Abstract.
Treatment against Plasmodium falciparum malaria includes blood schizonticides to clear asexual parasites responsible for disease. The addition of gametocytocidal drugs can eliminate infectious sexual stages with potential for transmission and the World Health Organization recommends a single dose (SD) of primaquine (PQ) to this end. The efficacy of PQ at 0.75 mg/kg to suppress gametocytemia when administered in single or fractionated doses was evaluated. A clinical controlled study with an open-label design was executed; three groups of 20 subjects were studied sequentially. All subjects were treated with the standard dose of artemether–lumefantrine plus the total dose of 0.75 mg/kg of PQ administered (without previous G6PD testing) in three different ways: Group “0.75d-3” received 0.75 mg/kg on day 3; Group “0.50d-1 + 0.25d-3” received 0.50 mg/kg on day 1 and 0.25 mg/kg on day 3; Group “0.25d-1,2,3” received 0.25 mg/kg on days 1, 2, and 3. Subjects were evaluated on days 1, 4, and 7 by thick smear microscopy and quantitative polymerase chain reaction to determine the carriage of immature and mature gametocytes. There were no adverse events. The three schemes caused a marked reduction (75–85%) in prevalence of gametocytes on day 4 compared with day 1, but only the group that received 0.75 mg/kg on day 3 maintained the reduced gametocyte burden until day 7. None of the three treatments were able to clear gametocyte carriage on days 4 or 7, but the group that received the SD had the lowest prevalence of gametocytes (15%). Further studies are needed to establish a PQ regimen with complete efficacy against gametocytes.
INTRODUCTION
Treatment against Plasmodium falciparum malaria includes blood schizonticide drugs to clear asexual circulating parasites responsible for disease and gametocytocidal drugs to eliminate infectious sexual stages with potential for transmission to mosquitoes.1,2 The standard regimen recommended by World Health Organization (WHO) includes administration of artemisinin-based combination therapy (ACT) plus a single dose (SD) of primaquine (PQ) at 0.75 mg base/kg (maximum 45 mg base in adults) in areas of low transmission.1 The WHO amended its recommendation to a dose of 0.25 mg/kg when G6PD deficiency testing is unavailable, due to concerns about safety.2–4 In Colombia, 0.75 mg/kg remains the recommended dose even without G6PD testing and is generally given as a SD on day 4 of treatment, 24 hours after completing schizonticide treatment.5 This PQ regimen is referred to here as the “SD”.
The SD has limited effectiveness because it reduces but does not eliminate gametocytes, and its cytotoxic activity is restricted to the mature gametocytes (stage V).6–13 PQ added to malaria treatments reduces gametocyte prevalence when given in doses greater than 0.4 mg/kg, but there are few reports that measured the effect of PQ treatment on transmission to mosquitoes.14,15 Although the SD reduces gametocytemia within 10 days,14–20 transmission is blocked before the number of gametocytes is lowered.18 The extent of this blocking effect has been proposed to depend on the dose of PQ, but more importantly, on the baseline gametocytemia, with complete efficacy observed when circulation of these parasite stages before treatment is low.18
There are three key considerations for the use of PQ as a gametocytocide against P. falciparum: 1) total dose per body weight; 2) single-dose administration (total dose administered in a SD) versus fractionated dose (total dose administered in several fractions or multidose); and 3) timing of PQ (beginning on day 1, 2, 3, or 4). There is no hard evidence to support the selection of 45 mg as the dose of PQ to eliminate the gametocytes of P. falciparum, since other doses also reduced gametocytemia.16,17,19,21–23 The dose of 0.25 mg/kg generated controversy because authors of a Cochrane review 2014 did not observe an effect on prevalence of gametocytes,14 whereas the review of NJ White in 2014 supported an effect20; that criticism led to the publication of the 2015 version of the Cochrane review to recognize the effects of low doses of PQ on gametocytes reduction.15 Doses of 0.25 mg/kg and 0.4 mg/kg had similar effects on gametocytemia, but lesser than the SD.24 Moreover, there is no evidence that a SD of PQ is superior to a multidose. Finally, limited data are available on the optimum timing for PQ administration following diagnosis. In studies where PQ was administered on day 1,20 on day 215 or on day 319,20,25–27 (i.e., together with the schizontocide treatment), there was an increased effect on gametocytemia compared with the SD.
