Abstract.
Plague is a fatal, primarily rodent-flea-borne zoonotic disease caused by Yersinia pestis. The identification of risk factors of plague was investigated through questionnaire interview and conducting focus group discussion (FGD) in Sinda and Nyimba districts of eastern Zambia. A total of 104 questionnaires were administered to individual respondents and 20 groups consisting of 181 discussants, which comprised FGD team in this study. The study revealed that trapping, transportation, and preparation of rodents for food exposed the community to rodent and their fleas suggesting that plague may have occurred primarily by either flea bites or contact with infected wild rodents. The study also revealed that most people in communities consumed rodents as part of their regular diet; therefore, contact with small wild mammals was a common practice. The mode of transportation of freshly trapped rodents, in particular, carcasses risked human to flea bites. Questionnaire respondents (75%) and FGD discussants (55%) indicated that trappers preferred to carry rodent carcasses in small bags, whereas 55.8% and 20% respectively, reported hunters carrying carcasses in their pockets. Carrying of carcass skewers on trappers’ shoulders was reported by 38.4% and 20% of individual respondents and FGD, respectively. All these activities were exposing humans to rodents and their fleas, the natural reservoirs and vectors of plague, respectively. This study also showed that there is a statistically significant (χ2 = 4.6878, P < 0.05), between digging of rodents from their burrows and the presence of fleas on the hunter’s bodies or clothes, which exposes humans to potentially flea bites in an enzootic cycle.
INTRODUCTION
Plague is a fatal flea-borne rodent-associated zoonotic disease caused by Yersinia pestis, a gram-negative, nonmotile, and non-spore-forming bacterium. The disease has been endemic in many parts of Africa including Zambia, which reported the first outbreak of the disease in the Luangwa valley in 1917.1–3 Since then, a number of outbreaks of the disease have occurred in various parts of the country, the latest being in Nyimba District, southern part of Luangwa valley in March 2015, in which three fatal cases were recorded.4 According to Low and Newman (1920), the spread of the disease from one village to another in Northern Luangwa valley was largely facilitated by sociocultural practices such as polygamy, where a man having more than one wife was shifting from one house or village to another and might have moved with infected fleas. Rodents hunting habits have also played a role in plague transmission.3 This scenario was also observed in Chitokoloki village in the Zambezi plain by Worsford.5
In the study area, communities developed traditional plague control methods, with limited success, such as burning of dwellings and killing of any escaping rodents from the deceased house, which was practiced in Tanzania and elsewhere.6 However, current plague control strategies in most countries usually involve the killing of fleas and rodents, chemotherapy for patients, chemoprophylaxis for contacts, environmental cleanliness and sanitary improvement, health education for leaders and communities in the affected areas, and where possible, quarantine of the affected localities. These approaches have been effective in most plague foci and are probably responsible for the decline of outbreaks of the disease in plague foci. This approach was equally practiced in Lushoto District of Tanzania during and soon after each outbreak of the disease since 1980; however, despite this control measure, plague outbreaks continued to occur sporadically every year, usually between the months of October and March. Kilonzo and others (1997), reported that despite these control measures, there were still other esoteric factors, which contributed to these sporadic disease outbreaks.6 These factors may include hunting behavior, mode of transportation of the carcasses, and method preparation of these carcasses, which may increase the contact with the flea vectors.7
In additional to these, others factors may include sociocultural, socioeconomic, and environmental, which may be responsible for transmission and maintenance of the disease in the focus for a long time, as other parameters such as biological and climatic factors including presence of suitable reservoirs, efficient vectors, and favorable climatic conditions, were similar in many other foci where the disease had been effectively controlled. However, in many African countries, some traditions and customs hinder control of not only plague but also other infectious diseases, which include people believing in witchcraft and/or evil spirits as causes of human plague.8 In Sinda District of Zambia, Ngulube and others (2006) reported that some plague deaths were associated with witchcraft and superstitious evil spirits.9 Likewise, the same concept was observed, that some cultural and social practices were partly responsible for outbreaks and maintenance of plague in some districts of Uganda.10
Plague is primarily transmitted through bites of infective oriental rat fleas, Xenopsylla cheopis (Rothschild), from living or dead animals either to noninfected animal or humans. The disease can also be transmitted by some other flea species including, Ctenocephalides felis (Bouche), Ctenocephalides canis (Curtis),11 Echidnophaga gallinacea (Westwood), and Pulex irritans (L).12 Basically, fleas effectively transmit plague when the former feed on opportunistic hosts after migrating or dropping off their primary hosts following death or self-grooming by the infested host.12 In the study area, communities hunt and catch rodents and use the same as protein source, thus facilitating close contacts with the animals, consequently, increasing risks of exposure to plague infection among such people.
