffi Z, 2015. Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect 143: 452–460. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24990510. Accessed August 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Requena-Méndez A, Buonfrate D, Bisoffi Z, Muñoz J, 2014. Advances in the diagnosis of human strongyloidiasis. Curr Trop Med Rep 1: 207–215. [Google Scholar]
- 9.Viney ME, Lok JB, 2015. The biology of Strongyloides spp. WormBook 16: 1–17. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26183912. Accessed December 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, Bisoffi Z, 2013. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis 13: 78 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3598958&tool=pmcentrez&rendertype=abstract. Accessed June 25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keiser PB, Nutman TB, 2004. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev 17: 208–217. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=321465&tool=pmcentrez&rendertype=abstract. Accessed July 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel MO, Simon GL, 2012. Is human immunodeficiency virus infection a risk factor for Strongyloides stercoralis hyperinfection and dissemination. PLoS Negl Trop Dis 6: e1581 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3409107&tool=pmcentrez&rendertype=abstract. Accessed December 16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotuzzo E, Terashima A, Alvarez H, Tello R, Infante R, Watts DM, Freedman DO, 1999. Strongyloides stercoralis hyperinfection associated with human T cell lymphotropic virus type-1 infection in Peru. Am J Trop Med Hyg 60: 146–149. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9988339. Accessed December 16, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Levi ME, Kumar D, Green M, Ison MG, Kaul D, Michaels MG, Morris MI, Schwartz BS, Echenique IA, Blumberg EA, 2014. Considerations for screening live kidney donors for endemic infections: a viewpoint on the UNOS policy. Am J Transplant 14: 1003–1011. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24636427. Accessed September 18, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton KW, Abt PL, Rosenbach MA, Bleicher MB, Levine MS, Mehta J, Montgomery SP, Hasz RD, Bono BR, Tetzlaff MT, Mildiner-Early S, Introcaso CE, Blumberg EA, 2011. Donor-derived Strongyloides stercoralis infections in renal transplant recipients. Transplantation 91: 1019–1024. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21358367. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Roseman DA, Kabbani D, Kwah J, Bird D, Ingalls R, Gautam A, Nuhn M, Francis JM, 2013. Strongyloides stercoralis transmission by kidney transplantation in two recipients from a common donor. Am J Transplant 13: 2483–2486. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3785548&tool=pmcentrez&rendertype=abstract. Accessed July 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le M, Ravin K, Hasan A, Clauss H, Muchant DG, Pasko JK, Cipollina G, Abanyie F, Montgomery SP, Loy M, Ahmed M, Mathur M, Chokkalingam Mani B, Mehr J, Kotru A, Varma C, Maksimak M, Schultz M, Obradovic G, Alvarez R, Toyoda Y, Birkenbach M, Brunner E, Nelson J, 2014. Single donor-derived strongyloidiasis in three solid organ transplant recipients: case series and review of the literature. Am J Transplant 14: 1199–1206. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24612907. Accessed August 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinkenberg E, Manissero D, Semenza JC, Verver S, 2009. Migrant tuberculosis screening in the EU/EEA: yield, coverage and limitations. Eur Respir J 34: 1180–1189. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19880618. Accessed April 24, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Qi J, Fu X, Meng S, Li C, Sun J, 2015. Case finding advantage of HIV rapid tests in community settings: men who have sex with men in 12 programme areas in China, 2011. Int J STD AIDS 26: 402–413. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25028452. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Buonfrate D, Baldissera M, Abrescia F, Bassetti M, Caramaschi G, Giobbia M, Mascarello M, Rodari P, Scattolo N, Napoletano G, Bisoffi Z; CCM Strongyloides Study Group, 2016. Epidemiology of Strongyloides stercoralis in northern Italy: results of a multicentre case-control study, February 2013 to July 2014. Euro Surveill 2016 Aug 4;21(31). doi:10.2807/1560-7917.ES.2016.21.31.30310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcaraz CO, Adell RI, Sánchez PS, Blasco MJ, Sánchez OA, Auñón AS, Calabuig DR, 2004. Characteristics and geographical profile of strongyloidiasis in healthcare area 11 of the Valencian community (Spain). J Infect 49: 152–158. Available at: http://www.ncbi.nlm.nih.gov/pubmed/?term=alcaraz+2004+strongyloides. Accessed April 24, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J, 2013. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7: e2002 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3547839&tool=pmcentrez&rendertype=abstract. Accessed June 25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Muñoz J, Nutman TB, 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: e2640 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3890421&tool=pmcentrez&rendertype=abstract. Accessed August 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Degani M, Tais S, Angheben A, Requena-Mendez A, Muñoz J, Nutman TB, Bisoffi Z, 2015. Accuracy of five serologic tests for the follow up of Strongyloides stercoralis infection. PLoS Negl Trop Dis 9: e0003491 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4323101&tool=pmcentrez&rendertype=abstract. Accessed August 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality, AHRQ’s National Guideline Clearinghouse Available at: https://www.guidelines.gov/. Accessed October 9, 2016.
