Abstract.
Malaria, a major global public health problem, is mainly caused by Plasmodium falciparum and Plasmodium vivax, and is responsible for nearly half a million deaths annually. Although P. vivax malaria was not believed to cause severe disease, recent robust studies have proved otherwise. However, the clinical spectrum and pathogenesis of severe vivax malaria and, especially, its respiratory complications remain poorly understood. A systematic search for articles reporting respiratory complications associated with vivax malaria was performed in Lilacs, Cochrane, Scielo, Web of Science, and Medline databases irrespective of publication date. Prevalence of acute respiratory distress syndrome (ARDS) and associated mortality among vivax patients were calculated from cross-sectional and longitudinal studies, whereas factors associated with mortality were calculated from data pooled from case reports and series of cases. A total of 101 studies were included (49 cross-sectional or longitudinal and 52 case reports or series of cases). Prevalence of ARDS was 2.8% and 2.2% in children and adults, respectively, with nearly 50% mortality. Moreover, female sex (P = 0.013), having any comorbidity (P = 0.036), lower body temperature (P = 0.032), lower hemoglobin (P = 0.043), and oxygen saturation (P = 0.053) values were significantly associated with mortality. Plasmodium vivax malaria respiratory complications included ARDS and were associated with high mortality. Demographics and clinical characteristics upon presentation to hospital were associated with mortality among patients with respiratory complications in vivax malaria. This study reaffirms the evidence of severe and fatal complications of P. vivax malaria and its associated respiratory complications.
INTRODUCTION
Malaria is a major global public health problem with 3.4 billion people at risk over 91 endemic countries and territories in 2016.1 Despite improvements since 2000, malaria still imposes a great burden causing 212 million cases and 429,000 deaths in 2015.1 Of five Plasmodium species causing human malaria, Plasmodium vivax is the most widespread2 with great impact in endemic regions,3 and responsible for 41% of malaria-related cases, 0.7% of world malaria deaths, and 9% of malaria deaths outside Africa.1 The World Health Organization (WHO) estimated 8.5 million cases and 3,100 deaths from vivax malaria in 2015.1
Although falciparum malaria is definitely considered more lethal, it is now evident that P. vivax infection also causes severe disease and death.4,5 The clinical spectrum of vivax malaria ranges from asymptomatic infection to severe disease.6,7
Recognizing and managing malaria respiratory complications is challenging for health-care practitioners in endemic areas. The spectrum ranges from cough and acute breathlessness, to pulmonary edema, acute respiratory distress syndrome (ARDS), and death even after malaria treatment initiation.8–13 In a recent review of severe falciparum malaria cases, respiratory distress (RD) was reported in 25% of adults and 40% in children, whereas ARDS was found in up to 25% of adults; associated mortality was nearly 50% with intensive care and 80% without ventilator support.14 Recently, respiratory complications have also been reported in non-falciparum malarias including P. vivax.15–22
The underlying pathogenic mechanisms of respiratory complications associated with vivax malaria are not entirely understood.23 Robust evidence for lung impairment in P. vivax malaria has only emerged recently. Therefore, the primary objective of this review was to describe the prevalence and mortality of respiratory complications in vivax malaria. Secondary objectives were to describe geographical variation, clinical characteristics, risk factors for mortality, and associated pathogenesis.
METHODS
A systematic review addressing respiratory complications caused by P. vivax malaria was conducted using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.24,25 We systematically identified studies reporting respiratory complications (Table 1). The last search was performed in November 2016. Additional studies were obtained through search of references. No date or language restrictions were applied. All study designs with primary data were included.
Table 1.
Search strategy
| Database | Search strategy |
|---|---|
| Lilacs | (vivax AND pulmonary) OR (vivax AND respiratory) OR (vivax AND ARDS) |
| Cochrane | |
| Scielo | |
| Web of Science | (Plasmodium vivax malaria AND pulmonary) OR (Plasmodium vivax malaria AND respiratory) OR (Plasmodium vivax malaria AND respiratory distress) OR (Plasmodium vivax malaria AND pulmonary oedema) OR (Plasmodium vivax malaria AND ards) OR (Plasmodium vivax malaria AND human ards) |
| Medline/PubMed | Plasmodium vivax malaria (MeSH Term) AND pulmonary; Plasmodium vivax malaria (MeSH Terms) AND respiratory; Plasmodium vivax malaria (MeSH Term) AND respiratory distress; Plasmodium vivax malaria (MeSH Term) AND pulmonary oedema; malaria AND ards; (Plasmodium vivax malaria [MeSH Terms]) AND human ards (MeSH Terms) |
We reviewed titles and abstracts to confirm they included data on malaria infection, human population, P. vivax mono-infection, and respiratory complications. Included studies were assessed for eligibility through full-text review and excluded when reporting inconclusive Plasmodium species, non-respiratory complications, or if these were secondary to other organ impairment. All reviews were conducted by two independent reviewers (Fernando Val and Kim Machado) and disagreements were resolved by consensus.
Measures and definitions.
In cross-sectional and longitudinal studies, the following data were retrieved: sample size, number of severe and pulmonary malaria (PM, defined as any evidence of respiratory complication detected by any clinical and/or laboratorial alteration), and mortality. In this study, pediatric population referred to individuals under 17 years of age, and adult population to those 18 years or older. The type of respiratory complication was used exactly as reported in the original study. Case reports and series of cases were used to describe demographic, clinical, laboratory, and disease characteristics and any histopathological findings. Nine additional cases with vivax PM from Manaus, Brazil,26 were included. The following definitions were used: presence of comorbidities and other alterations were any reported at medical examination; altered clinical status at baseline was any clinical alteration at admission; presence of signs and symptoms in previous days to hospital presentation; any disease symptom previous to admission; time of RD onset was defined by time between admission and RD; timing of RD onset and start of antimalarial treatment; whether RD started before or after antimalarial treatment initiation; noninvasive oxygen supplementation was any oxygen support excluding those via intubation.
Statistical analyses.
To compare PM proportion among pediatric and adult populations, type of respiratory complication, and age group data were pooled, categorized (1—prevalence of PM among all cases, 2—prevalence of PM among severe cases, and 3—mortality due to PM), and compared. Data from case reports were described as mean and one standard deviation or median and interquartile range and tested according to normality. Independent t tests, Wilcoxon Mann–Whitney (frequency of children versus adults; survivors versus non-survivors) or χ2 test were applied. Significance was set as P < 0.05. Analyses were performed using STATA version 12.1 (Stata Corp., Colege Station, TX).
RESULTS
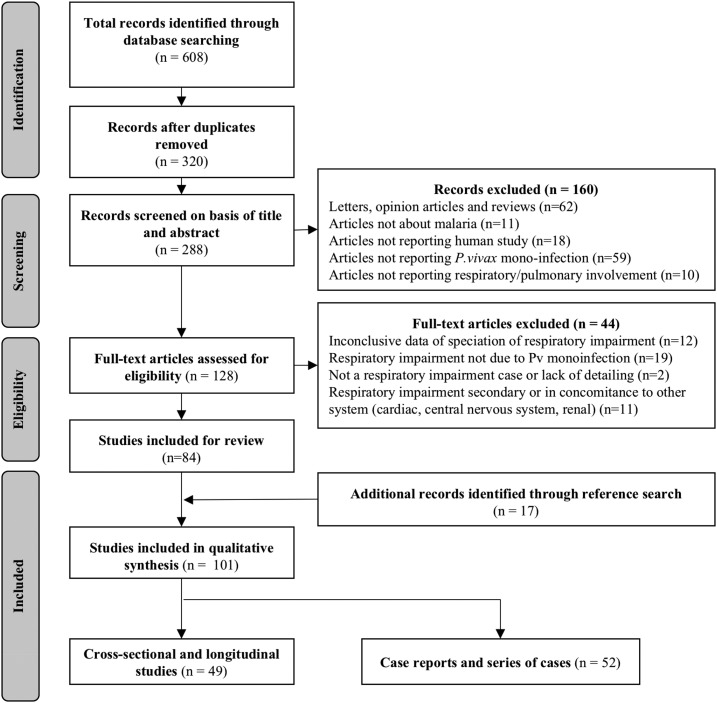
The original search returned 608 studies. After duplicate removal and review of titles and abstracts, 84 studies were included. After inclusion of 17 additional papers, a total of 101 studies were analyzed and separated in two major groups, the first containing 49 studies (cross-sectional or longitudinal studies) and the second 52 case reports and series of cases (Figure 1).
Figure 1.
Flow chart of inclusion of studies reporting respiratory complications Plasmodium vivax malaria.
Study characteristics.
