Abstract
Addiction is a chronic brain disorder that progressively invades all aspects of personal life. Accordingly, addiction to opiates severely impairs interpersonal relationships, and the resulting social isolation strongly contributes to the severity and chronicity of the disease. Uncovering new therapeutic strategies that address this aspect of addiction is therefore of great clinical relevance. We recently established a mouse model of heroin addiction in which, following chronic heroin exposure, ‘abstinent’ mice progressively develop a strong and long-lasting social avoidance phenotype. Here, we explored and compared the efficacy of two pharmacological interventions in this mouse model. Because clinical studies indicate some efficacy of antidepressants on emotional dysfunction associated with addiction, we first used a chronic 4-week treatment with the serotonergic antidepressant fluoxetine, as a reference. In addition, considering prodepressant effects recently associated with kappa opioid receptor signaling, we also investigated the kappa opioid receptor antagonist norbinaltorphimine (norBNI). Finally, we assessed whether fluoxetine and norBNI could reverse abstinence-induced social avoidance after it has established. Altogether, our results show that two interspaced norBNI administrations are sufficient both to prevent and to reverse social impairment in heroin abstinent animals. Therefore, kappa opioid receptor antagonism may represent a useful approach to alleviate social dysfunction in addicted individuals.
Keywords: abstinence, addiction, heroin, kappa opioid receptor, serotonin, sociability
INTRODUCTION
Addiction is a chronic brain disorder with devastating consequences for individuals and their social life (Volkow, Baler & Goldstein 2011; Everitt 2014). Natural history of the disease has been classically conceptualized as a vicious cycle. Drug intoxications initially produce positive subjective effects but are followed by aversive signs of withdrawal when pharmacological drug effects unfold. In turn, withdrawal feeds into a ‘preoccupation’ stage, where drug craving irresistibly drives drug-seeking behaviors and precipitates relapse (Koob & Volkow 2010). Escaping this vicious cycle and maintaining abstinence is a lifelong challenge for addicted individuals.
Several causes contribute to interrupt drug abstinence. External factors, such as stressful life experiences and drug-associated environments (Koob & Volkow 2010), are well-studied determinants for relapse. Alteration of emotional homeostasis during the course of the disease represents yet another key factor, which is less understood. Abstinence, notably from opiate abuse, is characterized by a symptomatology encompassing anxiety and depressive disorders, as well as social isolation (Grella et al. 2009). Emotional and social dysfunction in addicted subjects is a major concern that associates with a more severe and longer clinical course, as well as higher relapse rates during abstinence periods (Bakken, Landheim & Vaglum 2007). Several rodent paradigms have been used to study the various facets of emotional deficits associating with opiate abuse. In summary, chronic opiate exposure was found to potentiate stress vulnerability (Blatchford et al. 2005), defensive behaviors (Harris & Aston-Jones 1993, 2001) and depressive-like behaviors (Grasing & Ghosh 1998; Anraku et al. 2001; Hodgson et al. 2009; Jia et al. 2013). Recently, we established a novel mouse model of drug abstinence focused on social behaviors. We showed that abstinence from both morphine and heroin, two prototypical opiates, progressively leads over the course of 4 weeks to the emergence of a long-lasting, low sociability phenotype (corresponding to both decreased interactions and increased grooming when encountering a new congener), which also associates with depressive-like features (Goeldner et al. 2011; Lutz & Kieffer 2013; Lutz et al. 2014).
Rigorous clinical studies have been conducted to evaluate classical antidepressants in the context of emotional comorbidities of addiction (Nunes & Levin 2006). These studies reported mixed results, some positive but some negative, emphasizing the need to investigate other therapeutic options. Over the last few years, animal studies have identified the kappa opioid receptor (KOR) as a promising target for innovative antidepressant strategies (Bruchas, Land & Chavkin 2010; Knoll & Carlezon 2010; Lalanne et al. 2014), prompting clinicians to assess the potential of targeting this particular opioid receptor to treat emotional symptoms in drug abuse. Buprenorphine, for example, is a complex opiate drug that activates the mu opioid receptor (MOR), the main molecular mediator of reinforcing properties of opiates, but is also an antagonist at the KOR. Considering the treatment of addiction, buprenorphine was used primarily as a substitution to illegal opiates because of its MOR activity; interestingly, some authors reported beneficial effects on emotional distress (Gerra et al. 2004) possibly because of KOR blockade. Recent studies have also investigated combined administration of buprenorphine and MOR antagonists, in order to achieve KOR blockade while limiting the risk of abuse associated with MOR activation. Results have revealed significant improvement of depressive symptoms (Ehrich et al. 2014; Almatroudi et al. 2015), further reinforcing the possibility that KOR antagonism may have intrinsic antidepressant potential.
Because MOR and KOR exert complex and distinct controls over emotions and sociability, animal studies and clinical trials that target both receptor types may be difficult to interpret. Also, systemic KOR-targeting therapies have not been investigated in animal models of social comorbidities of opiate addiction. In the present study, we hypothesized that KOR signaling may play a role in the emergence of low sociability in opiate addicts and evaluated the efficacy of a pure KOR antagonist in a validated mouse model of social deficits in heroin abstinence. We examined the ability of norBNI to prevent low sociability in heroin abstinent mice and to reverse this phenotype after it has been established, using the antidepressant fluoxetine (FLX) as a comparison. Our results indicate that these two pharmacological interventions had protective effects during heroin abstinence, in prevention and reversion experiments, against both low social interactions and increased grooming.
METHODS
Animals
Male C57BL/6JCrl mice (Charles Rivers Laboratories, St.-Germain-sur-l’Arbresle, France) were habituated to housing conditions during 2 weeks and were 10-week old at the beginning of chronic heroin exposure. Animals were housed four/cage and maintained under standard laboratory conditions (12-hour light–dark cycle with lights on at 7 AM; food and water available ad libitum). All experimental procedures were performed according to standard ethical guidelines (European Union Council Directive of 22 September 2010, directive 2010/63/UE and IGBMC-ICS ethical comity, Com’Eth).
Heroin treatment
Heroin (Francopia, Gentilly, France) was administered intraperitoneally (i.p.) twice daily (8 AM and 6 PM) with escalating doses (10, 20, 30, 40, 50 mg/kg for 5 days, followed by a single 50 mg/kg injection on day 6) or saline solution as a control. We previously showed that this regimen induces a strong physical dependence, one of the hallmarks of opiate addiction in human (Lutz et al. 2014). Compared with our previous studies on morphine abstinence (20–100 mg/kg morphine doses) and based on systematic analyses of opiate-induced physical dependence across inbred mouse strains (Kest et al. 2002; Klein et al. 2008), we selected heroin doses divided by a factor of two (10–50 mg/kg). Although the signs of withdrawal qualitatively differ (refer to Lutz et al. 2014), the global scores for acute naloxone-precipitated withdrawal are comparable across these two morphine and heroin regimens. To avoid reciprocal social influences between saline- and heroin-treated mice (Cole et al. 2012), housing cages contained only saline-treated or only opiate-treated mice. Following the 6-day heroin regimen, animals experienced spontaneous withdrawal in their home cages and were maintained drug free (i.e. ‘abstinent’) for 4 or 8 weeks in prevention or reversion experiments, respectively.