- 26.Buonfrate D, Angheben A, Gobbi F, Munoz J, Requena-Mendez A, Gotuzzo E, Mena MA, Bisoffi Z, 2012. Imported strongyloidiasis: epidemiology, presentations, and treatment. Curr Infect Dis Rep 14: 256–262. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22322601. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Muennig P, Pallin D, Sell RL, Chan MS, 1999. The cost effectiveness of strategies for the treatment of intestinal parasites in immigrants. N Engl J Med 340: 773–779. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10072413. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Muennig P, Pallin D, Challah C, Khan K, 2004. The cost-effectiveness of ivermectin vs. albendazole in the presumptive treatment of strongyloidiasis in immigrants to the United States. Epidemiol Infect 132: 1055–1063. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2870196&tool=pmcentrez&rendertype=abstract. Accessed August 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisoffi Z, Buonfrate D, Angheben A, Boscolo M, Anselmi M, Marocco S, Monteiro G, Gobbo M, Bisoffi G, Gobbi F, 2011. Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoS Negl Trop Dis 5: e1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Checkley AM, Chiodini PL, Dockrell DH, Bates I, Thwaites GE, Booth HL, Brown M, Wright SG, Grant AD, Mabey DC, Whitty CJ, Sanderson F, 2010. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect 60: 1–20. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19931558. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Caruana SR, Kelly HA, Ngeow JY, Ryan NJ, Bennett CM, Chea L, Nuon S, Bak N, Skull SA, Biggs BA, 2006. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med 13: 233–239. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16884406. Accessed August 12, 2015. [DOI] [PubMed]
- 32.de Silva S, Saykao P, Kelly H, MacIntyre CR, Ryan N, Leydon J, Biggs BA, 2002. Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7–20 years after resettlement in Australia. Epidemiol Infect 128: 439–444. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2869840&tool=pmcentrez&rendertype=abstract. Accessed August 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mascarello M, Gobbi F, Angheben A, Gobbo M, Gaiera G, Pegoraro M, Lanzafame M, Buonfrate D, Concia E, Bisoffi Z, 2011. Prevalence of Strongyloides stercoralis infection among HIV-positive immigrants attending two Italian hospitals, from 2000 to 2009. Ann Trop Med Parasitol 105: 617–623. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4089805&tool=pmcentrez&rendertype=abstract. Accessed July 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baaten GG, Sonder GJ, van Gool T, Kint JA, van den Hoek A, 2011. Travel-related schistosomiasis, strongyloidiasis, filariasis, and toxocariasis: the risk of infection and the diagnostic relevance of blood eosinophilia. BMC Infect Dis 11: 84 Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21466667. Accessed August 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libman MD, MacLean JD, Gyorkos TW, 1993. Screening for schistosomiasis, filariasis, and strongyloidiasis among expatriates returning from the tropics. Clin Infect Dis 17: 353–359. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8218675. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Marcos LA, Terashima A, Dupont HL, Gotuzzo E, 2008. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg 102: 314–318. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18321548. Accessed June 25, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Asdamongkol N, Pornsuriyasak P, Sungkanuparph S, 2006. Risk factors for strongyloidiasis hyperinfection and clinical outcomes. Southeast Asian J Trop Med Public Health 37: 875–884. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17333728. Accessed June 25, 2013. [PubMed] [Google Scholar]
- 38.Fardet L, Généreau T, Cabane J, Kettaneh A, 2006. Severe strongyloidiasis in corticosteroid-treated patients. Clin Microbiol Infect 12: 945–947. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16961629. Accessed June 25, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui AA, Berk SL, 2001. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 33: 1040–1047. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11528578. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen PB, Mojon M, 1987. Improved diagnosis of Strongyloides stercoralis by seven consecutive stool specimens. Zentralbl Bakteriol Mikrobiol Hyg A 263: 616–618. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3604502. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Campo Polanco L, Gutiérrez LA, Cardona Arias J, 2014. Diagnosis of Strongyloides stercoralis infection: meta-analysis on evaluation of conventional parasitological methods (1980–2013) [in Spanish] Rev Esp Salud Publica 88: 581–600. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25327268. Accessed July 28, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Boscolo M, Gobbo M, Mantovani W, Degani M, Anselmi M, Monteiro GB, Marocco S, Angheben A, Mistretta M, Santacatterina M, Tais S, Bisoffi Z, 2007. Evaluation of an indirect immunofluorescence assay for strongyloidiasis as a tool for diagnosis and follow-up. Clin Vaccine Immunol 14: 129–133. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17135451. Accessed July 28, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huaman MC, Sato Y, Aguilar JL, Terashima A, Guerra H, Gotuzzo E, Kanbara H, 2003. Gelatin particle indirect agglutination and enzyme-linked immunosorbent assay for diagnosis of strongyloidiasis using Strongyloides venezuelensis antigen. Trans R Soc Trop Med Hyg 97: 535–538. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15307419. Accessed June 25, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Silva LP, Barcelos IS, Passos-Lima AB, Espindola FS, Campos DM, Costa-Cruz JM, 2003. Western blotting using Strongyloides ratti antigen for the detection of IgG antibodies as confirmatory test in human strongyloidiasis. Mem Inst Oswaldo Cruz 98: 687–691. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12973538. Accessed June 25, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Lindo JF, Conway DJ, Atkins NS, Bianco AE, Robinson RD, Bundy DA, 1994. Prospective evaluation of enzyme-linked immunosorbent assay and immunoblot methods for the diagnosis of endemic Strongyloides stercoralis infection. Am J Trop Med Hyg 51: 175–179. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8074251. Accessed June 25, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Neva FA, Gam AA, Burke J, 1981. Comparison of larval antigens in an enzyme-linked immunosorbent assay for strongyloidiasis in humans. J Infect Dis 144: 427–432. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7031142. Accessed June 25, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Mangali A, Chaicumpa W, Nontasut P, Chantavanij P, Tapchaisri P, Viravan C, 1991. Enzyme-linked immunosorbent assay for diagnosis of human strongyloidiasis. Southeast Asian J Trop Med Public Heal 22: 88–92. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1948267. Accessed June 25, 2013. [PubMed]
- 48.Loutfy MR, Wilson M, Keystone JS, Kain KC, 2002. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg 66: 749–752. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12224585. Accessed June 25, 2013. [DOI] [PubMed] [Google Scholar]
- 49.van Doorn HR, Koelewijn R, Hofwegen H, Gilis H, Wetsteyn JC, Wismans PJ, Sarfati C, Vervoort T, van Gool T, 2007. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J Clin Microbiol 45: 438–442. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17151215. Accessed June 25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sudarshi S, Stumpfle R, Armstrong M, Ellman T, Parton S, Krishnan P, Chiodini PL, Whitty CJ, 2003. Clinical presentation and diagnostic sensitivity of laboratory tests for Strongyloides stercoralis in travellers compared with immigrants in a non-endemic country. Trop Med Int Heal 8: 728–732. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12869094. Accessed June 25, 2013. [DOI] [PubMed]
- 51.Angheben A, Mistretta M, Gobbo M, Bonafini S, Iacovazzi T, Sepe A, Gobbi F, Marocco S, Rossanese A, Bisoffi Z, 2011. Acute strongyloidiasis in Italian tourists returning from southeast Asia. J Travel Med 18: 138–140. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21366799. Accessed June 20, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Luvira V, Trakulhun K, Mungthin M, Naaglor T, Chantawat N, Pakdee W, Phiboonbanakit D, Dekumyoy P, 2016. Comparative diagnosis of strongyloidiasis in immunocompromised patients. Am J Trop Med Hyg 95: 401–404. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27296387. Accessed August 4, 2016. [DOI] [PMC free article] [PubMed]
- 53.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN, 2016. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev CD007745 Available at: http://www.ncbi.nlm.nih.gov/pubmed/26778150. Accessed July 29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pene P, Mojon M, Garin JP, Coulaud JP, Rossignol JF, 1982. Albendazole: a new broad spectrum anthelmintic. Double-blind multicenter clinical trial. Am J Trop Med Hyg 31: 263–266. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7041665. Accessed August 13, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Singthong S, Intapan PM, Wongsaroji T, Maleewong W, 2006. Randomized comparative trial of two high-dose albendazole regimens for uncomplicated human strongyloidiasis. Southeast Asian J Trop Med Public Health 37 (Suppl 3): 32–34. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17547048. Accessed August 13, 2015. [PubMed] [Google Scholar]
