Abstract
Importance
Psychological stress contributes to numerous diseases and may do so in part through damage to telomeres, protective non-coding segments on the ends of chromosomes.
Objective
We conducted a systematic review and meta-analysis to determine the association between self-reported, perceived psychological stress (PS) and telomere length (TL).
Data Sources
We searched 3 databases (PubMed, PsycInfo, and Scopus), completed manual searches of published and unpublished studies, and contacted all study authors to obtain potentially relevant data.
Study Selection
Two independent reviewers assessed studies for original research measuring (but not necessarily reporting the correlation between) PS and TL in human subjects. 23 studies met inclusion criteria; 22 (totaling 8,948 subjects) could be meta-analyzed.
Data Extraction and Synthesis
We assessed study quality using modified MINORS criteria. Since not all included studies reported PS-TL correlations, we obtained them via direct calculation from author-provided data (7 studies), contact with authors (14 studies), or extraction from the published article (1 study).
Main Outcomes and Measures
We conducted random-effects meta-analysis on our primary outcome, the age-adjusted PS-TL correlation. We investigated potential confounders and moderators (sex, life stress exposure, and PS measure validation) via post hoc subset analyses and meta-regression.
Results
Increased PS was associated with a very small decrease in TL (n = 8,724 total; r = −0.06; 95% CI: −0.10, −0.008; p = 0.01; α = 0.025), adjusting for age. This relationship was similar between sexes and within studies using validated measures of PS, and marginally (nonsignificantly) stronger among samples recruited for stress exposure (r = −0.13; vs. general samples: b = −0.11; 95% CI: −0.27, 0.01; p = 0.05; α = 0.013). Publication bias may exist; correcting for its effects attenuated the relationship.
Conclusions and Relevance
Our analysis finds a very small, statistically significant relationship between increased PS (as measured over the past month) and decreased TL that may reflect publication bias. The association may be stronger with known major stressors and is similar in magnitude to that noted between obesity and TL. All included studies used single measures of short-term stress; the literature suggests long-term chronic stress may have a larger cumulative effect. Future research should assess for potential confounders and use longitudinal, multidimensional models of stress.
1. INTRODUCTION
Unmanaged psychological stress is pervasive in many modern societies. American adults consistently report moderately high levels of stress, with 53% believing they have experienced personal health problems as a result of stress and only 29% reporting that they are doing a ''very good'' or ''excellent'' job of managing or reducing stress1. Self-reported stress has increased in nearly every demographic between 1983 and 20092.
High levels of chronic stress are associated with numerous diseases and deleterious conditions, including obesity and abdominal fat deposition3, metabolic syndrome4, respiratory infection5, immune compromise6,7, cardiovascular disease8, systemic inflammation9–11, respiratory impairment12, tumor growth7, and dendritic shortening in the hippocampus and prefrontal cortex13. Mouse models have demonstrated that catecholamine stimulation (simulating the hormonal effects of chronic stress) causes systemic damage to chromosomes14.
Telomeres are non-coding, repetitive nucleotide segments on the ends of each mammalian chromosome that serve a protective role during DNA transcription. A small number of base pairs at the ends of a chromosome are lost during each transcription, resulting in an overall shortening of the chromosome after many duplications. Telomeres therefore serve as a protective “buffer” to prevent the truncation of functional coding segments during duplication. Although telomeres are routinely replenished by telomerase, their gradual attrition over the lifespan may contribute to disease. Recent studies have explored the relationship between telomere length and health15 and found short telomeres to be a risk factor for many diseases of aging, including cancer16, cardio-metabolic dysfunction17, and diabetes18.
Stressed, depressed, anxious, or previously traumatized individuals may have shorter telomeres than their psychologically healthy counterparts15,19–23. For example, recent stressor exposure within the last five years (but not earlier)24 as well as chronic social stress25 are associated with shorter telomeres. Since the first study documenting a relationship between telomere length and perceived stress15, many studies measuring telomere length have included a measure of psychological stress; however, relatively few have reported the effects of perceived stress on telomere length.
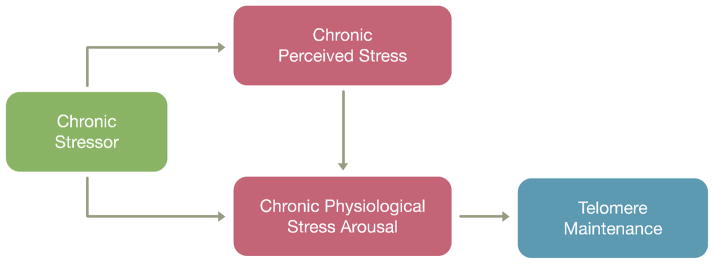
Stress is not a unitary construct, but rather comprises exposure to stressors, perception of stress, and the physiological stress response. Exposure to chronic stressors (such as domestic abuse) may provoke sustained physiological stress arousal, which in turn could impact telomere biology (Fig. 1). Indeed, experimental research in animal models and epidemiological research in humans suggests that central elements of the physiological stress response (namely cortisol exposure and individual cortisol reactivity) are associated with shortened telomeres26–28. The presence of a chronic stressor potentially indicates that an individual’s current perceived stress is reflective of a chronic, rather than short-lived, psychological state. Thus, the conceptual model in Fig. 1 predicts that perceived stress may be more strongly related to telomere length in the presence of a chronic stressor, a possibility addressed in the present analysis.
Fig 1. The Stress Triad and Telomere Maintenance.
Chronic major stressor exposures can lead to chronically high levels of perceived stress and subsequent stress arousal. In turn, chronic stress arousal is hypothesized to proximally impact telomere maintenance. To the extent that perceived stress over the month reflects a chronic state, and is related to stress arousal, there may be a relationship between perception and telomere length.
A previous meta-analysis including only a small number of studies that explicitly reported correlations between perceived stress and telomere length detected publication bias and called for additional research29. Additional methodological limitations motivate the present analysis. For example, 2 studies in the previous analysis shared subjects15,28, resulting in double-counting. Finally, statistically distinct effect sizes (for example, correlations adjusting for different sets of covariates) were synthesized, resulting in pooled point estimates with limited interpretability. We aimed to build on these preliminary results by conducting a more exhaustive review of the existing literature, addressing the methodological challenges of the prior analysis.
An additional, novel objective was to assess demographic and methodological factors that may confound or moderate the PS-TL relationship. First, psychometric validity and reliability vary across measures of PS; for example, conceptually distinct but associated constructs such as negative affect and trait neuroticism may contaminate PS measurement30. Second, because females tend to have longer telomeres31, but higher PS2, than males, sex could act as a suppressor or moderator variable. Third, the PS-TL relationship may differ substantially for subjects with a known major stressor or a physical health condition, both of which may be associated with PS and TL32–34. We quantitatively investigated these possible effects using meta-regressive methods and subset analyses.
In order to include both the growing published literature on perceived stress and telomere length as well as data not previously synthesized meta-analytically, we performed a systematic review using a comprehensive search strategy and inclusion criteria directed at capturing unreported correlations, unpublished data, and recent additions to the literature. We quantitatively meta-analyzed the association of perceived stress with telomere length.
2. METHODS
2.1. Data Sources and Searches
We systematically searched PubMed, PsycInfo, and Scopus from inception to April 2015 to identify all studies published in any language collecting any measure of telomere length (TL) and self-reported, perceived psychological stress (PS) in human subjects. We developed search strategiesa in consultation with a professional reference librarian; the search strings captured PS via terms including stress, dysthymia, anxiety, and trauma and captured TL via terms including telomere, oxidative stress, and cell aging. Search terms were deliberately broad in order to capture all potentially relevant articles; a later review process (detailed below) excluded the numerous articles failing to meet specific inclusion criteria.
