Abstract
We previously reported the bioaccumulation of contaminants of emerging concern (CECs), including pharmaceuticals and personal care products (PPCPs) and perfluorinated compounds, in field-collected juvenile Chinook salmon from urban estuaries of Puget Sound, WA (Meador et al., 2016). Although the toxicological impacts of CECs on salmon are poorly understood, several of the detected contaminants disrupt mitochondrial function in other species. Here, we sought to determine whether environmental exposures to CECs are associated with hepatic mitochondrial dysfunction in juvenile Chinook. Fish were exposed in the laboratory to a dietary mixture of 16 analytes representative of the predominant CECs detected in our field study. Liver mitochondrial content was reduced in fish exposed to CECs, which occurred concomitantly with a 24–32% reduction in expression of peroxisome proliferator-activated receptor (PPAR) ϒ coactivator-1a (pgc-1α), a positive transcriptional regulator of mitochondrial biogenesis. The laboratory exposures also caused a 40–70% elevation of state 4 respiration per unit mitochondria, which drove a 29–38% reduction of efficiency of oxidative phosphorylation relative to controls. The mixture-induced elevation of respiration was associated with increased oxidative injury as evidenced by increased mitochondrial protein carbonyls, elevated expression of glutathione (GSH) peroxidase 4 (gpx4), a mitochondrial-associated GSH peroxidase that protects against lipid peroxidation, and reduction of mitochondrial GSH. Juvenile Chinook sampled in a WWTP effluent-impacted estuary with demonstrated releases of CECs showed similar trends toward reduced liver mitochondrial content and elevated respiratory activity per mitochondria (including state 3 and uncoupled respiration). Interestingly, however, respiratory control ratios were greater in fish from the contaminated site relative to fish from a minimally-polluted reference site, which may have been due to differences in the timing of exposure to CECs under laboratory and field conditions. Our results indicate that exposure to CECs can affect both mitochondrial quality and content, and support the analysis of mitochondrial function as an indicator of the sublethal effects of CECs in wild fish.
Keywords: Contaminants of emerging concern, mitochondria, Chinook salmon, oxidative stress
1. Introduction
Municipal wastewater enters wastewater treatment plants (WWTPs) to undergo primary, secondary, and sometimes tertiary treatment processes that target the removal of biosolids, dissolved organic matter, and microbial contamination. Following these processes, the treated effluent is discharged into receiving waters such as rivers, lakes, and estuarine aquatic environments. Despite these treatment processes, reports over the past decade have indicated that WWTP effluent contain a broad range of structurally-diverse compounds which are subsequently introduced into aquatic environments (Brooks et al., 2005). These include the chemical components of common household products such as pharmaceuticals and personal care products (PPCPs), natural and synthetic hormones from contraceptive medications, alkylphenol surfactants, and perfluorinated compounds (PFCs) (Dickenson et al., 2011; Jasinska et al., 2015; Kim et al., 2012; Lubliner et al., 2010; Vidal-Dorsch et al., 2012). Compounds within the aforementioned chemical classes are termed “contaminants of emerging concern” (CECs) as there is currently little regulation of these chemicals in the environment (Diamond et al., 2011; Halden, 2015; Naidu et al., 2016). These emerging contaminants are frequently detected in environmental water samples at low ng/L concentrations, but can bioaccumulate in aquatic organisms (Meador et al., 2016; Vidal-Dorsch et al., 2012). Reports of adverse biological effects in aquatic biota resulting from accumulation and exposure to effluent-borne CECs at these levels underscore the need to clarify critical data gaps regarding their toxicity in non-target organisms.
Studies involving fish have indicated that behavior can be adversely modified by exposures to CECs at environmentally-relevant concentrations under controlled laboratory conditions. These adverse effects on behavior include reduced predator avoidance (Painter et al., 2009), altered predatory feeding behavior (Bisesi et al., 2016), decreased or increased aggression (Colman et al., 2009; Schultz et al., 2012), and reduced sociality (Brodin et al., 2013). Adverse impacts on fish reproductive function caused by CECs have also been reported (Crago et al., 2016; Lister et al., 2009; Niemuth et al., 2015). For example, a recent study in juvenile coho salmon (Oncorhynchus kisutch) demonstrated impacts on the hypothalamic-pituitary-gonad axis caused by exposure to 2 and 10 ng/L 17α-ethynylestradiol (EE2) and diluted WWTP effluent (Harding et al., 2016). Finally, exposure to CECs can inhibit metabolic function and rates of growth in several fish species (Ashfield et al., 1998; Niemuth and Klaper, 2015; Yang et al., 2014), which has implications on fish populations, as body mass has been linked to survival in wild fish (Meador, 2014; Spromberg and Meador, 2005). Despite these findings, however, there are currently no established biomarkers of exposure or effect of CECs on aquatic organisms (Harding et al., 2016; Jasinska et al., 2015).
A review of the literature led us to the a priori observation that some emerging contaminants cause dysfunction of mitochondria in humans and laboratory animal models. Mitochondria are sensitive targets of toxicity to numerous pharmaceuticals and environmental toxicants (Meyer et al., 2013; Wallace and Starkov, 2000). A common mechanism of mitochondrial toxicity is shared by a number of CECs, specifically, inhibiting function of the mitochondrial electron transport system (ETS). For example, the antidepressant medications fluoxetine (Souza et al., 1994) and sertraline (Li et al., 2012) inhibit mitochondrial state 3 respiration in isolated rat liver mitochondria and are priority CECs frequently detected in WWTP effluent (Kostich et al., 2014; Lubliner et al., 2010; Meador et al., 2016; Vidal-Dorsch et al., 2012). In general, a wide array of pharmaceutical medications, including anesthetics, antidiabetics, antidepressants, and nonsteroidal anti-inflammatory drugs, specifically inhibit the mitochondrial ETS in mammals (Chan et al., 2005). Perfluorooctane sulfonamide (PFOSA) is a high-priority perfluorinated compound that potently uncouples the mitochondrial ETS in isolated rat kidney mitochondria (Schnellmann and Manning, 1990). Similarly, perfluorooctanoate (PFOA) and perfluorodecanoic acid (PFDA) elevate mitochondrial state 4 respiration and reduce state 3 respiration in isolated rat liver mitochondria (Keller et al., 1992; Langley, 1990) and are frequently detected in WWTP effluent. Finally, the general-use antimicrobial agent triclosan is a component of numerous personal care products and is a potent mitochondrial uncoupler in rat and human mast cells, primary human keratinocytes (Weatherly et al., 2016), and in 24-hour post-fertilization zebrafish embryos (Shim et al., 2016). Although the aforementioned mitochondrial effects were mostly described in mammalian laboratory models, a recent study of 12 diverse fish species demonstrated that 65–86% of human drug targets were evolutionarily conserved in the studied fish species, suggesting that the mechanisms of action of many CECs may share commonalities with fish (Brown et al., 2014). Collectively, these reports along with the critical function of mitochondria in maintaining cellular energetics warrant the investigation of mitochondrial toxicity as a biomarker of sublethal CEC toxicity in aquatic species.
In the present study, we hypothesized that environmental exposures to CECs result in dysfunction of liver mitochondria in fish. We utilized juvenile Chinook salmon, an ecologically- and economically-critical species in the Pacific Northwest, and focused on hepatic mitochondria because the liver is the primary site of xenobiotic biotransformation and a target organ of toxicity for many CECs. Our approach was to characterize the potential impacts of exposure to CECs on mitochondrial ETS function and associated mitochondrial oxidative injury in a subchronic dietary study involving a complex mixture of the predominant analytes representative of field exposures. In a parallel field study, we compared mitochondrial content and function in juvenile Chinook collected from an estuarine field site that receives WWTP effluent, with those from a minimally-polluted reference site.
2. Materials and Methods
2.1. Chemicals and reagents
Amlodipine, azithromycin dehydrate, and diltiazem were purchased from Abcam (Cambridge, MA). Fluoxetine hydrochloride, gemfibrozil, metformin hydrochloride, miconazole nitrate, perluorodecanoic acid (PFDA), and sertraline hydrochloride were purchased from Cayman Chemical (Ann Arbor, MI). Diphenhydramine hydrochloride, fluocinonide, and heptadecafluorooctane sulfonic acid (PFOS) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Amitriptyline hydrochloride, perfluorooctane sulfonamide (PFOSA), 3,4,4’trichlorocarbanilide (triclocarban), and Irgasan (triclosan) were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Ethanol was purchased from Decon Labs, Inc. (King of Prussia, PA). RNAlater® solution was purchased from Ambion (Austin, TX). TRIzol® reagent was purchased from Invitrogen (Carlsbad, CA). Quantitative real time PCR (qPCR) primers were purchased from Eurofins MWG Operon (Huntsville, AL). Tricaine methanesulfonate (MS-222) was purchased from Argent Chemical Laboratories (Redmond, WA).