Fluoxetine treatment
The 10 mg/kg FLX dose was chosen based on our previous studies (Goeldner et al. 2011; Lutz et al. 2014). Briefly, the amount of FLX (Sigma-Aldrich, Lyon, France) supplemented to regular chow was based upon initial body weight of animals and daily average food intake: A 30 g mouse consuming 4 g/day (dry weight) of chow supplemented with 0.3 mg FLX received an approximate 10 mg/kg/24 hour dose. The 10 mg/kg was chosen as, in pilot experiments (refer to supplementary online material in Goeldner et al. 2011), it did not alter food intake during the 3 weeks of treatment and was ineffective on despair-related behaviors in naïve mice. In contrast, a higher (30 mg/kg) FLX dose was rejected as it severely reduced food intake and produced signs of serotonergic overdosage in some animals.
Norbinaltorphimine treatment
Norbinaltorphimine (norBNI) (Tocris Bioscience, Lille, France) is a long-acting antagonist with a greater than 100-fold selectivity for KOR over other opioid receptors (Metcalf & Coop 2005). Two injections of norBNI were administered i.p. over a 4-week period (Melief et al. 2011) and at a 10 mg/kg dose (McLaughlin et al. 2006; Bruchas et al. 2007a) with saline solution as a control. Housing cages contained only saline-treated or only norBNI-treated mice.
(trans)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl] benzene-acetamide treatment
(trans)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cycloxhexyl] benzene-acetamide (U50,488H; Sigma Aldrich, Lyon, France) was administered subcutaneously (s.c.), as previously described (Simonin et al. 1998), at a 20 mg/kg dose.
Behavioral testing
Following repeated heroin injections, mice were maintained drug free in their home cages. Social behaviors were investigated 4 or 8 weeks later in a familiar environment (open-field arena), as previously described (Goeldner et al. 2011; Lutz et al. 2014).
Social interactions
Pairs of unfamiliar mice, from different home cages but of similar weight, were placed simultaneously for 10 minutes in the open-field arena, indirectly lit at 50 lux. Prior habituation to the arena and dim lighting both favor social interactions in poorly anxiogenic conditions (Goeldner et al. 2011). While in the present study, social interactions were measured using pairs of mice from the same treatment condition, our previous work (Lutz et al. 2014) suggests that similar effects of abstinence are observed when heroin-treated mice interact with naïve, opiate-free animals. Using an ethological keyboard, we measured the number of occurrences and the total duration of social interaction behaviors (sniffing, following and pawing contact), as well as of the individual grooming behavior.
Tail immersion
The 52°C water is a commonly used nociceptive stimulus for opioid analgesia testing (Vaught & Takemori 1979; Mogil, Wilson & Wan 2001). Latency to withdraw the tail was measured by stopwatch. A cutoff time of 15 seconds was used to prevent heat-related tissue damage. For each mouse, pain-reflex latency was first measured at baseline. Then, U50,488H was administered with saline solution as a control, followed 30 minutes later (peak effect of the drug, refer to Bruchas et al. 2007b; Munro et al. 2012) by another measure of the pain reflex latency.
Statistical analyses
All data is expressed as mean ± SEM. Statistical analyses were performed using one- and two-way analyses of variance (ANOVA) with between-subjects (heroin abstinence, norBNI injections and FLX pellets) and within-subjects (U50,488H-induced analgesia) factors, in accordance with the experimental design. In case of significant interaction following ANOVA, multiple comparisons between groups were performed using Fischer’s post hoc. Statistical significance was defined as P < 0.05.
RESULTS
Low sociability during heroin abstinence can be both prevented and reversed by chronic antidepressant treatment with FLX
We showed previously that prolonged abstinence from chronic heroin exposure leads to the emergence of social withdrawal, a stable phenotype that can be detected at least from 4 to 7 weeks following the end of heroin treatment (Lutz et al. 2014). Here, in a first set of experiments (n = 128 mice, refer to timeline in Fig. 1a), we examined the ability of chronic antidepressant treatment to prevent (FLX administered during weeks 2 to 5) or reverse (FLX administered during weeks 5 to 9) heroin abstinence-induced social deficit. For each prevention or reversion experiment (n = 64 mice), two animal cohorts (n = 32 mice/cohort; n = 16 mice/saline or heroin group in each cohort) were processed independently, and results were pooled. FLX-supplemented pellets were replaced by normal pellets 48 hours before behavioral testing to avoid acute effects of the drug. Results of the prevention experiments were consistent with our previous findings (Fig. 1b, left panels) (Lutz et al. 2014) and showed that heroin abstinence decreases social behaviors while potentiating self-grooming. We note also that no aggressive behaviors could be detected in heroin abstinent mice under our experimental conditions. Statistical analyses (two-way ANOVA) showed that social exploration time (sum of the durations of sniffing, pawing contact and following between pairs of mice of same treatment group) was affected by heroin pre-exposure [F(1,21) = 61.7, P < 0.0001] as well as by FLX treatment [F(1,21) = 30.6, P < 0.0001], with no significant interaction [F(1,21) = 2.11, P > 0.05]. Post hoc analyses showed a decrease in social interactions in heroin-control food mice, as compared with both saline-control food (P < 0.0001), and heroin-FLX (P < 0.0001), mice. Heroin abstinence increased grooming [F(1,21) = 13.2, P < 0.005], a behavior that may represent an attempt to limit or avoid social contact (refer to our previous studies for a discussion). This effect was prevented by FLX [F (1,21) = 8.4, P < 0.05], with a significant interaction [F (1,21) = 7.4, P < 0.05]. Post hoc analysis showed that heroin-pretreated mice fed regular chow (heroin-control food) spent more time grooming than saline controls (saline-control food, P < 0.001) or than heroin abstinent mice fed FLX pellets (P < 0.001). Therefore, a chronic antidepressant treatment targeting the serotonergic (5-HT) system is able to promote social interactions and limit aberrant grooming during long-term withdrawal, consistent with our previous observation of dynamic adaptations within 5-HT circuits during this time period (Goeldner et al. 2011; Lutz et al. 2011). We next wondered whether chronic FLX treatment might be beneficial in abstinent mice when administered at a later stage, after neurochemical adaptations have developed and low sociability has established (Fig. 1b, right panels). In these reversion experiments, two-way ANOVA analysis of social behaviors duration found significant detrimental and beneficial effects of heroin [F(1,25) = 6.7, P < 0.05] and FLX [F (1,25) = 48.5, P < 0.0001], respectively, with a significant interaction [F(1,25) = 52.4, P < 0.0001]. Post hoc analyses showed a decrease in social interactions in heroin-control food mice, as compared with both saline-control food (P < 0.0001), and heroin-FLX (P < 0.0001), mice. Similar to results from prevention experiments, we also found that increased grooming induced by heroin pre-exposure [F (1,25) = 17.4, P < 0.0005] is reversed by FLX [F(1,25) = 16.6, P < 0.0005], with a significant interaction [F (1,25) = 14.1, P < 0.005]. Post hoc analyses further confirmed that heroin-control food mice exhibit significantly longer grooming behaviors compared with both saline-control food (P < 0.0001), and heroin-FLX (P < 0.0001), mice. Altogether, the data show that a chronic FLX treatment is able to both prevent and reverse social withdrawal, which has developed during heroin abstinence.
