Abstract.
Strongyloides stercoralis, although endemic in many countries, is not included in helminth control programs. Few data are available on the prevalence and morbidity linked to this infection. We compared data from two studies conducted in 1998 and 2013 on Pemba Island, Tanzania, involving 525 and 509 schoolchildren, respectively. In 1998, the diagnostic method used was Harada Mori, whereas in 2013 diagnosis was made by both Koga agar plate and Baermann methods. The prevalence registered was 41% in 1998 and 7% in 2013. This data suggest that the prevalence of S. stercoralis on Pemba was significantly reduced 7 years after the last ivermectin administration for preventive chemotherapy and underlines the importance and impact of large-scale preventive chemotherapy, which often goes beyond its actual target. Preventive chemotherapy with ivermectin should be recommended in areas where S. stercoralis is endemic.
Strongyloides stercoralis has been considered to be “the most neglected of the neglected tropical diseases (NTD).”1 In fact, although the parasite is endemic in many countries, and recent figures estimate a global prevalence of about 350 million infected people,2 it has not yet been included in control interventions for NTD. This is partly due to the fact that the resources needed for the diagnosis and treatment of S. stercoralis differ from the ones of the other soil-transmitted helminths (STH). First, the diagnosis of strongyloidiasis is more cumbersome compared with other STH, and diagnostic tools commonly used in the field are not sufficiently sensitive.3–5 Furthermore, treatment should not aim at the mere reduction of parasitic load, because of the potentially fatal risks of a persisting infection. Ivermectin is the drug of choice, which is superior in efficacy to albendazole.6 It should be noted that in countries endemic for Loa loa, ivermectin should be used with caution due to potentially severe side effects in patients with loiasis.7 However, ivermectin is often not easily available in S. stercoralis endemic areas, outside preventive chemotherapy (PC) programs. For these reasons, there is need for control programs specifically targeting S. stercoralis.
The potential benefit of PC with ivermectin in patients infected with S. stercoralis has been observed previously by a few studies that were reporting the results of control programs aiming at the elimination of either lymphatic filariasis (LF) or onchocercosis.8,9
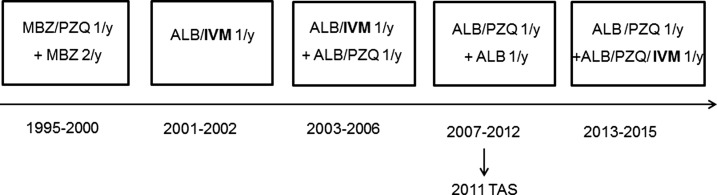
Pemba is together with Unguja one of the two main islands of the Zanzibar archipelago, where campaigns of PC including ivermectin in combination with albendazole for the elimination of LF have been periodically conducted since 2001. Several surveys assessing the prevalence and intensity of infections with STH were carried out in parallel.10–12 Zanzibar, in addition to LF, is also endemic for infections with STH and Strongyloides haematobium and PC either school-based and/or community-based has been ongoing as part of the Zanzibar National Helminth Control Program and LF Elimination Program since 1995. The time line of the different treatments with benzimidazoles, praziquantel, and/or ivermectin is illustrated in Figure 1. LF treatment was administered for 6 years both on Unguja and Pemba islands, and was discontinued in 2006 as prevalence in sentinel sites was below 1%.13 It was restarted in 2013, as the country did not pass the Transmission Assessment Survey carried out in 2011.14 In each of the six treatment rounds (1995–2014) (Figure 1), ivermectin was administered as a single dose of 200 μg/kg. We conducted a retrospective analysis of the results available from two studies conducted in schoolchildren in the study area to evaluate the impact of PC using ivermectin on S. stercoralis prevalence. The other drugs used in Pemba Island for helminth control in single dose have no (praziquantel) or only negligible (benzimidazoles) efficacy on S. stercoralis.
Figure 1.
Preventive chemotherapy timeline on Pemba Island from 1995 until 2015. The school-based national helminth control program started with administration of mebendazole (500 mg, single dose) and praziquantel (40 mg/kg, single dose) in 1995 and changed to albendazole (400 mg, single dose) and praziquantel (40 mg/kg, single dose) in 2003. The program to eliminate lymphatic filariasis started in 2001 with ivermectin (200 μg/kg, single dose) plus albendazole (400 mg, single dose) at community level. In 2006, lymphatic filariasis treatment was discontinued and restarted in 2013. ALB = albendazole; IVM = ivermectin; MBZ = mebendazole; PZQ = praziquantel; TAS = transmission assessment survey; y = year.