We reviewed included articles' references for potentially relevant articles that had not been captured in the database search and conducted manual searches for published and unpublished studies in consultation with an experienced researcher in the field (EE). We conducted the final search on April 3, 2015.
2.2. Study Selection
Among the articles of any design and written in any language retrieved from the initial, broad database search, we included in analysis studies that: (1) represented original research (excluding systematic reviews, narratives, meta-analyses, etc.); (2) used human subjects (excluding animal and in vitro studies); (3) measured both TL and self-reported PS for at least a subset of subjects. We included studies measuring PS only as a covariate, even when the association between PS and TL was not reported.
2.3. Data Extraction and Quality Assessment
We performed article screening using web-based systematic review software DistillerSR (Evidence Partners, Ottawa, Canada). Two investigators (MM and SK) independently assessed each article against the inclusion criteria, resolving disagreements through re-review, discussion, and arbitration by a referee (NK). Both investigators first assessed only titles and abstracts in order to exclude articles lacking a measure of TL. For the remaining articles, the investigators then obtained full texts as necessary to assess the remainder of the inclusion criteria. We assessed methodological quality of eligible studies using modified MINORS criteria35, assessing clarity of aims and inclusion criteria, description of a priori data collection and analysis plans, prospective calculation of sample sizes, quality of the PS and TL variables, blinded assessment of TL, and reporting of missing data.
2.4. Data Collection
Two investigators (MM and EE) contacted authors for each eligible study to request summary statistics or preferably raw data, including measures of TL, PS, age, and sex. For the studies for which we obtained raw data, MM re-analyzed the raw data to confirm published statistics related to the PS-TL relationship, resolving any discrepancies through discussion with the authors. Summary measures included the raw correlation between PS and TL, the partial correlation adjusting for age (henceforth “age-adjusted”), and the age- and sex-adjusted correlation, as well as the age-adjusted, sex-stratified correlation. When no endpoints of interest nor mathematically equivalent statistics were available, we searched for other relevant statistics on the relationship between PS and TL (such as rank correlations) for qualitative description. Finally, for eligible studies sharing an author, we verified with authors whether there was any duplication of subjects between studies and used this information to eliminate duplicated data.
2.5. Protocol Modifications
We clarified the inclusion criteria post hoc in the following instances: (1) 3 studies measured domain-specific stress (stress specifically related to the duties of a schoolteacher36, stress in 8 domains [such as career- and relationships-related]37, and instantaneous state stress immediately before exposure to a laboratory stressor38). Because a priori inclusion criteria did not adequately address eligibility of such measures, we excluded these studies, further refining the inclusion criterion to include only studies measuring global perceived stress; (2) 1 study39 employed the Global Health Questionnaire (GHQ-12) measure. Upon reviewing and excluding a later article that included a more detailed description of the GHQ-12 measure, reviewers agreed that this measure did not meet inclusion criteria, and this study was excluded; (3) 1 article identified via manual search40 made no mention of PS, but was included because author EE was aware the study had collected PS measures based on other publications from the study.
2.6. A Priori Endpoints
The primary outcome and predictor variables of interest were TL and PS; the primary analytic goal was to synthesize data on their relationship. Because TL declines with age41,42, we assessed age as a covariate and likely confounder by additionally estimating the age-adjusted partial correlation between PS and TL through meta-analytic methods. Thus, we specified 2 endpoints a priori: (1) the age-adjusted Pearson correlation between PS and TL (primary); and (2) the raw, unadjusted Pearson correlation between PS and TL (secondary).
2.7. Moderator and Subgroup Analyses
As described in the Introduction, demographic variables such as sex and past exposure to a major stressor, as well as methodological factors such as psychometric quality of PS measures, may confound or moderate the PS-TL relationship. Based on these hypothesized effects, we investigated 4 post hoc endpoints and subset analyses: (1) We examined the age-adjusted PS-TL correlation among only studies employing an empirically validated measure of PS. All subsequent secondary analyses were also conducted among only this subset of studies; (2) We examined the age- and sex-adjusted PS-TL correlation and the moderation effect of sex; (3) We investigated possible effects of sample heterogeneity by characterizing the PS-TL relationship according to the type of sample enrolled using 3 mutually exclusive categories: "General samples" included those not specifically selected for physical health conditions or stress exposures (these samples mostly comprised healthy adults, but subjects with physical health conditions or stress exposures were not excluded); "stress-exposed samples" included those selected for exposure (past or present) to a major stressor such as traumatic events or caregiving responsibilities for those with medical illness (this category included studies enrolling both stressed and control subjects); “physical condition samples” included those selected for the presence of a disease or other physical condition.
We did not exclude control subjects from studies recruiting stressed samples and samples with physical conditions. An alternative approach of including only samples with homogenous stressor exposure would severely limit power and could produce artificial range restriction43. The ideal approach, namely using subject-level data to classify subjects by stressor exposure, was not possible given limitations in data availability. A caveat of our classification approach is potentially increased heterogeneity among “stressor-exposed” samples due to the inclusion of control subjects. Additionally, limited availability of raw data precluded assessment of the subject-level relationship between stressor exposure and PS.
Many such studies involved diseases known to be comorbid with stress or depression. In addition, in the samples that did not recruit specifically for a disease group, we coded whether there were exclusion criteria to rule out major diseases such as cardiovascular disease, diabetes, or cancer. Because specific physical conditions may have strong effects on telomere biology, these samples were not included in the stress-exposed category (which in most cases were healthy samples where major diseases were excluded; Table 1).
Table 1.