Plague can also be transmitted by direct contact with infected materials as reported in Libya and Kenya, where human beings got infected after skinning and eating the suspected animal carcasses of camels and goats, respectively.13,14 Furthermore, a case of human plague infection that occurred after conducting an autopsy of a dead lion in the United States has been documented.15 A similar case has also been reported in Russia, where a 10-year-old boy contracted plague after skinning a marmot.16 Despite the aforegoing reports on sociocultural factors associated with plague epidemiology in several areas, there are a few studies on human behavioral factors determinant of plague outbreaks in Zambia. Traditions and cultural practices differ from one ethnic group to another and these may have an impact on the transmission of plague in Zambia. The possibility that some cultural beliefs and practices, favor maintenance, and outbreaks of the disease in some localities cannot be ruled out.6 In view of this, it was considered important to determine social cultural behavioral factors, which are possibly responsible for facilitating plague transmission and outbreaks in the study areas. To substantiate such possibility, it was fact desirable to determine risk factors, which may promote the spread and maintenance of the disease in the area and establish the knowledge, attitudes, and practices (KAP) of local communities with respect to plague in eastern Zambia. The aim of this study was, therefore, to investigate potential risk factors that are possibly associated with facilitating, maintaining, and transmitting sylvatic and murine plague to humans in the study area.
MATERIALS AND METHODS
Study site.
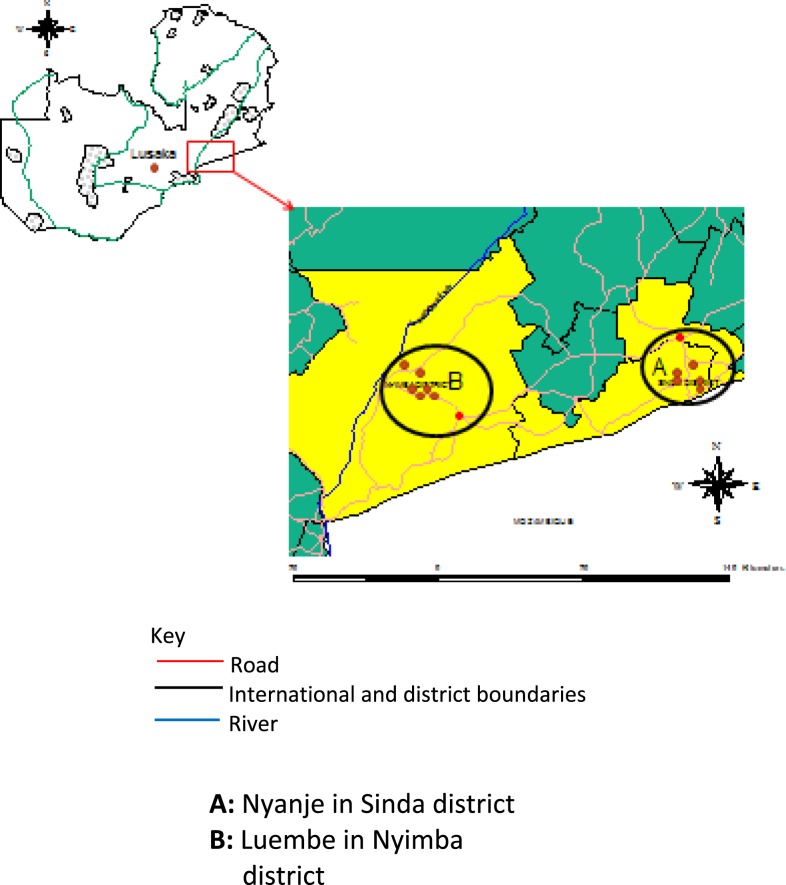
The study was conducted in the eastern Zambia, where plague has been reported in the recent past. In this area, four plague foci have been identified namely Tembwe in Chama District in 1917 and 1956,2,3 Luembe in Nyimba District in 2015,4 both are in northern and southern Luangwa valley. Others are Mukomba in Lundazi District in 19562 and Nyanje in Sinda District in 2001 and 2007.9,17 The study mostly focused on the two most recently affected areas in Nyimba and Sinda districts (Figure 1) and took place between March 2015 and November 2016.