Figure 1.
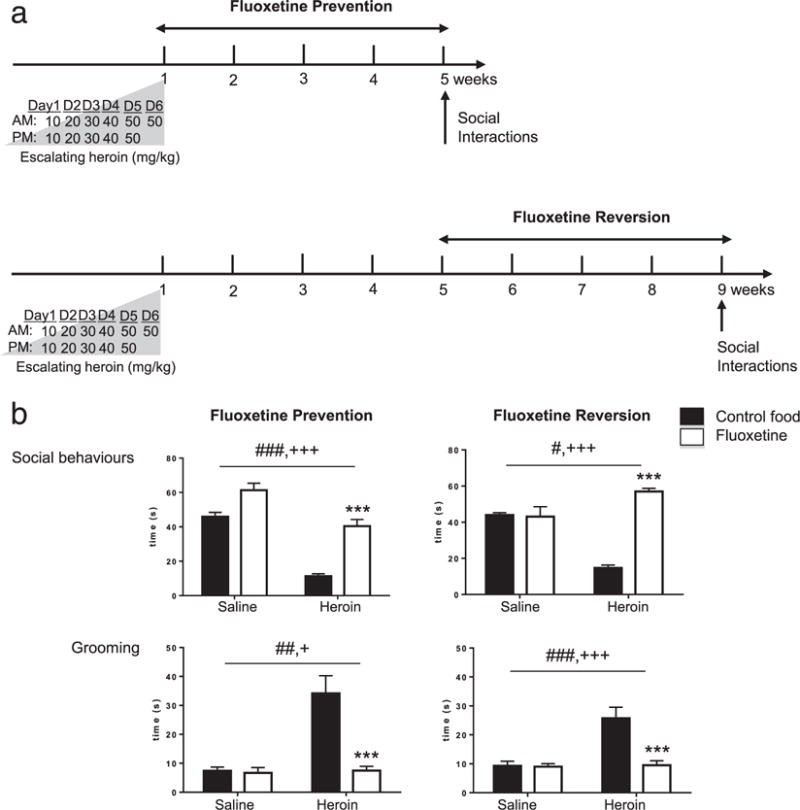
Chronic FLX treatment can both prevent and reverse low sociability in heroin abstinent mice. (a) Timeline for ‘Fluoxetine Prevention’ (upper panel) and ‘Fluoxetine Reversion’ (lower panel) experiments. Following heroin treatment, mice were maintained drug-free to experience spontaneous withdrawal for 4 weeks in prevention or 8 weeks in reversion experiments, followed by behavioral testing (Social Interactions). Mice were fed FLX-supplemented pellets (10 mg/kg/24 hours) during weeks 2 to 5 or weeks 5 to 8, in prevention or reversion experiments, respectively. (b) Social Interactions in ‘Fluoxetine Prevention’ (left panel) and ‘Fluoxetine Reversion’ (right panel) experiments. Consistent with our previous findings, heroin abstinence reduced social behaviors and potentiated grooming. Both of these deficits were prevented (left panel) and reversed (right panel) by 4 weeks of per os FLX treatment. Data represented as mean ± SEM. #P < 0.05, ##P < 0.01, ###P < 0.001, ANOVA, main effect of heroin. +P < 0.05, +++P < 0.001, ANOVA main effect of FLX. ***P < 0.001, post hoc FLX effect in heroin-FLX food mice as compared with heroin-control food mice
Two norBNI injections are sufficient to significantly block KOR signaling during a 4-week period
The long-lasting KOR antagonist activity of norBNI was described originally by several groups of investigators to last in vivo around 14 to 21 days (in both rodents, Bruchas et al. 2007b; Munro et al. 2012; Patkar et al. 2013, and rhesus monkeys, Butelman et al. 1993). While extremely long compared with most opiates, the reported duration of norBNI effects is shorter than the 4-week abstinence period in our model. We therefore determined the number of injections necessary to block KOR signaling during the entire 4-week abstinence period.
To this aim, we tested the analgesic effect of the KOR agonist U50,488H 4 weeks after a single i.p. norBNI injection (refer to timeline in Fig. 2a and results in Fig. 2b, left bottom panel). As expected, two-way ANOVA showed a significant analgesic effect of U50,488H, with an increase in the tail withdrawal latency [F(1,10) = 11.87, P < 0.01]. We noted, however, that norBNI had no statistically significant effect [F(1,10) = 1.99, P = 0.18], with no interaction between U50,488H and norBNI [F (1,10) = 1.9, P = 0.19], indicating that the later compound no longer significantly antagonized the KOR 4 weeks after a single injection. We then tested KOR-dependent antinociception following two norBNI injections separated by a 2-week interval (refer to timeline in Fig. 2a and results in Fig. 2b, right bottom panel). Results again confirmed the effect of U50,488H [F(1,9) = 11.74, P < 0.01]. Importantly, we also found a significant effect of norBNI [F(1,9) = 7.3, P < 0.05] with a tendency for an interaction [F(1,9) = 4.23, P = 0.06]. Post hoc analyses confirmed a significant analgesic effect of U50,488H in saline-treated controls (P < 0.005) but not in norBNI-treated mice (P = 0.37). Accordingly, we decided to use a two-injection paradigm for later experiments in heroin abstinence mice.
Figure 2.
