Abstract.
Mass administration of azithromycin (MDA) is integral to trachoma control. Recent studies suggest that MDA may increase drug-resistant pathogens, yet findings from prior studies suggest little long-term impact on resistance. This disparity may be linked to differences in pre-MDA community-level resistance patterns. We describe carriage prevalence and antibiotic resistance patterns for Streptococcus pneumoniae (Spn) (nasopharyngeal swab collection), Staphylococcus aureus (SA) (nasopharyngeal swabs), and Escherichia coli (EC) (rectal swabs) in 1,047 children ages 1–59 months in a district with MDA cessation 4 years ago. Antibiotic susceptibility was evaluated by disk diffusion and Etest. The carriage rates for Spn, SA, and EC were 43.5% (455/1,047), 13.2% (138/1,047), and 61.7% (646/1,047), respectively. Resistance to AZM was observed in 14.3%, 29.0%, and 16.6% of the Spn, SA, and EC isolates, respectively. Spn resistance was variable (0–67%) by hamlet. Future analyses will assess the influence of pre-MDA antibiotic resistance patterns on those observed following MDA.
INTRODUCTION
Trachoma, an ocular infection caused by Chlamydia trachomatis is the leading infectious cause of blindness.1 Mass treatment with azithromycin (AZM) in trachoma-endemic communities is part of trachoma control programs. Annual, mass distribution of AZM (MDA) is effective in reducing trachoma and chlamydial infections as well as slowing rate of reemergence.2,3 Collateral benefits from MDA may be ascribed to its efficacy against a diverse array of pathogens including Streptococcus pyogenes, Streptococcus pneumoniae (Spn), Mycoplasma pneumoniae, and Chlamydia pneumoniae,1 thus mitigating against diarrheal disease,2 respiratory infection,3 and impetigo.2 However, there is concern that MDA may promote antibiotic resistance. In Ethiopia, the prevalence of macrolide-resistant Spn colonization was greater than 70% following biannual mass AZM treatment and remained significantly elevated for 2 years following cessation of treatment.4 In Kongwa District, Tanzania, over the 6-month follow-up, there was more than 2-fold rise in the proportion of children colonized with macrolide-resistant Spn following MDA.5 In contrast, populations with little exposure to AZM demonstrate small, transient increases in the prevalence of antimicrobial-resistant (Spn) carriage in treated children.2 These findings suggest that pre-MDA antibiotic resistance patterns modify the impact of MDA on antibiotic resistance. We sought to determine the prevalence of antibiotic resistance prior to MDA in a district where MDA had ceased 4 years before, in children born after the last MDA.
METHODS
Setting and population.
A cross-sectional, population-based assessment was conducted in 32 randomly selected communities in Kilosa District, Tanzania, from a total of 706 communities. Kilosa had active trachoma, underwent 4 years of annual MDA until 2010 when trachoma prevalence had decreased to < 5% and MDA was no longer indicated. A surveillance study in June 2014 found no evidence of reemergence of trachoma.6 This study was carried out from November 2014 to July 2015 as part of the initial assessment for a clinical trial.
A complete census of each community was conducted and 40 children were randomly assigned to participate in the survey. Children aged between 1 and 59 months, who lived in the randomly selected community, and had a guardian who was capable of providing consent, were eligible to participate. If a community had < 40 eligible children, all the children were invited to participate.
Data collection.
A trained field team collected data on current symptoms of acute lower respiratory illness and diarrhea, and any current treatments. Both nasopharyngeal and rectal swabs were collected for culture. A nasopharyngeal specimen was obtained using a sterile, calcium alginate swab (Puritan Medical Products, Guilford, ME). The swab was inserted in media containing skim milk, tryptone, glucose, and glycerin (STGG) and stored at −20°C pending shipment on ice to the Muhimbili National Hospital Microbiology laboratory (MHML), within 1 month of collection. Rectal specimens were collected using sterile cotton swabs, placed into Amies Transport Media, and stored at −20°C for up to 1 month before they were shipped on ice to the MHML for Escherichia coli (EC) isolation and resistance testing. Pilot testing with swabs inoculated with EC showed that the organisms were still viable after storage at −20°C for approximately 7 weeks.