Two additional factors are important when measuring the efficacy of PQ: 1) the time point of evaluation and 2) the diagnostic test used for gametocyte detection. Most studies measured gametocyte reduction on day 7, that is, 4 days after completing the schizonticide treatment,7–13,17,19,25,28 whereas a few studies measured gametocyte reduction on day 4, that is, 24 hours after completing the schizonticide treatment.19,27,29 There is no consensus on the appropriate day to measure gametocytemia posttreatment. Some authors argue that it should not be beyond day 4 because on that day the patient has completed the schizonticide and is asymptomatic.18,20,26 Therefore, the ideal drug against P. falciparum gametocytes should eliminate gametocytes on day 4 or earlier.26
The diagnostic test commonly used to measure gametocytemia is thick blood smear.7–10,13,17,25–28 However, molecular tests are more sensitive and detect lower levels of gametocytes, including submicroscopic gametocytemia. Molecular assays typically detect mRNA of pfs25, a marker for mature gametocytes.11,12,19
This study aimed to assess the efficacy of PQ at 0.75 mg/kg to suppress gametocytemia on days 4 and 7, when administered in single (nonfractionated) or fractionated doses together with the schizonticidal treatment (artemether + lumefantrine).
METHODS
Study sites.
The study was carried out in three municipalities in Colombia: Puerto Libertador in the Department of Córdoba and Río Iró and Lloró in the Department of Chocó. During 2010–2014, the total malaria cases reported in Colombia varied between 39,762 and 115,884, with a mean of 65,070, and a median of 59,403 cases. Plasmodium vivax accounted for 60–70% of malaria cases until 2014, and during 2014 the frequency of P. falciparum was 50%. Both species cocirculate in the three selected municipalities, but P. vivax predominates in Puerto Libertador (ratio 8:1), whereas P. falciparum predominates in Río Iró and Lloró (ratio 2:1). During 2014, the annual parasitic index (malaria cases/1,000 inhabitants) was 1.46 in Puerto Libertador and 32 in Chocó.30
Study design.
A controlled trial was carried out with an open-label design. Three groups were studied sequentially; Group 0.75-d3 was studied first, followed by Group 0.25-d1,2,3, and then Group 0.50-d1 + 0.25-d3 (see group definitions below). The size sample was established by convenience with at least 20 subjects per group.
Inclusion and exclusion criteria.
Volunteers were recruited after fulfillment of the following criteria: thick blood smear positive exclusively for P. falciparum, absence of signs or symptoms of severe malaria (less than 50,000 asexual parasites/µL)5 or concomitant disease, absence of pregnancy, and agreement to return for follow-up. Subjects provided informed consent. Subjects were excluded if they developed severe malaria, failed to attend follow-up visits, or withdrew their consent.
Antimalarial treatment and study groups.
All subjects were treated with AL (1.5 mg/kg [artemether] and 12 mg/kg [lumefantrine]) twice a day for three consecutive days (days 1, 2, and 3), according to the guidelines for nonsevere P. falciparum malaria from the Ministry of Public Health of Colombia.5 In addition, all subjects received PQ at a total dose of 0.75 mg/kg (maximum 45 mg), but administered in three different ways: 1) Group 0.75-d3 received 0.75 mg/kg on day 3; 2) Group 0.50-d1 + 0.25-d3 received 0.50 mg/kg on day 1 and 0.25 mg/kg on day 3; and 3) Group 0.25-d1,2,3 received 0.25 mg/kg on days 1, 2, and 3. The treatment was supervised directly by researchers. Previous testing for G6PD deficiency is not required in Colombia for administration of PQ5 and was not performed. Each study group included at least 10 subjects from the Córdoba region and 10 subjects from the Chocó region.
Follow-up, sample collection, and processing.
At enrolment, a questionnaire was completed which recorded sociodemographic data, malaria history, and antimalarial treatment. Subjects were evaluated on day 1 (enrolment and first day of treatment), day 4 (24 hours after treatment with PQ), and day 7. At each evaluation visit, 3 mL of venous blood were collected to prepare thick blood smears and blood spots (approximately 100 µL of blood) on filter paper Whatman grade 3 (Merck KGaA, Darmstadt, Germany), and 100 µL of blood was added to 900 µL of Trizol™ (for RNA analysis) (Thermo Fisher Scientific, Waltham, MA) which was stored in liquid nitrogen. Moreover, a questionnaire about adverse events possibly associated with the treatment was completed on days 4 and 7.
Microscopy and molecular diagnosis.
Field stained thick blood smears were examined with a ×100 objective to identify plasmodial species and quantify asexual parasitemia and gametocytemia. Parasite density (asexual and sexual forms) was measured by counting parasites per 200 leukocytes, based on a mean count of 8,000 leukocytes per microliter of blood (theoretical value). A slide was considered negative after examination of all fields suitable for reading.