Longitudinal and cross-sectional studies: Data were extracted from 49 studies reporting respiratory complications in P. vivax malaria26–74 (Supplemental Table 1). There was heterogeneity in the terminology used to describe respiratory complications. A total of 29 studies (59.1%) reported ARDS, 10 (20.4%) RD, two (4%) respiratory failure, one (2%) lung injury, one (2%) pulmonary edema, one (2%) acute respiratory distress, and the remaining five (10.2%) a combination between ARDS, RD, acute lung injury, and pulmonary edema. A total of 42 studies (85.7%) adopted WHO definitions for PM, whereas the remaining used general or country-specific definitions or did not report the adopted definition (Table 2).
Table 2.
Respiratory impairment and severe malaria definitions according to the literature
| Author, year (reference) | Name | Definition |
|---|---|---|
| Bernard, 1994146 | AECC definition | Timing: Acute onset; Radiograph: Bilateral infiltrates (frontal chest radiograph); PAWP: ≤ 18 mmHg or no clinical evidence of left atrial hypertension; Oxygenation: ALI criteria: PaO2/FiO2 ≤ 300/ARDS criteria: PaO2/FiO2 ≤ 200 (regardless of PEEP level) |
| The ARDS Definition Task Force, 2012127 | Berlin definition | Timing: Within 1 week of a known clinical insult or new or worsening respiratory symptoms; Chest imaging: Bilateral opacities—not fully explained by effusions, lobar/lung collapse, or nodules*; Origin of edema: Respiratory failure not fully explained by cardiac failure or fluid overload†; Oxygenation: Mild (200 mmHg < PaO2/FiO2 ≤ 300 mmHg); Moderate (100 mmHg < PaO2/FiO2 ≤ 200 mmHg); Severe (PaO2/FiO2 ≤ 100 mmHg). Obs.: PEEP or CPAP ≥ 5 cm H2O (Mild) or PEEP ≥ 5 cm H2O (moderate and severe) |
| Riviello, 2016128 | Kibali modification | Timing: Within 1 week of a known clinical insult or new or worsening respiratory symptoms; Imaging: Bilateral opacities not fully explained by effusions, lobar/lung collapse, or nodules by chest radiograph or ultrasound; PEEP requirement: No PEEP requirement, consistent with AECC definition; Origin of edema: respiratory failure not fully explained by cardiac failure or fluid overload†; Oxygenation: SpO2/FiO2 ≤ 315 |
| WHO definitions and clinical features of respiratory impairment in malaria | ||
| WHO, 1990123 | Severe and complicated malaria | Pulmonary edema: Sudden development after one or two days of treatment in adults. Evidence may include fluid overload, raised central venous or pulmonary artery wedge pressures and grossly positive fluid balance. It may also develop suddenly after delivery in pregnant women in positive fluid balance. Risk factors include hyperparasitemia, renal failure, pregnancy. Metabolic acidosis and hypoglycemia may be associated |
| WHO, 2000124 | Severe falciparum malaria | RD (associated acidosis): Raised RR, deep breathing and increased depth of respiration accompanied by a degree of intercostal indrawing (lower chest wall); pulmonary edema: later in course of disease with acute development. Presence of increased RR and chest x-ray evidencing pulmonary edema. PAWP may be normal. |
| WHO, 2012147 | Severe malaria | RD (acidosis)—Clinical features: Deep breathing, with indrawing of the bony structures of the lower chest wall, in the absence of localizing chest signs, suggests metabolic acidosis. Indrawing of the intercostal spaces alone is a less useful sign; pulmonary edema – Clinical features: increase in the respiratory rate, preceded by chest signs (radiograph resembling ARDS), reduced PaO2. Hypoxia may cause convulsions and consciousness deterioration, and the patient may die within a few hours. In pregnant, it may develop suddenly and unexpectedly several days after admission or may occur immediately after childbirth |
| WHO, 2014126 | Severe malaria | Bedside clinical classification of severe malaria (children and adults). RD (acidotic breathing): Mild—sustained nasal flaring and/or mild intercostal indrawing; Severe—either marked indrawing of the bony structure of the lower chest wall or deep (acidotic) breathing. Adults—Pulmonary edema: Radiologically confirmed, or oxygen saturation < 92% on room air with a respiratory rate > 30/minutes, often with chest indrawing and crepitation on auscultation; Acidosis: Severe acidosis manifests clinically as RD—rapid, deep, and labored breathing |
AECC = American-European Consensus Conference; ALI = acute lung injury; ARDS = acute respiratory distress syndrome; FiO2 = fraction of inspired oxygen; PaO2 = partial pressure of oxygen in arterial blood; PAWP = pulmonary artery wedge pressure; PEEP = positive end expiratory pressure; RD = respiratory distress; RR = respiratory rate; SpO2 = peripheral capillary oxygen saturation; WHO = World Health Organization.
Chest radiograph or computed tomography scan.
Need objective assessment, as echocardiography, to exclude hydrostatic edema if no risk factor present.
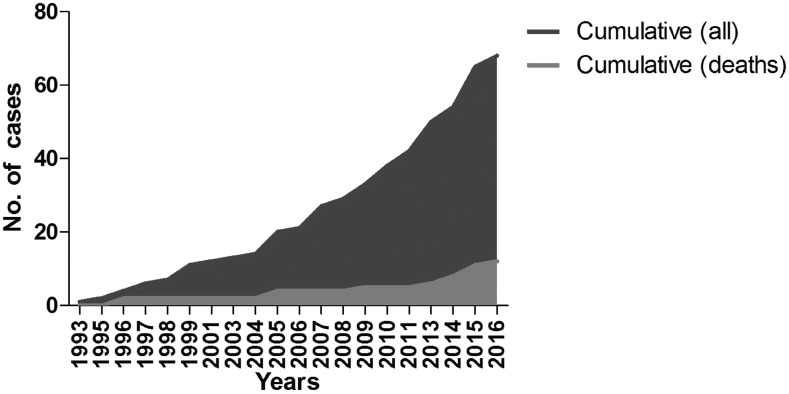
Case reports and series of cases: A total of 67 cases were analyzed (58 cases from included case reports and series of cases,11–13,58,59,75–118 and additional nine cases from Manaus, Brazil26). The earliest identified report was from 1993 (Figure 2). Cases were predominantly from India (Table 3) and diagnosed mainly by peripheral blood films (Table 4).
Figure 2.
Cumulative number of cases and deaths from case reports.
Table 3.
Number of pulmonary malaria cases and associated deaths, from case reports and series of cases of vivax malaria, by country of publication
| Country of publication | No. of cases (% of total) | No. of deaths (% of deaths) |
|---|---|---|
| India | 21 (31.4) | 4 (33.3) |
| United States | 10 (15) | 0 (0) |
| Brazil | 10 (15) | 3 (25) |
| England | 5 (7.6) | 0 (0) |
| Colombia | 4 (5.9) | 3 (25) |
| Greece | 2 (2.9) | 0 (0) |
| Pakistan | 2 (2.9) | 0 (0) |
| Spain | 2 (2.9) | 0 (0) |
| Thailand | 2 (2.9) | 1 (8.3) |
| Germany | 1 (1.5) | 0 (0) |
| Italy | 1 (1.5) | 0 (0) |
| Malaysia | 1 (1.5) | 0 (0) |
| Mexico | 1 (1.5) | 0 (0) |
| Peru | 1 (1.5) | 1 (8.3) |
| Singapore | 1 (1.5) | 0 (0) |
| South Korea | 1 (1.5) | 0 (0) |
| Turkey | 1 (1.5) | 0 (0) |
| Venezuela | 1 (1.5) | 0 (0) |
| Total | 67 (100) | 12 (100) |
Table 4.
Methods used in case reports and series of cases for malaria diagnosis and types of parasitemia presentation
| Method(s) | No. (% of total) |
|---|---|
| PBF + PCR | 23 (34.8) |
| PBF + RDT | 20 (30.3) |
| PBF | 16 (24.2) |
| PBF + RDT + PCR | 5 (7.6) |
| QBC + PCR | 1 (1.5) |
| PBF + QBC + RDT | 1 (1.5) |
| Total | 66 (97.05) |
PBF = peripheral blood film; PCR = polymerase chain reaction; RDT = rapid diagnostic test; QBC = quantitative buffy coat.
Respiratory complications frequency and mortality.
Overall, 450,115 patients were pooled from longitudinal and cross-sectional studies; 5,448 with severe disease, 453 developing PM, and 74 deaths. The overall prevalence of respiratory complications was 0.1% among patients and 8.3% among severe cases, with a mortality of 16.3% (Supplemental Table 1).
According to WHO-defined regions,1 the highest frequency of PM (8.7%) occurred in southeast Asia. More details are found in Supplemental Table 2.