Laboratory procedures.
For Spn isolation, swabs were inoculated onto blood agar containing 5% sheep blood and 5 mg/L gentamicin (Becton Dickinson, Cockeysville, MD), then incubated at 37°C in 5% CO2 for 18–24 hours. Colonies were confirmed by Optochin disk (Taxo, Becton Dickinson) inhibition or bile solubility testing. A pneumococcal reference strain was used for quality control. Culture-positive specimens were subcultured and frozen in STGG at −70°C until antibiotic susceptibility testing.
For Staphylococcus aureus (SA), nasal swabs were streaked onto blood and chocolate agar and incubated at 35°C for 24 hours. Organisms were identified based on color and evidence of beta hemolysis. Isolates were subcultured onto tryptic soy media and incubated at 37°C for 24 hours. Isolates were confirmed based on growth on mannitol, DNAse, and the Coagulase testing. Isolates were frozen in STGG at −70°C prior to antibiotic susceptibility testing.
The Kirby–Bauer disk diffusion method was used to test Spn and SA isolates for sensitivity to AZM (15 µg disk) on Mueller–Hinton media with 5% lysed sheep blood. Inhibitory zone diameters were used to classify isolates as “sensitive,” “intermediately resistant,” or “resistant” according to the recommendations of the Clinical and Laboratory Standards Institute. AZM-resistant isolates were subsequently screened using Etest (AB Biodisk, Solna, Sweden) to determine minimum inhibitory concentrations (MICs). AZM-resistant Spn isolates with MICs ≥ 16 µg/mL were defined as being clinically resistant5,7; AZM-resistant SA isolates were defined as clinically resistant with MICs ≥ 8µg/mL.7 Budgetary limitations precluded Spn isolate serotyping.
For EC, swabs were streaked on MacConkey agar and cultured overnight at 37°C. Up to three lactose-fermenting colonies were inoculated into nutrient agar stabs and grown overnight at 37°C followed by storage at room temperature. Indole-positive, citrate-negative isolates were considered to be EC. AZM MICs were determined using Etest strips. Breakpoints to define macrolide resistance in EC have not been established. We identified 32 mg/mL as an epidemiologically meaningful cutoff to designate resistance in community-based screening.8,9
Outcomes.
The outcomes of interest were 1) carriage prevalence of Spn, SA, and EC and 2) the proportion of resistant isolates for AZM as determined by Kirby–Bauer classification and Etest.
Data management and statistical analyses.
Data were entered into custom-built Access databases with range checks. Percentages and confidence intervals (CIs) were calculated. Data were analyzed with SAS software (SAS, Raleigh, NC). Intra class coefficients were used to assess clustering of resistance in communities.
Ethical review.
Ethical approval was obtained from the Tanzanian National Institute for Medical Research and the Institutional Review Board of the Johns Hopkins School of Medicine. Children were included in the study on the basis of documented written informed consent from guardians.
RESULTS
A total of 1,049 participated in the study of whom 1,047 contributed both nasopharyngeal and rectal swabs (Table 1). At the time of the survey, 4% of children were diagnosed with diarrhea and 2% were symptomatic with acute respiratory infection.
Table 1.
Characteristics of Children in the 32 communities
| Characteristic | n (%) |
|---|---|
| Total number of children | 1049 |
| Age in months | |
| 1–11 | 181 (17.3) |
| 12–23 | 224 (21.4) |
| 24–35 | 209 (19.9) |
| 36–47 | 213 (20.3) |
| 48–59 | 222 (21.2) |
| Females | 516 (49.4) |
| Child diagnosed with acute respiratory infection | 21 (2.0) |
| Child diagnosed with diarrhea | 41 (3.9) |
| Rectal swab taken | 1048 (99.9) |
| Nasal swab taken | 1047 (99.8) |
The nasopharyngeal carriage rate for Spn and SA was 43.5% (455/1,047) and 13.2% (138/1,047), respectively. The rectal carriage rate of EC was 61.7% (646/1,047). In those nasopharyngeal swabs in which Spn isolates were obtained, 14.3% were resistant to AZM (Table 2); 29% of recovered SA isolates (nasopharyngeal swabs) and 17% of EC isolates (rectal swabs) were resistant to AZM.