Molecular diagnosis was carried out for confirmation of P. falciparum monoinfection. Blood spots were used for DNA extraction using the saponin-Chelex method.31 The extracted DNA was resuspended in 50 μL of water and real-time quantitative polymerase chain reaction (qPCR) was performed as described elsewhere.32 DNA copies were quantified in copies/μL of template based on a plasmid standard curve.
Gametocytemia assessment by molecular assays (qPCR).
Total RNA was isolated from blood stored in Trizol. cDNA was synthesized with the first strand cDNA Synthesis kit using Superscript™ III (Invitrogen™, Carlsbad, CA), followed by amplification of pfs16 (for immature gametocytes, stages I–IV) and pfs25 (for mature gametocytes, stage V), using primers previously described with some modifications.33 The sequences of primers and probes used in the assays are listed in Table 1. Real-time PCR was performed using TaqMan Universal Master Mix (Invitrogen) in an ABI 7500 (Applied Biosystems®, Thermo Fisher Scientific, Waltham, MA). Amplifications with the following parameters: 10 minutes at 95°C, 40 cycles of 30 seconds annealing at 60.8°C for pfs16 and at 55.3°C for pfs25, and 30 seconds final extension at 72°C. Samples were quantified using a standard curve of a plasmid containing each amplicon (pfs16 or pfs25) diluted at 10, 102, 103, 104, 105, and 106 copies/μL in Tris-EDTA buffer. Each qPCR included two no template controls (water instead of DNA), a no RT control (minus reverse transcriptase), and a positive control consisting of cDNA obtained from P. falciparum in vitro culture (strain 3D7).
Table 1.
Primers (forward [F] and reverse [R]) and probes (P) used to assess gametocytemia by qPCR
| Gene | Sequence (5′-3′) | Product size (pb) | GeneBank accession no. |
|---|---|---|---|
| pfs16 | F: AGTTCTTCAGGTGCCTCTCTTCA | 111 | M64705 |
| R: AGCTAGCTGAGTTTCTAAAGGCA | |||
| P: dFAM-TCTCAAGGTCTTTCTGGAAAAGATTCTGCTGACAA-BHQ-1 | |||
| pfs25 | F: AGACTGTAAATAAACCATGTGGAGA | 156 | AE014185.2 |
| R: ACCGTTACCACAAGTTACATTCT | |||
| P: dFAM-TGGAAATCCCGTTTCATACGCTTGTAAATGTAATC-BHQ-1 |
qPCR = quantitative polymerase chain reaction.
Statistical analysis.
Data were analyzed using Epi Info 6.04 (Centers for Disease, Control and Prevention, Atlanta, GA) and SPSS 19 (IBM Corp., Armonk, NY). Significance was set at P < 0.05. Qualitative variables were compared using χ2 test and quantitative variables were compared using the Kruskal–Wallis (KW) test. Analysis of independent groups was used for comparison of prevalence (χ2 test) and density (KW test) of gametocytes with each PQ scheme (0.75-d3, 0.50-d1 + 0.25-d3, and 0.25-d1,2,3) in each day (1, 4, and 7), that is, comparison of three groups each day. In addition, analysis of dependent or paired groups was performed to compare the net relative change in gametocytemia (increase or decrease) in each study group over time, that is, comparison of the same group over time; Wilcoxon (only two groups) or Friedman (greater than two groups) tests were used.
Ethics.
Participants signed a voluntary consent form. The study involved minor risk, and approval was granted by the Comité de Ética of Instituto de Investigaciones Médicas (ethical approval number IIM0803), from the Universidad de Antioquia.
RESULTS
A total 183 samples were collected from 61 volunteers; 20 for group 0.75-d3, and 21 each for groups 0.50-d1 + 0.25-d3 and 0.25-d1,2,3. The general characteristics of subjects are listed in Table 2. On admission (day 1), asexual parasitemia quantified by microscopy was similar in the three study groups; however, based on qPCR, the asexual parasitemia in patients receiving PQ at 0.5 mg/kg on day 1 and 0.25 mg/kg on day 3 (group 0.5-d1 + 0.25-d3) was significantly higher (Table 3). No volunteers had adverse events possibly associated with the antimalarial treatment. The signs and symptoms observed in all patients were indistinguishable from the common malaria symptoms. Treatment was not suspended in any volunteer.
Table 2.