The two studies available for the current analyses had been approved by the relevant institutional review boards and ethics committees and were conducted according to international ethics standards (for details, see individual publications).10,15
The first study was carried out in 1998 at the beginning of the helminth control program, before starting PC interventions. It aimed to assess differences in iron status in children affected by the two species of hookworm (Ancylostoma duodenale and Necator americanus).10 Although it did not target S. stercoralis specifically, data were collected also on this parasite. Children were enrolled in 10 primary schools on Pemba Island. The diagnostic methods used in this study were Kato-Katz and Harada Mori, on a single stool sample. The second study was conducted in 2013, 7 years after the last distribution of ivermectin; data on prevalence of S. stercoralis on Pemba Island were obtained in the framework of a clinical trial on efficacy of oxantel–pamoate against Trichuris trichiura infection.15 Samples were collected in two primary schools. The diagnostic methods used were quadruplicate Kato-Katz slides on two stool samples, two Koga agar plates and two Baermann tests for each individual.
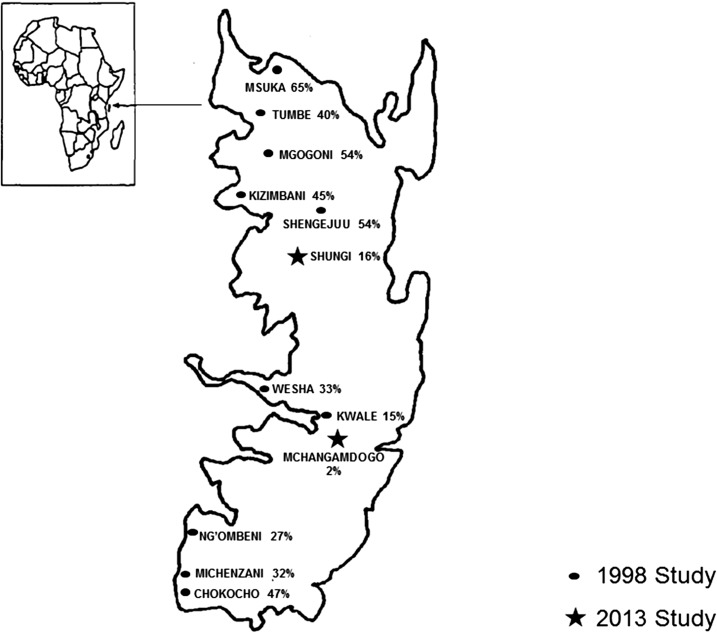
The two studies comprised a total of 1,034 individuals.10,15 Prevalence of S. stercoralis on Pemba Island was significantly reduced after 7 years of yearly ivermectin treatment (six treatments in total). In 1998, the overall prevalence of S. stercoralis was 41%, whereas in 2013, it was as low as 7%. In more detail, S. stercoralis prevalence in 1998 ranged from 15.3% to 65.3%, whereas in 2013 the range was 2–16% (Figure 2). Worth of notice, the second study was carried out using double Koga agar plate and Baermann, techniques which have a better sensitivity than Harada–Mori (on a single stool sample) used in the 1998 study, hence the true prevalence in 1998 might have been even higher. These data underline the importance and extended impact of PC, which goes beyond the targeted parasitic infections.
Figure 2.
Strongyloides stercoralis prevalence on Pemba Island in 1998 and 2013.
Our result is consistent with the findings from Unguja reported by Knopp and others.8 In Unguja 1 year after six rounds of treatment with ivermectin, prevalence of S. stercoralis infection was reduced by 80% even though the diagnostic methods used during the last assessment were more sensitive than the one used at baseline. The same trend has been observed in Ecuador9: a significant decrease was observed in S. stercoralis prevalence in areas where PC for Onchocerca volvulus with ivermectin has been carried out for several years.
Our study has two limitations. First of all we identified only two data sets that included S. stercoralis, highlighting the neglect of this parasitic infection. Furthermore, the schools selected in 2013 were different from the ones screened in 1998. However, it is worth highlighting that Pemba is a small island and the schools studied are close, sharing the same environment and likely similar transmission potential (Figure 2). In more detail, in Mchangamdogo the prevalence was 2% in 2013 compared with Kwale, which had revealed a prevalence of 15% in 1998. The same has been recorded for Shungui, close to Shengejuu, where the prevalences were determined as 54% (1998) versus 16% (2013). It is worth noticing that sanitation interventions have been limited in the study area and the prevalence of STH15 is still rampant in the Island, making unlikely that reduction of S. stercoralis prevalence was due to improved sanitary conditions and other factors in addition to PC campaigns. In conclusion, data from this retrospective study confirms the high efficacy and long-lasting impact of ivermectin for LF and onchocerciasis control on S. stercoralis infection. In agreement with other surveys,8 we emphasize that ivermectin should be added to ongoing PC programs to control S. stercoralis in areas where this neglected parasite is endemic.