2.2. Dietary subchronic exposures of juvenile Chinook to a mixture of CEC
All methods associated with salmon husbandry, collection, and exposures were approved by the University of Washington Institutional Animal Care and Use Committee. Juvenile Chinook salmon (1 year of age, approximately 45 g) were obtained from the Wallace River Hatchery (Sultan, WA) under Washington State Scientific Collection Permit 13–046 and ESA Section 10(a)(1)(A) permit 17798. The hatchery-reared Chinook were housed in large cylindrical tanks (n=20 fish per tank) receiving ~12 °C recirculating freshwater from Lake Washington, Seattle, under a natural photoperiod at the UW fish hatchery. Following acclimation, the juvenile Chinook were exposed via diet to a mixture of 16 of the predominant CECs detected in field samples based on data from the analytical field study (Meador et al., 2016). The rationale used to prioritize the 16 mixture analytes for the feeding study is presented in Supplementary Table 1. Calculated amounts of stock solutions of each analyte (dissolved in ethanol) were added to three separate volumes of 4 L of ethanol in order to generate three mixture concentrations intended to mimic 0.3-, 1-, and 10-fold concentrations of each analyte detected in field-collected fish. BioClark’s Fry 2.5 mm low-fat food pellets (Bio-Oregon, Longview, WA) were dosed with the contaminant mixtures as described previously (Meador et al., 2006). The feeding study consisted of four groups, including the aforementioned three concentrations of the mixture of emerging contaminants, and solvent control diets. Replicates of the experimental treatments included 4 replicate tanks for solvent control feed (n=80 fish), 3 replicate tanks for 0.3× CEC feed (n=60 fish), 4 replicate tanks for 1× CEC feed (n=80 fish), and 3 replicate tanks for 10× CEC feed (n=60 fish). Twice daily feedings (morning and afternoon) were conducted five days per week, for a total of 50 feedings over the 32-day dosing period starting on February 16, 2015. The mass of mixture-treated feed administered to fish was increased each week by a quantity assuming a growth rate of 2.2% body weight (bw)•day−1 based on day 0 of the experiment (Meador et al., 2005). Following exposures, AXYS Analytical Services Ltd. (Sidney, British Columbia, Canada) employed multi-analyte HPLC/MS/MS techniques to measure whole-body concentrations of CECs in laboratory-exposed fish. A total of four composite samples (control, 0.3×, 1×, and 10×–treated fish) were analyzed, consisting of one fish from each tank replicate resulting in a pool of n=3–4 whole-bodies of fish per composite sample. Details regarding analytical methods of whole body residue analysis with limits of detection of each compound were reported previously (Meador et al., 2016).
2.3. Field sampling of Chinook
Juvenile Chinook were collected from field sites as described previously (Meador et al., 2016). Briefly, fish were collected by beach seine from a WWTP effluent-impacted site and a reference site in Puget Sound, WA. The effluent-impacted Puyallup River estuary (PE) contains eight WWTPs with a combined permitted effluent volume of 63 million liters/day, with flows generally running much lower (Pierce County, 2010). By contrast, our reference site in the nearby Nisqually river estuary (NE) does not receive inputs of WWTP effluent, and has been utilized as a reference site in studies conducted in the Pacific Northwest (Mccain et al., 1990; Meador, 2014; Myers et al., 1994; Varanasi et al., 1993). Live Chinook were transported to the laboratory in aerated coolers filled with site water chilled with ice packs in order to maintain the temperature of the water column measured at the field sites (13 °C). Water quality parameters measured at each site on the date of sampling, as well as body weights, lengths, and condition factors of the field-sampled fish assessed in subsequent laboratory experiments are reported in Supplementary Table 2.
2.4. Assessment of mitochondrial function in field-sampled and laboratory-exposed fish
Liver mitochondrial function was assessed in 6–8 individual Chinook salmon (two fish from each tank replicate) exposed to the mixture of CECs as part of the dietary exposure study, and six individual Chinook per field site. All fish from the field and laboratory studies were sacrificed using 250 mg/L MS-222 prior to removal of liver tissues. Oxygen consumption rates (OCRs) were measured in saponin-permeabilized liver samples via high-resolution respirometry (Oxygraph-2k, Oroboros Instruments, Innsbruck, Austria). Briefly, approximately 20 mg of liver tissue was gently homogenized with a glass tissue homogenizer in 150 µL ice-cold respiration buffer (distilled H2O, 0.25 M mannitol, 10 mM MgCl2, 10 mM KHPO4 buffer, pH=7.2). Homogenized liver samples were transferred to a 1.5 mL centrifuge tube containing an additional 135 µL ice-cold respiration buffer, and centrifuged at 400 rpm at 4 °C for 5 min. Centrifuged liver samples were then permeabilized on ice by addition of 15 µL permeabilization buffer (50 µg/mL saponin in respiration buffer, 20 min, 4 °C). The total volume of permeabilized liver sample in 300 µL buffer was then transferred to an oxygraph chamber for analysis.
Experimental procedures for the assessment of ETS functional parameters were performed as described (Yeh et al., 2015). Briefly, respiratory substrates and inhibitors were sequentially added to permeabilized liver samples in the following order: 1) 5 mM pyruvate, 2 mM malate, 10 mM glutamate, and 2.5 mM ADP to induce state 3 respiration with complex I substrates only, 2) 10 mM succinate to maximally induce state 3 respiration with substrates of complexes I and II, 3) 2.5 µM oligomycin to induce state 4, or proton leak, respiration, 4) 2.5 µM CCCP to induce maximum respiratory capacity, or uncoupled respiration, 5) 0.5 µM rotenone to measure uncoupled respiration with complex I inhibition, i.e., uncoupled flux through complex II, 6) 2.5 µM antimycin A to determine non-mitochondrial respiration, 7) 0.5 mM N,N,N′,N′-tetramethyl-ρ-phenylenediamine (TMPD) and 2 mM ascorbate to determine flux through complex IV, and 8) 1 mM potassium cyanide to inhibit complex IV. The non-mitochondrial rate of oxygen consumption was subtracted from all measured functional parameters before reporting final values. Similarly, the rate of oxygen consumption after addition of potassium cyanide was subtracted from the rate of flux through complex IV for normalization purposes. All experimentally-determined mitochondrial OCRs were measured as pmoles O2/sec/mg wet weight of liver sample. All mitochondrial function experiments were conducted at 13 °C, the average temperature of the water column in the field sites (Meador et al., 2016), and comparable to that of the recirculating freshwater from Lake Washington used in the laboratory exposure study (~12 °C).
Livers from each fish assessed in the respirometry experiments were further divided into three sections of approximately 20 mg each. Two sections were snap frozen and stored at −80 °C for assessment of oxidative injury in isolated liver mitochondria (described in the subsequent section), and quantification of mitochondrial content. Liver mitochondrial content was assessed in whole tissue homogenates by measurement of activity of citrate synthase (an enzymatic marker of the mitochondrial matrix) in a spectrophotometric plate reader assay according to the manufacturer’s protocol (Citrate Synthase Assay Kit, Sigma, St. Louis, MO). A third liver section was preserved in RNAlater® solution and stored at −80 °C for gene expression experiments (described in section 2.6).
2.5. Analysis of oxidative injury in liver mitochondria
In the fish exposed to the contaminant mixtures in the laboratory, mitochondrial fractions were isolated from liver tissue sections as previously described (Gallagher et al., 1992). Citrate synthase activity was measured in the subcellular fractions to verify purity of intact mitochondria. Oxidative modification of mitochondrial proteins was determined by measurement of protein carbonylation in a fluorescent plate reader assay according to the manufacturer’s protocol (OxiSelect Protein Carbonyl Fluorometric Assay kit, Cell Biolabs, San Diego, CA). Protein concentrations in the mitochondrial samples were determined by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Total mitochondrial GSH concentrations were assessed in a fluorescent plate reader assay as previously described (Yeh et al., 2015). Mitochondrial 4-HNE-protein adducts were assessed in a competitive ELISA assay according to the manufacturer’s protocol (HNE Adduct Competitive ELISA, Cell Biolabs, San Diego, CA).
2.6. Measurement of antioxidant and mitochondrial function gene expression in laboratory-exposed fish
Procedures for isolation of total RNA from liver tissue, cDNA synthesis, and PCR primer product validation were conducted as previously described (Ramsden and Gallagher, 2016). Prior to gene expression determination, the concentrations and quality of isolated liver RNA were determined via Agilent 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA). Quantification of RNA transcript levels for selected antioxidant and mitochondrial marker genes was then determined in 300 ng of RNA per fish (n=6–8 individuals per treatment) according to the manufacturer’s recommended procedure for isolated RNA.