General characteristics of patients in each study group on admission
| Variable | Study group | P | ||
|---|---|---|---|---|
| 0.75-d3 (N = 20) | 0.50d1 + 0.25-d3 (N = 21) | 0.25-d1,2,3 (N = 20) | ||
| Age (years), median (IQR) | 23 (17–42) | 21 (14–44) | 24 (18–38) | 0.86 (KW) |
| Male:female (%) | 65:35 | 57:43 | 62:38 | 1.00 (χ2) |
| Days of illness before diagnosis, median (IQR) | 2 (2–3) | 2 (3–7) | 5 (2–8) | 0.34 (KW) |
IQR = interquartile range; KW = Kruskal–Wallis.
Table 3.
Asexual and sexual parasitemia on admission (day 1) per study group
| Variable | Study group | P | ||
|---|---|---|---|---|
| 0.75-d3 (N = 20) | 0.50-d1 + 0.25-d3 (N = 21) | 0.25-d1,2,3 (N = 20) | ||
| Asexual parasitaemia [parasites/µL] by microscopy median (IQR); geometric mean | 968 (274–2,909); 1,119 | 2,620 (918–3,974); 1,896 | 686 (70–1,646); 544 | 0.071 (KW) |
| Asexual parasitaemia [DNA copies/µL] by qPCR median (IQR); geometric mean | 10,377 (1,122–12,574); 7,052 | 22,815 (14,488–30,019); 19,799 | 10,561 (6,263–10,561); 4,147 | 0.002 (KW) |
| Gametocyte prevalence by microscopy % (n) [CI 95%] | 10 (2) [1–32] | 5 (1) [0.1–24] | 0 (0) [0–17] | 0.352 (χ2) |
| Gametocyte prevalence by qPCR % (n) [CI 95%] | 60 (12) [36–81] | 24 (5) [8–47] | 50 (10) [27–73] | 0.054 (χ2) |
| Gametocytes density by qPCR [cDNA copies/µL] median (IQR); geometric mean | 12 (0–202); 55 | 0 (0–2); 166 | 2 (0–16); 19 | 0.117 (KW) |
qPCR = quantitative polymerase chain reaction; IQR = interquartile range; KW = Kruskal–Wallis. CI 95% = exact binomial confidence interval of 95%, geometric mean was calculated excluding negative samples.
Bold values indicate a P value < 0.05.
The prevalence of gametocytemia (proportion of subjects with gametocytes) on admission, that is, before treatment, was similar in all three study groups, according to microscopy and qPCR. The gametocyte prevalence ranged from 0% to 10% (mean for the three groups: 5%) by microscopy and from 24% to 60% (mean 45%) by qPCR. In addition, the density of gametocytes measured by qPCR was similar across all three groups (Table 3). In general, qPCR detected eight times more gametocytes than microscopy.
In general, mature gametocytes (detected with the pfs25 marker) were scarce in all three groups and during the complete follow up (days 1, 4, and 7). Henceforth, the prevalence and density of gametocytes by qPCR refers to the total gametocytemia, that is, immature (pfs16 marker) plus mature (pfs25 marker). The gametocytemia by qPCR on admission was similar in all three study groups (Table 4).
Table 4.
Prevalence and density of gametocytemia (by qPCR) per maturation state on day 1, according to the study group
| Study group | Prevalence % (n) | Density (geometric mean of cDNA copies/µL)* | ||||
|---|---|---|---|---|---|---|
| Immature | Mature | Total | Immature | Mature | Total | |
| pfs16 | pfs25 | pfs16 + pfs25 | pfs16 | pfs25 | pfs16 + pfs25 | |
| 0.75-d3 | 60 (12) | 15 (3) | 60 (12) | 50 | 33 | 55 |
| 0.50-d1 + 0.25-d3 | 25 (5) | 5 (1) | 24 (5) | 166 | 9 | 166 |
| 0.25-d1,2,3 | 48 (10) | 5 (1) | 50 (10) | 19 | 1 | 19 |
| Total | 44 (27) | 8 (5) | 44 (27) | 44 | 12 | 56 |
| P (χ2) | P (KW) | |||||
| 0.041 | 0.400 | 0.055 | 0.119 | 0.356 | 0.117 | |
qPCR = quantitative polymerase chain reaction; KW = Kruskal–Wallis.
Bold values indicate a P value < 0.05.
Density was calculated for patients with gametocytes (excluding those without gametocytes).