Frequency of ARDS was 2.4% in 23 studies, mostly in hospitalized patients (N = 3,574 patients) (Table 5). A total of 1,156 developed severe malaria, 85 presenting ARDS (7.3%). Mortality among patients with ARDS was 49.5%. In children, ARDS frequency was 2.8% (48.3% mortality). In adults, 2.2% presented ARDS (50% mortality). There was no statistical difference in ARDS prevalence (P = 0.315) and mortality (P = 0.787) between children and adults.
Table 5.
Frequency and case fatality rate of ARDS among patients with Pv malaria according to age groups in hospital-based studies
| First author, year (reference) | Location | Study population | No. of Pv patients | Frequency (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Severe | ARDS | Deaths | ARDS /total | ARDS /severe | ARDS mortality | |||
| Studies with pediatric population (children under 18 years) | |||||||||
| Kochar, 201027 | India | Malaria hospitalizations | 103 | 65 | 1 | – | 0.9 | 1.5 | – |
| Singh, 201133 | India | Patient hospital presentations | 108 | 23 | 0 | 0 | 0 | 0 | 0 |
| Lança, 201228 | Brazil | Pv pediatric hospitalizations | 24 | 24 | 3 | 0 | 12.5 | 12.5 | 0 |
| Sharma, 201229 | India | Patients admitted to hospital | 105 | 46 | 9 | 7 | 8.5 | 19.5 | 77.8 |
| Yadav, 201230 | India | Severe vivax malaria | 131 | 131 | 3 | 2 | 2.2 | 2.2 | 66.7 |
| Gehlawat, 201332 | India | Severe vivax in children | 18 | 18 | 1 | 1 | 5.5 | 5.5 | 100 |
| Bhattacharjee, 201334 | India | Pv pediatric hospitalizations | 168 | 168 | 2 | 0 | 1.1 | 1.1 | 0 |
| Singh, 201335 | India | Patients admitted to hospital | 61 | 38 | 5 | 3 | 8.2 | 13.1 | 60 |
| Sharma, 201336 | India | Pv pediatric hospitalizations | 261 | 54 | 3 | – | 1.1 | 5.5 | 0 |
| Goyal, 201437 | India | Pv hospital admissions | 47 | – | 2 | 1 | 4.2 | – | 50 |
| Total | 1026 | 567 | 29 | 14 | 2.8 | 5.1 | 48.2 | ||
| Studies with adult population | |||||||||
| Kotwal, 200557 | Afghanistan | American soldiers | 38 | 1 | 1 | 0 | 2.6 | 100 | 0 |
| Sharma, 200960 | India | Records of malaria cases | 221 | – | 3 | 3 | 1.3 | – | 100 |
| Kochar, 200961 | India | Patients admitted to hospital | 456 | 40 | 4 | 2 | 0.8 | 100 | 50 |
| George, 201071 | India | Severe Pv admissions | 30 | 30 | 1 | 1 | 3.3 | 3.3 | 100 |
| Nayak, 201162 | India | Severe malaria cases | 80 | 80 | 4 | 4 | 5 | 5 | 100 |
| Srivastava, 201163 | India | Patient hospital presentations | 50 | 41 | 3 | 0 | 6 | 7.3 | 0 |
| Limaye, 201264 | India | Patients admitted to hospital | 336 | 50 | 10 | 6 | 2.9 | 20 | 60 |
| Mehmood, 201265 | Pakistan | Pv cases in Emergency Dept. | 97 | – | 1 | 0 | 1 | – | 0 |
| Singh, 201356 | India | Patients admitted to hospital | 140 | 63 | 3 | 2 | 2.1 | 4.7 | 66.7 |
| Rizvi, 201367 | India | Patients admitted to hospital | 172 | 62 | 6 | 2 | 3.5 | 9.7 | 33.3 |
| Muley, 201470 | India | Hospital admissions | 100 | – | 6 | 1 | 6 | – | 16.6 |
| Siqueira, 201526 | Brazil | Patients admitted to hospital | 316 | 40 | 7 | 1 | 22.2 | 17.5 | 14.2 |
| Siqueira, 201526 | India | Patients admitted to hospital | 462 | 157 | 5 | 5 | 1.1 | 3.1 | 100 |
| Jain, 201672 | India | Patients in Emergency Dept. | 48 | 25 | 2 | 1 | 4.1 | 8 | 50 |
| Total | 2548 | 589 | 56 | 28 | 2.2 | 9.5 | 50 | ||
ARDS = acute respiratory distress syndrome; Dept. = department; Pv = Plasmodium vivax. Blank spaces mean no data was available in original paper.
On the other hand, among patients who died due to vivax-associated respiratory complications, pulmonary malaria was present in 52.9%, in a series of 17 autopsies cases, the best reference for that purpose, despite the limited sample. Out of these, two had confirmed ARDS on histopathology.13 In Papua, 26 out of 65 patients with P. vivax infection who died had any respiratory complication, that is, 40%, similar to the autopsy series.119
Clinical and epidemiological case characteristics.
Among individual case reports, there was a slight predominance of male sex (57.6%). Most cases (73.1%) aged between 19 and 49 years. Comorbidities and other relevant medical conditions were described in 33.3%, (i.e., hypertension, diabetes, hypercholesterolaemia, β-thalassaemia, parasites coinfections, previous histoplasmosis, previous myocardial infarction, malignant vasovagal syndrome, occasional tobacco use, and chronic obstructive pulmonary disease). Two patients were pregnant. Most patients presented with respiratory complications on hospital admission (55.2%), predominantly increased respiratory rate (75%) and heart rate (62.2%), and/or reduced pulse oximetry (46.6%). Anemia (81.8%), thrombocytopenia (91.6%), and elevated liver enzymes (55.1%) were also observed. Median parasite density was 14,780 per µL (Table 6).
Table 6.
Characterization of selected characteristics according to outcome
| Parameters | All | Survival | Non-survival | Completeness n (%) | |
|---|---|---|---|---|---|
| No. (% of total) | No. (% of total) | No. (% of total) | P value* | (Survival and non-survival) | |
| Demographics | |||||
| Total with individual information | 67 (100) | 54 (81.8) | 12 (18.2) | – | 66 (98.5) |
| Age (years) | 0.371 | 65 (97) | |||
| Mean (1 SD) | 39 (15) | 40 (±14) | 36 (±18) | ||
| IQR (Range) | 29–50 (15–75) | 30–50 (15–74) | 20–30 (19–75) | ||
| Gender | |||||
| Male | 38 (57.6) | 34 (64.2) | 3 (25) | 0.013 | 65 (97) |
| Female | 28 (42.4) | 19 (35.8) | 9 (75) | ||
| Imported malaria case | 27 (40.3) | 22 (40.7) | 4 (33.3) | 0.635 | 65 (97) |
| Clinical and laboratory parameters at hospital admission | |||||
| Presence of comorbidities and other alterations | 18 (33.3) | 11 (25.6) | 6 (60) | 0.036 | 53 (79) |
| Altered clinical status at baseline | 55 (91.7) | 45 (90) | 10 (100) | 0.296 | 60 (89.5) |
| Malaria signs and symptoms in last days | 58 (96.7) | 48 (96) | 10 (100) | 0.520 | 60 (89.5) |
| Presence of respiratory complication | 32 (55.2) | 24 (50) | 8 (88.9) | 0.031 | 57 (85) |
| Respiratory rate (irpm)† | 30 (± 10) | 30 (±10) | 29 (±10) | 0.429 | 46 (68.5) |
| Heart rate (bpm)† | 107 (± 20) | 107 (±21) | 107 (±15) | 0.532 | 45 (67.2) |
| O2 Saturation (%)‡ | 94 (82–98) | 95 (86–99) | 78 (75–88) | 0.053 | 30 (44.8) |
| Body temperature (°C)† | 38.36 (±1.4) | 38.5 (±1.2) | 37.5 (±1.7) | 0.032 | 45 (67.2) |
| Hemoglobin (g/dL)† | 10.3 (± 2.9) | 10.63 (±2.8) | 8.67 (±3.15) | 0.043 | 44 (65.7) |
| Leukocytes (×103/mm3)‡ | 6.6 (3.9–10) | 6 (3.9–10) | 8.7 (4–9.9) | 0.921 | 47 (70.1) |
| Platelet count (×103/mm3)‡ | 60 (35.5–95) | 59 (36–91) | 81 (34–120) | 0.447 | 48 (71.6) |
| AST (IU/L)‡ | 56 (38–98) | 60 (47–98) | 36 (31–83) | 0.118 | 29 (43.3) |
| ALT (IU/L)‡ | 62 (36–77) | 67 (42–77) | 34 (28–66) | 0.092 | 29 (43.3) |
| Parasitemia (×103/µL)‡ | 14.8 (1.8–34) | 13.1 (13–34) | 15.4 (8–28.7) | 0.811 | 22 (32.8) |
| Disease progression | |||||
| Duration of malaria symptoms (days)‡ | 6 (4–7) | 5 (4–7) | 7 (7–7) | 0.289 | 53 (79.1) |
| P. vivax diagnosis on day 1 | 50 (84.7) | 41 (83.7) | 9 (90) | 0.612 | 59 (88) |
| Time of onset of RD | |||||
| Before AM initiation | 22 (37.3) | 19 (38) | 3 (33.3) | 0.790 | 59 (88) |
| After AM initiation | 37 (62.7) | 31 (62) | 6 (66.7) | ||
| After RD, change of AM | 12 (27.9) | 10 (27.7) | 2 (28.6) | 0.966 | 43 (62.4) |
| Time of parasite clearance | |||||
| Before RD onset | 5 (12.8) | 3 (8.8) | 2 (40) | 0.052 | 39 (58.2) |
| After RD onset | 34 (87.2) | 31 (91.2) | 3 (60) | ||
| Supplementary oxygen use | 56 (98.2) | 47 (100) | 9 (90) | 0.029 | 57 (85) |
| Oxygen support use (days)‡ | 6 (3–10) | 6 (4–10) | 3 (1–5) | 0.026 | 37 (55.2) |
| Type of oxygen support | |||||
| Noninvasive | 18 (33.9) | 17 (39.5) | 1 (10) | 0.076 | 53 (79.1) |
| Invasive (intubated) | 35 (66.1) | 26 (60.5) | 9 (90) | ||
| Time hospitalized (days)‡ | 13 (7–21) | 14 (9–21) | 4 (2–10) | 0.007 | 43 (62.4) |
ALT = alanine aminotransferase; AM = antimalarials; AST = aspartate aminotransferase; bpm = beats per minute; brpm = breaths per minute; IQR = interquartile range; RD = respiratory distress; SD = standard deviation. The bold highlights signify P values.