Expression of hepatic genes involved in the cellular antioxidant response and mitochondrial function were quantified in fish from the dietary exposure laboratory study using a customized Quantigene® plex 2.0 (QGP) panel (Affymetrix, Fremont, CA) (Mills and Gallagher, 2017). The antioxidant response genes included glutamate-cysteine ligase catalytic subunit (GCLC), phospholipid hydroperoxide glutathione peroxidase (GPx4), and mitochondrial superoxide dismutase (SOD2). Genes involved in mitochondrial function included peroxisome proliferator-activated receptor (PPAR) ϒ coactivator-1a (PGC-1α), and nuclear respiratory factor-1 (NRF-1). All target genes were normalized to the geometric mean of the expression of beta actin (β-actin), and 60S ribosomal protein L8 (RPL8). Primer sequence information (Supplementary Table 3) determined by preliminary PCR experiments was used by Affymetrix to design custom QGP probe sets against the antioxidant and mitochondrial function genes.
2.7. Statistical analyses
All data sets were examined for potential outliers using the Grubb’s test, and outlier values were excluded at significance level p<0.05. In the laboratory study, data reflecting mitochondrial content, ETS functional parameters, concentrations of mitochondrial protein carbonyls, total glutathione, HNE-protein adducts, and expression of antioxidant and mitochondrial function genes were assessed for homogeneity of variance by Bartlett’s test and comparison of means assessed by One-Way ANOVA followed by Fisher’s Least Significant Difference post-hoc test. Differences were considered significant at p<0.05. In experiments involving juvenile Chinook collected from the field, morphological measurements, mitochondrial content, and ETS functional parameters were assessed for homogeneity of variance by F test, followed by comparison of means by Unpaired t-test. Differences between means measured in reference and polluted site fish were considered significant at p<0.05. All statistical analyses were conducted in GraphPad Prism Ver. 5.0 (Graph Pad Software Inc., San Diego, CA, USA), with the exception of Fisher’s Least Significant Difference post-hoc test which was conducted using the Analysis ToolPak in Microsoft Excel (Microsoft ® Excel ® 2016). We also performed log-transformed analyses of our data, as well as analyses by linear regression. Results of these analyses were similar to those of the non-transformed data and are not discussed.
3. Results
3.1. Results of the feeding study
Bioaccumulation of CECs in laboratory-exposed fish
A summary of concentrations of CECs measured in Chinook salmon fed the dietary mixtures is presented in Table 1. Of note was that the sum of all observed compounds in whole-body fish was relatively low, ranging from 51 ng/g wet weight (ww) for the low dose treatment to 795 ng/g ww for the high dose. The analytes bioaccumulated at roughly the proportion intended between treatments, with 14 of the 16 CECs in the mixture bioaccumulating in Chinook salmon following the 32-day dietary exposure. Eleven of the compounds, including the PPCPs amitriptyline, amlodipine, azithromycin, diltiazem, diphenhydramine, fluoxetine, metformin, miconazole, and the perfluorinated compounds PFDA, PFOS, and PFOSA, were detected in whole-body samples from all three of the experimental contaminant mixture groups. Three other analytes, gemfibrozil, sertraline, and triclocarban, were detected in the high concentration (10×) treatment group only. Only two analytes, fluocinonide and triclosan, were below detection limits in all whole-body samples. None of the studied CECs were detected in control fish. No fish mortalities occurred as a result of the 32-day dietary exposure to the mixture of emerging contaminants.
Table 1.
Whole body concentrations of CECs measured in laboratory-exposed Chinook salmon.
| CEC analyte | Field study data (Meador et al., 2016) |
Laboratory exposure study Concentrations after day 32 of feeding study |
|||
|---|---|---|---|---|---|
| Fish tissue ng/g |
Control ng/g (RL) |
0.3× ng/g (RL) |
1× ng/g (RL) |
10× ng/g (RL) |
|
| Amitriptyline | 0.63 | U (0.13) | 0.13 (0.13) | U (0.12) | 0.96 (0.1) |
| Amlodipine | 0.81 | U (0.59) | 1.2 (0.59) | 1.1 (0.59) | 20 (0.58) |
| Azithromycin | 1.7 | U (1.81) | 2.8 (2.42) | 1.8 (0.65) | 29 (0.733) |
| Diltiazem | 1.5 | U (0.24) | 17 (0.32) | 11 (0.23) | 100 (1.1) |
| Diphenhydramine | 2.6 | U (0.24) | 1.8 (0.23) | 1.2 (0.24) | 14 (0.23) |
| Fluocinonide | 6.5 | U (2.4) | U (2.9) | U (3.8) | U (3.0) |
| Fluoxetine | 4.8 | U (0.59) | 1.4 (0.59) | 1.0 (0.59) | 20 (0.58) |
| Gemfibrozil | 1.3 | U (0.59) | U (0.59) | U (0.59) | 3.3 (0.58) |
| Metformin | 28 | U (2.88) | 4.5 (3.03) | U (4.7) | 40 (2.61) |
| Miconazole | 1.6 | U (0.73) | 1.2 (0.75) | U (0.68) | 13 (0.63) |
| PFDA | 0.78 | U (0.50) | 1.3 (0.44) | 2.2 (0.50) | 31 (0.48) |
| PFOS | 35 | U (0.99) | 5.7 (0.88) | 7.5 (0.99) | 130 (0.95) |
| PFOSA | 2.2 | U (0.59) | 6.0 (0.53) | 9.1 (0.59) | 190 (0.57) |
| Sertraline | 11 | U (0.52) | U (0.52) | U (0.52) | 9.1 (0.52) |
| Triclocarban | 5.2 | U (1.18) | U (1.17) | U (1.18) | 8.7 (1.16) |
| Triclosan | 25 | U (23.5) | U (23.4) | U (23.5) | U (23.3) |
Table legend. Fish tissue concentrations from the field study represent mean analyte concentrations measured in field samples. “RL” reporting limit; “U” below detection limit.
Mitochondrial function and content in laboratory-exposed fish
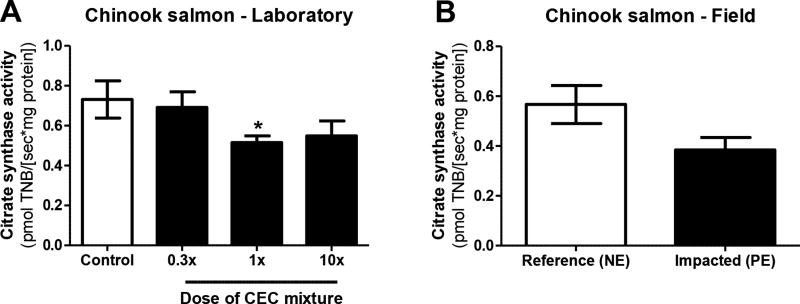
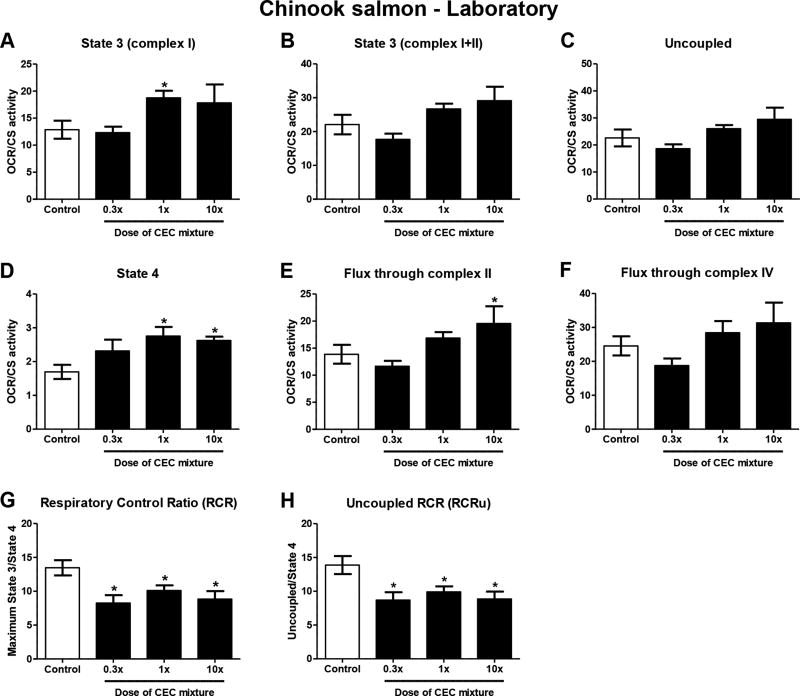
Liver CS activity was significantly reduced by 29% relative to controls in fish exposed to the medium (1×) dose of the contaminant mixture (Figure 1A). Fish exposed to the low (0.3×) and high dose (10×) mixture diets also trended toward lower CS activity relative to controls, but values did not reach statistical significance at p<0.05. Respirometry experiments determined similar rates of state 3 respiration (induced by complex I, and complex I+II substrates), uncoupled respiration, and fluxes through complexes II and IV per liver homogenate in control and mixture-exposed fish (Table 2). By contrast, state 4 respiration was significantly elevated by all tested doses of the contaminant mixture; specifically, 40 and 70% elevation relative to controls in the low and medium dose groups, and high dose group, respectively (Table 2). Subsequently, OCRs measured in respirometry experiments were normalized to CS activity, and indicated that exposure to CECs caused an overall elevation of respiration per unit mitochondria. State 3 respiration (complex I substrates only) was significantly elevated by 46% in fish exposed to the medium dose of the mixture (Figure 2A). Similarly, oxygen flux through complex II was significantly elevated by 42% in fish exposed to the high dose of the mixture (Figure 2E). Mixture-exposed fish also trended toward elevated rates of state 3 respiration with complex I+II substrates (Figure 2B), uncoupled respiration (Figure 2C), and flux through complex IV (Figure 2F) per mitochondria. Moreover, the dietary exposure to CECs induced significant 40 and 70% increases in the rates of state 4 respiration per unit mitochondria in the medium and high dose groups, respectively (Figure 2D), which drove the significant reduction of RCR and RCRu in all mixture-exposed fish relative to controls (Figures 2G and H).