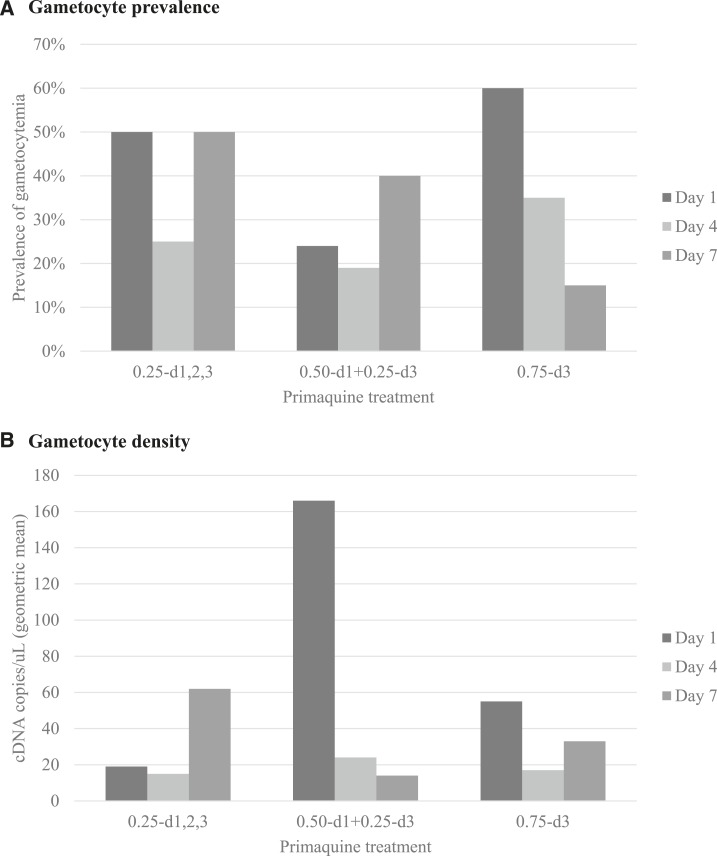
On day 4 (24 hours after completion of the antimalarial treatment [schizonticide plus PQ]), the prevalence and gametocyte density were statistically similar among the three study groups; prevalence ranged from 19% to 35% (P (χ2) = 0.504) and the geometric mean of cDNA for both molecular markers (pfs16 + pfs25) ranged from 15 and 24 copies/µL, without statistical difference among the groups (P [KW] = 0.525) (Figure 1).
Figure 1.
Prevalence and gametocyte density on days 1, 4, and 7, per study group. (A) Gametocyte prevalence, that is, proportion of subjects with gametocytes, in each study group and day. (B) Gametocyte density, that is, geometric mean of cDNA copies per microliter, in each study group and day. Geometric mean was calculated excluding negative samples.
On day 7, the prevalence and gametocyte density were also statistically similar among the three study groups. However, the lowest prevalence (15%) was observed in group 0.75-d3, whereas group 0.25-d1,2,3 had the highest prevalence (P [χ2] = 0.057). The latter group (0.25-d1,2,3) also had the highest gametocyte density on day 7 (P [KW] = 0.055) (Figure 1).
The three schemes of PQ analyzed here caused a marked reduction in prevalence of gametocytes on day 4 compared with day 1; the net relative change ranged from 21% to 50% (Table 5, Figure 1) without statistical difference (P = 0.266) among study groups. The prevalence of gametocytes continued to decrease until day 7 only in the group 0.75-d3, but even in that group, the gametocytemia was never reduced to zero (Table 5, Figure 1). All three schemes resulted in a decrease in gametocyte density on day 4 compared with day 1, but the gametocytes density continued decreasing until day 7 with the scheme 0.50-d1 + 0.25-d3. The schemes 0.75-d3 and 0.50-d1 + 0.25-d3 had lower gametocyte densities on day 7 compared with day 1, whereas the gametocyte density with the scheme 0.25-d1,2,3 was higher on day 7 than day 1 (Table 5, Figure 1).
Table 5.
Net relative change in gametocytemia per study group
| Prevalence | ||||||
|---|---|---|---|---|---|---|
| Treatment | Prevalence (%) | Net relative change (%)* | ||||
| Day 1 | Day 4 | Day 7 | Days 1–4 | Days 1–7 | Days 4–7 | |
| 0.75-d3 | 60 | 35 | 15 | −42 | −75 | −57 |
| 0.50-d1 + 0.25-d3 | 24 | 19 | 40 | −21 | +66 | +110 |
| 0.25-d1,2,3 | 50 | 25 | 50 | −50 | 0 | +100 |
| Total | 44 | 26 | 35 | −41 | −20 | +35 |
| Density | ||||||
|---|---|---|---|---|---|---|
| Treatment | Density (geometric mean, cDNA copies/µL) | Net relative change (%)* | ||||
| Day 1 | Day 4 | Day 7 | Days 1–4 | Days 1–7 | Days 4–7 | |
| 0.75-d3 | 55 | 17 | 33 | −69 | −40 | +94 |
| 0.50-d1 + 0.25-d3 | 166 | 15 | 14 | −91 | −91 | −7 |
| 0.25-d1,2,3 | 19 | 24 | 62 | +26 | +226 | +158 |
| Total | 56 | 18 | 30 | −68 | −46 | +66 |
Net relative change indicates the proportion of increase (positive value) or decrease (negative value) of gametocytemia (prevalence or density) over time with each scheme. These calculations were performed to obtain the net relative change of day 4 or 7 with respect to day 1: 100 − (prevalence or density on day 4 or 7 × 100/prevalence or density on day 1). Geometric mean was calculated excluding samples without gametocytes.