Characteristics of All Eligible Studies
| Study | Sample demogra phicsa |
Sex es enr olle d |
Sam ple class ificat ion |
Ma jor dis eas e exc lud ed |
Main endpo int |
Perc eived stres s meas ure |
Dat a sou rce |
T L as sa y ty p e |
TL cell type |
TL assay CV |
Age - adj uste d corr elati on |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bersani, et al. (in prep) | Male combat veterans, some with PTSD (n=76) | Males | Stressed | No | Association of psychiatric measures with TL | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (PBMC) | 4.00% | −0.3 |
| Buss J, et al. (2014) | Females, overweight or obese (n=42) | Females | Physical condition | No | Associations of eating behaviors and metabolic profile with TL | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (leukocyte) | 4.00% | 0.01 |
| Carlson, LE, et al. (2014) | Female breast cancer survivors, distressed (n=87) | Females | Physical condition | No | Effect of psychosocial interventions (randomized) on TL | Symptoms of Stress Scale (C- SOSI , 56- item) | Summary statistics from author | PCR | Blood (leukocyte) | Not calculated | −0.1 |
| Chen, X, et al. (2015) | Adults, caregivers of disabled children (n=89) | Both | Stressed | No | Association of smoking and PS with TL | Perceived Stress Scale (14- item) | Summary statistics from author | PCR | Salivary | 11.00% | − 0.07 |
| Entringer, S, et al. (2011) | Adults, some whose mothers were psychologically stressed during pregnancy (n=98) | Both | Stressed | Yes | Association of maternal stress status with offspring’s TL | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (leukocyte) | 4.00% | − 0.15 |
| Epel, ES, et al. (2004) | Females, premenopausal mothers with chronically ill child and premenopausal normal mothers (n=57) | Females | Stressed | Yes | Association of PS and caregiving status with TL | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (PBMC) | Not calculated | − 0.31 |
| Epel, ES, et al. (2012) | Females, healthy with high education levels and low stress (n=258) | Females | General | No | Association of mind- wandering with TL | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (PBMC) | 4.00% | − 0.09 |
| Georgin-Lavialle, S, et al. (2013) | Adults, mastocytosis patients, 79% with some grade of depression (n=19) | Both | Physical condition | No | Association of negative emotionality with TL | Perceived Stress Scale (14- item) | Data from author | PCR | Blood (PBMC) | Not calculated | − 0.73 |
| Geronimus, A, et al. (2010) | Females, middle- aged, premenopausal, half Black (n=215) | Females | General | No | Differences in TL between black and white subjects | Perceived Stress Scale (4- item) | Published article | PCR | Blood (PBMC) | 4.50% | N/A |
| Hassett, AL, et al. (2012) | Females, fibromyalgia (n=61) | Females | Physical condition | Yes | Association of pain with TL | Perceived Stress Scale (4- items ) | Summary statistics from author | PCR | Blood (leukocyte) | 3.00% | − 0.21 |
| Hoen, PW, et al. (2010) | Adults, stable coronary heart disease, many with major depression (n=949) | Both | Physical condition | No | Association of depression with TL | Perceived Stress Scale (4- item) | Summary statistics from author | PCR | Blood (leukocyte) | 3.70% | − 0.06 |
| Humphreys, J, et al. (2012) | Females, formerly abused and non- abused (n=102) | Females | Stressed | Yes | Association of abuse status with TL | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (PBMC) | 3.50% | − 0.025 |
| Ludlow, AT, et al. (2008) | Adults, middle- to older- aged (n=60) | Both | General | No | Association of physic al activity with TL | Perceived Stress Scale (10- item) | Data from author | PCR | Blood (PBMC) | 5% | − 0.03 |
| NIEHS Sister Study 1 (b) | Females, middle- aged, sisters have breast cancer (n=1,085) | Females | General | No | Causes and sequelae of breast cancer | Perceived Stress Scale (4- item) | Data from author | PCR | Blood (leukocyte) | 11.00% | 0 |
| NIEHS Sister Study 2 (b) | Females, middle- aged, sisters have breast cancer (n=632) | Females | General | No | Causes and sequelae of breast cancer | Perceived Stress Scale (4- item) | Data from author | PCR | Blood (leukocyte) | 8.50% | − 0.04 |
| Prather, A, et al. (2015) | Adults, obese (n=87) | Both | Physical condition | Yes | Association of sleep quality with multiple TL measures | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (PBMC) | 4.00% | 0.03 |
| Puterman, E, et al. (2010) | Females, post- menopausal, some dementia caregiver s (n=58) | Females | Stressed | Yes | Interaction of exercise with PS-TL relationship | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (leukocyte) | 4.00% | − 0.26 |
| Sibille, KT, et al. (2012) | Adults, chronic pain from knee osteoarthritis and controls (n=36) | Both | Physical condition | No | Association of chronic pain and PS with TL | Perceived Stress Scale (10- item) | Summary statistics from author | PCR | Blood (PBMC) | 7.50% | − 0.13 |
| Surtee s, PG, et al. (2011) | Females, middle- aged, White, probability sample of Norfolk residents (n=4,353) | Females | General | No | Association of PS and emotional health with TL | Single 5- point item: perceived stress over past 10 years | Data from author | PCR | Blood (lymphocyte) | Not calculated | − 0.02 |
| Tyrka, AR, et al. (2009) | Adults, some with severe childhood abuse and non- abused controls (n=31) | Both | Stressed | Yes | Association of childhood abuse with TL | Perceived Stress Scale (14- item) | Data from author | PCR | Blood (leukocyte) | 4.30% | 0.17 |
| Tyrka, AR, et al. (2015) | Adults, history of psychopathology and/or childhood adversity and controls (n=289) | Both | Stressed | Yes | Association of psychopathology and childhood adversity with TL | Perceived Stress Scale (14- item) | Summary statistics from author | PCR | Blood (leukocyte) | 4.54% | −0.1 |
| Uchino, BN, et al. (2012) | Adults, middle- to older- age (n=136) | Both | General | No | Association of social relationship type with TL | Perceived Stress Scale (10- item) | Data from author | PCR | Blood (PBMC) | 3.10% | 0.02 |
| Wikgren, M, et al. (2012) | Adults, national probability sample of Swedish general population (n=129) | Both | General | No | Association of depression status and hypocortisolemic state with TL | Perceived Stress Questionnaire (30- item) | Published article (Sp ear man correlationonl y) | PCR | Blood (leukocyte) | 6.00% | − 0.26 |
TL assay CV = inter-assay coefficient of variation of T/S ratio.
Sample sizes and demographics represent only those subjects with data for both PS and TL. For example, some studies enrolled from additional demographics but collected PS and TL on only a subset of subjects, while others collected PS and TL for a superset of subjects analyzed in the parent article.
2.8. Comparative Analysis for Limitations of PS Measures
A gold standard predictor variable for stress would accurately measure physiological stress as the most proximal stress-related influence on telomere degradation. Discrepancies between perceived, physiological, and reported stress could mask a stronger effect of proximal physiological factors on telomere shortening. We planned to conduct an exploratory analysis comparing the magnitudes of PS-TL relationships to physiological stress-TL relationships among studies collecting both measures of stress. We reviewed Google Scholar and PubMed using combinations of the search terms psychological stress, perceived stress, physiological stress, and cortisol.
2.9. Statistical Analysis
We conducted all statistical analyses in R (Version 3.1.0, multiple contributors, Vienna, Austria)b. We adjusted analyses for multiple comparisons via the Šidák method, applying a standard family-wise error rate of α = 0.05 to the 2 primary analyses and to the 4 post hoc analyses separately. This yielded an adjusted α = 0.025 for each primary analysis and α = 0.013 for each post hoc analysis. We adjusted all confidence intervals accordingly; p-values are unadjusted and reported with corresponding adjusted α levels. We did not apply multiplicity adjustments to sensitivity analyses.
We preferentially computed effect size measures from raw data; for studies without available raw data, we used summary correlations provided by authors or published effect size measures. Common measures of PS are many-item composite scores that can reasonably be treated as continuous. 1 study44 with available raw data included a covariate corresponding to assay plate; we additionally adjusted for this variable in all correlation measures for this study. We used Fisher's r-to-z transformation for variance stabilization and normalization45 and reconverted all reported results to r scale. We pooled point estimates via linear mixed-effects modeling (allowing random effects by study) estimated via restricted or unrestricted maximum likelihood estimation. Models employed inverse-variance weighting, and we based inference on pooled estimates on the t-distribution using Knapp-Hartung adjusted standard errors (an adjustment to the DerSimonian-Laird method with improved statistical properties)46.
We estimated and tested for between-study effect heterogeneity using (1) Cochran's Q, a weighted sum of squares on a standardized scale and the associated chi-square statistic, and (2) T, the estimated standard deviation of true effects across studies45. Finally, using the available raw data, we visually examined scatterplots to evaluate model assumptions – for example, by assessing the linearity of the relationship between PS and TL and the possible presence of systematically occurring influential outliers.
We used the same modeling approach in post hoc analyses as in main analyses but excluded studies using an unvalidated stress measure. We made the distinction between validated and unvalidated measures post hoc. Therefore, in keeping with our a priori analysis plan, we included both types of measures in primary analyses to avoid inflation of a levels due to post hoc changes to analyses47. For secondary analyses, we also report results of sensitivity analyses in which no studies were excluded, as in primary analyses.
We estimated the pooled, age-adjusted PS-TL correlation among this subset of studies. We further investigated the effect of sex as a confounder by estimating the age- and sex-adjusted pooled correlation. To investigate whether sex might moderate the PS- TL relationship, we stratified study samples by sex and used meta-regression (introducing a fixed covariate effect to the random-effects model); this coefficient represents the estimated difference in PS-TL correlation for females versus males. We used a similar approach to assess whether sample type (general population, samples selected for a physical condition, or samples selected for stress exposure) moderated the PS-TL relationship; the corresponding coefficients represent the difference in point estimates across the 3 types of samples. For each meta-regressive model as well as for a comparable “reduced” model not containing the moderator of interest, we computed Higgins’ residual I2 statistic, which estimates the proportion of residual variance attributable to true inter-study heterogeneity in effect sizes45. A much smaller residual I2 in the full model, compared to the reduced model, would suggest that the moderator variable of interest may have contributed strongly to inter-study effect heterogeneity.