Survival vs. non-survival groups.
Mean (one standard deviation).
IQR.
Vivax malaria diagnosis was performed in the first day in almost all cases. RD started before hospitalization in 27.1% and occurred after the start of antimalarial treatment in 62.7%. Parasite clearance occurred after RD onset in 87.2%. Median of hospitalization was 13 days (Table 6).
Almost all patients were reported to have received supplementary oxygen (98.2%): 33.9% were managed by noninvasive strategies, with positive pressure ventilation strategies in some cases. In the remaining 66.1%, invasive strategies were necessary with some reporting use of low-tidal volumes, moderate levels of positive end expiratory pressure, and other protective ventilation strategies. Other approaches adopted were prone positioning and extracorporeal membrane oxygenation (Table 6).
Survivors and non-survivors.
Female sex was associated with higher mortality (P = 0.013). One female was pregnant. Comorbidities (P = 0.036) and respiratory complications at admission (P = 0.031) were more frequent among deceased patients. Body temperature (P = 0.032), hemoglobin level (P = 0.043), and oxygen saturation (P = 0.053) were lower at hospital presentation among non-survivors. Non-survivors were kept on oxygen support for less time (P = 0.026), with a great predominance of cases being reported to use invasive oxygen delivery strategies (P = 0.076). Moreover, these patients had shorter hospitalizations (P = 0.007) (Table 6).
Histopathological and functional studies.
Few studies provided histopathological findings. A study published in 1993 revealed bronchiolitis obliterans organizing pneumonia.103 Further studies reported the presence of monocytes, lymphocytes, and neutrophils in pulmonary microvasculature along with phagocytosed pigment and diffuse alveolar damage110,115 accompanied by hyaline membrane formation.13 Severe alveolar edema and congestion with infiltrates containing mononuclear cells and suggestive images of adhesion of Plasmodium-infected red cells to lung microvasculature were reported in an autopsy series.120 Functional studies reported gas flow impairments with reduction of vascular component at presentation and improvement by day 7, and also with alveolar-capillary membrane component decreased at presentation with progressive deterioration after 14 days from treatment in severe vivax malaria.121 Of note, a case without any clinical or laboratory evidence of vivax induced respiratory complications was reported in 1989 and showed a reversible diffuse lung uptake of technetium-99m sulfur colloid during acute and recovery periods, which could indicate, according to the authors, a malaria-induced increase in lung macrophages.122
DISCUSSION
Pulmonary edema and ARDS are features of severe falciparum malaria adopted since 1990,123 and have been used also to define severe vivax malaria afterward. In this study, we proposed to estimate prevalence and mortality of respiratory complications in vivax malaria along with risk factors associated to death. The analysis of longitudinal and cross sectional studies showed that respiratory complications and ARDS occur in lower frequencies in vivax malaria, both in children and adults, but with a mortality resembling falciparum disease. Female gender, presence of comorbidities, and respiratory complications at hospital admission along with lower hemoglobin and body temperature measurements were associated with death.
Most of the studies were published before 2012 and used previous WHO definitions for respiratory complications.124,125 Bedside clinical classification of RD has two degrees according to WHO with similar clinical features among children and adults.126 Lung edema is a criterium for severe falciparum disease and is defined through clinical and radiological criteria.126 ARDS, with a definition update in 2012,127 was also commonly reported in studies analyzed in this review. Despite advances in ARDS diagnosis and treatment, most malaria-endemic areas are resource-poor settings. Therefore, misdiagnosis, underreporting, and incorrect management may occur. A more inclusive ARDS diagnostic tool, which withdraws positive end-expiratory pressure requirements and keeps only imaging and a single oxygen saturation cutoff value, has been developed, and despite lacking validation, it seems a promising epidemiological tool for ARDS.128
The presence of comorbidities and altered clinical and laboratory parameters at hospital admission may serve as early indicators of disease severity and risk of death. Underlying diseases, such as hypertension and diabetes, may indicate a greater hemodynamic compromise with an exacerbated inflammatory profile.129 These might contribute to vivax mortality through disturbances in cytokine production, endothelial activation, altered thrombostasis, and further organ specific damage,130 which may further explain findings from histopathological and functional studies.
Although vivax malaria rarely develops hyperparasitemia,13 a hallmark of severe falciparum malaria,126 greater inflammatory and endothelial activation per parasite occurs in vivax malaria when compared with falciparum disease.131–133 Inflammatory exacerbation may cause pulmonary injury,130 whereas elevated cytokines and other inflammatory-inducing molecules may be responsible for alveolar-capillary barrier loss and increased permeability.134
Several reports from different Plasmodium species show that respiratory impairment begins several hours after antimalarials start, even in the presence of declining or negative parasitemia. In a falciparum malaria study, 79 patients developed respiratory failure, in the first 48 hours in 42% and afterward in 46%.135 Lung function studies revealed small airway obstructions and decreased diffusion capacity in both uncomplicated falciparum and vivax malaria.121,136,137 The capillary volume compartment in vivax patients was reduced at admission, returning to normal after treatment, whereas the membranous compartment was normal at presentation but progressively deteriorated. According to the authors, the uncoupling of both compartments at different time points is consistent with Plasmodium-infected red cells sequestration and a progressive alveolar-capillary injury due to a posttreatment inflammatory response to parasite killing.121 In vivax malaria, cytoadhesion of infected red cells to the endothelial cell layer is supported by recent studies.138,139 In an autopsy series, seven out of 13 patients in which vivax was the probable or contributing cause of death presented pulmonary complications.120 A lung tissue section from one of these patients presented images suggestive of sequestration of P. vivax-infected red cells within pulmonary vasculature. Interestingly, this sample was taken from a patient with negative peripheral parasitemia but with positive polymerase chain reaction for vivax in the lung.120
The high number of patients needing mechanical ventilation and other advanced life-support strategies, which are not always available in resource-poor settings, may have impacted mortality. Early malaria diagnosis accompanied by effective antimalarial therapy, rapid identification of oxygen saturation deterioration with prompt support, and identification of complications could avoid progression to death.140
The time of oxygen support and length of hospitalization were lower among those who died, evidencing a more severe clinical deterioration than in survivors. Similarly, in a clinical characterization of vivax malaria patients suffering from ARDS and acute lung injury, older age, use of invasive mechanical ventilation, hypoxemia, presence of other organ involvement and comorbidities were considered risk factors for mortality.141 The higher presence of comorbidities found among non-survivors, along with higher presentation to hospital with respiratory impairment, probably explain the outcome in this group.