DISCUSSION
This study evaluated the antigametocyte efficacy of three schemes of PQ through a controlled open-label design using a molecular test to detect immature and mature gametocytes. This study confirms once again the greater sensitivity of molecular tests to detect gametocytes compared with the conventional thick blood smear; whereas the mean prevalence of gametocytemia on admission was 5% by microscopy, it was 45% by qPCR (ratio 1:9). Other studies in Colombia reported low gametocytemia on admission, based on thick blood smear,26,34–40 and it is also consistent with the short duration of symptoms before diagnosis, which in general lasted between 4 and 6 days, as in this study. In addition, the low gametocytemia correlated with the low level of asexual parasitemia reported generally in Colombia, approximately 5,000 parasites/µL.26,34–40
Most previous studies evaluated the antigametocyte effect of antimalarial treatment using microscopy,13,26,27,40 which is based on observation of mature gametocytes in peripheral blood. Few studies included the detection of immature gametocytes because they were presumed absent in peripheral blood due to sequestration in the microvasculature. This study and one other41 confirm that immature gametocytes are detected in peripheral blood and are in fact present at higher densities than mature stages.
It is important to note that all patients in this study received the same treatment with AL (1.5 and 12 mg/kg, twice a day for three consecutive days), which is an ACT and the recommended treatment by the Colombian health authorities.5 Therefore, differences in treatment per study group consisted in daily dose and time of administration of PQ (0.75 mg/kg on day 3 versus 0.50 mg/kg on day 1 and 0.25 mg/kg on day 3 versus 0.25 mg/kg on days 1, 2, and 3). Gametocyte prevalence and density were not reduced to zero at any day of follow up (7 days) in the three study groups; hence, the findings from this study demonstrate the lack of efficacy of the recommended treatment (AL plus PQ) to eliminate parasites implicated in malaria transmission.
Of the three regimens, PQ administered as a SD of 0.75 mg/kg on day 3 was most effective at controlling gametocytemia on days 4 and 7. Although the number of subjects included in each study group was low and this likely reduced the possibility of finding statistical differences of gametocytemia among the study groups during the follow-up, our results are consistent with a study of 113 children from Uganda who received the same dose of PQ on day 3 and had a gametocyte prevalence on day 7 similar to the present study.19
WHO has recommended the administration of a SD of PQ at 0.25 mg/kg in low-transmission areas, because that dose is both effective in blocking transmission and unlikely to cause serious toxicity in individuals with any of the G6PD-deficiency variants.4 However, findings of this study indicate that 0.25 mg/kg of PQ even administered three times (instead of once as WHO recommends) is insufficient to control gametocytemia of P. falciparum.
The reduction of gametocytemia after PQ treatment has been evaluated mainly on day 7,7–13,17,19,25,28 and few studies have measured that reduction on day 4.19,27,29 We consider that the adequate moment to evaluate the antigametocyte effect of an antimalarial treatment is on day 4 when the patient has completed the schizonticide and is asymptomatic.18,20,26
In conclusion, the currently recommended treatment of noncomplicated falciparum malaria in Colombia (AL [1.5 and 12 mg/kg] plus PQ [0.75 mg/kg]) is unable to eliminate gametocytes by days 4 and day 7 posttreatment. However, this treatment was slightly more effective than two alternate treatments evaluated here. Further studies are needed to establish a PQ regimen with complete efficacy against gametocytes within the first four days of treatment.
Acknowledgments:
We thank the participating patients, field assistants, employees and managers of the local hospitals for their collaboration. We also thank Sédami Gnidehou for her help in the laboratory.