2.10. Sensitivity Analyses for Publication Bias
We used a funnel plot and Egger's test45, a meta-regressive estimate of the association of a study's point estimate with its standard error (SE), to assess for possible publication bias or other systematic effects of sample variability. If the PS-TL relationship is truly stronger for samples recruited for a physical condition or major stressor (which are likely to be smaller in size and higher in SE) than for the general population, such an effect could spuriously produce the appearance of publication bias. Therefore, we also conducted a modified Egger's regression containing fixed-effects of both study SE and sample type. We then used the likelihood-ratio chi-square test to assess whether removing the coefficient for study SE significantly worsened model fit; a significant result would suggest that any tendency of smaller studies to report larger correlations cannot be attributed to differences in sample demographics alone, and would more strongly indicate publication bias. Finally, we used the Duval approach48 to estimate a trimmed-and-filled, age-adjusted point estimate. We planned to conduct an exploratory analysis comparing physiological to perceived stress measures, but limitations of the published literature made this unfeasible.
3. RESULTS
3.1. Study Eligibility and Data Collection
Our literature search retrieved 2,192 potentially relevant articles across all 4 databases (Fig. A.1). We removed 115 duplicated articles. Of the remaining 2,077 unique articles, we excluded 1,620 articles that clearly did not measure TL after abstract and title screening. We found an additional 3 potentially relevant articles40,49,50 via manual search. After abstract or full-text article review of the remaining 460 articles, we excluded 431 that failed to meet all inclusion criteria, leaving 29 relevant articles15,20,21,40,44,49,51–72 that met all inclusion criteria. Inter-rater agreement for study eligibility was 99.5% (κ = 0.86); we resolved 10 disagreements through discussion.
In correspondence with study authors, we identified instances of subject duplication in included studies and excluded an additional 6 articles20,54,55,61,64,70. 1 included study62 used a subset of data from the National Institute of Environmental Health Sciences (NIEHS) "Sister Study." We obtained data directly from NIEHS for all Sister Study subjects with data for PS and TL, resulting in a larger sample size in our analysis than was used in the corresponding paper. Data obtained from the NIEHS represented 2 heterogeneous sub-studies within the Sister Study, which were treated as separate studies ("Sister Study 1"62 and "Sister Study 2"73) in analysis.
We thus identified 23 eligible studies (Table 1). We were able to obtain both of the 2 primary endpoints (age-adjusted or raw PS-TL correlation) for 21 of these, and 1 of the primary endpoints for 1 other study. We could obtain neither endpoint of interest, nor a mathematical equivalent, for 1 study59, which enrolled 129 subjects and reported a significant negative Spearman age-adjusted correlation between PS and TL (r = −0.26, p = 0.0003). Thus, we were able to obtain and meta-analyze at least 1 of the 2 primary summary measures for 22 studies, comprising a total of 8,948 subjects.
3.2. Study Characteristics
Eligible studies enrolled subjects representing a variety of demographics, including special populations such as subjects with past or current psychological stressors (e.g., caregiving duties for an ill relative, childhood or adulthood abuse, or intrauterine stress exposure), subjects with physical health conditions (e.g., knee osteoarthritis, fibromyalgia, or mastocytosis), and subjects with mood disorders (e.g., anxiety or depression). 10 of the studies (45%) enrolled subjects of both sexes, 11 (50%) enrolled only females, and 1 (5%) enrolled only males (Table 1). Study quality was variable; most did not report a priori analysis plans and sample size calculations or occurrence of missing data (Table A.1.). Studies measured telomeres in leukocyte cells (11 studies), peripheral blood mononuclear cells (10 studies), lymphocytes (1 study), and salivary cells including an unspecified combination of cell types (1 study). (To justify pooling across cell types in analysis, we conducted a sensitivity analysis in which we meta-regressed the age-adjusted correlation on cell type [leukocytes vs. PBMC]; this analysis suggested no moderation by cell type.)
All eligible studies measured PS and TL cross-sectionally, although some studies measured additional variables retrospectively or prospectively or involved randomization to an intervention. All but 3 of the studies44,59,67 measured PS using a full or abridged version of the validated Perceived Stress Scale (Methods A.1), in which respondents consider their experiences and feelings over the past month74. 2 studies used other validated measures: the Calgary SOSI index67 or the Perceived Stress Questionnaire59. Another44 used a single 5-point item: "All things considered, how stressful do you believe that your life has been over the past ten years?" All studies measured TL using polymerase chain reaction (PCR) methods75.
3.3. Unadjusted Correlation Between PS and TL
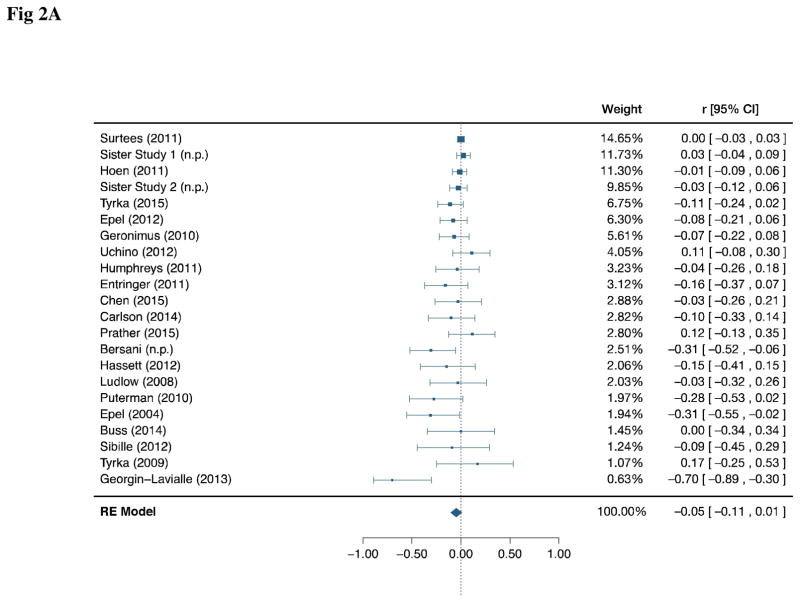
The unadjusted correlation was available for 22 studies and a total of 8,948 subjects (Fig. 2A). 3 studies included in quantitative analysis reported significant negative correlations15,49,58, as well as 1 included in qualitative description59. The rest had nonsignificant point estimates. Visual assessments of available raw data supported modeling the PS-TL relationship as linear. Effect estimates showed significant heterogeneity (Q = 43.0; df = 21; p = 0.003; T = 0.05), suggesting that the true effect may have differed across studies due to, for example, inherent differences in the population sampled. The pooled correlation estimate did not indicate a significant linear relationship between PS and TL (r = −0.05; 95% CI: −0.11, 0.01; p = 0.07; α = 0.025).
Fig 2.