Figure 1.
Mitochondrial content in livers of Chinook salmon as measured by activity of citrate synthase (CS) (pmoles TNB/[sec*mg tissue]). (A) CS activity measured in Chinook salmon following 32-day dietary exposure to a mixture of CECs in the laboratory. *p≤0.05 relative to control fish. Data are mean CS activity ± SEM of n = 6–8 individuals. (B) CS activity measured in livers of juvenile Chinook salmon from the NE reference site and PE impacted site. Data are mean CS activity ± SEM of n = 6 individuals.
Table 2.
Liver oxygen consumption rates (OCR) measured in laboratory-exposed juvenile Chinook salmon
| Liver oxygen consumption rates (pmoles O2/[sec*mg tissue]) ± SEM |
||||
|---|---|---|---|---|
| ETS functional parameter | Control | 0.3× | 1× | 10× |
| State 3 (complex I) | 8.4 ± 0.3 | 8.1 ± 0.4 | 9.4 ± 0.5 | 8.6 ± 0.8 |
| State 3 (complex I+II) | 14 ± 0.6 | 12 ± 0.8 | 13 ± 0.4 | 14 ± 1.4 |
| Uncoupled | 15 ± 0.6 | 12 ± 1.0 | 13 ± 0.8 | 15 ± 1.2 |
| State 4 | 1.0 ± 0.0 | 1.4 ± 0.2 | 1.4 ± 0.1 | 1.7 ± 0.1 |
| Flux through complex II | 9.1 ± 0.4 | 7.8 ± 0.6 | 8.6 ± 0.6 | 9.6 ± 0.8 |
| Flux through complex IV | 16 ± 1.2 | 12 ± 0.8 | 14 ± 1.6 | 15 ± 2.2 |
Values in bold indicate p≤0.05 relative to the control group. Data are mean liver mitochondrial oxygen consumption rates (OCR) ± SEM of n = 6–8 individuals per treatment group.
Figure 2.
Liver mitochondrial oxygen consumption rates (OCRs, pmoles O2/[sec*mg tissue]) normalized to mitochondrial content as measured by citrate synthase activity (CS activity, pmoles TNB/[sec*mg tissue]) in liver of hatchery-reared juvenile Chinook salmon (O. tshawytscha) exposed to increasing doses of the Puget Sound mixture of CECs for 32 days. Experimental conditions to measure mitochondrial OCRs were: (A) state 3 respiration with complex I substrates; (B) maximal state 3 respiration with substrates of complexes I and II; (C) maximum uncoupled respiration; (D) state 4 respiration induced by oligomycin. Also, flux through (E) complex II and (F) complex IV were determined. Efficiency of oxidative phosphorylation as measured by (G) respiratory control ratio (RCR) and (H) uncoupled RCR (RCRu) were calculated as the ratio of maximal state 3/state 4 respiration, and uncoupled/state 4 respiration, respectively. *p≤0.05 relative to the control group. Data are means ± SEM of n = 6–8 individuals.
Effect of dietary exposure to CECs on mitochondrial oxidative injury
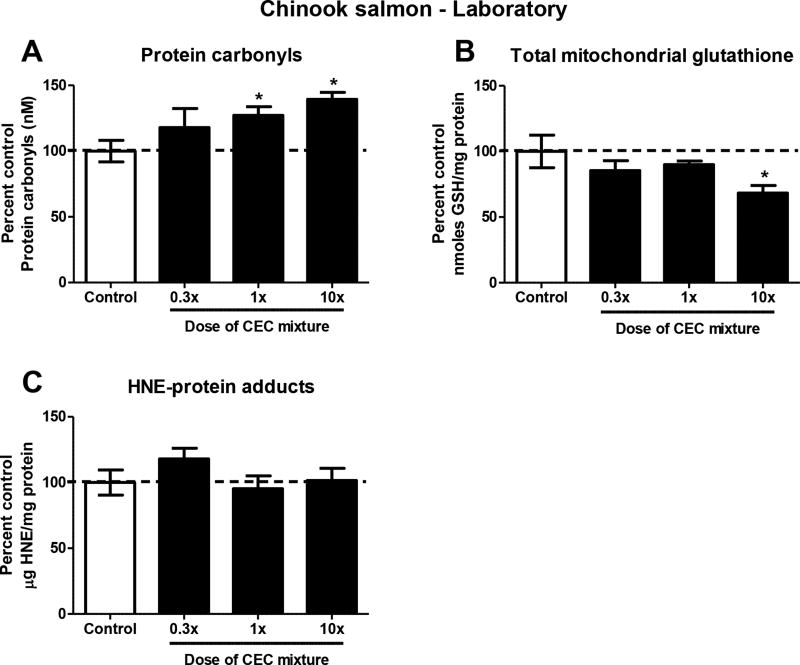
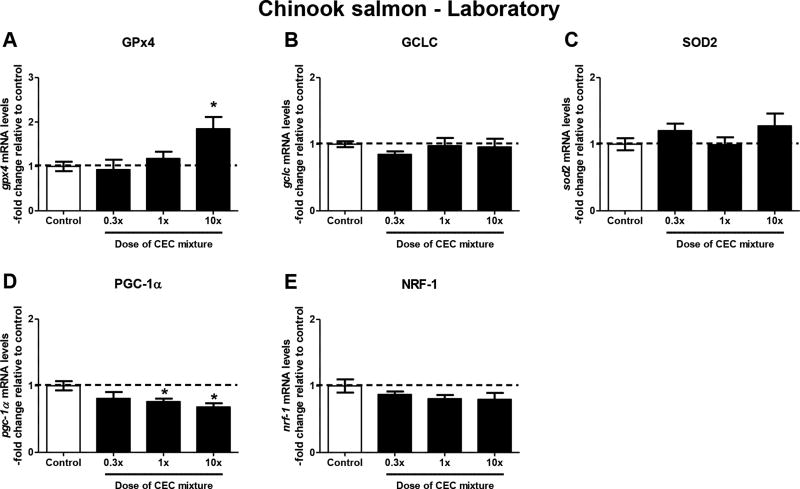
Mitochondrial protein carbonyl formation was significantly elevated by 27–39% in the livers of fish exposed to the medium and high doses of the contaminant mixture diets (Figure 3A). The increase in mitochondrial protein carbonyl formation occurred concomitantly with a 32% reduction in the liver mitochondrial GSH pool in fish exposed to the high dose mixture (Figure 3B). By contrast, the levels of HNE-protein adduct formation (Figure 3C) did not differ between control and mixture-exposed fish. Expression of the mitochondrial antioxidant response gene gpx4 was significantly elevated by 85% in fish exposed to the high dose of the contaminant mixture (Figure 4A). However, expression of the other antioxidant response genes gclc and sod2 was not different among control and exposed fish (Figures 4B and 4C). Measurement of genes involved in mitochondrial function revealed significant 24–32% reduction of pgc1-α in the medium and high dose-exposed fish (Figure 4D). Expression of nrf-1 also trended toward reduction in mixture-exposed fish, but did not reach statistical significance at p<0.05 (Figure 4E).
Figure 3.