REFERENCES
- 1.World Health Organization, 2010. Guidelines for the Treatment of Malaria, 2nd edition. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.World Health Organization, 2015. Guidelines for the Treatment of Malaria, 3rd edition. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 3.World Health Organization, 2012. WHO Evidence Review Group: The Safety and Effectiveness of Single Dose Primaquine as a P. falciparum gametocytocide. Meeting Report. Pullman Hotel, Bangkok, Thailand, August 13–15, 2012, World Health Organization. [Google Scholar]
- 4.World Health Organization, 2015. Policy Brief on Single-Dose Primaquine as Gametocytocide in Plasmodium falciparum Malaria. Geneva, Switzerland: World Health Organization, 8. [Google Scholar]
- 5.Padilla J, Montoya R, 2011. Guía de atención clínica de malaria. Infectio 15: 302–323. [Google Scholar]
- 6.Bunnag D, Harinasuta T, Pinichpongse S, Suntharasami P, 1980. Effect of primaquine on gametocytes of Plasmodium falciparum in Thailand. Lancet 2: 91. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko A, Kamei K, Suzuki T, Ishii A, Siagian R, Panjaitan W, 1989. Gametocytocidal effect of primaquine in a chemotherapeutic malaria control trial in North Sumatra, Indonesia. Southeast Asian J Trop Med Public Health 20: 351–359. [PubMed] [Google Scholar]
- 8.Singhasivanon V, Chongsuphajaisiddhi T, Sabchareon A, Attanath P, Webster HK, Edstein MD, Lika ID, 1994. Pharmacokinetic study of mefloquine in Thai children aged 5–12 years suffering from uncomplicated falciparum malaria treated with MSP or MSP plus primaquine. Eur J Drug Metab Pharmacokinet 19: 27–32. [DOI] [PubMed] [Google Scholar]
- 9.Kamtekar KD, Gogtay NJ, Dalvi SS, Karnad DR, Chogle AR, Aigal U, Kshirsagar NA, 2004. A prospective study evaluating the efficacy of a single, 45-mg dose of primaquine, as a gametocytocidal agent, in patients with Plasmodium falciparum malaria in Mumbai, India. Ann Trop Med Parasitol 98: 453–458. [DOI] [PubMed] [Google Scholar]
- 10.Gogtay NJ, Kamtekar KD, Dalvi SS, Mehta SS, Chogle AR, Aigal U, Kshirsagar NA, 2006. A randomized, parallel study of the safety and efficacy of 45 mg primaquine versus 75 mg bulaquine as gametocytocidal agents in adults with blood schizonticide-responsive uncomplicated falciparum malaria [ISCRTN50134587] BMC Infect Dis 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Sayed B, El-Zaki SE, Babiker H, Gadalla N, Ageep T, Mansour F, Baraka O, Milligan P, Babiker A, 2007. A randomized open-label trial of artesunate-sulfadoxine-pyrimethamine with or without primaquine for elimination of sub-microscopic P. falciparum parasitaemia and gametocyte carriage in eastern Sudan. PLoS One 2: e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shekalaghe S, Drakeley C, Gosling R, Ndaro A, van Meegeren M, Enevold A, Alifrangis M, Mosha F, Sauerwein R, Bousema T, 2007. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One 2: e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smithuis F, et al. , 2010. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis 10: 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves PM, Gelband H, Garner P, 2014. Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission. Cochrane Database Syst Rev 6: CD008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graves PM, Gelband H, Garner P, 2015. Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission. Cochrane Database Syst Rev 6: CD008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pukrittayakamee S, Chotivanich K, Chantra A, Clemens R, Looareesuwan S, White NJ, 2004. Activities of artesunate and primaquine against asexual- and sexual-stage parasites in falciparum malaria. Antimicrob Agents Chemother 48: 1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolaczinski K, Leslie T, Ali I, Durrani N, Lee S, Barends M, Beshir K, Ord R, Hallett R, Rowland M, 2012. Defining Plasmodium falciparum treatment in South West Asia: a randomized trial comparing artesunate or primaquine combined with chloroquine or SP. PLoS One 7: e28957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White NJ, 2013. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis 13: 175–181. [DOI] [PubMed] [Google Scholar]
- 19.Eziefula AC, et al. , 2014. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis 14: 130–139. [DOI] [PubMed] [Google Scholar]
- 20.White NJ, et al. , 2014. Assessment of therapeutic responses to gametocytocidal drugs in Plasmodium falciparum malaria. Malar J 13: 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieckmann KH, McNamara JV, Frischer H, Stockert TA, Carson PE, Powell RD, 1968. Gametocytocidal and sporontocidal effects of primaquine and of sulfadiazine with pyrimethamine in a chloroquine-resistant strain of Plasmodium falciparum. Bull World Health Organ 38: 625–632. [PMC free article] [PubMed] [Google Scholar]