Figure 1.
Study site showing two districts in eastern Zambia. This figure appears in color at www.ajtmh.org.
In this study, both qualitative and quantitative approaches were applied using open- and closed-ended questionnaires and focus group discussion (FGD). There were > 300 villages in the two districts and about 150 villages were affected with plague disease. Participants, both individuals and in FGD, were selected at random from systematically selected affected villages and households. Informed consent was obtained from the participants either verbally or in writing through their village leaders or their community health workers. In each FGD, four participants (two males and two females) were selected at random from each affected village and one from each household. These participants were convened at a central place, where the FGD (between 8 and 12 participants) were formed from different villages. All participants were informed of the objectives of the FGD and requested to sign the consent to take part in the discussion. The flow of the discussion was controlled by the moderator and was recorded, both electronically and manually, by his assistant. The questions in the questionnaire were designed to seek information on the KAP related to plague transmission, epidemiology and control, and sociocultural factors associated with the disease.
The major questions sought information on personal details (including age, sex, occupation, and level of education); the small wild mammals mostly preferred to be hunted for food, method of trapping and hunting small wild animals, mode of transporting their carcasses, methods of preparation before eating, experience and knowledge on causes, and symptoms of plague; type and numbers of livestock kept and where they were kept, biting insects they experienced (especially fleas), health and environmental status in the household. The questionnaires were instituted in English and the local language (Chichewa/Nsenga), commonly used by the community. The study did not recruit residents who came from another district in the past 6 months.
Data analysis.
Data were compiled and entered in the Microsoft Access and analyzed using the Epi info statistical package 7.2.0.1 (Center for Disease Control and Prevention, Atlanta, GA).
RESULTS
Personal characteristics of respondents.
A total of 104 individual respondents and 20 FGD (FGD of 181 members) made up between 8 and 12 members in each group, were recruited. Of the individual respondents, 29.8% (N = 31) and 70.2% (N = 73) were females and males, respectively. Their ages ranged from 16 to 69 years, with the majority being between 30 and 52 years. Most of the respondents were peasant farmers 85.6% (N = 89), followed by pupils 3.8% (N = 4) and housewives 2.9% (N = 3). Others were businessmen, religious leaders, teachers, a radiographer, and university students. The educational level of the respondents was mostly primary 35.6% (N = 37), whereas 1.9% (N = 2) had attended adult literacy classes and 12.5% (N = 13) had completed senior secondary school. On the contrary, 30.8% (N = 32) attended junior secondary school and the remaining 6.7% (N = 7) were illiterate.
KAP of respondents on plague.
A total of 72.1% (N = 75) of individual respondents claimed to have noticed symptoms related to plague disease in the community such as inflammation of lymph nodes (Buboes), headache, and fever, whereas 11.5% (N = 12) had noticed symptoms such as a sore throat while the rest could not define the symptoms (Table 1). When asked about possible factors responsible for the causation of the disease in the area, 57.7% (N = 60) indicated that rodent borne and fleas were responsible, 25.7% (N = 27) reported dirty environment was responsible, 5.7% (N = 6) attributed plague to both rodents and dirty environment, and the rest did not know.
Table 1.
Conditions expressed by the respondents as a result of flea infestation
| Disease experienced | No. of respondents | Percentage (%) |
|---|---|---|
| Lymphadenopathy/tonsillitis | 75 | 72.1 |
| Itching and sores | 5 | 4.8 |
| Rash | 4 | 3.8 |
| Sore throats | 8 | 7.6 |
| Not sure | 12 | 11.5 |
For the FGD, of the 20 groups, 85% (N = 17 groups) said they had noticed the disease symptoms of swollen lymph nodes, whereas 10% (N = 2 groups) noticed itching and 5% (N = 1 group) noticed sore throats. The groups also attributed these symptoms to rodents and fleas.
Hunting and use of small wild animals for food.