Indices of oxidative injury assessed in isolated liver mitochondria from Chinook salmon exposed to the mixture of CECs in the laboratory. Concentrations of: (A) protein carbonyls, (B) total reduced and oxidized glutathione, and (C) HNE-protein adducts were determined in isolated liver mitochondria. *p≤0.05 relative to the control group. Data are expressed as percent of control values and are mean concentrations ± SEM for n=6–8 individuals.
Figure 4.
Effect of exposure to the mixture of CECs on antioxidant response and mitochondrial function-related genes. mRNA transcript levels of the antioxidant response genes (A) gpx4, (B) gclc, (C) sod2, and mitochondrial function genes (D) pgc-1α, and (E) nrf-1 were measured in livers of juvenile Chinook salmon exposed to the mixture of CECs for 32 days. Gene expression was normalized to the geometric mean of expression of reference genes β-actin and rpl8. *p≤0.05 relative to the control group. Data represent-fold change in mRNA levels normalized to the reference genes and are mean ± SEM of n=6–8 individuals.
3.2. Results of the field study
Field site water quality parameters and morphological data for field-collected fish utilized in the respirometry experiments are reported in Supplementary Table 2. Similar temperature, pH, and dissolved oxygen content of the water column were observed at the reference and contaminated sites. Salinity of the water column at the polluted site (23.5 ppt) was higher than that measured at the reference site (15.5 ppt), but values at both sites were within the range of salinity previously reported for Puget Sound estuaries (Department of Ecology, 2014). Condition factors of fish from the two field sites did not significantly differ (Supplementary Table 2).
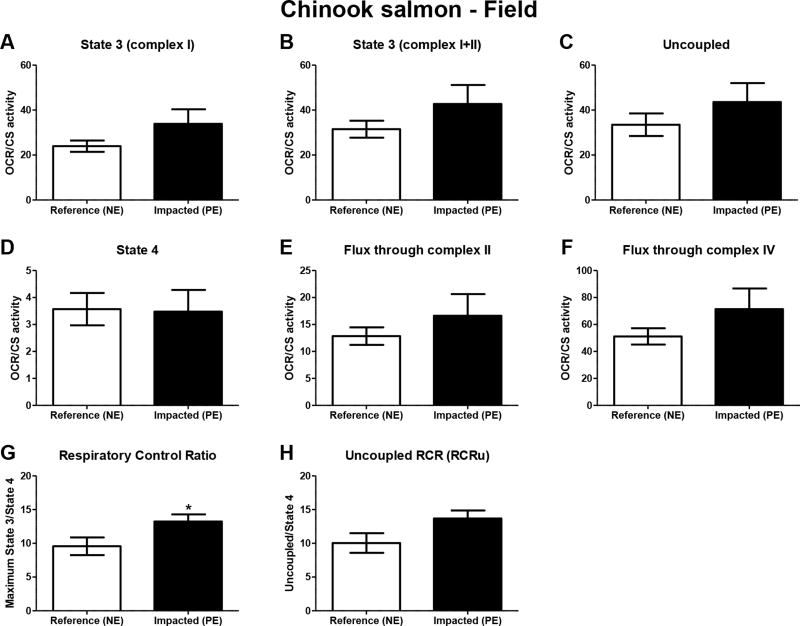
Fish from the contaminated site had 33% lower liver citrate synthase (CS) activity relative to reference site fish, but these differences were not statistically significant (p=0.09) (Figure 1B). Similarly, no significant site differences were observed with respect to rates of state 3 respiration (induced by complex I, and complex I+II substrates), uncoupled respiration, state 4 respiration, and fluxes through complexes II and IV per liver homogenate (Table 3). Subsequently, mitochondrial OCRs were normalized to CS activity to determine rates of respiratory activity per unit mitochondria. Upon normalization, contaminated site fish trended toward elevated respiratory activity per mitochondria relative to reference site fish. Specifically, fish from the contaminated site trended toward elevated State 3 (complex I, and complex I+II) and uncoupled respiration per unit mitochondria (Figures 5A–C), and elevated rates of flux through complexes II and IV per unit mitochondria (Figures 5E–F). By contrast, rates of State 4 respiration per mitochondria were similar among reference and polluted site fish (Figure 5D). As a result, respiratory control ratios (RCR) tended to be higher in contaminated site Chinook relative to reference fish (p=0.053, Figure 5G). Uncoupled respiratory control ratios (RCRu) also tended to be higher in Chinook from the contaminated site (p=0.08, Figure 5 H).
Table 3.
Liver oxygen consumption rates measured in juvenile Chinook salmon from the reference and WWTP effluent-impacted field sites
| Liver oxygen consumption rates (pmoles O2/[sec*mg tissue]) ± SEM |
||
|---|---|---|
| ETS functional parameter | Reference site | Impacted site |
| State 3 (complex I) | 13 ± 0.8 | 13 ± 1.5 |
| State 3 (complex I+II) | 17 ± 0.9 | 16 ± 2.0 |
| Uncoupled | 17 ± 1.0 | 17 ± 2.1 |
| State 4 | 1.9 ± 0.3 | 1.3 ± 0.2 |
| Flux through complex II | 6.9 ± 0.6 | 6.3 ± 1.1 |
| Flux through complex IV | 27 ± 1.8 | 28 ± 4.8 |
Data are mean liver mitochondrial oxygen consumption rates (pmoles O2/[sec*mg tissue]) ± SEM of n = 6 individuals per field site.
Figure 5.
Liver mitochondrial oxygen consumption rates (OCRs, pmoles O2/[sec*mg tissue]) normalized to mitochondrial content as measured by citrate synthase activity (CS activity, pmoles TNB/[sec*mg tissue]) in liver of wild juvenile Chinook salmon (O. tshawytscha) from the reference Nisqually estuary (NE) and CEC-impacted Puyallup estuary (PE) field sites. Experimental conditions to measure mitochondrial OCR were: (A) state 3 respiration with complex I substrates; (B) maximal state 3 respiration with substrates of complexes I and II; (C) maximum uncoupled respiration; (D) state 4 respiration induced by oligomycin. Also, flux through (E) complex II and (F) complex IV were determined. Efficiency of oxidative phosphorylation as measured by (G) respiratory control ratio (RCR) and (H) uncoupled RCR (RCRu) were calculated as the ratio of maximal state 3/state 4 respiration, and uncoupled/state 4 respiration, respectively. Data are means ± SEM of n = 6 individuals.
4. Discussion
Effects of exposure to CECs in field-collected Chinook salmon
In the field study, a trend toward reduced liver mitochondrial content was observed in fish collected from the contaminated field site. It is important to note that in addition to chemical exposures, nonchemical stressors such as temperature (Lucassen et al., 2006) and dissolved oxygen (DO) content (Cooper et al., 2002; Mandic et al., 2014; Zhou et al., 2000) can affect mitochondrial content in fish tissues. However, at the time of sampling, similar temperature and DO content of the water columns were measured at the reference and impacted field sites, and thus we can largely discount the potential effect of these variables on our results. By contrast, salinity at the CEC-impacted site was higher than that measured at the reference site, although within the range of normal values reported for Puget Sound. Relatively few studies have reported the effects of salinity on mitochondrial content in fish tissues (Marshall et al., 1999; Mccormick et al., 1989), and to our knowledge, the effects of salinity on CS activity specifically in liver tissue of fish have not been characterized, with the exception of a report involving hatchery-reared juvenile coho salmon (Oncorhynchus kisutch) exposed to increased salinity having reduced CS activity in gill tissues (Shrimpton et al., 1994). The fact that we observed a similar reduction in liver CS activity in Chinook exposed to the mixture of CECs in the laboratory strongly suggests that the observed field effects were due to these chemical exposures. However, it is important to note that legacy contaminants such as polycyclic aromatic hydrocarbons, polychlorinated biphenyls and heavy metals may have also been present at the field sites and could also have contributed to the observed effects.
As discussed, OCRs measured per liver homogenate were similar between fish from the two field sites, with the notable exception of lower rates of state 4 respiration in impacted site fish. As a result, the RCRs measured in impacted site fish were higher than those of reference fish, suggesting that mitochondrial function was more efficient in fish from the CEC-impacted site. This finding was unexpected, as we hypothesized that fish from the impacted site would present with elevated state 4 respiration and lower RCR, indicative of mitochondrial dysfunction. However, Du et al. (2015) compared liver mitochondrial ETS function of Fundulus heteroclitus from a field site historically-contaminated with persistent organic pollutants to fish collected from a reference site and observed lower rates of state 4 respiration in hepatocytes from reference site fish hepatocytes, but no difference in rates of state 3 respiration (Du et al., 2015). These findings are in concordance with the results of the current study, and may indicate a cellular or physiological adaptive response of fish in polluted environments toward more efficient mitochondrial respiration.