- 22.Baird JK, 2012. Reinventing primaquine for endemic malaria. Expert Opin Emerg Drugs 17: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess RW, Bray RS, 1961. The effect of a single dose of primaquine on the gametocytes, gametogony and sporogony of Laverania falciparum. Bull World Health Organ 24: 451–456. [PMC free article] [PubMed] [Google Scholar]
- 24.Gonçalves BP, et al. , 2016. Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lederman ER, Maguire JD, Sumawinata IW, Chand K, Elyazar I, Estiana L, Sismadi P, Bangs MJ, Baird JK, 2006. Combined chloroquine, sulfadoxine/pyrimethamine and primaquine against Plasmodium falciparum in Central Java, Indonesia. Malar J 5: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arango EM, Upegui YA, Carmona-Fonseca J, 2012. Efficacy of different primaquine-based antimalarial regimens against Plasmodium falciparum gametocytemia. Acta Trop 122: 177–182. [DOI] [PubMed] [Google Scholar]
- 27.Vásquez AM, Sanín F, Alvarez LG, Tobón A, Ríos A, Blair S, 2009. Therapeutic efficacy of a regimen of artesunate-mefloquine-primaquine treatment for Plasmodium falciparum malaria and treatment effects on gametocytic development [in Spanish] Biomedica 29: 307–319. [PubMed] [Google Scholar]
- 28.Sutanto I, Endawati D, Ling LH, Laihad F, Setiabudy R, Baird JK, 2010. Evaluation of chloroquine therapy for vivax and falciparum malaria in southern Sumatra, western Indonesia. Malar J 9: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah NK, Schapira A, Juliano JJ, Srivastava B, MacDonald PD, Poole C, Anvikar A, Meshnick SR, Valecha N, Mishra N, 2013. Nonrandomized controlled trial of artesunate plus sulfadoxine-pyrimethamine with or without primaquine for preventing posttreatment circulation of Plasmodium falciparum gametocytes. Antimicrob Agents Chemother 57: 2948–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daza Camelo L, 2015. Informe Final del Evento Malaria, Colombia, 2015. Bogotá, Columbi: Instituto Nacional de Salud. Subdirección Prevención, Vigilancia y Control en Salud Pública. Dirección de Vigilancia y Análisis del Riesgo en Salud Pública. [Google Scholar]
- 31.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE, 1995. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 52: 565–568. [DOI] [PubMed] [Google Scholar]
- 32.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK, 2009. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol 47: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider P, Schoone G, Schallig H, Verhage D, Telgt D, Eling W, Sauerwein R, 2004. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol Biochem Parasitol 137: 35–41. [DOI] [PubMed] [Google Scholar]
- 34.Carmona-Fonseca J, Arango E, Blair S, 2008. Gametocitemia en malaria por Plasmodium falciparum tratada con amodiaquina o artesunato. Biomedica 28: 195–212. [PubMed] [Google Scholar]
- 35.Osorio L, Pérez Le P, González IJ, 2007. Assessment of the efficacy of antimalarial drugs in Tarapacá, in the Colombian Amazon basin [in Spanish] Biomedica 27: 133–140. [PubMed] [Google Scholar]
- 36.Blair S, Carmona-Fonseca J, Piñeros J, Ríos A, Alvarez T, Alvarez G, Tobón A, 2006. Therapeutic efficacy test in malaria falciparum in Antioquia, Colombia. Malar J 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arango E, Alvarez T, Carmona J, Blair S, 2004. Gametocitemia de Plasmodium falciparum según la respuesta terapéutica a sulfadoxina-pirimetamina y cloroquina en dos municipios de Antioquia, Colombia. Biomedica 24: 79–88. [PubMed] [Google Scholar]
- 38.Osorio L, Ferro B, Castillo C, 2002. Effects of chloroquine and sulfadoxine/pyrimethamine on gametocytes in patients with uncomplicated Plasmodium falciparum malaria in Colombia. Mem Inst Oswaldo Cruz 97: 1221–1223. [DOI] [PubMed] [Google Scholar]
- 39.Méndez F, Muñoz A, Carrasquilla G, Jurado D, Arévalo-Herrera M, Cortese J, Plowe C, 2002. Determinants of treatment response to sulfadoxine-pyrimethamine and subsequent transmission potential in falciparum malaria. Am J Epidemiol 156: 230–238. [DOI] [PubMed] [Google Scholar]
- 40.Bousema T, Drakeley C, 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24: 377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kast K, Berens-Riha N, Zeynudin A, Abduselam N, Eshetu T, Löscher T, Wieser A, Shock J, Pritsch M, 2013. Evaluation of Plasmodium falciparum gametocyte detection in different patient material. Malar J 12: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]