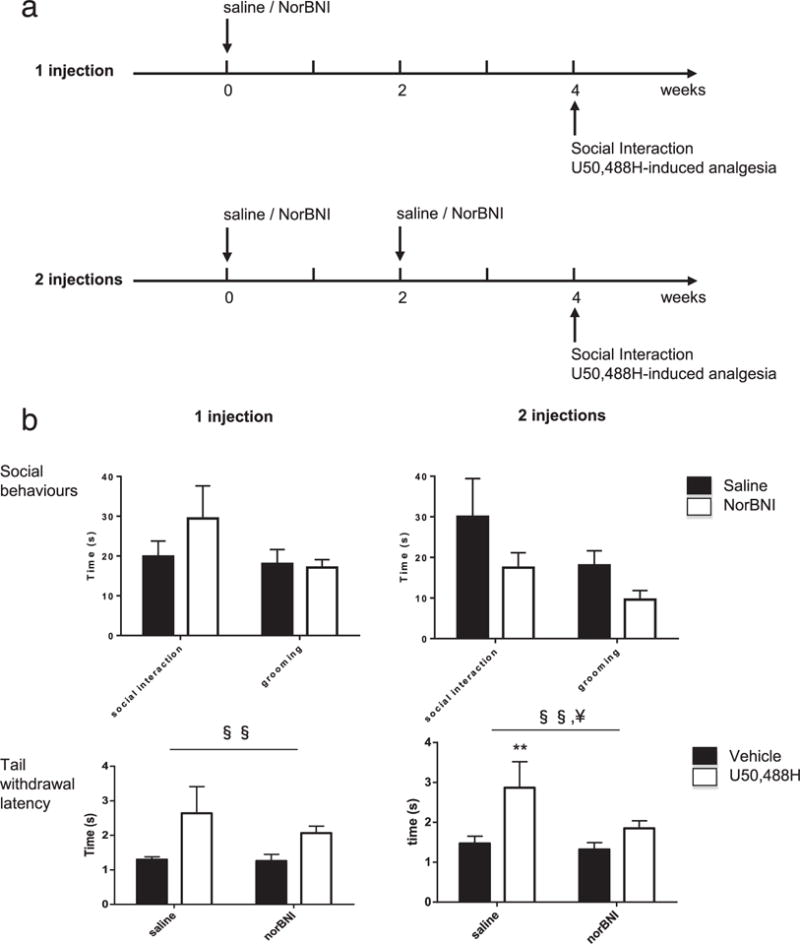
Two norBNI injections are sufficient to achieve chronic blockade of the kappa opioid receptor over 4 weeks. (a) Experimental time-line to assess chronic KOR blockade by one (upper panel) or two (lower panel) norBNI injections. Mice received one or two injections of norBNI (10 mg/kg, i.p.) over a 4-week time period. Four weeks after the first norBNI injection, mice underwent social interaction testing followed by U50,488H-induced analgesia (20 mg/kg, i.p.). (b) Social interactions (upper panels) and tail immersion (lower panels) in mice after one (left panels) or two (right panels) norBNI injections. Neither one nor two norBNI injections had significant effect on social behaviors and grooming. While the analgesic effect of U50,488H was still detectable 4 weeks after a single norBNI injection, two norBNI injections were able to prevent U50,488H-induced analgesia, in the 52°C tail immersion assay. Data represented as mean ± SEM. §§P < 0.01, ANOVA, main effect of U50,488H. ¥P < 0.01, ANOVA, main effect of norBNI. **P < 0.01, post hoc comparisons for the effect of U50,488H
In this experimental series, we also examined whether long-term blockade of endogenous KOR signaling might impact on the expression of social behaviors in naive mice. Social interactions were tested prior analgesic testing to limit stress effects of the pain assay. Results showed that in the single norBNI injection paradigm (Fig. 2b, left upper panel), norBNI had no significant effect on either social interactions (P = 0.32) or self-grooming (P = 0.82). Similarly, two injections of norBNI (Fig. 2b, right upper panel) had no effect on social (P = 0.11) or grooming (P = 0.10) behaviors. We conclude that, under basal conditions, sociability levels are not controlled by endogenous KOR signaling in adult mice, a finding that is consistent with our previous results in KOR knockout animals (Lutz et al. 2014).
Low sociability during heroin abstinence is both prevented and reversed with two systemic injections with the KOR antagonist norBNI
We next examined whether the two-injection norBNI regimen, which is sufficient to block KOR signaling over a 4-week period, prevents the emergence of low sociability in heroin abstinent animals. Similar to our experiments using FLX (n = 128 mice, refer to timeline in Fig. 3a), we examined whether chronic blockade of the KOR would prevent or reverse abstinence-induced deficits.
Figure 3.
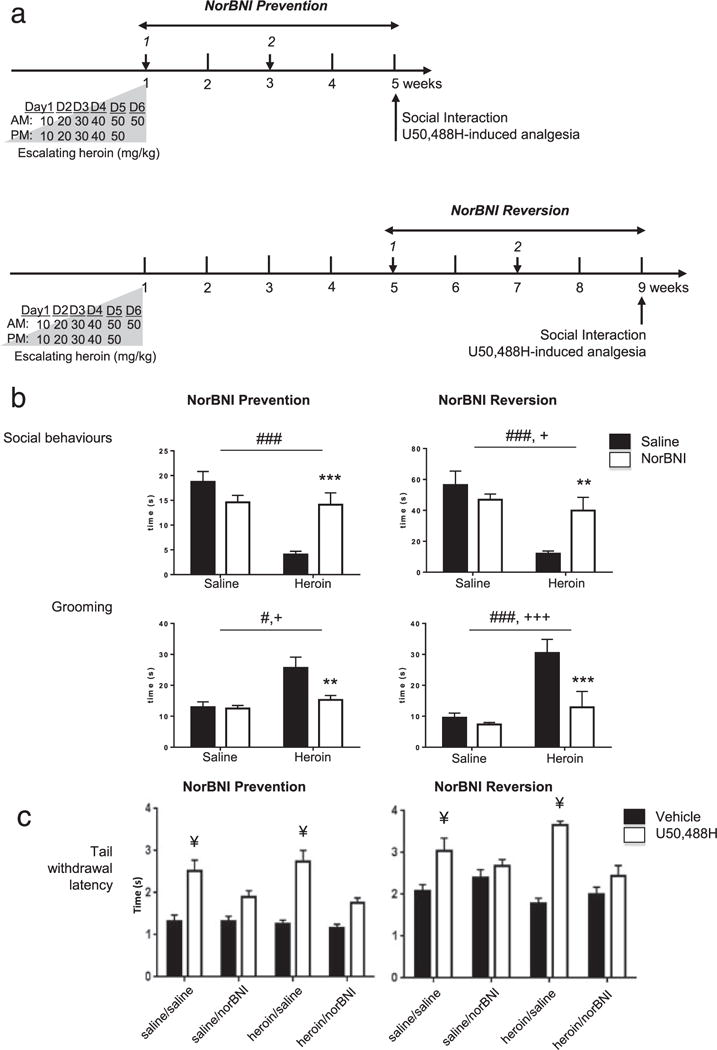
Chronic kappa opioid receptor norBNI antagonism prevents and reverses low sociability in heroin abstinent mice. (a) Timeline for ‘norBNI Prevention’ (upper panel) and ‘norBNI Reversion’ (lower panel) experiments. Following heroin treatment, mice experienced spontaneous withdrawal for 4 or 8 weeks, in prevention or reversion experiments, respectively. Two norBNI i.p. injections were used to achieve KOR blockade over a 4-week period. In ‘norBNI Prevention’ experiments, norBNI was administered 24 hours and 2 weeks after the last heroin injection. In ‘norBNI Reversion’ experiments, injections were 4 and 6 weeks after heroin treatment. (b) Social Interactions in ‘norBNI Prevention’ (left panels) and ‘norBNI Reversion’ (right panels) experiments. In the absence of norBNI, heroin abstinence decreased social behaviors and increased grooming. Two norBNI injections were sufficient to prevent and reverse these deficits. (c) Tail immersion in ‘norBNI Prevention’ (left panel) and ‘norBNI Reversion’ (right panel) experiments. To confirm chronic KOR blockade, mice were tested for U50,488H-induced analgesia (20 mg/kg, i. p.) in the 52°C tail immersion test, after social interaction testing. norBNI significantly blocked U50,488H-induced analgesia in both heroin abstinent and control mice, in prevention as well as in reversion experiments. Data represented as mean ± SEM. #P < 0.05, ###P < 0.001, ANOVA, main effect of heroin; +P < 0.05, +++P < 0.001, ANOVA, main effect of norBNI; **P < 0.01, ***P < 0.001, post hoc norBNI effect in heroin-norBNI mice as compared with heroin-control mice; ¥P < 0.005, post hoc comparisons for the effect of U50,488H