A critical aspect of our mitochondrial function analysis was the normalization of mitochondrial OCRs per homogenate to mitochondrial content, as this led to the finding that respiration per unit mitochondria tended to be greater in fish from the polluted site. Without normalization to mitochondrial content, mitochondrial respiratory functional parameters in liver homogenates were similar among fish from the reference and impacted sites. However, as discussed, mitochondrial content tended to be lower in fish from the contaminated site, suggesting that liver mitochondria of contaminated site fish were respiring at a greater rate than reference site fish. Our findings demonstrate the importance of measuring mitochondrial ETS function as well as mitochondrial content, as this methodology allows for the determination of whether a chemical exposure-induced change in mitochondrial respiratory activity was caused by a change in ETS function, or rather a change in the quantity of mitochondria in tissue.
Bioaccumulation of CECs in laboratory-exposed Chinook
Accumulated concentrations of the emerging contaminants were measured in fish whole bodies rather than in isolated livers. Our rationale was that measuring whole-body concentrations, rather than tissue-specific concentrations, are more relevant for the assessment of bioaccumulation and for comparing toxicity among species, particularly for a given species at the same life history stage (i.e. juveniles in the current study) (Meador, 2006). Measuring whole-body concentrations also helped to mitigate potential allosteric effects due to differences in body size between the laboratory-exposed and field-collected fish, as internal chemical concentrations were used as a common dose metric among the two groups. It should be noted that allosteric effects are indeed important for determination of external exposure concentrations due to differences in uptake and elimination kinetics (Meador et al., 2008), but may be less important to consider when internal concentrations are being used as the dose metric.
Differences in body burdens of CECs between the low (0.3×), medium (1×), and high (10×) dose treatment groups were smaller than expected. In fact, bioaccumulation of CECs in the low dose group was similar or higher than in the medium dose group for eight of the mixture analytes, specifically, amitriptyline, amlodipine, azithromycin, diltiazem, diphenhydramine, fluoxetine, metformin, and miconazole. This may have been due to differences in reporting limits (RLs), which varied slightly between treatment groups due to the total mass of whole bodies that were analyzed, as many of the aforementioned analytes were detected close to their RLs in the low dose group. Alternatively, these differences in bioaccumulation may have been due to individual variance with respect to rates of feeding or respiration, but further experiments are required to validate these hypotheses. It should be noted that pharmacokinetic data for these compounds in fish is largely unavailable, and thus our experimental design regarding dosing and mixture concentrations of analytes was largely based on compound half-lives reported in humans. Clinical data from humans indicate that the half-lives of the tested compounds are short, specifically, 1.5–24 hrs for diphenhydramine, gemfibrozil, metformin, miconazole, sertraline, triclocarban, and triclosan, and 10–60 hrs for amitriptyline, amlodipine, azithromycin, and fluoxetine (Law et al., 2014; National Center for Biotechnology Information). With the exception of the perfluorinated compounds, whose half-lives are estimated to be years, these data support the hypothesis that biotransformation and excretion of the tested compounds occurred in the laboratory-exposed fish, resulting in smaller differences in accumulated concentrations at the end of the 32-day exposure period. However, further studies are required to validate this hypothesis.
Effects of CECs in laboratory-exposed Chinook
In contrast to the somewhat ambiguous trends in certain mitochondrial function parameters observed in field-collected fish, dysfunction of liver mitochondria was more clearly evident upon exposure to CECs in the laboratory. Significant reduction of liver CS activity was observed upon exposure to the medium dose of the mixture of CECs, and gene expression data showed reduced pgc1- expression by dietary exposure to CECs, underlying inhibition of mitochondrial biogenesis, and reduced expression of nrf-1, which regulates the expression of mitochondrial ETS subunits. Collectively, these results strongly suggest that environmental exposures to mixtures of CECs can cause a reduction in liver mitochondrial content in juvenile Chinook salmon. Other molecular markers of mitochondrial content, such as mitochondrial DNA (mtDNA), cardiolipin content, protein expression of ETS complexes I-V, and activities of complexes I-IV are of utility in future studies to elucidate the impact of environmental exposure to CECs on mitochondrial content in other tissues and species.
Similar to field-collected fish, the laboratory-exposed fish fed the contaminant mixture diets had no difference in rates of mitochondrial state 3 respiration per liver homogenate relative to controls, but trended toward reduced liver CS activity. Hence, normalization of OCRs to CS activity determined that exposure to CECs caused the respiration rates per mitochondria in liver to increase. The fact that this trend was also observed in field-collected fish provides further evidence to suggest that environmental exposures to mixtures of CECs may increase the respiratory activity per mitochondria, but additional analyses are warranted to verify this hypothesis. Given that the impacts of exposure to CECs (both in the field and laboratory) were sublethal, this finding suggests that the exposed fish were able to maintain homeostasis and cellular ATP demand despite a reduction of liver mitochondria, and therefore possessed fewer but more functionally-efficient liver mitochondria. This could have occurred via stimulation of mitophagy, increased expression of ETS complexes, morphological changes in the mitochondrial inner membrane and cristae, or stimulation of other pathways resulting in increased mitochondrial respiratory capacity. These and other physiological and cellular adaptive responses to maintain homeostasis and cellular ATP demand will be of interest in future experiments.
In contrast to the field-collected fish, exposure to the contaminant mixtures elevated state 4 respiration in the laboratory-exposed fish, which indicated reduced efficiency of oxidative phosphorylation as measured by RCR and RCRu. Similar elevation of state 4 respiration may not have occurred in field-collected fish due to the controlled nature of the laboratory study, which provided a consistent, daily dietary exposure to the mixture of CECs. Differences in the timing of exposures may have also played a role in the differential effect on state 4 respiration. The elevation of state 4 respiration in laboratory-exposed fish may indicate an early response to exposure to CECs that was not observed in the field-exposed fish. Alternatively, the laboratory exposure involved a mixture of only 16 representative CECs, whereas a complex mixture of chemicals is present in the Puget Sound aquatic environment. Indeed, thousands of compounds with varying modes of action are present that can ultimately cause protective or inhibitory effects on liver mitochondria. The dietary exposure-induced elevation of state 4 respiration may have been due to modulation of pathways associated with regulated, inducible proton conductance via adenine nucleotide translocase, or uncoupling proteins, which were not investigated in the current study. Several CECs in the mixture, including the pharmaceutical fluoxetine, (Souza et al., 1994), the PPCP triclosan (Shim et al., 2016), and the perfluorinated compound PFOSA (Schnellmann and Manning, 1990) uncouple mitochondrial respiration and increase rates of state 4 respiration in laboratory animal models. Both fluoxetine and PFOSA bioaccumulated in Chinook salmon; however, triclosan was below detection limits in whole-body samples but may have been present. All 16 analytes comprising the experimental mixture were detected in samples of field-collected fish, including the aforementioned chemicals that are potential mitochondrial uncouplers.
The physiological consequences of elevated mitochondrial state 4 respiration, which is a measure of respiration attributable to proton leak across the inner mitochondrial membrane, has been an area of interest to several investigators (Jastroch et al., 2010; Rolfe et al., 1999). Proton leak across mitochondrial membranes can vary between cell and tissue types. Across species, leak respiration is influenced by body size and whether an organism is an endotherm or ectotherm (Hulbert et al., 2002). Our results demonstrated that laboratory exposure to the mixture of CECs increased the rates of state 4 respiration in liver mitochondria, suggesting a reduction in the synthesis of ATP via oxidative phosphorylation and reduced metabolic capacity in the liver. This could suggest effects on growth and metabolic deficits. Indeed, our results indicated that exposure to the emerging contaminants caused deficits in growth, over the 32-day exposure period (unpublished results). One of the CECs in the experimental mixture was the pharmaceutical metformin, which is a known metabolic disruptor and inhibitor of ETS complex I. Recent reports suggest that metformin may impact growth in fish (Niemuth and Klaper, 2015). Other recent studies have demonstrated that survival of juvenile Chinook salmon, particularly during the first year spent in marine waters, is directly dependent on growth. Furthermore, juvenile Chinook salmon migrating through Puget Sound estuaries impacted by WWTP effluent exhibited significant reduction in survival compared to those migrating through uncontaminated estuaries (Meador, 2014). In this way, the sublethal effects of exposure to CECs may indirectly cause mortalities as a result of reduced population fitness (Spromberg and Meador, 2005).