Fig 2A: Forest Plots of Unadjusted Correlation between Perceived Stress and Telomere Length
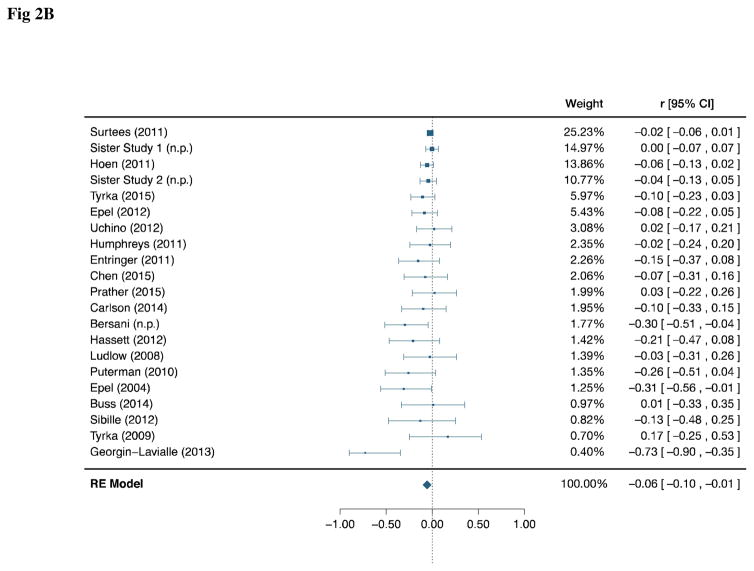
Fig 2B: Forest Plot of Age-Adjusted Correlation between Perceived Stress and Telomere Length
n.p. = not published.
Fig 2A displays the unadjusted correlation. Fig 2B displays the age-adjusted correlation. Studies are displayed in descending order of weight (inverse variance). The pooled confidence interval is corrected for multiplicity between the 2 a priori endpoints.
3.4. Age-Adjusted Correlation Between PS and TL
The age-adjusted correlation was available for 21 studies and a total of 8,724 subjects (Fig. 2B). The same 3 studies reporting a statistically significant unadjusted correlation also reported significant age-adjusted correlations15,49,58, while the rest were null. Unadjusted and age-adjusted point estimates were similar in all studies for which both were available. As in the unadjusted analysis, there was evidence of between-study heterogeneity (Q = 35.1; df = 20; p = 0.02; T = 0.03). Adjusting for age, higher PS was associated with reduced TL (r = −0.06; 95% CI: −0.10, −0.008; p = 0.01; α = 0.025), with a similar effect size to the unadjusted estimate.
3.5. Moderator and Subgroup Analyses
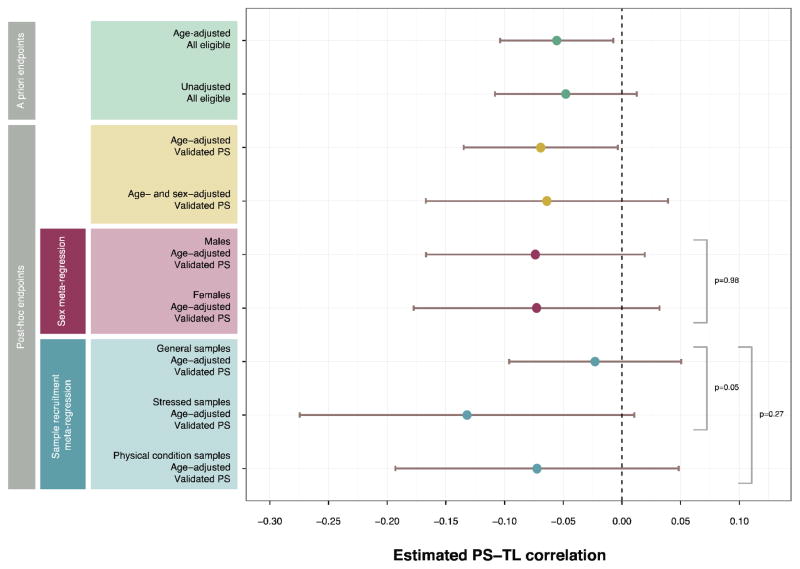
Results of post hoc analyses are displayed in Fig. 3. 1 study44 used an unvalidated measure of PS and was not included in the post hoc analyses. Removing this study did not substantively affect the age-adjusted point estimate (n = 4,371 total, r = −0.07; 95% CI −0.13, −0.004; p = 0.01; α = 0.013). All subsequent post hoc analyses included only studies using validated PS measures.
Figure 3. Pooled Point Estimates from A Priori, Subset, and Moderation Analyses.
For consistency, estimates from meta-regressive models are presented as fitted values rather than coefficients and represent the estimated PS-TL correlation for the relevant group. Thus, plotted confidence intervals correspond to testing for a nonzero correlation within the group of interest rather than a comparison of effect sizes across groups. Bracketed p-values correspond to meta-regressive tests of differences across groups.
To assess potential confounding by sex, we meta-analyzed the age- and sex-adjusted point estimates among the 10 studies enrolling both sexes (n = 1,787 total), again yielding a similar, though nonsignificant, point estimate to primary analyses (r = −0.06; 95% CI −0.17, 0.04; p = 0.09; α = 0.013). A sensitivity analysis including both unvalidated and validated PS measures (as in primary analyses) would yield exactly the same result for this outcome because the single study using an unvalidated measure enrolled only females and therefore would not have contributed a sex-adjusted estimate.
We meta-regressively assessed possible moderation by sex, finding that the PS-TL relationship was similar between sexes (male: r = -0.07; 95% CI: -0.17, 0.02; female: r = −0.07, 95% CI: −0.18, 0.03; female vs. male: b = 0.001; p = 0.98; α = 0.013). The latter coefficient represents the estimated difference in PS-TL correlation between female subsamples and male subsamples. Consistent with the lack of moderation by sex, Higgins’ residual I2 (the estimated proportion of “unexplained” variance that is attributable to true effect heterogeneity) was similar in the null model not containing sex (28.8%) and in the model containing sex (28.5%). A sensitivity analysis in which we included both unvalidated and validated PS measures yielded similar results (male: r = −0.07; 95% CI: −0.17, 0.02; female: r = −0.06, 95% CI: −0.16, 0.05; female vs. male: b = 0.01; p = 0.75; α = 0.013).
Finally, meta-regressing on sample type suggested that the PS-TL relationship was comparable across samples recruited from the general population (r = −0.02; 95% CI: −0.10, 0.05) and in those recruited for a physical condition (r = −0.07; 95% CI: −0.19, 0.05; vs. general: b = −0.05; p = 0.27; α = 0.013). The correlation was marginally, but nonsignificantly, stronger in samples recruited for psychological stress exposure (r = −0.13; 95% CI: −0.27, 0.01; vs. general: b = −0.11; p = 0.05; α = 0.013). Reported correlations and CIs represent the fitted estimates, while coefficients and p-values represent the estimated difference from general samples. Higgins’ residual I2 was reduced from 47.7% in the null model (not containing sex)c to 32.9% in the model containing sex. A sensitivity analysis in which we included both unvalidated and validated PS measures again yielded comparable results (general population: r = −0.02; 95% CI: −0.06, 0.02; physical condition samples: r = −0.13; 95% CI: −0.17, 0.03; vs. general: b = −0.05; p = 0.19; α = 0.013; stress samples: r = −0.13; 95% CI: −0.25, −0.01; vs. general: b = -0.11; p = 0.03; α =0.013).
3.6. Publication Bias
The funnel plot (Fig. A.2) and traditional Egger's test indicated significantly larger point estimates among smaller studies with larger SEs (b = −1.03; 95% CI −1.78, −0.28; p = 0.01). However, as expected, sample size was strongly associated with sample type, with general samples tending to be much larger (median n = 258) than samples recruited for a physical condition (median n = 61) or psychological stressor (median n = 83). Removing study SE from a modified Egger's model did not significantly worsen model fit with the inclusion of fixed effects of sample type (LR = 2.60; p = 0.11), suggesting that the association of SE with effect size may be partly related to systematic differences in sample demographics between small and large studies. Using the Duval trim-and-fill method to correct for publication bias attenuated the correlation to nonsignificance (r = −0.03; 95% CI −0.06, 0.005; p = 0.09; α = 0.025). This suggests that the primary finding may be partly attributable to publication bias or other sample-size effects.