In the prevention experiment (n = 64 mice), the two norBNI injections were performed 24 hours and 2 weeks following the last heroin injection. Results revealed a significant effect of heroin abstinence [F(1.28) = 18.4, P < 0.001] and a significant interaction between abstinence and norBNI [F(1,28) = 16.1, P < 0.001], on the total duration of social behaviors (Fig. 3b, left upper panel). NorBNI had no main effect [F(1,28) = 2.8, P = 0.11]. Post hoc analyses confirmed that, in the absence of any norBNI exposure, social behaviors were decreased in heroin-pretreated compared with saline-pretreated abstinent animals (P < 0.0001). Importantly, two norBNI injections were sufficient to restore sociability to normal values in heroin-pretreated groups (P < 0.001), a striking effect that matches the efficacy of chronic FLX. As for grooming (Fig. 3b, left bottom panel), we found significant effects of heroin [F(1,28) = 13.3, P < 0.005] and norBNI [F(1,28) = 6.5, P < 0.05], with an interaction [F(1.28) = 5.54, P < 0.05]. Post hoc analyses revealed that, in the absence of norBNI exposure, heroin pretreatment significantly increased grooming behaviors (P < 0.0005). This effect was completely reversed by KOR blockade, as shown when comparing norBNI- and saline-treated mice in the two heroin abstinent groups (P < 0.005). Altogether, these results clearly demonstrate that two norBNI injections are sufficient to completely block heroin abstinence effects on sociability, as well as on self-centered, grooming behaviors.
Next, we explored the efficacy of norBNI in reversing the effects of heroin. In these reversion experiments (n = 64 mice), the two norBNI injections were performed 4 and 6 weeks following the last heroin injection, when impaired sociability is already prominent (Lutz et al. 2014). Results from the two-way ANOVA analysis showed that heroin abstinence [F(1,17) = 21.1, P < 0.0005] and norBNI [F(1,17) = 5.5, P < 0.05] had opposed and significant effects on social behaviors (Fig. 3b, right upper panel), with a strong interaction between the two factors [F(1,17) = 13.1, P < 0.005]. Post hoc group comparisons confirmed that, in the absence of norBNI administration, sociability was strongly impaired following a 8-week abstinence period (P < 0.0001). In addition, comparing the two heroin-pretreated groups showed that this deficit was potently reversed by norBNI (P < 0.0001). We also analyzed the duration of grooming (Fig. 3b, right bottom panel) and found effects of both heroin [F(1,17) = 20.9, P < 0.0005] and norBNI [F (1,17) = 29.7, P < 0.0001], as well as a significant interaction [F(1,17) = 21.6, P < 0.001]. Post hoc analyses further demonstrated that heroin abstinent mice show increased grooming compared with saline controls (P < 0.0001), an effect that was not observed following norBNI injections (P < 0.0001). Altogether, our results indicate that norBNI can be administered in long-term abstinent mice to reverse heroin-induced social withdrawal.
Compared with our preliminary analyses in naive mice (refer to Fig. 2), here norBNI was administered to animals experiencing significantly higher levels of stress (with repeated i.p. injections), which may potentially modify (and likely potentiate, Bruchas et al. 2010; Knoll & Carlezon 2010; Lalanne et al. 2014) endogenous KOR activity. To confirm that the two-injection norBNI regimen was still antagonizing KOR activity at the time of behavioral testing, U50,488H-induced antinociception was measured in both prevention and reversion experiments, following social interaction testing. Results showed that in prevention experiments (Fig. 3c, left panel), heroin pre-exposure had no effect [F(1,56) = 3.1, P = 0.09], suggesting that chronic MOR activation does not interfere with later KOR-mediated analgesia. In contrast, we found that norBNI [F(1,56) = 25.1, P < 0.0001] and U50,488H [F(2,56) = 109.0, P < 0.0001] had strong effects, with a significant interaction between these two treatments [F(1,56) = 25.6, P < 0.0001]. Post hoc group comparisons further indicated that U50,488H significantly increased tail withdrawal latency in saline-saline and heroin-saline groups (P < 0.0005) but not in saline-norBNI or heroin-norBNI groups (P > 0.05). In the reversion experiments (Fig. 3c, right panel), U50,488H had a significant effect [F(1,46) = 159.7, P < 0.0001], with a tendency for an effect of norBNI [F(1,46) = 3.5, P = 0.065], but not of heroin [F (1,46) = 2.8, P = 0.10]. We finally observed a strong interaction between U50,488H and norBNI [F(2,46) = 42.9, P < 0.0001], and post hoc comparisons found that U50,488H had significant effects in saline-saline (P < 0.005) and heroin-saline (P < 0.0005) groups but not in saline-norBNI or heroin-norBNI groups (P > 0.05). Overall, these results indicate that norBNI effectively blocked the KOR throughout abstinence.