Mitochondrial respiration is a major source of intracellular reactive oxygen species (ROS) (Brand, 2016). These ROS generated via oxidative phosphorylation by cross-reactions occurring at ETS complexes include superoxide, hydrogen peroxide, and hydroxyl radical, which can cause oxidative damage to proteins and nuclear and mitochondrial DNA, peroxidation of lipids, and depletion of mitochondrial and cytosolic glutathione. This is consistent with our laboratory study that showed an elevation of protein carbonylation in isolated liver mitochondria, induction of the mitochondrial-protective antioxidant gene gpx4, and a reduction of total mitochondrial glutathione in salmon exposed to emerging contaminants. The fact that expression of gclc mRNA, a driver of GSH biosynthesis, was not induced by exposure to CECs is consistent with the strict cellular control of mitochondrial GSH (Mari et al., 2009). However, we should also note that we did not discriminate reduced from oxidized mitochondrial GSH in the present study, which may better inform the effects of CECs on mitochondrial redox status. Interestingly, others have proposed that increased proton leak respiration is a cellular adaptive response against oxidative injury from mitochondrial ROS production, i.e., the “uncoupling to survive” hypothesis (Brand, 2000). Because oxidative injury was also associated with elevated state 4 respiration, our results may indicate a compensatory uncoupling of mitochondria in fish exposed to the mixture of emerging contaminants.
5. Conclusion
In the present study we observed modulation of both the function and content of mitochondria in liver of juvenile Chinook salmon after exposure to a mixture of CECs at environmentally-relevant concentrations. It is important to note that hatchery-reared, actively migrating Chinook salmon are different from laboratory-reared Chinook salmon with respect to a number of physiological parameters, including nutritional status (Congleton and Wagner, 2006). Despite these differences, both field-collected and laboratory-exposed fish exhibited similar trends and effects on liver mitochondrial content and with respect to a number of parameters related to mitochondrial function. As a critical aspect of molecular and physiological function, the liver mitochondrial OCRs reported herein may aid in future studies involving other salmonids or fish species. Importantly, the effects on liver mitochondrial content and function characterized in fish from the contaminated estuary were largely replicated under controlled laboratory conditions in an exposure study involving a representative mixture of CECs, underscoring the utility of Chinook salmon as a relevant biomonitoring species.
Supplementary Material
Highlights.
Elevated respiratory activity per mitochondria in Chinook salmon liver resulted from dietary exposure to a mixture of CECs.
Elevation of state 4 (proton leak) respiration suggested reduced efficiency of oxidative phosphorylation.
Mitochondrial metabolic changes were also associated with oxidative injury in liver mitochondria after dietary exposure.
Field-collected fish exposed to CECs showed similar but less severe trends in mitochondrial metabolism.
Results indicate that exposure to environmentally-relevant mixtures of CECs affects mitochondrial function in fish.
Acknowledgments
This work was supported by Washington Department of Ecology G1300089, UW NIEHS Superfund Research Program (NIEHS P42ES004696), UW NIEHS training grant in Environmental Pathology and Toxicology (T32 ES007032), and NIH grant RC2 AG036606.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashfield LA, Pottinger TG, Sumpter JP. Exposure of female juvenile rainbow trout to alkylphenolic compounds results in modifications to growth and ovosomatic index. Environ Toxicol Chem. 1998;17:679–686. [Google Scholar]
- Bisesi JH, Jr, Sweet LE, van den Hurk P, Klaine SJ. Effects of an antidepressant mixture on the brain serotonin and predation behavior of hybrid striped bass. Environ Toxicol Chem. 2016;35:938–945. doi: 10.1002/etc.3114. [DOI] [PubMed] [Google Scholar]
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Brodin T, Fick J, Jonsson M, Klaminder J. Dilute Concentrations of a Psychiatric Drug Alter Behavior of Fish from Natural Populations. Science. 2013;339:814–815. doi: 10.1126/science.1226850. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ. Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem. 2005;24:464–469. doi: 10.1897/04-081r.1. [DOI] [PubMed] [Google Scholar]
- Brown AR, Gunnarsson L, Kristiansson E, Tyler CR. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Philos T R Soc B. 2014:369. doi: 10.1098/rstb.2013.0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Truong D, Shangari N, O'Brien PJ. Drug-induced mitochondrial toxicity. Expert opinion on drug metabolism & toxicology. 2005;1:655–669. doi: 10.1517/17425255.1.4.655. [DOI] [PubMed] [Google Scholar]
- Colman JR, Baldwin D, Johnson LL, Scholz NL. Effects of the synthetic estrogen, 17alpha-ethinylestradiol, on aggression and courtship behavior in male zebrafish (Danio rerio) Aquat Toxicol. 2009;91:346–354. doi: 10.1016/j.aquatox.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Congleton JL, Wagner T. Blood-chemistry indicators of nutritional status in juvenile salmonids. J Fish Biol. 2006;69:473–490. [Google Scholar]
- Cooper RU, Clough LM, Farwell MA, West TL. Hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fish Leiostomus xanthurus. J Exp Mar Biol Ecol. 2002;279:1–20. [Google Scholar]
- County P. Pierce County Public Works and Utilities - Sewer Utility Unified Sewer Plan Update 2010 [Google Scholar]
- Crago J, Bui C, Grewal S, Schlenk D. Age-dependent effects in fathead minnows from the anti-diabetic drug metformin. General and comparative endocrinology. 2016;232:185–190. doi: 10.1016/j.ygcen.2015.12.030. [DOI] [PubMed] [Google Scholar]
- Department of Ecology SoW. Long-term marine water quality data 2014 [Google Scholar]
- Diamond JM, Latimer HA, Munkittrick KR, Thornton KW, Bartell SM, Kidd KA. Prioritizing Contaminants of Emerging Concern for Ecological Screening Assessments. Environ Toxicol Chem. 2011;30:2385–2394. doi: 10.1002/etc.667. [DOI] [PubMed] [Google Scholar]
- Dickenson ERV, Snyder SA, Sedlak DL, Drewes JE. Indicator compounds for assessment of wastewater effluent contributions to flow and water quality. Water Res. 2011;45:1199–1212. doi: 10.1016/j.watres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Du X, Crawford DL, Oleksiak MF. Effects of Anthropogenic Pollution on the Oxidative Phosphorylation Pathway of Hepatocytes from Natural Populations of Fundulus heteroclitus. Aquat Toxicol. 2015;165:231–240. doi: 10.1016/j.aquatox.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Hasspieler BM, Digiulio RT. Effects of Buthionine Sulfoximine and Diethyl Maleate on Glutathione Turnover in the Channel Catfish. Biochemical pharmacology. 1992;43:2209–2215. doi: 10.1016/0006-2952(92)90180-q. [DOI] [PubMed] [Google Scholar]
- Halden RU. Epistemology of contaminants of emerging concern and literature meta-analysis. J Hazard Mater. 2015;282:2–9. doi: 10.1016/j.jhazmat.2014.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding LB, Schultz IR, da Silva DA, Ylitalo GM, Ragsdale D, Harris SI, Bailey S, Pepich BV, Swanson P. Wastewater treatment plant effluent alters pituitary gland gonadotropin mRNA levels in juvenile coho salmon (Oncorhynchus kisutch) Aquat Toxicol. 2016;178:118–131. doi: 10.1016/j.aquatox.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Else PL, Manolis SC, Brand MD. Proton leak in hepatocytes and liver mitochondria from archosaurs (crocodiles) and allometric relationships for ectotherms. J Comp Physiol B. 2002;172:387–397. doi: 10.1007/s00360-002-0264-1. [DOI] [PubMed] [Google Scholar]
- Jasinska EJ, Goss GG, Gillis PL, Van Der Kraak GJ, Matsumoto J, Machado AAD, Giacomin M, Moon TW, Massarsky A, Gagne F, Servos MR, Wilson J, Sultana T, Metcalfe CD. Assessment of biomarkers for contaminants of emerging concern on aquatic organisms downstream of a municipal wastewater discharge. Sci Total Environ. 2015;530:140–153. doi: 10.1016/j.scitotenv.2015.05.080. [DOI] [PubMed] [Google Scholar]
- Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller BJ, Marsman DS, Popp JA, Thurman RG. Several nongenotoxic carcinogens uncouple mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 1992;1102:237–244. doi: 10.1016/0005-2728(92)90105-b. [DOI] [PubMed] [Google Scholar]