The pathogenesis of respiratory complications is historically better characterized in P. falciparum infection with studies revealing important lung vascular alterations: congested capillaries, thickened alveolar septum, diffuse edema, and hyaline membrane formation142; endothelial cell swelling with narrowed capillary lumen, macrophages within interstitium and hemozoin143; and presence of inflammatory and Plasmodium-infected red cells within capillaries.144 Essentially, and despite the progression and outcome of lung deterioration, the major difference between P. falciparum and P. vivax ARDS pathogenesis is the presence of more sequestration of parasitized red blood cells in the former145 and scanty evidence in the second.13,120
Only studies containing respiratory complications were included, which may increase the prevalence estimates of respiratory complications. Furthermore, severe cases are more likely to be published, leading to a clear bias of publication. Also, calculating disease prevalence or clinical outcome among several studies published with different objectives and lack of standardization on data reporting were expected limitations. The publications’ time range, different localities, and different case definitions may have affected, therefore, disease management and data reporting priorities, which may further have influenced the results of the present study. This lack of systematic reporting hampers broad data analysis and completeness.
CONCLUSIONS
To our knowledge, this is the first systematic review and meta-analysis attempting to estimate the frequency of respiratory complications in different vivax malaria populations, its disease characteristics, factors associated with death, and histopathology. As in falciparum malaria, vivax disease also presents an important rate of respiratory impairment among severe cases, with elevated mortality among children and adults. Female sex, presence of comorbidities, respiratory status at hospital admission, and lower hemoglobin values were found to statistically differ between survivors and non-survivors. Multicenter studies are needed using standardized protocols with adequate data registering, confirmation of mono-infections through molecular methodologies, exclusion of comorbidities, and other concurrent infections to better characterize vivax clinical spectrum and, specifically, respiratory impairment.
Supplementary Material
Note: Supplemental tables appear at www.ajtmh.org.
Disclaimer: Data included in this study were not previously presented or published elsewhere.
REFERENCES
- 1.WHO, 2016.. World Malaria Report 2016. WHO. [Google Scholar]
- 2.Gething PW, Elyazar IRF, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HFL, Price RN, Müeller I, Baird JK, Hay SI, 2012. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis 6: e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, Hay SI, 2016. Global epidemiology of Plasmodium vivax. Am J Trop Med Hyg 95(Suppl 6): 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W, 2014. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J 13: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird JK, 2013. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev 26: 36–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacerda MVG, Mourão MPG, Alexandre MAA, Siqueira AM, Magalhães BML, Martinez-Espinosa FE, Filho FSS, Brasil P, Ventura AMRS, Tada MS, Couto VSCD, Silva AR, Silva RSU, Alecrim MGC, 2012. Understanding the clinical spectrum of complicated Plasmodium vivax malaria: a systematic review on the contributions of the Brazilian literature. Malar J 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anstey NM, Douglas NM, Poespoprodjo JR, Price RN, 2012. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv Parasitol 80: 151–201. [DOI] [PubMed] [Google Scholar]
- 8.Punyagupta S, Srichaikul T, Nitiyanant P, Petchclai B, 1974. Acute pulmonary insufficiency in falciparum malaria: summary of 12 cases with evidence of disseminated intravascular coagulation. Am J Trop Med Hyg 23: 551–559. [DOI] [PubMed] [Google Scholar]
- 9.Deaton JG, 1970. Fatal pulmonary edema as a complication of acute falciparum malaria. Am J Trop Med Hyg 19: 196–201. [DOI] [PubMed] [Google Scholar]
- 10.Martell RW, Kallenbach J, Zwi S, 1979. Pulmonary oedema in the falciparum malaria. BMJ 1: 1763–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam N, Qamruddin K, 1995. Unusual complications in benign tertian malaria. Trop Geogr Med 47: 141–143. [PubMed] [Google Scholar]
- 12.Pukrittayakamee S, Chantra A, Vanijanonta S, White NJ, 1998. Pulmonary oedema in vivax malaria. Trans R Soc Trop Med Hyg 92: 421–422. [DOI] [PubMed] [Google Scholar]
- 13.Valecha N, Pinto RGW, Turner GDH, Kumar A, Rodrigues S, Dubhashi NG, Rodrigues E, Banaulikar SS, Singh R, Dash AP, Baird JK, 2009. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg 81: 758–762. [DOI] [PubMed] [Google Scholar]
- 14.Taylor WRJ, Hanson J, Turner GDH, White NJ, Dondorp AM, 2012. Respiratory manifestations of malaria. Chest 142: 492–505. [DOI] [PubMed] [Google Scholar]
- 15.Lau Y-L, Lee W-C, Tan L-H, Kamarulzaman A, Omar SFS, Fong M-Y, Cheong F-W, Mahmud R, 2013. Acute respiratory distress syndrome and acute renal failure from Plasmodium ovale infection with fatal outcome. Malar J 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strydom K-A, Ismail F, Frean J, 2014. Plasmodium ovale: a case of not-so-benign tertian malaria. Malar J 13: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EY, Maguire JH, 1999. Acute pulmonary edema complicating ovale malaria. Clin Infect Dis 29: 697–698. [DOI] [PubMed] [Google Scholar]
- 18.Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW, 2012. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar J 11: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, Wong KT, Adem P, Zaki SR, Singh B, Krishna S, 2010. Severe malaria - a case of fatal Plasmodium knowlesi infection with post-mortem findings: a case report. Malar J 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, Khoo S, Frederick C, Jelip J, Anstey NM, Yeo TW, 2011. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg Infect Dis 17: 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daneshvar C, Davis TME, Cox‐Singh J, Rafa’ee MZ, Zakaria SK, Divis PCS, Singh B, 2009. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis 49: 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Descheemaeker P-N, Mira J-P, Bruneel F, Houzé S, Tanguy M, Gangneux J-P, Flecher E, Rousseau C, Le Bras J, Mallédant Y, 2009. Near-fatal multiple organ dysfunction syndrome induced by Plasmodium malariae. Emerg Infect Dis 15: 832–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor WRJ, Cañon V, White NJ, 2006. Pulmonary manifestations of malaria: recognition and management. Treat Respir Med 5: 419–428. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D, 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Med 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siqueira AM, Lacerda MV, Magalhães BML, Mourão MP, Melo GC, Alexandre MA, Alecrim MG, Kochar D, Kochar S, Kochar A, Nayak K, del Portillo H, Guinovart C, Alonso P, Bassat Q, 2015. Characterization of Plasmodium vivax-associated admissions to reference hospitals in Brazil and India. BMC Med 13: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochar DK, Tanwar GS, Khatri PC, Kochar SK, Sengar GS, Gupta A, Kochar A, Middha S, Acharya J, Saxena V, Pakalapati D, Garg S, Das A, 2010. Clinical features of children hospitalized with malaria–a study from Bikaner, northwest India. Am J Trop Med Hyg 83: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lança EFC, Magalhães BML, Vitor-Silva S, Siqueira AM, Benzecry SG, Alexandre MAA, O’Brien C, Bassat Q, Lacerda MVG, 2012. Risk factors and characterization of Plasmodium vivax-associated admissions to pediatric intensive care units in the brazilian Amazon. PLoS One 7: e35406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma R, Gohain S, Chandra J, Kumar V, Chopra A, Chatterjee S, Aneja S, Kumar Dutta A, 2012. Plasmodium vivax malaria admissions and risk of mortality in a tertiary-care children’s hospital in north India. Paediatr Int Child Health 32: 152–157. [DOI] [PubMed] [Google Scholar]