DISCUSSION
Interactions with congeners represent major determinants of emotional well-being and resilience in both animal species and human. Disruption of social relationships is a hallmark of addiction (whatever the drug of abuse considered) and has been recognized as one of the diagnostic criteria most strongly associated with disease severity (Hasin et al. 2013). From a therapeutic perspective, medications that could help in restoring proper social functioning in addicted individuals are of great interest. Recently, we established an animal model of opiate abstinence in which impaired sociability (decreased interactions and increased grooming during a new social encounter) stands as a robust and long-lasting phenotype. While this model rely on experimentally delivered opiate injections and does not recapitulate important core dimensions of addiction (such as loss of control over drug seeking), our data indicate that it has both face (strong physical dependence, low sociability and negative affective state during withdrawal) and predictive (beneficial effects of antidepressant medication) validities for the human condition (American Psychiatric Association 1994; Hyman, Malenka & Nestler 2006; George, Koob & Vendruscolo 2014). Here, building on this model, we further demonstrate that abstinence-induced social dysfunction is reversible and can be normalized by the 5-HT antidepressant FLX after it has established. Importantly, results from the present study further indicate that, in the same mouse model, two systemic injections with the KOR antagonist norBNI are sufficient to completely restore social behaviors to normal levels. Therefore, we propose that chronic blockade of the KOR may represent a powerful strategy for the management of social withdrawal in addicted individuals, alone or in combination with classical antidepressants.
Previous studies from our group (Goeldner et al. 2011) and others (Fadda et al. 2005; Tao & Auerbach 2005; Ferreira & Menescal-de-Oliveira 2012) indicate that chronic opiate exposure strongly activates the 5-HT system (an essential mediator of social behaviors), as revealed by increased 5-HT release and turnover across several brain regions, and also potently activates the hypothalamus–pituitary–adrenal stress axis. Our previous study showed that, with the exception of 5-HT levels in the dorsal raphe nucleus (DRN), all these effects attenuated with time while, in contrast, emotional deficits gradually developed along abstinence (Goeldner et al. 2011; Lutz et al. 2011). In particular, social avoidance strengthened to become significant after 4 weeks of drug withdrawal and persisted during at least three more weeks in the case of heroin (Lutz et al. 2011). Importantly, social withdrawal could not be attributed to changes in motor activity, anxiety-like behavior or hedonic tone (Lutz et al. 2014; Ayranci et al. 2015) and occurred before the emergence of despair-like behavior at 7 weeks of abstinence (Lutz et al. 2014), suggesting that deficient sociability precedes mood disruption during ongoing heroin abstinence. Further, we found that low sociability in abstinent mice was prevented when a chronic FLX treatment, known to potentiate 5-HT signaling, was initiated immediately after the last heroin injection. In the present study, we reproduced this finding and also examined whether delayed FLX administration, that is, after the 4-week abstinence period, would also be beneficial and reduce social behavior perturbations. Our results clearly indicate a complete reversion of the aberrant phenotype, with similar efficacy compared with prevention experiments, indicating that FLX has the capacity to restore normal functioning even after social avoidance has fully developed. Neurochemical and circuit mechanisms of FLX effects are unknown at this stage. For both prevention and reversion experiments, FLX could produce a true reversion of neuroadaptations that have developed either upon chronic opiate exposure (prevention experiment) or during abstinence (reversion experiment). Alternatively, FLX may trigger new adaptations that counteract the former modifications to restore normal behaviors. Future studies will be required to determine whether FLX-mediated restoration of social behaviors solely rely on the modulation of serotonergic neurons or recruit other neurotransmitters such as glutamatergic or noradrenergic signaling (the latter being tightly coupled with 5-HT neurons and known to be regulated by the KOR; Al-Hasani et al. 2013).
Because the KOR has prodepressant properties and has been recently suggested to inhibit social behaviors (Lutz & Kieffer 2013; Bilkei-Gorzo et al. 2014; Robles et al. 2014), we hypothesized that KOR signaling may play a role in the emergence of low sociability during opiate abstinence. We recently obtained some evidence supporting this hypothesis, as knockout (KO) mice for the KOR developed a strikingly lower social withdrawal upon heroin abstinence, compared with controls (Lutz et al. 2014). In the later experiment, however, we noted that the intensity of physical dependence to heroin was also attenuated in the KOR mutant line, as shown in precipitated withdrawal experiments. Considering that physical withdrawal represents an aversive experience, it is possible that the milder withdrawal syndrome experienced by KOR KO mice during heroin exposure may contribute, at least partly, to the absence of social avoidance following abstinence in these mutants. In the present study, we used a pharmacological strategy to gain temporal control over KOR blockade. NorBNI was administered 24 hours after the end of intermittent heroin injections, thereby leaving unchanged the severity of physical withdrawal episodes that occurred after each heroin challenge. After establishing that two interspaced norBNI injections are sufficient to block KOR over 4-week duration (Fig. 2), we found that this two-injection regimen completely prevents the emergence of social impairment in abstinent mice. Therefore, we conclude that this phenotype does not result from the recruitment of KOR activities during repeated cycles of heroin intoxication and withdrawal but rather originates from KOR signaling during the abstinence period. Finally, we note that norBNI had no impact on sociability in control animals, indicating that this treatment specifically influenced long-term neuroadaptations that result from chronic heroin exposure and incubate during abstinence.
Similar to our reasoning with 5-HT signaling and FLX, we hypothesized that blockade of KOR activity could be beneficial both to prevent the development of social withdrawal during abstinence and also to reverse low sociability that has been established in abstinent animals. The KOR antagonist treatment was delayed and initiated after 4 weeks of abstinence, when the social phenotype is already constituted. Results indicate that norBNI injections fully reverse the heroin-induced phenotype with similar efficacy compared with the preventive norBNI intervention. These findings suggest that systemic norBNI administration may prove useful even in addicted subjects who already show signs of severe social isolation.
Results from these norBNI experiments strikingly mirror those obtained using FLX. A robust body of literature has recently demonstrated that KOR-dependent signaling pathways within DRN 5-HT neurons (such as the p38α kinase) mediate aversive responses in models of acute stress and stress-induced reinstatement of cocaine or nicotine drug seeking (Bruchas et al. 2007a; Land et al. 2009; Bruchas et al. 2011). Data also indicate that the nucleus accumbens (NAc) may be an important endpoint of KOR-dependent regulation of 5-HT neurons, which would ultimately affect dopaminergic signaling to produce behavioral effects (Bruchas et al. 2011; Schindler et al. 2012). Within this line, Zan et al. very recently provided interesting evidence in the specific context of opiate abstinence (Zan et al. 2015). Following chronic morphine exposure in mice, the authors showed that KOR blockade in the NAc could prevent abstinence-induced depressive-like behaviors and anhedonia (in the forced swim and sucrose preference tests, respectively). Together with our own results, the later findings therefore suggest that the NAc is critically located at the interplay of 5-HT and dopaminergic systems to control emotional homeostasis during opiate abstinence.