Table 2.
Resistance of organisms to azithromycin
| Streptococcus pneumoniae | Streptococcus aureus | Escherichia coli | |
|---|---|---|---|
| Number isolated (%) | 454/1046 (43.4%) | 138/1046 (13.2%) | 646/1047 (61.7%) |
| Samples with further testing | 454 | 138 | 644 |
| Sensitive | (ZD ≥ 18) | (ZD ≥ 18) | (ZD ≥ 10) |
| n/N (%) | 353/454 (77.7) | 81/138 (58.7) | 537/644 (83.4) |
| Intermediate | (ZD 14–17) | (ZD 14–17) | – |
| n/N (%) | 36/454 (7.9) | 17/138 (12.3) | |
| Resistant | (ZD ≤ 13) | (ZD ≤ 13) | (ZD ≤ 9) |
| n/N (%) | 65/454 (14.3) | 40/138 (29.0) | 107/644 (16.6) |
| Etest done | 65/65 | 40/40 | 103/107 |
| Range of MICs (μg/mL) | (0.02–256) | (0.05–256) | (0.96–968) |
| % of specimens greater than cutoff for resistance | 37/65 (57% ≥ 16 µg/mL) | 32/40 (80% ≥ 8µg/mL) | 86/103 (83.4% ≥ 32 µg/mL) |
MIC = minimum inhibitory concentration; ZD = Zone diameter.
Of the 65 Spn resistant isolates, 57% had MICs ≥ 16 µg/mL. For SA, 80% of the 40 resistant isolates had MICs ≥ 8 µg/mL. For EC, 83% of the 103 tested had MICs ≥ 32 µg/mL.
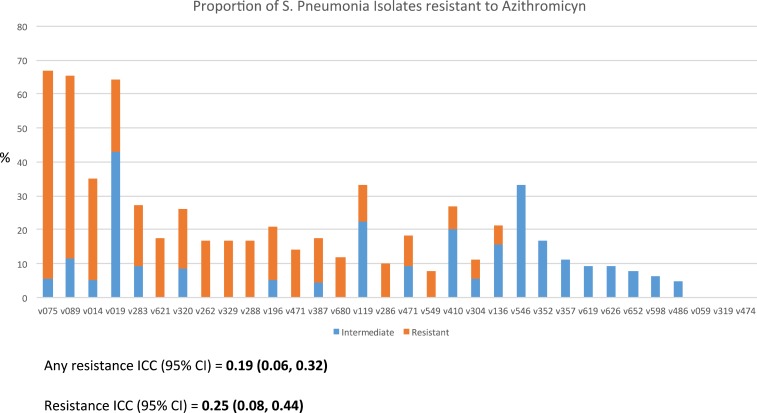
The Spn resistance was clustered: three communities had no resistant isolates, while over 50% of isolates in three communities were resistant (ICC = 0.19, 95% CI = 0.06–0.32). (Figure 1)
Figure 1.
Quantification of Streptococcus Pneumonia resistance to AZM by community. This figure appears in color at www.ajtmh.org.
DISCUSSION
The findings suggest moderate rates of AZM resistance in Spn, SA, and EC isolates among children aged 1 month to < 5 years in a district that had four rounds of MDA that had last been administered 4 years previously. The rates of Spn resistance are similar to those reported by Haug and others (21%) in Ethiopia, 2 years following the last round of six biannual MDA, where resistance immediately after MDA was more than 70%.4 The rates are lower than the 35% found in communities in Kongwa District, Tanzania, that had previous rounds of MDA 4 years prior, although surrounding communities had recently undergone MDA.5 However, the rates are higher than those reported in communities with no prior exposure to MDA. In Nepal, virtually no resistance was found at 6 and 12 months following one to two rounds of MDA.2 In Australia, a baseline resistance of 1.5% was observed in an Aboriginal community; after increasing, this fell to 6% at 6 months postsingle round MDA.10 In Rombo District, Tanzania, where the baseline rate of AZM resistance rates in Spn isolates was 0%, resistance was only 1% at 6 months postsingle round MDA.11 Collectively, the data suggest that the effect of repeated MDA may result in more prolonged resistance; this could depend on the baseline resistance pattern. The finding that Spn resistance clusters in certain communities merits further investigation, although some resistance was found in all but three of the 32 communities examined. In addition, 41% of SA and 17% of EC isolates were found to be nonsusceptible to AZM. The findings underscore the need for continued monitoring of resistance patterns.