- 30.Yadav D, Chandra J, Aneja S, Kumar V, Kumar P, Dutta AK, 2012. Changing profile of severe malaria in north Indian children. Indian J Pediatr 79: 483–487. [DOI] [PubMed] [Google Scholar]
- 31.Ketema T, Bacha K, 2013. Plasmodium vivax associated severe malaria complications among children in some malaria endemic areas of Ethiopia. BMC Public Health 13: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gehlawat VK, Arya V, Kaushik JS, Gathwala G, 2013. Clinical spectrum and treatment outcome of severe malaria caused by Plasmodium vivax in 18 children from northern India. Pathog Glob Health 107: 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh H, Parakh A, Basu S, Rath B, 2011. Plasmodium vivax malaria: is it actually benign? J Infect Public Health 4: 91–95. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharjee P, 2013. The clinicopathologic manifestations of Plasmodium vivax malaria in children: a growing menace. J Clin Diagn Res 7: 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh R, 2013. A comparative study of clinical profiles of vivax and falciparum malaria in children at a tertiary care centre in Uttarakhand. J Clin DIAGNOSTIC Res 7: 2234–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma S, 2013. The unusual presentation of a usual organism—the changing spectrum of the clinical manifestations of Plasmodium vivax malaria in children: a retrospective study. J Clin Diagn Res 7: 1964–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal JP, Makwana AM, 2014. Comparison of clinical profile between P. vivax and P. falciparum malaria in children: a tertiary care centre perspective from India. Malar Res Treat 2014: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumari M, Ghildiyal R, 2014. Clinical profile of Plasmodium vivax malaria in children and study of severity parameters in relation to mortality: a tertiary care centre perspective in Mumbai, India. Malar Res Treat 2014: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genton B, D’Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, Muller I, 2008. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med 5: e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manning L, Laman M, Law I, Bona C, Aipit S, Teine D, Warrell J, Rosanas-Urgell A, Lin E, Kiniboro B, Vince J, Hwaiwhanje I, Karunajeewa H, Michon P, Siba P, Mueller I, Davis TME, 2011. Features and prognosis of severe malaria caused by Plasmodium falciparum, Plasmodium vivax and mixed Plasmodium species in Papua New Guinean children. PLoS One 6: e29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arboleda M, Pérez MF, Fernández D, Usuga LY, Meza M, 2012. Clinical and laboratory profile of Plasmodium vivax malaria patients hospitalized in Apartadó, Colombia [in Spanish] Biomédica Rev del Inst Nac Salud 32 (Suppl 1): 58–67. [DOI] [PubMed] [Google Scholar]
- 42.Nadkar MY, Huchche AM, Singh R, Pazare AR, 2012. Clinical profile of severe Plasmodium vivax malaria in a tertiary care centre in Mumbai from June 2010–January 2011. J Assoc Physicians India 60: 11–13. [PubMed] [Google Scholar]
- 43.Aatif S, Jamal Q, Altaf A, 2013. Salimullah. Is vivax malaria really benign? A Karachi-based study. J Pak Med Assoc 63: 721–724. [PubMed] [Google Scholar]
- 44.Jain V, Agrawal A, Singh N, 2013. Malaria in a tertiary health care facility of central India with special reference to severe vivax: implications for malaria control. Pathog Glob Health 107: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nayak KC, Meena SL, Gupta BK, Kumar S, Pareek V, 2013. Cardiovascular involvement in severe vivax and falciparum malaria. J Vector Borne Dis 50: 285–291. [PubMed] [Google Scholar]
- 46.Mendonca VRR, Souza LCL, Garcia GC, Magalhaes BML, Lacerda MVG, Andrade BB, Goncalves MS, Barral-Netto M, 2014. DDX39B (BAT1), TNF and IL6 gene polymorphisms and association with clinical outcomes of patients with Plasmodium vivax malaria. Malar J 13: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alexandre MA, Ferreira CO, Siqueira AM, Magalhães BL, Mourão MPG, Lacerda MV, Alecrim MDGC, 2010. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis 16: 1611–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN, 2008. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 5: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barcus MJ, Basri H, Picarima H, Manyakori C, Sekartuti, Elyazar I, Bangs MJ, Maguire JD, Baird JK, 2007. Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am J Trop Med Hyg 77: 984–991. [PubMed] [Google Scholar]
- 50.Chaparro-Narvaez PE, Lopez-Perez M, Marcela Rengifo L, Padilla J, Herrera S, Arevalo-Herrera M, 2016. Clinical and epidemiological aspects of complicated malaria in Colombia, 2007–2013. Malar J 15: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang J, Cullen KA, Kachur SP, Arguin PM, Baird JK, 2014. Severe morbidity and mortality risk from malaria in the United States, 1985–2011. Open Forum Infect Dis 1: ofu034–ofu034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demissie Y, Ketema T, 2016. Complicated malaria symptoms associated with Plasmodium vivax among patients visiting health facilities in Mendi town, northwest Ethiopia. BMC Infectious Dis 16: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh J, Purohit B, Desai A, Savardekar L, Shanbag P, Kshirsagar N, 2013. Clinical manifestations, treatment, and outcome of hospitalized patients with Plasmodium vivax malaria in two Indian states: a retrospective study. Malar Res Treat 2013: 341862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrade BB, Reis-Filho A, Souza-Neto SM, Clarêncio J, Camargo LMA, Barral A, Barral-Netto M, 2010. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nurleila S, Syafruddin D, Elyazar IRF, Baird JK, 2012. Serious and fatal illness associated with falciparum and vivax malaria among patients admitted to hospital at west Sumba in eastern Indonesia. Am J Trop Med Hyg 87: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh S, Singh R, Ahmad N, 2013. Complications of vivax malaria in Uttarakhand, India. Int J Res Med Sci 1: 532–535. [Google Scholar]
- 57.Kotwal RS, Wenzel RB, Sterling RA, Porter WD, Jordan NN, Petruccelli BP, 2005. An outbreak of malaria in US Army Rangers returning from Afghanistan. JAMA 293: 212–216. [DOI] [PubMed] [Google Scholar]
- 58.Habib AG, Singh KS, 2004. Respiratory distress in nonimmune adults with imported malaria. Infection 32: 356–359. [DOI] [PubMed] [Google Scholar]
- 59.Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A, 2005. Plasmodium vivax malaria. Emerg Infect Dis 11: 132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma A, Khanduri U, 2009. How benign is benign tertian malaria? J Vector Borne Dis 46: 141–144. [PubMed] [Google Scholar]
- 61.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, Kochar A, Khatri MP, Gupta V, 2009. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg 80: 194–198. [PubMed] [Google Scholar]
- 62.Nayak KC, Kumar S, Tanwar RS, Kulkarni V, Gupta A, Sharma P, Sirohi P, Ratan P, 2011 A study on pulmonary manifestations in patients with malaria from northwestern India (Bikaner). J Vector Borne Dis 48: 219–223. [PubMed] [Google Scholar]
- 63.Srivastava S, Ahmad S, Shirazi N, Kumar Verma S, Puri P, 2011. Retrospective analysis of vivax malaria patients presenting to tertiary referral centre of Uttarakhand. Acta Trop 117: 82–85. [DOI] [PubMed] [Google Scholar]
- 64.Limaye CS, Londhey VA, Nabar ST, 2012. The study of complications of vivax malaria in comparison with falciparum malaria in Mumbai. J Assoc Physicians India 60: 15–18. [PubMed] [Google Scholar]
- 65.Mehmood A, Ejaz K, Ahmed T, 2012. Severity of Plasmodium vivax malaria in Karachi: a cross-sectional study. J Infect Dev Ctries 6: 664–670. [DOI] [PubMed] [Google Scholar]
- 66.Barber BE, William T, Grigg MJ, Menon J, Auburn S, Marfurt J, Anstey NM, Yeo TW, 2013. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis 56: 383–397. [DOI] [PubMed] [Google Scholar]
- 67.Rizvi I, Tripathi D, Zaman S, Zaidi N, Chughtai A, Beg M, 2013. Complications associated with Plasmodium vivax malaria: a retrospective study from a tertiary care hospital based in western Uttar Pradesh, India. Ann Afr Med 12: 155. [DOI] [PubMed] [Google Scholar]