Several rodent studies have explored the effects of chronic MOR activation on the expression and function of the KOR and its endogenous ligands, the dynorphins A and B peptides. Results suggest a complex pattern of responses, including different and even opposite changes in dynorphin levels depending on (i) the brain region, with an increase in the striatum and a decrease in the hippocampus and pituitary gland (Nylander, Vlaskovska & Terenius 1995a, 1995b); (ii) the timepoint, with an increase in the NAc after 4, but not 2 weeks, of morphine withdrawal in mouse (Zan et al. 2015); and also (iii) genetic factors, with increased and decreased dynorphins in the VTA of Fischer and Lewis rats, respectively (Nylander et al. 1995a). A complete description of both dynorphin/KOR and 5-HT systems in the present heroin abstinence model, including FLX and norBNI treatment effects, will be instrumental to understand interactions between the two systems and design further molecular manipulations at targeted brain sites, including NAc as well as DRN or other brain sites involved in the social brain.
The present study is not without limitations, as heroin was delivered by the experimenter, during a relatively short period and at doses that likely do not produce acute reinforcing effects (Schlussman et al. 2008). Additional studies will be necessary to explore whether similar social dysfunction emerges following distinct drug exposure regimens (e.g. during longer periods, Williams et al. 2012, at doses that classically produce conditioned place preference, Schlussman et al. 2008, or in self-administration paradigms, Picetti et al. 2012) and its sensitivity to antidepressant or KOR antagonist medications. Beyond negative affective consequences of drug exposure, these additional paradigms have the potential to recapitulate other aspects of human addiction (such as loss of control over drug seeking).
In conclusion, previous studies focusing on rodent models of cocaine, nicotine, ethanol and opiate addictions consistently indicated that KOR antagonists may help prevent relapse episodes driven by stressful experiences or even drug-associated contexts (refer to Lalanne et al. 2014 for a recent review). Our study further expands the utility of KOR targeting compounds and suggests that they may also promote higher social functioning in addicted individuals. Importantly, two norBNI administrations only were sufficient to match the efficacy of a prolonged FLX administration. Therefore, the well-established long-acting properties of available KOR antagonists may help to improve compliance in opiate-dependent patients, who typically prove difficult to retain over long-term follow-ups. Two clinical trials recently reported mixed results on the tolerability of two opiates with antagonist activity at the KOR (JDTic, Buda et al. 2015, buprenorphine, Karp et al. 2014), with evidence for a potential cardiac toxicity (Buda et al. 2015). Future studies will be necessary to characterize the risk/benefit balance of these compounds when used as antidepressants in clinical populations, notably in the context of opiate addiction.
Acknowledgments
We thank Pr André Dufour for his help with statistical analyses.
ROLE OF FUNDING SOURCES
We thank the Mouse Clinical Institute (Illkirch France) for help with behavioral experiments and protocols. This work was supported by Fondation Fyssen (PEL), Fondation Bettencourt-Schueller (PEL), Canadian Institutes of Health Research (PEL), American Foundation of Suicide Prevention (PEL), Ministère de l’Enseignement Supérieur et de la Recherche (GA and PEL), Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Agence Nationale de la Recherche (ANR-12-BSV4-0028 ABSTINENCE, PEL and BLK), Frame program Investissements d’Avenir (ANR-10-IDEX-0002-02 and ANR-10-LABX-0030-INRT; LL, GA, DF, CGR, KB, BLK), and the National Institutes of Health (NIH-NIDA #005010).
References
- Al-Hasani R, McCall JG, Foshage AM, Bruchas MR. Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:2484–2497. doi: 10.1038/npp.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almatroudi A, Husbands SM, Bailey CP, Bailey SJ. Combined administration of buprenorphine and naltrexone produces antidepressant-like effects in mice. J Psychopharmacol. 2015;29:812–821. doi: 10.1177/0269881115586937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berl) 2001;157:217–220. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- Ayranci G, Befort K, Lalanne L, Kieffer BL, Lutz PE. Dissociation of heroin-induced emotional dysfunction from psychomotor activation and physical dependence among inbred mouse strains. Psychopharmacology (Berl) 2015;232:1957–1971. doi: 10.1007/s00213-014-3826-5. [DOI] [PubMed] [Google Scholar]
- Bakken K, Landheim AS, Vaglum P. Axis I and II disorders as long-term predictors of mental distress: a six-year prospective follow-up of substance-dependent patients. BMC Psychiatry. 2007;7:29. doi: 10.1186/1471-244X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Mauer D, Michel K, Zimmer A. Dynorphins regulate the strength of social memory. Neuropharmacology. 2014;77:406–413. doi: 10.1016/j.neuropharm.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Blatchford KE, Diamond K, Westbrook RF, McNally GP. Increased vulnerability to stress following opiate exposures: behavioral and autonomic correlates. Behav Neurosci. 2005;119:1034–1041. doi: 10.1037/0735-7044.119.4.1034. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci: Off J Soc Neurosci. 2007a;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007b;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda JJ, Carroll FI, Kosten TR, Swearingen D, Walters BB. A double-blind, placebo-controlled trial to evaluate the safety, tolerability, and pharmacokinetics of single, escalating oral doses of JDTic. Neuropsychopharmacology: Off Publ Am College Neuropsychopharmacology. 2015;40:2059–2065. doi: 10.1038/npp.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Cole SL, Hofford RS, Evert DJ, Wellman PJ, Eitan S. Social influences on morphine conditioned place preference in adolescent mice. Addict Biol. 2012;18:274–285. doi: 10.1111/j.1369-1600.2011.00426.x. [DOI] [PubMed] [Google Scholar]
- Ehrich E, Turncliff R, Du Y, Leigh-Pemberton R, Fernandez E, Jones R, Fava M. Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology: Off Publ the Am College Neuropsychopharmacology. 2014;40:1448–1455. doi: 10.1038/npp.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories—indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163–2182. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Dopamine and serotonin release in dorsal striatum and nucleus accumbens is differentially modulated by morphine in DBA/2 J and C57BL/6 J mice. Synapse. 2005;56:29–38. doi: 10.1002/syn.20122. [DOI] [PubMed] [Google Scholar]