3.7. Comparative Analysis for Limitations of PS Measures
We intended to conduct a comparative analysis of perceived, self-reported PS measures versus physiological measures. However, we found very few relevant studies20,28,61,76, and these reported inconsistent relationships between physiological measures of stress and TL, making our planned analysis unfeasible.
4. DISCUSSION
Given burgeoning scientific interest in relationships between fundamental cellular physiology and psychology, we conducted a systematic review and meta-analysis using both published and unpublished data to examine the relationship between perceived stress and telomere length. We find a very small, significant, negative age-adjusted correlation that was not significant prior to adjustment for age. The relationship may be marginally, but nonsignificantly, stronger in samples with a known major stressor. Post hoc analyses suggest that results are similar when limited to studies using an empirically validated stress measure, between sexes, and between general population samples and those recruited for a medical condition.
A previous meta-analysis on this topic found a stronger negative correlation between perceived stress and telomere length29. As discussed previously, this preliminary analysis had several methodological limitations; our more exhaustive search strategy allowed us to include more than 7,000 additional eligible subjects and resolved the double-counting and statistical issues of the previous analysis. The present, more comprehensive analysis resulted in a smaller pooled effect size than that reported previously.
Our results should be interpreted in light of several statistical and methodological limitations. We found statistically significant heterogeneity in effect estimates across studies, possibly arising from differences in sample demographics. Pooled statistical estimates must therefore be interpreted cautiously, as they average over the entire population from which all the studies are drawn and therefore may not appropriately represent the potentially unique “true effect” within any single study population. Additionally, we characterized the relationship between perceived stress and telomere length using the Pearson correlation because of its widespread availability and because limited raw data suggested its assumptions were generally fulfilled. Using directly comparable effect measures across studies is important for valid quantitative pooling and minimizes subjective influences that could occur with post hoc definition of categories or elimination of apparent outliers. However, this approach means that Pearson assumptions may occasionally have been violated in individual studies. We noted substantial inter-assay coefficients of variation in telomere length measures.
Our results are consistent with publication bias (the “file-drawer effect”)45. To address this, we performed sensitivity analyses correcting for the effect of publication bias, which attenuated the observed age-adjusted relationship. There may be other mechanisms, not reflective of true publication bias, by which sample variability can be associated with effect size. For example, it is possible that smaller studies were less affected by statistical confounding or suppression, as they were more likely to recruit samples homogeneous on confounders such as health conditions17,18. The “p-curve”77, a more precise test of publication bias that does not rely on sample standard error, was not feasible in this case due to the small number of positive findings.
Although we were able to assess moderation effects of several demographic and methodologic factors, other variables known to be associated with telomere length, such as lifestyle factors56,72, health conditions17,18, medications78, and clinical depression79, were reported too infrequently in the literature for meta-analysis. Indeed, individual studies included in our analysis suggested moderation by factors such as smoking66 and physical activity56. We used partial correlations to adjust for age as a known confounder of the PS-TL relationship, but were not able to assess its effect as a possible moderator due to limited availability of individual participant data. Moderation by age could occur if stress effects are cumulative over the lifespan, causing a stronger relationship between perceived stress and telomere length among older versus younger subjects. Alternatively, many age-related diseases are associated with a heightened cortisol response to challenge; thus, older subjects may be more physiologically susceptible to a given stressor than are younger subjects80. Indeed, a study included in this meta-analysis found a relationship between perceived stress and telomere length only among subjects aged at least 55 years61.
Another theoretically challenging extraneous variable is clinical depression. While perceived stress and depression are strongly associated, depression is a more severe state characterized by substantial neurobiological alterations. A past review found clinical diagnosis of depression, but not self-reported depression, to be associated with telomere length, suggesting the possibility of a threshold effect rather than a continuous response81. Additionally, past research has suggested that a history of major depression mediates the relationship between perceived stress and telomere length79; simple covariate adjustment for depression status aimed at reducing confounding may therefore aggravate rather than alleviate bias due to depression82. We recommend that future work use more sophisticated modeling approaches such as structural equation modeling to address bias due to depression.
The very small magnitude of our finding may reflect limitations of perceived stress measures. One of the largest studies of telomere length79 found that stressful event exposure alone (not accounting for perceived stress) predicts telomere length. Additionally, short-term stress may impact telomere biology only briefly79, and longitudinal measures of perceived stress may better capture chronic effects83; elevated perceived stress over a lifetime may play an important role not fully reflected in current telomere literature. In addition to chronic stress effects, severe life stressors and events across the life course (including early life) appear to have long-lasting associations with health effects, including telomere length84–87. Thus, perceived stress over the past month may be limited as a single measure. Ideally, future research should increasingly adopt longitudinal designs rather than the current cross-sectional designs. By measuring multiple stress constructs – perceived stress, physiological stress, and stressful life events – as well as telomere length repeatedly within each subject, such designs would clarify the temporal ordering of the integrated stress response, changes in telomere length, and changes in aforementioned extraneous variables (Fig. 1).
In context of these limitations, our findings indicate that 2 subjects differing on perceived stress by a full standard deviation differ on average by 6% of a standard deviation on telomere length. Equivalently, variations in perceived stress appeared to account for less than 1% of variability in telomere length. Our finding of an effect size of r = −.06 is similar to the effect size of obesity on TL (r = -.057)88, approximately 18–35% that of blood pressure on TL (r = −0.34 and r = −0.17 for males and females, respectively)41, approximately 30% that of incident coronary heart disease on TL (OR = 1.44 for highest- versus lowest-tertile TLd)42, and approximately 30% that of depression on TL (r = −0.205)90. As noted previously, this very small effect size may, in theory, belie aggregate effects of practical impact. If reflective of a true causal relationship between short-term stress and telomere biology, the observed effect could potentially translate over the lifespan into cumulatively divergent cellular health among individuals with different levels of chronic stress. Such a divergence could culminate in clinically relevant differences in telomere biology by old age.
In completing what we believe to be the most comprehensive meta-analysis on this topic to date, we find a very small age-adjusted decrease in telomere length with increases in perceived stress that appears to be approximately equivalent to that seen in the relationship between obesity and telomere length. Emerging research on this topic, such a large new study finding an effect size similar to our pooled estimate91, will help verify our findings as well as improve statistical power to more precisely assess confounders and moderators. Our finding is qualified by likely publication bias, although fully parsing the effects of true publication bias from other sample-size correlates is challenging. Overall, our analysis indicates that the literature does not currently support a strong role of perceived stress (as measured over the past month) in shortening telomeres, though the relationship may be stronger among individuals facing adversity.
In light of the high incidence of reported stress as well as the complex interplay between life events, perceptions of their importance, and development of disease, our findings highlight the need for additional longitudinal research. Development of multidimensional lifespan models of reported, perceived, and physiological stress, use of standardized telomere assays, and incorporation of known extraneous variables (such as medications, health conditions, lifestyle factors, and clinical depression) would strengthen such future work.
Supplementary Material
Telomere shortening is a risk factor for numerous diseases and may be a general biomarker of aging.
Preliminary observational evidence suggests that psychological stress may be related to telomere shortening.
Meta-analysis of 8,724 individuals suggests a very small association between increased psychological stress and shorter telomeres, but publication bias is a likely problem.
Overcoming limitations of current measures of perceived stress and publication bias is an important consideration for future research.
Acknowledgments
We thank Christopher Stave for providing expertise on database search terms and Charlotte Chae for her invaluable assistance in creating graphics. We are particularly grateful to those authors who shared raw data with us (Jack A. Taylor, Nicholas Wainwright, Bert Uchino, Sophie Georgin-Lavialle, Andrew Ludlow, Kristin Litzelman and Corinne Engelman, and Roland Känel) and to those who computed summary statistics (Afton Hassett, Kimberly Sibille, Eli Puterman, Janice Humphreys, Sonja Entringer, Michelle Williams, Linda Carlson, Audrey Tyrka, Jennifer Daubenmier, Aric Prather, Francesco Saverio Bersani, Mary Whooley, and Andrew Steptoe).