- 68.Quispe AM, Pozo E, Guerrero E, Durand S, Baldeviano GC, Edgel KA, Graf PCF, Lescano AG, 2014. Plasmodium vivax hospitalizations in a monoendemic malaria region: severe vivax malaria? Am J Trop Med Hyg 91: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saravu K, Rishikesh K, Kamath A, Shastry AB, 2014. Severity in Plasmodium vivax malaria claiming global vigilance and exploration—a tertiary care centre-based cohort study. Malar J 13: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muley A, Lakhani J, Bhirud S, Patel A, 2014. Thrombocytopenia in Plasmodium vivax malaria: how significant? J Trop Med 2014: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.George P, Alexander LM, 2010. A study on the clinical profile of complicated Plasmodium vivax mono-infections. Asian Pac J Trop Med 3: 560–562. [Google Scholar]
- 72.Jain A, Kaushik R, Kaushik RM, 2016. Malarial hepatopathy: clinical profile and association with other malarial complications. Acta Trop 159: 95–105. [DOI] [PubMed] [Google Scholar]
- 73.Chung SJ, Low JGH, Wijaya L, 2014. Malaria in a tertiary hospital in Singapore–clinical presentation, treatment and outcome: an eleven year retrospective review. Travel Med Infect Dis 12 (6 Pt B): 738–744. [DOI] [PubMed] [Google Scholar]
- 74.Nayak KC, Khatri MP, Gupta BK, Sirohi P, Choudhary V, Verma SK, Beniwal S, 2009. Spectrum of vivax malaria in pregnancy and its outcome: a hospital-based study. J Vector Borne Dis 46: 299–302. [PubMed] [Google Scholar]
- 75.Gupta H, Afsal MP, Shetty SM, Satyamoorthy K, Umakanth S, 2015. Plasmodium vivax infection causes acute respiratory distress syndrome: a case report. J Infect Dev Ctries 9: 910–913. [DOI] [PubMed] [Google Scholar]
- 76.McGready R, Wongsaen K, Chu CS, Tun NW, Chotivanich K, White NJ, Nosten F, 2014. Uncomplicated Plasmodium vivax malaria in pregnancy associated with mortality from acute respiratory distress syndrome. Malar J 13: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gougoutsi A, Karageorgopoulos DE, Dimitriadou A, Melas N, Kranidiotis G, Voutsinas D, Melidonis A, 2014. Severe Plasmodium vivax malaria complicated with acute respiratory distress syndrome: a case associated with focal autochthonous transmission in Greece. Vector Borne Zoonotic Dis 14: 378–381. [DOI] [PubMed] [Google Scholar]
- 78.Lee H-J, Baek J-H, Chae M-H, Joo H, Lee J-S, Chung M-H, Park Y-K, Kim J-T, 2013. A case of vivax malaria complicated by adult respiratory distress syndrome and successful management with extracorporeal membrane oxygenation. Korean J Parasitol 51: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chalkias A, Aridas S, Karageorgopoulos DE, Stratiotis G, Mystrioti D, Mallios A, Nakos I, Mpellos N, Ganotopoulou A, Xanthos T, 2013. Severe sepsis and septic shock due to Plasmodium vivax infection. Am J Emerg Med 31: 761.e1–761.e2. [DOI] [PubMed] [Google Scholar]
- 80.Ab Rahman AK, Sulaiman FN, 2013. Plasmodium vivax malaria presenting as acute respiratory distress syndrome: a case report. Trop Doct 43: 83–85. [DOI] [PubMed] [Google Scholar]
- 81.Atam V, Singh AS, Yathish BE, Das L, 2013. Acute pancreatitis and acute respiratory distress syndrome complicating Plasmodium vivax malaria. J Vector Borne Dis 50: 151–154. [PubMed] [Google Scholar]
- 82.Sonkar SK, Uniyal R, Sonkar GK, 2011. Three unusual presentations of Plasmodium vivax malaria. Trop Doct 41: 240–241. [DOI] [PubMed] [Google Scholar]
- 83.Smereck J, 2011. Malaria in pregnancy: update on emergency management. J Emerg Med 40: 393–396. [DOI] [PubMed] [Google Scholar]
- 84.Flower B, Armstrong-James D, Dance C, Bremner F, Doherty T, 2011. Blind, breathless, and paralysed from benign malaria. Lancet 377: 438. [DOI] [PubMed] [Google Scholar]
- 85.Sarkar S, Saha K, Das CS, 2010. Three cases of ARDS: an emerging complication of Plasmodium vivax malaria. Lung India Off organ Indian Chest Soc 27: 154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gera C, Dhanoa J, 2010. Vivax-induced ARDS: report of two cases. J Assoc Physicians India 58: 48–50. [PubMed] [Google Scholar]
- 87.Kaneko-Wada FT, Muñoz-Monroy OE, Hernández-Díaz S, Pescina-Casas J, 2009. Severe neutropenia and acute respiratory distress syndrome secondary to Plasmodium vivax [in Spanish]. Med intensiva 33: 100–101. [DOI] [PubMed] [Google Scholar]
- 88.Fernandez-Becerra C, Pinazo MJ, Gonzalez A, Alonso PL, del Portillo HA, Gascon J, 2009. Increased expression levels of the pvcrt-o and pvmdr1 genes in a patient with severe Plasmodium vivax malaria. Malar J 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kasliwal P, Rao MS, Kujur R, 2009. Plasmodium vivax malaria: an unusual presentation. Crit Care Med 13: 103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar S, Melzer M, Dodds P, Watson J, Ord R, 2007. P. vivax malaria complicated by shock and ARDS. Scand J Infect Dis 39: 255–256. [DOI] [PubMed] [Google Scholar]
- 91.Illamperuma C, Allen BL, 2007. Pulmonary edema due to Plasmodium vivax malaria in an American missionary. Infection 35: 374–376. [DOI] [PubMed] [Google Scholar]
- 92.Agarwal R, Nath A, Gupta D, 2007. Noninvasive ventilation in Plasmodium vivax related ALI/ARDS. Intern Med 46: 2007–2011. [DOI] [PubMed] [Google Scholar]
- 93.Price L, Planche T, Rayner C, Krishna S, 2007. Acute respiratory distress syndrome in Plasmodium vivax malaria: case report and review of the literature. Trans R Soc Trop Med Hyg 101: 655–659. [DOI] [PubMed] [Google Scholar]
- 94.Maguire JD, Fenton ME, Susanti AI, Walker JB, 2007. Plasmodium vivax-associated acute respiratory distress syndrome after extended travel in Afghanistan. Travel Med Infect Dis 5: 301–305. [DOI] [PubMed] [Google Scholar]
- 95.Saleri N, Gulletta M, Matteelli A, Caligaris S, Tomasoni LR, Antonini B, Perandin F, Castelli F, Rodriguez-Morales AJ, Benitez JA, Franco-Paredes C, 2006. Acute respiratory distress syndrome in Plasmodium vivax malaria in traveler returning from Venezuela. J Travel Med 13: 112–113. [DOI] [PubMed] [Google Scholar]
- 96.Lomar A V, Vidal JE, Lomar FP, Barbas CV, de Matos GJ, Boulos M, 2005. Acute respiratory distress syndrome due to vivax malaria: case report and literature review. Braz J Infect Dis 9: 425–430. [DOI] [PubMed] [Google Scholar]
- 97.Lawn SD, Krishna S, Jarvis JN, Joet T, Macallan DC, 2003. Case reports: pernicious complications of benign tertian malaria. Trans R Soc Trop Med Hyg 97: 551–553. [DOI] [PubMed] [Google Scholar]
- 98.Tanios MA, Kogelman L, McGovern B, Hassoun PM, 2001. Acute respiratory distress syndrome complicating Plasmodium vivax malaria. Crit Care Med 29: 665–667. [DOI] [PubMed] [Google Scholar]
- 99.Carlini ME, White AC, Atmar RL, 1999. Vivax malaria complicated by adult respiratory distress syndrome. Clin Infect Dis 28: 1182–1183. [DOI] [PubMed] [Google Scholar]
- 100.Curlin ME, Barat LM, Walsh DK, Granger DL, 1999. Noncardiogenic pulmonary edema during vivax malaria. Clin Infect Dis 28: 1166–1167. [DOI] [PubMed] [Google Scholar]
- 101.Torres JR, Perez H, Postigo MM, Silva JR, 1997. Acute non-cardiogenic lung injury in benign tertian malaria. Lancet 350: 31–32. [DOI] [PubMed] [Google Scholar]
- 102.Munteis E, Mellibovsky L, Márquez MA, Mínguez S, Vázquez E, Díez A, 1997. Pulmonary involvement in a case of Plasmodium vivax malaria. Chest 111: 834–835. [DOI] [PubMed] [Google Scholar]
- 103.Yale SH, Adlakha A, Sebo TJ, Ryu JH, 1993. Bronchiolitis obliterans organizing pneumonia caused by Plasmodium vivax malaria. Chest 104: 1294–1296. [DOI] [PubMed] [Google Scholar]
- 104.Abideen ZU, Qadeer A, Akhtar A, Rasheed A, 2016. Severe acute respiratory distress syndrome secondary to Plasmodium vivax malaria. J Pak Med Assoc 66: 351–353. [PubMed] [Google Scholar]
- 105.Arróspide N, Espinoza MM, Miranda-Choque E, Mayta-Tristán P, Legua P, Cabezas C, 2016. Maternal death from severe malaria due to Plasmodium vivax. Rev Peru Med Exp Salud Publica 33: 368–372. [PubMed] [Google Scholar]
- 106.Tan LKK, Yacoub S, Scott S, Bhagani S, Jacobs M, 2008. Acute lung injury and other serious complications of Plasmodium vivax malaria. Lancet Infect Dis 8: 449–454. [DOI] [PubMed] [Google Scholar]
- 107.Bamanikar A, Dhobale S, Shinde K, 2013. Plasmodium vivax-induced adult respiratory distress syndrome. Med J Dr DY Patil Univ 6: 486. [Google Scholar]
- 108.Günbatar H, Yıl Y, Odabaş ÜD, Kampüsü TM, Van T, Sertoğullarından B, Ekin S, Özbay B, Sünnetcioğlu A, 2013. A case of Plasmodium vivax malaria with respiratory failure. J Clin Exp Invest 4: 226–228. [Google Scholar]