- Ferreira MD, Menescal-de-Oliveira L. Opioidergic, GABAergic and serotonergic neurotransmission in the dorsal raphe nucleus modulates tonic immobility in guinea pigs. Physiol Behav. 2012;106:109–116. doi: 10.1016/j.physbeh.2012.01.005. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology (Berl) 2014;231:3911–3917. doi: 10.1007/s00213-014-3623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Borella F, Zaimovic A, Moi G, Bussandri M, Bubici C, Bertacca S. Buprenorphine versus methadone for opioid dependence: predictor variables for treatment outcome. Drug Alcohol Depend. 2004;75:37–45. doi: 10.1016/j.drugalcdep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasing K, Ghosh S. Selegiline prevents long-term changes in dopamine efflux and stress immobility during the second and third weeks of abstinence following opiate withdrawal. Neuropharmacology. 1998;37:1007–1017. doi: 10.1016/s0028-3908(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Grella CE, Karno MP, Warda US, Niv N, Moore AA. Gender and comorbidity among individuals with opioid use disorders in the NESARC study. Addict Behav. 2009;34:498–504. doi: 10.1016/j.addbeh.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate somatic and aversive signs of opiate withdrawal. Neuropsychopharmacology: Off PublAm College Neuropsychopharmacology. 1993;9:303–311. doi: 10.1038/npp.1993.66. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology: Off Publ Am College Neuropsychopharmacology. 2001;24:75–85. doi: 10.1016/S0893-133X(00)00184-6. [DOI] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sci. 2009;84:52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jia W, Liu R, Shi J, Wu B, Dang W, Du Y, Zhou Q, Wang J, Zhang R. Differential regulation of MAPK phosphorylation in the dorsal hippocampus in response to prolonged morphine withdrawal-induced depressive-like symptoms in mice. PLoS One. 2013;8:e66111. doi: 10.1371/journal.pone.0066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, Mulsant BH, Reynolds CF., 3rd Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry. 2014;75:e785–793. doi: 10.4088/JCP.13m08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: a survey of 11 inbred mouse strains. Pharmacol Biochem Behav. 2002;73:821–828. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- Klein G, Juni A, Waxman AR, Arout CA, Inturrisi CE, Kest B. A survey of acute and chronic heroin dependence in ten inbred mouse strains: evidence of genetic correlation with morphine dependence. Pharmacol Biochem Behav. 2008;90:447–452. doi: 10.1016/j.pbb.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: Off Publ Am College Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: from addiction to depression, and back. Front Psychiatry. 2014;5:170. doi: 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Ayranci G, Chu-Sin-Chung P, Matifas A, Koebel P, Filliol D, Befort K, Ouagazzal AM, Kieffer BL. Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence. Neuropsychopharmacology: Off Publ Am College Neuropsychopharmacology. 2014;39:2694–2705. doi: 10.1038/npp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol. 2013;23:473–479. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Pradhan AA, Goeldner C, Kieffer BL. Sequential and opposing alterations of 5-HT(1A) receptor function during withdrawal from chronic morphine. Eur neuropsychopharmacology: J Eur College Neuropsychopharmacology. 2011;21:835–840. doi: 10.1016/j.euroneuro.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology: Off Publ Am College Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Beguin C, Carlezon WA, Jr, Cohen BM, Grimwood S, Mitch CH, Rorick-Kehn L, Chavkin C. Duration of action of a broad range of selective kappa-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol. 2011;80:920–929. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. AAPS J. 2005;7:E704–722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Wan Y. Assessing nociception in murine subjects. In: Kruger L, editor. Methods in Pain Research. CRC Press; Boca Raton, FL: 2001. pp. 11–39. [Google Scholar]
- Munro TA, Berry LM, Van’t Veer A, Beguin C, Carroll FI, Zhao Z, Carlezon WA, Jr, Cohen BM. Long-acting kappa opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol. 2012;12:5. doi: 10.1186/1471-2210-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EV, Levin FR. Treating depression in substance abusers. Curr Psychiatry Rep. 2006;8:363–370. doi: 10.1007/s11920-006-0037-8. [DOI] [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain Res. 1995a;683:25–35. doi: 10.1016/0006-8993(95)00279-y. [DOI] [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. The effects of morphine treatment and morphine withdrawal on the dynorphin and enkephalin systems in Sprague-Dawley rats. Psychopharmacology (Berl) 1995b;118:391–400. doi: 10.1007/BF02245939. [DOI] [PubMed] [Google Scholar]
- Patkar KA, Wu J, Ganno ML, Singh HD, Ross NC, Rasakham K, Toll L, McLaughlin JP. Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J Pharmacol Exp Ther. 2013;346:545–554. doi: 10.1124/jpet.113.206086. [DOI] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology (Berl) 2012;220:163–172. doi: 10.1007/s00213-011-2464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles CF, McMackin MZ, Campi KL, Doig IE, Takahashi EY, Pride MC, Trainor BC. Effects of kappa opioid receptors on conditioned place aversion and social interaction in males and females. Behav Brain Res. 2014;262:84–93. doi: 10.1016/j.bbr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Messinger DI, Smith JS, Shankar H, Gustin RM, Schattauer SS, Lemos JC, Chavkin NW, Hagan CE, Neumaier JF, Chavkin C. Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. J Neurosci: Off J Soc Neurosci. 2012;32:17582–17596. doi: 10.1523/JNEUROSCI.3220-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Hsu NM, Allen JM, Ho A, Kreek MJ. Heroin-induced locomotor activity and conditioned place preference in C57BL/6 J and 129P3/J mice. Neurosci Lett. 2008;440:284–288. doi: 10.1016/j.neulet.2008.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. mu-Opioids disinhibit and kappa-opioids inhibit serotonin efflux in the dorsal raphe nucleus. Brain Res. 2005;1049:70–79. doi: 10.1016/j.brainres.2005.04.076. [DOI] [PubMed] [Google Scholar]
- Vaught JL, Takemori AE. Differential effects of leucine and methionine enkephalin on morphine-induced analgesia, acute tolerance and dependence. J Pharmacol Exp Ther. 1979;208:86–90. [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Goldstein RZ. Addiction: pulling at the neural threads of social behaviors. Neuron. 2011;69:599–602. doi: 10.1016/j.neuron.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, Kloss ND, Bilimoria JL, Smith CE, Walker BM. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology (Berl) 2012;223:75–88. doi: 10.1007/s00213-012-2691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan GY, Wang Q, Wang YJ, Liu Y, Hang A, Shu XH, Liu JG. Antagonism of kappa opioid receptor in the nucleus accumbens prevents the depressive-like behaviors following prolonged morphine abstinence. Behav Brain Res. 2015;291:334–341. doi: 10.1016/j.bbr.2015.05.053. [DOI] [PubMed] [Google Scholar]