REFERENCES
- 1.Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P, 2009. Strongyloidiasis: the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 103: 967–972. [DOI] [PubMed] [Google Scholar]
- 2.Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Muñoz J, Krolewiecki AJ, Gotuzzo E, Mena MA, Chiodini PL, Anselmi M, Moreira J, Albonico M, 2013. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 7: e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqui AA, Berk SL, 2001. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis Off Publ Infect Dis Soc Am 33: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 4.Krolewiecki AJ, Ramanathan R, Fink V, McAuliffe I, Cajal SP, Won K, Juarez M, Di Paolo A, Tapia L, Acosta N, Lee R, Lammie P, Abraham D, Nutman TB, 2010. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol 17: 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keiser J, Utzinger J, 2010. The drugs we have and the drugs we need against major helminth infections. Adv Parasitol 73: 197–230. [DOI] [PubMed] [Google Scholar]
- 6.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN, 2016. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev CD007745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Provisional Strategy for Interrupting Lymphatic Filariasis Transmission in Loiasis-Endemic Countries: Report of the Meeting on Lymphatic Filariasis, Malaria and Integrated Vector Management. Geneva, Switzerland: WHO; Available at: http://www.who.int/lymphatic_filariasis/resources/who_htm_ntd_pct_2012.6/en/. Accessed March 1, 2017. [Google Scholar]
- 8.Knopp S, Mohammed KA, Rollinson D, Stothard JR, Khamis IS, Utzinger J, Marti H, 2009. Changing patterns of soil-transmitted helminthiases in Zanzibar in the context of national helminth control programs. Am J Trop Med Hyg 81: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 9.Anselmi M, Buonfrate D, Guevara Espinoza A, Prandi R, Marquez M, Gobbo M, Montresor A, Albonico M, Racines Orbe M, Martin Moreira J, Bisoffi Z, 2015. Mass administration of ivermectin for the elimination of onchocerciasis significantly reduced and maintained low the prevalence of Strongyloides stercoralis in Esmeraldas, Ecuador. PLoS Negl Trop Dis 9: e0004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albonico M, Stoltzfus RJ, Savioli L, Tielsch JM, Chwaya HM, Ercole E, Cancrini G, 1998. Epidemiological evidence for a differential effect of hookworm species, Ancylostoma duodenale or Necator americanus, on iron status of children. Int J Epidemiol 27: 530–537. [DOI] [PubMed] [Google Scholar]
- 11.Albonico M, Rinaldi L, Sciascia S, Morgoglione ME, Piemonte M, Maurelli MP, Musella V, Utzinger J, Ali SM, Ame SM, Cringoli G, 2013. Comparison of three copromicroscopic methods to assess albendazole efficacy against soil-transmitted helminth infections in school-aged children on Pemba Island. Trans R Soc Trop Med Hyg 107: 493–501. [DOI] [PubMed] [Google Scholar]
- 12.Speich B, Moser W, Ali SM, Ame SM, Albonico M, Hattendorf J, Keiser J, 2016. Efficacy and reinfection with soil-transmitted helminths 18-weeks post-treatment with albendazole-ivermectin, albendazole-mebendazole, albendazole-oxantel pamoate and mebendazole. Parasit Vectors 9: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization, 2011. Global Programme to Eliminate Lymphatic Filariasis: Monitoring and Epidemiological Assessment of Mass Drug Administration: A Manual for National Elimination Programs. Geneva, Switzerland: WHO. [Google Scholar]
- 14.Rebollo MP, Mohammed KA, Thomas B, Ame S, Ali SM, Cano J, Escalada AG, Bockarie MJ, 2015. Cessation of mass drug administration for lymphatic filariasis in Zanzibar in 2006: was transmission interrupted? PLoS Negl Trop Dis 9: e0003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speich B, Ali SM, Ame SM, Bogoch II, Alles R, Huwyler J, Albonico M, Hattendorf J, Utzinger J, Keiser J, 2015. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: a four-arm, randomised controlled trial. Lancet Infect Dis 15: 277–284. [DOI] [PubMed] [Google Scholar]