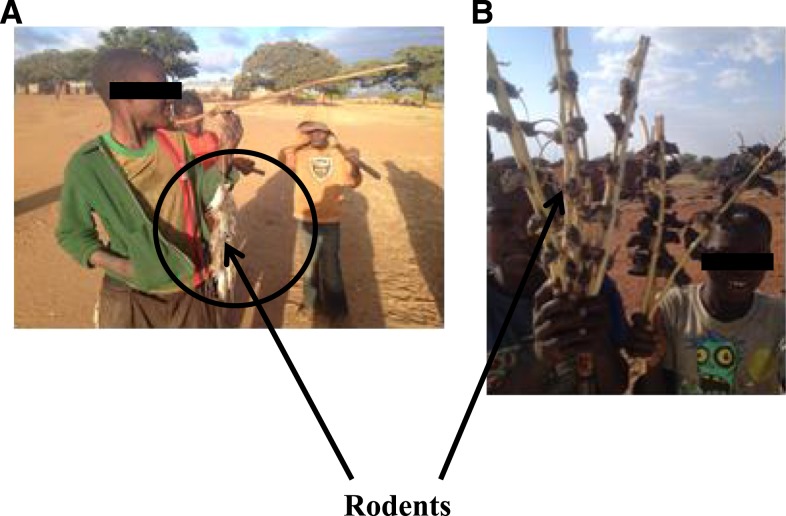
All the respondents (both individuals and in FGD) were aware of the different types of rodents used by the community for food (Table 2) and various methods used for hunting or catching the animals. These include digging the rodent burrows 78.8% (N = 82), using local traps 59.6% (N = 62), using water buckets traps 38.3% (N = 42), using hand or head flashlights (torch) 15.4% (N = 22), and using hunting dogs 13.4% (N = 14). As regard to a number of rodents killed, the respondents indicated that they could kill between eight and 450 animals per day, most of them were rats. When asked about carcass handling and transportation procedures, respondents said that several methods were used for carrying the dead rats, including the use of small bags or sacks or simply hanging them on a string around their necks or shoulders 75% (N = 78). Other methods included carrying them in pockets 55.8% (N = 58), using stick skewers or threads and carrying them on the shoulder or on the head 38.5% (N = 40) (Figure 2), using buckets carried on the head, 12.5% (N = 13). They also reported that methods of preparation differed from person to person. Some people boiled the animals with its fur after eviscerating them 12.5% (N = 13) although the majority of trappers preferred flaming off the fur before eviscerating the carcasses 91.3% (N = 95). Others reported that trappers skinned the bigger species before eviscerating 24% (N = 25). For the FGD, 55% (N = 11 groups) of them killed the rodents after digging them out of their burrows, whereas 25% (N = 5 groups) used local snares, and yet other 20% used flashlights at night (N = 4 groups) and bucket traps with water 5% (N = 1 group). The groups also explained that different methods were used to transport the rats including sacks 11 (55%), pockets 5 (25%), and pinning the rodents on stick skewers and carrying them on their shoulders or on the head 20% (N = 4 groups) (Figure 2). They also reported the most commonly used preparation technique was to flame the fur followed by eviscerating 75% (N = 15 groups), skinning and eviscerate 15% (N = 3 groups), eviscerating then boiling with fur 10% (N = 2 groups).
Table 2.
Most commonly preferred and hunted small animals
| Small animals | No. of respondents | Percentage (%) |
|---|---|---|
| Rats/mice | 104 | 100 |
| Hare/rabbit | 64 | 61.5 |
| (Kunda) Cricetomys spp. | 39 | 37.5 |
| (Tondo) Petrodomus spp. | 14 | 13.5 |
| Birds | 51 | 49.0 |
| Impala | 43 | 41.3 |
Figure 2.
(A) A boy carrying rodents on a string and a stick and (B) boys carrying rodents using stick technique. This figure appears in color at www.ajtmh.org.
Observation of fleas moving from hunted small wild animals to people.
A total of 32.7% (N = 34) respondents reported having observed fleas during trapping or killing of the animals, whereas 29.8% (N = 30) observed fleas during transportation of carcasses to the village or to the designated place for preparation. Sixteen (15.4%) respondents reported that they mainly noticed fleas during carcass preparation, especially during skinning, whereas 23.08% (N = 24) noticed fleas during transportation and preparation (Table 3). These reports broadly suggest the presence of strong association of contact between humans and fleas and rodents during digging (χ2 = 4.6878, P < 0.05).
Table 3.
Activities associated with flea transfer from captured animals to humans
| Activity on carcasses | No. of respondents | Percentage (%) |
|---|---|---|
| During killing or hunting | 34 | 32.7 |
| During transportation | 30 | 28.8 |
| During preparation | 16 | 15.4 |
| During transportation and preparation | 24 | 23.1 |
Although 45% (N = 9 groups) of the FGD said they noticed fleas during preparation only, while 30% (N = 6 groups) noticed fleas during trapping and killing and 25% (N = 5 groups) reported fleas during transportation and preparation. These reports show evident of contact between human beings and rodent fleas.