Although several of the studies have tried to determine exposure to antibiotics prior to MDA, this remains a shared limitation of the studies. The identities of the antibiotics are not easily resolved and may contribute both to baseline and sustained resistance. Few children were diagnosed with concurrent illness at the time of survey, but parental recall of medication (use and type) was unreliable. Indeed, we found no difference in resistance in children purporting to have taken medication for infection versus those who did not. In general, AZM is not widely used in Kilosa but we have found AZM in a few of the local pharmacies. We did not evaluate clindamycin resistance and did not characterize the mechanism of AZM resistance in Spn isolates. Finally, the rates of recovery for EC were lower than expected; this could signify issues with sample collection or processing, and may have impacted the estimated resistance patterns.
In conclusion, our study provides population-based estimates of AZM resistance in a district that previously received four annual rounds of MDA, 4 years prior. Although MDA is an effective intervention for trachoma, further work on AZM resistance in the setting of prior MDA is warranted. Future analyses of this cohort will assess the impact of pre-MDA antibiotic resistance patterns on the circulation of antibiotic resistance isolates in communities following MDA.
REFERENCES
- 1.Gray GC, McPhate DC, Leinonen M, Cassell GH, Deperalta EP, Putnam SD, Karcher JA, Sawyer MH, Laurila A, Connor JD, 1998. Weekly oral azithromycin as prophylaxis for agents causing acute respiratory disease. Clin Infect Dis 26: 103–110. [DOI] [PubMed] [Google Scholar]
- 2.Fry AM, Jha HC, Lietman TM, Chaudhary JS, Bhatta RC, Elliott J, Hyde T, Schuchat A, Gaynor B, Dowell SF, 2002. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis 35: 395–402. [DOI] [PubMed] [Google Scholar]
- 3.Coles CL, Levens J, Seidman JC, Mkocha H, Munoz B, West S, 2012. Mass distribution of azithromycin for trachoma control is associated with short-term reduction in risk of acute lower respiratory infection in young children. Pediatr Infect Dis J 31: 341–346. [DOI] [PubMed] [Google Scholar]
- 4.Haug S, et al. , 2010. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin Infect Dis 51: 571–574. [DOI] [PubMed] [Google Scholar]
- 5.Coles CL, Mabula K, Seidman JC, Levens J, Mkocha H, Munoz B, Mfinanga SG, West S, 2013. Mass distribution of azithromycin for trachoma control is associated with increased risk of azithromycin-resistant Streptococcus pneumoniae carriage in young children 6 months after treatment. Clin Infect Dis 56: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 6.West SK, Munoz B, Weaver J, Mrango Z, Dize L, Gaydos C, Quinn TC, Martin DL, 2016. Can We Use Antibodies to Chlamydia trachomatis as a Surveillance Tool for National Trachoma Control Programs? Results from a District Survey. PLoS Negl Trop Dis 10: e0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI, 2014. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. Wayne, PA: CLSI document M100-S24.
- 8.Seidman JC, Coles CL, Silbergeld EK, Levens J, Mkocha H, Johnson LB, Munoz B, West SK, 2014. Increased carriage of macrolide-resistant fecal E. coli following mass distribution of azithromycin for trachoma control. Int J Epidemiol 43: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, Rodloff A, Steinbakk M, Urbaskova P, Vatopoulos A, 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J Antimicrob Chemother 52: 145–148. [DOI] [PubMed] [Google Scholar]
- 10.Leach AJ, Shelby-James TM, Mayo M, Gratten M, Laming AC, Currie BJ, Mathews JD, 1997. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis 24: 356–362. [DOI] [PubMed] [Google Scholar]
- 11.Batt SL, Charalambous BM, Solomon AW, Knirsch C, Massae PA, Safari S, Sam NE, Everett D, Mabey DC, Gillespie SH, 2003. Impact of azithromycin administration for trachoma control on the carriage of antibiotic-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 47: 2765–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]