- 56.Datry A, Hilmarsdottir I, Mayorga-Sagastume R, Lyagoubi M, Gaxotte P, Biligui S, Chodakewitz J, Neu D, Danis M, Gentilini M, 1994. Treatment of Strongyloides stercoralis infection with ivermectin compared with albendazole: results of an open study of 60 cases. Trans R Soc Trop Med Hyg 88: 344–345. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7974685. Accessed August 13, 2015. [DOI] [PubMed]
- 57.Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, Hatz C, 1996. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg 55: 477–481. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8940976. Accessed August 13, 2015. [DOI] [PubMed] [Google Scholar]
- 58.Toma H, Sato Y, Shiroma Y, Kobayashi J, Shimabukuro I, Takara M, 2000. Comparative studies on the efficacy of three anthelminthics on treatment of human strongyloidiasis in Okinawa, Japan. Southeast Asian J Trop Med Public Health 31: 147–151. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11023084. Accessed August 13, 2015. [PubMed] [Google Scholar]
- 59.Nontasut P, Claesson BA, Dekumyoy P, Pakdee W, Chullawichit S, 2005. Prevalence of Strongyloides in northern Thailand and treatment with ivermectin vs albendazole. Southeast Asian J Trop Med Public Health 36: 442–444. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15916052. Accessed August 13, 2015. [PubMed] [Google Scholar]
- 60.Suputtamongkol Y, Premasathian N, Bhumimuang K, Waywa D, Nilganuwong S, Karuphong E, Anekthananon T, Wanachiwanawin D, Silpasakorn S, 2011. Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis. PLoS Negl Trop Dis 5: e1044 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3091835&tool=pmcentrez&rendertype=abstract. Accessed July 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitisuttithum P, Supanaranond W, Chindanond D, 1995. A randomized comparative study of albendazole and thiabendazole in chronic strongyloidiasis. Southeast Asian J Trop Med Public Health 26: 735–738. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9139386. Accessed August 13, 2015. [PubMed] [Google Scholar]
- 62.Gann PH, Neva FA, Gam AA, 1994. A randomized trial of single- and two-dose ivermectin versus thiabendazole for treatment of strongyloidiasis. J Infect Dis 169: 1076–1079. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8169394. Accessed August 13, 2015. [DOI] [PubMed] [Google Scholar]
- 63.Adenusi A, Oke A, Adenusi A, 2003. Comparison of ivermectin and thiabendazole in the treatment of uncomplicated human Strongyloides stercoralis infection. Afr J Biotechnol 2: 465–469. [Google Scholar]
- 64.Bisoffi Z, Buonfrate D, Angheben A, Boscolo M, Anselmi M, Marocco S, Monteiro G, Gobbo M, Bisoffi G, Gobbi F, 2011. Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoS Negl Trop Dis 5: e1254 Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21814588. Accessed August 13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mejia R, Nutman TB, 2012. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis 25: 458–463. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22691685. Accessed August 13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization, 2008. WHO Model Formulary Available at: http://apps.who.int/medicinedocs/documents/s16879e/s16879e.pdf. Accessed August 13, 2015.
- 67.Multiple versus Single Dose of Ivermectin for the Treatment of Strongyloidiasis (STRONG TREAT). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01570504?term=ivermectin&rank=10. Accessed October 31, 2015.
- 68.Roxby AC, Gottlieb GS, Limaye AP, 2009. Strongyloidiasis in transplant patients. Clin Infect Dis 49: 1411–1423. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2913967&tool=pmcentrez&rendertype=abstract. Accessed October 31, 2015. Accessed October 31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biggs BA, Caruana S, Mihrshahi S, Jolley D, Leydon J, Chea L, Nuon S, 2009. Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am J Trop Med Hyg 80: 788–791. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19407125. Accessed August 13, 2015. [PubMed] [Google Scholar]
- 70.Salvador F, Sulleiro E, Sánchez-Montalvá A, Saugar JM, Rodríguez E, Pahissa A, Molina I, 2014. Usefulness of Strongyloides stercoralis serology in the management of patients with eosinophilia. Am J Trop Med Hyg 90: 830–834. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4015573&tool=pmcentrez&rendertype=abstract. Accessed August 13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Page WA, Dempsey K, McCarthy JS, 2006. Utility of serological follow-up of chronic strongyloidiasis after anthelminthic chemotherapy. Trans R Soc Trop Med Hyg 100: 1056–1062. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16551471. Accessed October 31, 2015. [DOI] [PubMed] [Google Scholar]
- 72.Suputtamongkol Y, Kungpanichkul N, Silpasakorn S, Beeching NJ, 2008. Efficacy and safety of a single-dose veterinary preparation of ivermectin versus 7-day high-dose albendazole for chronic strongyloidiasis. Int J Antimicrob Agents 31: 46–49. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18023151. Accessed September 18, 2015. [DOI] [PubMed] [Google Scholar]