DISCUSSION
The results revealed that most people in the studied communities commonly eat rodents, which are the most preferred hosts for flea vectors and potentially plague carriers. The rodent provides a protein source in their diet as other protein sources may either be scarce or expensive. In view of this habit, communities use various methods for catching, handling, and preparing the animals for the culinary behavior of the communities in the study area are closely associated with plague transmission in eastern Zambia16 (Figure 2). These rodent handling practices inevitably facilitate fleas to infest humans and other susceptible animals in the vicinity and spread plague if they are carrying Y. pestis. The study also revealed that a good number of younger males were mostly involved in the rodents hunting, transportation, and preparing. The younger males prepare the delicious meals on their own at a place (traditionally called Sangweni or Mphara), a place where males gather, eat together, and discuss how best they can live in the society. That could be the reason as to why most affected people with plague were younger males than adult males in most plague disease outbreaks.18 The behavioral deeds are closely consistent with observation elsewhere including Kenya where herdsmen contracted plague after carrying goat kids on their shoulders.19 Wang and others (2006) reported a case of a person infected with pneumonic plague after carrying a dead dog on his shoulders for burying it at the distance20 and in Russia, a Former Soviet Union, where a 10-year-old boy got infected with Y. pestis after skinning a marmot.16
The current observation that most people used the flame for removing the fur of the captured animals is probably not effective against flea ectoparasites of such animals since the process is done long after the animal dies and most of the flea ectoparasites leave carcass hours before subjecting the latter to fire. Indeed, culinary activities would be less risky in plague transmission if the carcasses were flamed and processed immediately after the captured animal dies or while still on the warm carcass, thus killing the fleas and other ectoparasites before they could leave the carcass and seek an alternative host.
Furthermore, the observation that the community was aware of plague being transmitted by rodent fleas and 50% of the respondent’s associated lymphadenitis of the axillary inguinal and submaxillary region as signs of the disease, suggesting that residents in the area have some indigenous knowledge about bubonic plague. It was also reported that heavy flea infestations occurred most commonly during rainy season, suggest that the communities are knowledgeable about seasonality of plague outbreaks, and is consistent with other findings in Zambia and elsewhere.1,21,22
CONCLUSION
It can be justifiably concluded from the current observation that sociocultural human behavioral factors especially hunting, transportation, and preparation of rodents before their use for food expose most villagers in the study area to flea bites and consequently the risk of being infected with the plague. It can also be conclusively argued that the current observations are at least partly responsible for the current presence of suspected plague cases in the study area.
Instituting of health education and sustainable campaign on plague epidemiology, prevention and control, and the importance of avoiding contacts with flea and rodent carcasses is strongly recommended to the stakeholders.
Acknowledgments:
We thank the Director Department of Livestock in the Ministry of Fisheries and Livestock for his support of this work, members of staff of Nyanje RCZ Mission Hospital, Chinambi Health Center, members of staff of district veterinary offices and community health personnel in Nyimba and Sinda districts for organizing the participants, and any other the assistance rendered to the sampling team. We also thank the villagers for their cooperation during the interviews and focus group discussion sessions. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Disclaimer: Ethical approval to conduct studies was sought from the Institutional Ad hoc committee from Sokoine University of Agriculture, Tanzania, with approval from the study area in Zambia (Assurance No. FWA00000338). Approving board is the Biomedical Research Ethics Committee (BREC) in Lusaka, Zambia. Informed consent was sought and given by respondents prior to administering the questionnaires and conducting FGD meetings. Research was conducted in compliance with the Animal Welfare Act and the Guidelines for the care and use of laboratory animals of the country