FUNDING SOURCES
This research was supported by the Agency for Healthcare Research and Policy (1 K08 HS019816, Dr. Khazeni). The Survey of the Health of Wisconsin, from which we obtained raw data, was funded by the Wisconsin Partnership Program PERC Award (233 PRJ 25DJ), the National Institutes of Health's Clinical and Translational Science Award (5UL1RR025011), and the National Heart, Lung, and Blood Institute (1 RC2 HL101468). The Sister Study was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ZO1 ES044005), and telomere assays supported in part through by Department of Defense Breast Cancer Research Concept Award (BC045286, Dr. Parks). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Specific search strings were as follows
PubMed: (dysthym* [ti] OR pessim* [ti] OR “anxiety” [mesh] OR “anxiety” [tiab] OR “bipolar disorder” [mesh] OR “bipolar disorder” [tiab] OR depress* [ti] OR adversity* [ti] OR traum* [ti] OR stress* [ti] OR “stress, psychological” [mesh] OR “mood disorders” [mesh] OR “mental disorders” [mesh] OR “psychology” [sh] OR cognit* [ti]) AND (telomere* [ti] OR “telomere” [mesh] OR “chromosome breakage” [mesh] OR “matched pair analysis” [mesh] OR “cell aging” [mesh]) NOT (oxidative [ti] OR "oxidative stress" [mesh] OR editorial [pt] OR letter [pt] OR "review" [pt]) NOT ("animals" [mesh] NOT "humans" [mesh]).
PsycInfo: (telomer*.mp. or telomere length.id. or telomere biology.id.) and (exp stress/or stress*.mp. or exp mental health/)
Scopus: KEY(telomer*) AND KEY(chronic stress OR mental stress)
We used the following packages: xlsx, reshape2, ggplot2, metafor, lme4, lmerTest, Amelia, car.
The null models used as comparators for the meta-regressive model containing sex and that containing sample type were not identical. The sex meta-regression model contained multiple observations for some studies and a corresponding random intercept by study; thus its null model contained these as well.
This OR is equivalent to Cohen’s d effect size (which is comparable to the Pearson correlation)89, of .
AUTHOR CONTRIBUTIONS
MM and NK conceived the study. MM, NK, EE, and MD contributed to the analysis plan. MM, SK, and DS collected data. MM performed statistical analyses. MM, NK, EE, MD, CP, and DS wrote the manuscript.
RESEARCH TRANSPARENCY
Analysis code and other study materials are publicly available at https://osf.io/e9tg8/.
COMPETING INTERESTS
The authors declare that they have no competing financial interests.
ETHICS
This research was reviewed and granted a waiver by the appropriate ethics committee (Stanford University IRB).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson N, Johnson S, Belar C, et al. Stress in America: Our health at risk. American Psychological Association. 2012 [Google Scholar]
- 2.Cohen S, Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. Journal of Applied Social Psychology. 2012;42(6):1320–1334. [Google Scholar]
- 3.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food”. Proceedings of the National Academy of Sciences. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332(7540):521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 6.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proceedings of the National Academy of Sciences. 1996;93(7):3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iso H, Date C, Yamamoto A, et al. Perceived mental stress and mortality from cardiovascular disease among Japanese men and women: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk Sponsored by Monbusho (JACC Study) Circulation. 2002;106(10):1229–1236. doi: 10.1161/01.cir.0000028145.58654.41. [DOI] [PubMed] [Google Scholar]
- 9.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 10.Miller GE, Blackwell E. Turning up the heat: inflammation as a mechanism linking chronic stress, depression, and heart disease. Current Directions in Psychological Science. 2006;15(6):269–272. [Google Scholar]
- 11.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 12.Lehrer P. Anger, stress, dysregulation produces wear and tear on the lung. Thorax. 2006;61(10):833–834. doi: 10.1136/thx.2006.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen B. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. European journal of pharmacology. 2008;583(2):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara MR, Kovacs JJ, Whalen EJ, et al. A stress response pathway regulates DNA damage through beta-2-adrenoreceptors and beta-arrestin-1. Nature. 2011;477(7364):349–353. doi: 10.1038/nature10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. PNAS. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage S. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Mello MJJ, Ross SA, Briel M, Anand SS, Gerstein H, Paré G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circulation: Cardiovascular Genetics. 2015;8(1):82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Miao K, Wang H, Ding H, Wang DW. Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PLOS One. 2013 doi: 10.1371/journal.pone.0079993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okereke OI, Prescott J, Wong JYY, Han J, Rexrode KM, De Vivo I. High phobic anxiety is related to lower leukocyte telomere length in women. PLOS One. 2012;7(7):e40516. doi: 10.1371/journal.pone.0040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epel ES, Lin J, Wilhelm FH, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Tyrka AR, Price LH, Kao H-T, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon NM, Smoller JW, McNamara KL, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.O’Donovan A, Epel E, Lin J, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biological Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhoeven JE, van O, Patricia, Puterman E, Elzinga B, Penninx BWJH. The Association of Early and Recent Psychosocial Life Stress With Leukocyte Telomere Length. Psychosomatic medicine. 2015;77(8):882–891. doi: 10.1097/PSY.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira B, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, Guerra R. Systematic review of the association between chronic social stress and telomere length: A life course perspective. Ageing research reviews. 2015 doi: 10.1016/j.arr.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Haussmann MF, Heidinger BJ. Telomere dynamics may link stress exposure and ageing across generations. Biology letters. 2015;11(11):20150396. doi: 10.1098/rsbl.2015.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotlib IH, LeMoult J, Colich NL, et al. Telomere length and cortisol reactivity in children of depressed mothers. Molecular psychiatry. 2015;20(5):615–620. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomiyama AJ, O’Donovan A, Lin J, et al. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav. 2012;106(1):40–45. doi: 10.1016/j.physbeh.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schutte NS, Malouff JM. The Relationship Between Perceived Stress and Telomere Length: A Meta-analysis. Stress and Health. 2014 doi: 10.1002/smi.2607. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Williamson G. The social psychology of health: Claremont symposium on applied social psychology. Newbury Park, CA: Sage; 1988. Perceived stress in a probability sample of the US; pp. 31–67. [Google Scholar]
- 31.Gardner M, Bann D, Wiley L, et al. Gender and telomere length: systematic review and meta-analysis. Experimental Gerontology. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11(2):213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 33.Dohrenwend BS, Dohrenwend BP. Handbook of Clinical Health Psychology. Springer; 1982. Some issues in research on stressful life events; pp. 91–102. [Google Scholar]
- 34.Turner RJ, Wheaton B, Lloyd DA. The epidemiology of social stress. American Sociological Review. 1995:104–125. [Google Scholar]
- 35.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ Journal of Surgery. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 36.von Känel RM, Nico T, Hamer M, Malan L. Comparison of telomere length in black and white teachers from South Africa: the Sympathetic Activity and Ambulatory Blood Pressure in Africans study. Psychosomatic Medicine. 2015;77(1):26–32. doi: 10.1097/PSY.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 37.Litzelman K, Skinner HG, Gangnon RE, Nieto FJ, Malecki K, Witt WP. Role of global stress in the health-related quality of life of caregivers: evidence from the Survey of the Health of Wisconsin. Quality of Life Research. 2014;23(5):1569–1578. doi: 10.1007/s11136-013-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zalli A, Carvalho LA, Lin J, et al. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proceedings of the National Academy of Sciences. 2014;111(12):4519–4524. doi: 10.1073/pnas.1322145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kananen L, Surakka I, Pirkola S, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLOS One. 2010;5(5):e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoen PW, de Jonge P, Na BY, et al. Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosom Med. 2011;73(7):541–547. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benetos A, Okuda K, Lajemi M, et al. Telomere length as an indicator of biological aging the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37(2):381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 42.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. The Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 43.Hunter JE, Schmidt FL, Le H. Implications of direct and indirect range restriction for meta-analysis methods and findings. Journal of Applied Psychology. 2006;91(3):594. doi: 10.1037/0021-9010.91.3.594. [DOI] [PubMed] [Google Scholar]