- 109.Gupta S, Bajwa SS, Singh A, Parmar S, 2013. Acute respiratory distress syndrome: a rare clinical presentation of pulmonary involvement in Plasmodium vivax infection. Ann Trop Med Public Heal 6: 361. [Google Scholar]
- 110.Bustos MM, Gómez R, Álvarez CA, Valderrama S, Támara JR, 2014. Adult acute respiratory distress syndrome by Plasmodium vivax. Acta Med Colomb 39: 211–215. [Google Scholar]
- 111.Cogollo González M, Julio Narváez LC, Alvarado Cueto DE. Síndrome de distrés respiratorio agudo por Plasmodium vivax Acta Colombiana de Cuidado Intensivo 15: 330–332. [Google Scholar]
- 112.Gupta P, Talreja V, Dhananjaya M, Mittal S, 2014. A case of acute respiratory distress syndrome with Plasmodium vivax malaria. Tanta Med J 42: 151. [Google Scholar]
- 113.Sundriyal D, Kumar N, Chandrasekharan A, Sharma B, Patnaik I, Kamble U, 2013. Fatal complications of Plasmodium vivax malaria: a series of three case reports. Ann Trop Med Public Heal 6: 578. [Google Scholar]
- 114.Mukherjee T, Lavania AK, 2008. Acute respiratory distress syndrome due to vivax malaria. Med J Armed Forces India 64: 365–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martínez O, 1996. Síndrome de dificultad respiratoria aguda en por Plasmodium vivax. Acta Médica Colomb 21: 146–150. [Google Scholar]
- 116.Spudick JM, Garcia LS, Graham DM, Haake DA, 2005. Diagnostic and therapeutic pitfalls associated with primaquine-tolerant Plasmodium vivax. J Clin Microbiol 43: 978–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mohin G, Gupta A, 2007. Rare case of multiorgan failure associated with Plasmodium vivax malaria. Infect Dis Clin Pract 15: 209–212. [Google Scholar]
- 118.Perren A, Beretta F, Schubarth P, 1998. ARDS in Plasmodium vivax malaria Schweiz Med Wochenschr 128: 1020–1023. [PubMed] [Google Scholar]
- 119.Douglas NM, Pontororing GJ, Lampah DA, Yeo TW, Kenangalem E, Poespoprodjo J, Ralph AP, Bangs MJ, Sugiarto P, Anstey NM, Price RN, 2014. Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua, Indonesia. BMC Med 12: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lacerda MVG, Fragoso SCP, Alecrim MGC, Alexandre MAA, Magalhães BML, Siqueira AM, Ferreira LCL, Araújo JR, Mourão MPG, Ferrer M, Castillo P, Martin-Jaular L, Fernandez-Becerra C, Del Portillo H, Ordi J, Alonso PL, Bassat Q, 2012. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis an Off Publ Infect Dis Soc Am 55: 67–74. [DOI] [PubMed] [Google Scholar]
- 121.Anstey NM, Handojo T, Pain MCF, Kenangalem E, Tjitra E, Price RN, Maguire GP, 2007. Lung injury in vivax malaria: pathophysiological evidence for pulmonary vascular sequestration and posttreatment alveolar-capillary inflammation. J Infect Dis 195: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leclerc Y, Verreault J, Bisson G, 1989. Diffuse lung uptake of technetium-99m sulfur colloid in malaria. J Nucl Med 30: 117–119. [PubMed] [Google Scholar]
- 123.WHO—World Health Organization, 1990. Severe and complicated malaria. World Health Organization, Division of Control of Tropical Diseases. Trans R Soc Trop Med Hyg 84 (Suppl 2): 1–65. [PubMed] [Google Scholar]
- 124.WHO, 2000. Severe falciparum malaria. Trans R Soc Trop Med Hyg 94 (Suppl 1): S1–S90. [PubMed] [Google Scholar]
- 125.WHO, 2010.. Guidelines for the Treatment of Malaria, 2nd edition. Geneva, Switzerland: WHO. [Google Scholar]
- 126.WHO, 2014. Severe malaria. Trop Med Int Health 19 (Suppl 1): 7–131. [DOI] [PubMed] [Google Scholar]
- 127.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS; ARDS Definition Task Force, 2012. Acute respiratory distress syndrome. JAMA 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 128.Riviello ED, Kiviri W, Twagirumugabe T, Mueller A, Banner-Goodspeed VM, Officer L, Novack V, Mutumwinka M, Talmor DS, Fowler RA, 2016. Hospital incidence and outcomes of the acute respiratory distress syndrome using the kigali modification of the Berlin definition. Am J Respir Crit Care Med 193: 52–59. [DOI] [PubMed] [Google Scholar]
- 129.Han C, Rice MW, Cai D, 2016. Neuroinflammatory and autonomic mechanisms in diabetes and hypertension. Am J Physiol Endocrinol Metab 311: E32–E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Anstey NM, Russell B, Yeo TW, Price RN, 2009. The pathophysiology of vivax malaria. Trends Parasitol 25: 220–227. [DOI] [PubMed] [Google Scholar]
- 131.Yeo TW, Lampah DA, Tjitra E, Piera K, Gitawati R, Kenangalem E, Price RN, Anstey NM, 2010. Greater endothelial activation, Weibel-Palade body release and host inflammatory response to Plasmodium vivax, compared with Plasmodium falciparum: a prospective study in Papua, Indonesia. J Infect Dis 202: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barber BE, William T, Grigg MJ, Parameswaran U, Piera KA, Price RN, Yeo TW, Anstey NM, 2015. Parasite biomass-related inflammation, endothelial activation, microvascular dysfunction and disease severity in vivax malaria. PLoS Pathog 11: e1004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hemmer CJ, Holst FGE, Kern P, Chiwakata CB, Dietrich M, Reisinger EC, 2006. Stronger host response per parasitized erythrocyte in Plasmodium vivax or ovale than in Plasmodium falciparum malaria. Trop Med Int Health 11: 817–823. [DOI] [PubMed] [Google Scholar]
- 134.Mokra D, Kosutova P, 2015. Biomarkers in acute lung injury. Respir Physiol Neurobiol 209: 52–58. [DOI] [PubMed] [Google Scholar]
- 135.Krishnan A, Karnad DR, 2003. Severe falciparum malaria: an important cause of multiple organ failure in Indian intensive care unit patients. Crit Care Med 31: 2278–2284. [DOI] [PubMed] [Google Scholar]
- 136.Anstey NM, Jacups SP, Cain T, Pearson T, Ziesing PJ, Fisher DA, Currie BJ, Marks PJ, Maguire GP, 2002. Pulmonary manifestations of uncomplicated falciparum and vivax malaria: cough, small airways obstruction, impaired gas transfer, and increased pulmonary phagocytic activity. J Infect Dis 185: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 137.Maguire GP, Handojo T, Pain MCF, Kenangalem E, Price RN, Tjitra E, Anstey NM, 2005. Lung injury in uncomplicated and severe falciparum malaria: a longitudinal study in papua, Indonesia. J Infect Dis 192: 1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carvalho BO, Lopes SCP, Nogueira PA, Orlandi PP, Bargieri DY, Blanco YC, Mamoni R, Leite JA, Rodrigues MM, Soares IS, Oliveira TR, Wunderlich G, Lacerda MVG, del Portillo H a, Araújo MOG, Russell B, Suwanarusk R, Snounou G, Rénia L, Costa FTM, 2010. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis 202: 638–647. [DOI] [PubMed] [Google Scholar]
- 139.Lopes SCP, Albrecht L, Carvalho BO, Siqueira AM, Thomson-Luque R, Nogueira PA, Fernandez-Becerra C, Del Portillo HA, Russell BM, Rénia L, Lacerda MVG, Costa FTM, 2014. Paucity of Plasmodium vivax mature schizonts in peripheral blood is associated with their increased cytoadhesive potential. J Infect Dis 209: 1403–1407. [DOI] [PubMed] [Google Scholar]
- 140.Marks M, Gupta-Wright A, Doherty JF, Singer M, Walker D, 2014.. Managing malaria in the intensive care unit. Br J Anaesth 113: 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Londhe C, Ganeriwal A, deSouza R, 2014. Study of clinical profile of acute respiratory distress syndrome and acute lung injury in Plasmodium vivax malaria. J Vector Borne Dis 51: 339–342. [PubMed] [Google Scholar]
- 142.Brooks MH, Kiel FW, Sheehy TW, Barry KG, 1968. Acute pulmonary edema in falciparum malaria. N Engl J Med 279: 732–737. [Google Scholar]
- 143.Duarte MI, Corbett CE, Boulos M, Amato Neto V, 1985. Ultrastructure of the lung in falciparum malaria. Am J Trop Med Hyg 34: 31–35. [DOI] [PubMed] [Google Scholar]
- 144.Macpherson GG, Warrell MJ, 1985. Human cerebral malaria: a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol 119: 385–401. [PMC free article] [PubMed] [Google Scholar]
- 145.Taylor WRJ, Hanson J, Turner GDH, White NJ, Dondorp AM, 2012. Respiratory manifestations of malaria. Chest 142: 492–505. [DOI] [PubMed] [Google Scholar]
- 146.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R, 1994. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824. [DOI] [PubMed] [Google Scholar]
- 147.WHO, 2012. Management of Severe Malaria: A Practical Handbook, 3rd edition. Geneva, Switzerland: WHO Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.