REFERENCES
- 1.Nyirenda SS, Hang’ombe BM, Kilonzo B, 2016. Factors that precipitated human plague in Zambia from 1914 to 2014: an overview for a century (100 years). J. Zoonotic Dis. 1: 1–14. [Google Scholar]
- 2.Davis DH, Fisher BW, Goldring F, 1960. The Luangwa Valley plague outbreaks and their significance in relation to Savannah plague in Central Africa. Bull World Health Organ 23: 405–408. [PMC free article] [PubMed] [Google Scholar]
- 3.Low RB, Newman SG, 1920. The Progress and Diffusion of Plague, Cholera and Yellow Fever Throughout the World, 1914–1917. London, United Kingdom: His Majesty Stationery Office. [Google Scholar]
- 4.Nyirenda SS, Hang’ombe BM, Kilonzo BS, Kabeta MN, Mundia C, Sinkala Y, 2017. Molecular, serological and epidemiological observations after a suspected outbreak of plague in Nyimba, eastern Zambia. Trop Doct 47: 38–43. [DOI] [PubMed] [Google Scholar]
- 5.Worsfold JT, 1955. An outbreak of plague at Chitokoloki, Balovale. Cent Afr J Med 1: 116–117. [PubMed] [Google Scholar]
- 6.Kilonzo BS, Mvena ZSK, Machangu RS, Mbise TJ, 1997. Preliminary observations on factors responsible for long persistence and continued outbreaks of plague in Lushoto district, Tanzania. Acta Trop 68: 215–227. [DOI] [PubMed] [Google Scholar]
- 7.Eisen RJ, MacMillan K, Atiku LA, Mpanga JT, Zielinski-Gutierrez E, Graham CB, Boegler KA, Enscore RE, Gage KL, 2014. Identification of risk factors for plague in the West Nile Region of Uganda. Am J Trop Med Hyg 90: 1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilonzo BS, Mhina J, Sabuni C, Mgode G, 2005. The role of rodents and small carnivores in plague endemicity in Tanzania. Belgium J Zool 135: 119–125. [Google Scholar]
- 9.Ngulube TJ, Mwanza K, Njobvu CA, Muula AS, 2006. Knowledge, attitudes and public health response towards plague in Petauke, Zambia. Trop Doct 36: 223–225. [DOI] [PubMed] [Google Scholar]
- 10.Ogen-Odoi AA, 1993. A Report on Plague Outbreaks in Nebbi District, Uganda. An official document submitted by the Ministry of Health, Enttebe to the WHO Representative (Uganda) regarding an outbreak of plague in the country in early. Enttebe, Uganda: Uganda Ministry of Health. [Google Scholar]
- 11.Eisen RJ, et al. , 2008. Early-phase transmission of Yersinia pestis by cat fleas (Ctenocephalides felis) and their potential role as vectors in a plague-endemic region of Uganda. Am J Trop Med Hyg 78: 949–956. [PubMed] [Google Scholar]
- 12.Ratovonjato J, Rajerison M, Rahelinirina R, Boyer S, 2014. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis 20: 1414–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bin Saeed A, Al-Hamdan N, Fontaine RE, 2005. Plague from eating raw camel liver. Emerg Infect Dis 11: 1456–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie B, Chen TH, Elberg SS, 1980. Plague in camels and goats: their role in human epidemics. J Infect Dis 141: 724–726. [DOI] [PubMed] [Google Scholar]
- 15.Wong D, et al. , 2009. Primary pneumonic plague contracted from a mountain lion carcass. Clin Infect Dis 49: e33–e38. [DOI] [PubMed] [Google Scholar]
- 16.Will S, 2016. Fears of a mass bubonic plague outbreak in Russia: Thousands given emergency vaccinations after boy contracts the disease. Available at: http://www.dailymail.co.uk/news/article-3689697/Fears-mass-BUBONIC-PLAGUE-outbreak-Russia.html. Accessed July 7, 2017. [Google Scholar]
- 17.Neerinckx S, Bertherat E, Leirs H, 2010. Human plague occurrences in Africa: an overview from 1877 to 2008. Trans R Soc Trop Med Hyg 104: 97–103. [DOI] [PubMed] [Google Scholar]
- 18.Davis DH, Fisher BW, Goldring F, 1960. The Luangwa Valley plague outbreaks and their significance in relation to Savannah plague in Central Africa. Bull World Health Organ 23: 405–408. [PMC free article] [PubMed] [Google Scholar]
- 19.Kenya, 1978. Communicable Disease Centre, Ministry of Health. Plague in Kenya. Heal Inf Bull 2: 1–2. [Google Scholar]
- 20.Wang H, et al. , 2011. A dog-associated primary pneumonic plague in Qinghai Province, China. Clin Infect Dis 52: 185–190. [DOI] [PubMed] [Google Scholar]
- 21.Hang’ombe BM, Nakamura I, Samui KL, Kaile D, Mweene AS, Kilonzo BS, Sawa H, Sugimoto C, Wren BW, 2012. Evidence of Yersinia pestis DNA from fleas in an endemic plague area of Zambia. BMC Res Notes 5: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilonzo B, 1976. A survey of rodents and their flea ectoparasites in north-eastern Tanzania. E African J Med Res 3: 117–126. [Google Scholar]