- 44.Surtees PG, Wainwright NW, Pooley KA, et al. Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. J Gerontol A Biol Sci Med Sci. 2011;66(11):1152–1162. doi: 10.1093/gerona/glr112. [DOI] [PubMed] [Google Scholar]
- 45.Borenstein M, Hedges LV. Introduction to meta-analysis. Wiley; 2009. p. 1. [Google Scholar]
- 46.IntHout J, Ioannidis JPA, Borm GM. The Hartung–Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Medical Research Methodology. 2014;14(1):25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmons JP, Nelson LD, Simonsohn U. False-positive psychology undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological science. doi: 10.1177/0956797611417632. 20110956797611417632. [DOI] [PubMed] [Google Scholar]
- 48.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 49.Bersani FS, Lindqvist D, Mellon SH, et al. Association of dimensional psychopathological indices with telomere length in war veterans. doi: 10.1016/j.jad.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 50.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 51.Uchino BN, Cawthon RM, Smith TW, et al. Social relationships and health: Is feeling positive, negative, or both (ambivalent) about your social ties related to telomeres? Health Psychology. 2012;31(6):789. doi: 10.1037/a0026836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hassett AL, Epel E, Clauw DJ, et al. Pain is associated with short leukocyte telomere length in women with fibromyalgia. J Pain. 2012;13(10):959–969. doi: 10.1016/j.jpain.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Sibille KT, Langaee T, Burkley B, et al. Chronic pain, perceived stress, and cellular aging: an exploratory study. Mol Pain. 2012;8(1):12. doi: 10.1186/1744-8069-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Donovan A, Lin J, Dhabhar FS, et al. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain, behavior, and immunity. 2009;23(4):446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Donovan A, Tomiyama AJ, Lin J, et al. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain, Behavior, and Immunity. 2012;26(4):573–579. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLOS One. 2010;5(5):e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humphreys J, Epel ES, Cooper BA, Lin J, Blackburn EH, Lee KA. Telomere shortening in formerly abused and never abused women. Biological research for nursing. doi: 10.1177/1099800411398479. 20111099800411398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Georgin-Lavialle S, Moura DS, Bruneau J, et al. Leukocyte telomere length in mastocytosis: correlations with depression and perceived stress. Brain Behav Immun. 2014;35:51–57. doi: 10.1016/j.bbi.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 59.Wikgren M, Maripuu M, Karlsson T, et al. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012;71(4):294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Entringer S, Epel ES, Kumsta R, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108(33):E513–E518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parks CG, Miller DB, McCanlies EC, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18(2):551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parks CG, DeRoo LA, Miller DB, McCanlies EC, Cawthon RM, Sandler DP. Employment and work schedule are related to telomere length in women. Occup Environ Med. 2011;68(8):582–589. doi: 10.1136/oem.2010.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US Black Women Experience Stress-Related Accelerated Biological Aging?: A Novel Theory and First Population-Based Test of Black-White Differences in Telomere Length. Hum Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiefer A, Lin J, Blackburn E, Epel E. Dietary restraint and telomere length in pre- and postmenopausal women. Psychosom Med. 2008;70(8):845–849. doi: 10.1097/PSY.0b013e318187d05e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc. 2008;40(10):1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen X, Velez JC, Barbosa C, et al. Smoking and perceived stress in relation to short salivary telomere length among caregivers of children with disabilities. Stress. 2015;18(1):20–28. doi: 10.3109/10253890.2014.969704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121(3):476–484. doi: 10.1002/cncr.29063. [DOI] [PubMed] [Google Scholar]
- 68.Tyrka AR, Parade SH, Price LH, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buss J, Havel PJ, Epel E, Lin J, Blackburn E, Daubenmier J. Associations of ghrelin with eating behaviors, stress, metabolic factors, and telomere length among overweight and obese women: preliminary evidence of attenuated ghrelin effects in obesity? Appetite. 2014;76:84–94. doi: 10.1016/j.appet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uchino BN, Cawthon RM, Smith TW, Kent RG, Bowen K, Light KC. A cross-sectional analysis of the association between perceived network social control and telomere length. Health Psychology. 2015;34(5):531. doi: 10.1037/hea0000148. [DOI] [PubMed] [Google Scholar]
- 71.Epel ES, Puterman E, Lin J, Blackburn E, Lazaro A, Mendes WB. Wandering minds and aging cells. Clinical Psychological Science. 20122167702612460234. [Google Scholar]
- 72.Prather AA, Gurfein B, Moran P, et al. Tired telomeres: Poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain, Behavior, and Immunity. 2014 doi: 10.1016/j.bbi.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim S, Sandler DP, Carswell G, et al. Telomere length in peripheral blood and breast cancer risk in a prospective case-cohort analysis: results from the Sister Study. Cancer Causes & Control. 2011;22(7):1061–1066. doi: 10.1007/s10552-011-9778-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983:385–396. [PubMed] [Google Scholar]
- 75.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30(10):e47–e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savolainen K, Eriksson JG, Kajantie E, Lahti J, Räikkönen K. Telomere length and hypothalamic-pituitary-adrenal axis response to stress in elderly adults. Psychoneuroendocrinology. 2015;53:179–184. doi: 10.1016/j.psyneuen.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 77.Simonsohn U, Nelson LD, Simmons JP. P-curve: A key to the file-drawer. Journal of Experimental Psychology: General. 2014;143(2):534. doi: 10.1037/a0033242. [DOI] [PubMed] [Google Scholar]
- 78.Saliques S, Teyssier J-R, Vergely C, et al. Circulating leukocyte telomere length and oxidative stress: a new target for statin therapy. Atherosclerosis. 2011;219(2):753–760. doi: 10.1016/j.atherosclerosis.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 79.Cai N, Chang S, Li Y, et al. Molecular signatures of major depression. Curr Biol. 2015;25(9):1146–1156. doi: 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30(1):80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Lindqvist D, Epel ES, Mellon SH, et al. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neuroscience \& Biobehavioral Reviews. 2015 doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. American Journal of Epidemiology. 2002;155(2):176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 83.Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110(3):406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- 84.Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 85.Blaze J, Asok A, Roth TL. The long-term impact of adverse caregiving environments on epigenetic modifications and telomeres. Front Behav Neurosci. 2015;9:79. doi: 10.3389/fnbeh.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price LH, Kao H-T, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biological Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen S, Janicki-Deverts D, Turner RB, et al. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults: telomere length and respiratory viral infection. JAMA. 2013;309(7):699–705. doi: 10.1001/jama.2013.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes Rev. 2014;15(3):192–201. doi: 10.1111/obr.12126. [DOI] [PubMed] [Google Scholar]
- 89.Sánchez-Meca J, Marín-Martínez F, Chacón-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychological Methods. 2003;8(4):448. doi: 10.1037/1082-989X.8.4.448. [DOI] [PubMed] [Google Scholar]
- 90.Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. Depression and telomere length: A meta-analysis. Journal of affective disorders. 2016;191:237–247. doi: 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lynch SM, Peek MK, Mitra N, et al. Race, Ethnicity, Psychosocial Factors, and Telomere Length in a Multicenter Setting. PloS one. 2016;11(1) doi: 10.1371/journal.pone.0146723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.