- Kim SK, Im JK, Kang YM, Jung SY, Kho YL, Zoh KD. Wastewater treatment plants (WWTPs)-derived national discharge loads of perfluorinated compounds (PFCs) J Hazard Mater. 2012;201:82–91. doi: 10.1016/j.jhazmat.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Kostich MS, Batt AL, Lazorchak JM. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ Pollut. 2014;184:354–359. doi: 10.1016/j.envpol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Langley AE. Effects of perfluoro-n-decanoic acid on the respiratory activity of isolated rat liver mitochondria. Journal of toxicology and environmental health. 1990;29:329–336. doi: 10.1080/15287399009531395. [DOI] [PubMed] [Google Scholar]
- Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu YF, Maciejewski A, Arndt D, Wilson M, Neveu V, Tang A, Gabriel G, Ly C, Adamjee S, Dame ZT, Han BS, Zhou Y, Wishart DS. DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 2014;42:D1091–D1097. doi: 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Couch L, Higuchi M, Fang JL, Guo L. Mitochondrial Dysfunction Induced by Sertraline, an Antidepressant Agent. Toxicol Sci. 2012;127:582–591. doi: 10.1093/toxsci/kfs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister A, Regan C, Van Zwol J, Van Der Kraak G. Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: a mechanistic evaluation. Aquat Toxicol. 2009;95:320–329. doi: 10.1016/j.aquatox.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lubliner B, Redding M, Ragsdale D. Pharmaceuticals and personal care products in municipal wastewater and their removal by nutrient treatment technologies 2010 [Google Scholar]
- Lucassen M, Koschnick N, Eckerle LG, Portner HO. Mitochondrial mechanisms of cold adaptation in cod (Gadus morhua L.) populations from different climatic zones. J Exp Biol. 2006;209:2462–2471. doi: 10.1242/jeb.02268. [DOI] [PubMed] [Google Scholar]
- Mandic M, Ramon ML, Gracey AY, Richards JG. Divergent transcriptional patterns are related to differences in hypoxia tolerance between the intertidal and the subtidal sculpins. Mol Ecol. 2014;23:6091–6103. doi: 10.1111/mec.12991. [DOI] [PubMed] [Google Scholar]
- Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxid Redox Sign. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WS, Emberley TR, Singer TD, Bryson SE, McCormick SD. Time course of salinity adaptation in a strongly euryhaline estuarine teleost, Fundulus heteroclitus: A multivariable approach. J Exp Biol. 1999;202:1535–1544. doi: 10.1242/jeb.202.11.1535. [DOI] [PubMed] [Google Scholar]
- Mccain BB, Malins DC, Krahn MM, Brown DW, Gronlund WD, Moore LK, Chan SL. Uptake of Aromatic and Chlorinated Hydrocarbons by Juvenile Chinook Salmon (Oncorhynchus-Tshawytscha) in an Urban Estuary. Arch Environ Con Tox. 1990;19:10–16. doi: 10.1007/BF01059807. [DOI] [PubMed] [Google Scholar]
- Mccormick SD, Moyes CD, Ballantyne JS. Influence of Salinity on the Energetics of Gill and Kidney of Atlantic Salmon (Salmo-Salar) Fish Physiol Biochem. 1989;6:243–254. doi: 10.1007/BF01875027. [DOI] [PubMed] [Google Scholar]
- Meador JP. Rationale and procedures for using the tissue-residue approach for toxicity assessment and determination of tissue, water, and sediment quality guidelines for aquatic organisms. Hum Ecol Risk Assess. 2006;12:1018–1073. [Google Scholar]
- Meador JP. Do chemically contaminated river estuaries in Puget Sound (Washington, USA) affect the survival rate of hatchery-reared Chinook salmon? Can J Fish Aquat Sci. 2014;71:162–180. [Google Scholar]
- Meador JP, McCarty LS, Escher BI, Adams WJ. 10th Anniversary Critical Review: The tissue-residue approach for toxicity assessment: concepts, issues, application, and recommendations. J Environ Monitor. 2008;10:1486–1498. doi: 10.1039/b814041n. [DOI] [PubMed] [Google Scholar]
- Meador JP, Sommers FC, Kubin L, Wolotira RJ. Techniques in Aquatic Toxicology. CRC Press; 2005. Conducting dose-response feeding studies with salmonids: growth as an endpoint; pp. 93–115. [Google Scholar]
- Meador JP, Sommers FC, Ylitalo GM, Sloan CA. Altered growth and related physiological responses in juvenile Chinook salmon (Oncorhynchus tshawytscha) from dietary exposure to polycyclic aromatic hydrocarbons (PAHs) Can J Fish Aquat Sci. 2006;63:2364–2376. [Google Scholar]
- Meador JP, Yeh A, Young G, Gallagher EP. Contaminants of emerging concern in a large temperate estuary. Environ Pollut. 2016;213:254–267. doi: 10.1016/j.envpol.2016.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Leung MCK, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a Target of Environmental Toxicants. Toxicol Sci. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills MG, Gallagher EP. A targeted gene expression platform allows for rapid analysis of chemical-induced antioxidant mRNA expression in zebrafish larvae. PloS one. 2017;12:e0171025. doi: 10.1371/journal.pone.0171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MS, Stehr CM, Olson OP, Johnson LL, Mccain BB, Chan SL, Varanasi U. Relationships between Toxicopathic Hepatic-Lesions and Exposure to Chemical Contaminants in English Sole (Pleuronectes-Vetulus), Starry Flounder (Platichthys-Stellatus), and White Croaker (Genyonemus-Lineatus) from Selected Marine Sites on the Pacific Coast, USA. Environ Health Persp. 1994;102:200–215. doi: 10.1289/ehp.94102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu R, Jit J, Kennedy B, Arias V. Emerging contaminant uncertainties and policy: The chicken or the egg conundrum. Chemosphere. 2016;154:385–390. doi: 10.1016/j.chemosphere.2016.03.110. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Database [Google Scholar]
- Niemuth NJ, Jordan R, Crago J, Blanksma C, Johnson R, Klaper RD. Metformin exposure at environmentally relevant concentrations causes potential endocrine disruption in adult male fish. Environmental toxicology and chemistry/SETAC. 2015;34:291–296. doi: 10.1002/etc.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemuth NJ, Klaper RD. Emerging wastewater contaminant metformin causes inters ex and reduced fecundity in fish. Chemosphere. 2015;135:38–45. doi: 10.1016/j.chemosphere.2015.03.060. [DOI] [PubMed] [Google Scholar]
- Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL. Antidepressants at Environmentally Relevant Concentrations Affect Predator Avoidance Behavior of Larval Fathead Minnows (Pimephales Promelas) Environ Toxicol Chem. 2009;28:2677–2684. doi: 10.1897/08-556.1. [DOI] [PubMed] [Google Scholar]
- Ramsden R, Gallagher EP. Dual NRF2 paralogs in Coho salmon and their antioxidant response element targets. Redox Biol. 2016;9:114–123. doi: 10.1016/j.redox.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe DFS, Newman JMB, Buckingham JA, Clark MG, Brand MD. Contribution of mitochondrial proton leak to respiration rate in working skeletal muscle and liver and to SMR. Am J Physiol-Cell Ph. 1999;276:C692–C699. doi: 10.1152/ajpcell.1999.276.3.C692. [DOI] [PubMed] [Google Scholar]
- Schnellmann RG, Manning RO. Perfluorooctane Sulfonamide - a Structurally Novel Uncoupler of Oxidative-Phosphorylation. Biochimica Et Biophysica Acta. 1990;1016:344–348. doi: 10.1016/0005-2728(90)90167-3. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Bartell SE, Schoenfuss HL. Effects of Triclosan and Triclocarban, Two Ubiquitous Environmental Contaminants, on Anatomy, Physiology, and Behavior of the Fathead Minnow (Pimephales promelas) Arch Environ Con Tox. 2012;63:114–124. doi: 10.1007/s00244-011-9748-x. [DOI] [PubMed] [Google Scholar]
- Shim J, Weatherly LM, Luc RH, Dorman MT, Neilson A, Ng R, Kim CH, Millard PJ, Gosse JA. Triclosan is a mitochondrial uncoupler in live zebrafish. Journal of Applied Toxicology. 2016;36:1662–1667. doi: 10.1002/jat.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrimpton JM, Bernier NJ, Iwama GK, Randall DJ. Differences in Measurements of Smolt Development between Wild and Hatchery-Reared Juvenile Coho Salmon (Oncorhynchus-Kisutch) before and after Saltwater Exposure. Can J Fish Aquat Sci. 1994;51:2170–2178. [Google Scholar]
- Souza HEJ, Polizello ACM, Uyemura SA, Castrosilva O, Curti C. Effect of Fluoxetine on Rat-Liver Mitochondria. Biochemical pharmacology. 1994;48:535–541. doi: 10.1016/0006-2952(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Spromberg JA, Meador JP. Relating Results of Chronic Toxicity Responses to Population-Level Effects: Modeling Effects on Wild Chinook Salmon Populations. Integr Environ Asses. 2005;1:9–21. doi: 10.1897/ieam_2004a-005.1. [DOI] [PubMed] [Google Scholar]
- Varanasi U, Casillas E, Arkoosh MR, Hom T, Misitano DA, Brown DW, Chan SL, Collier TK, Mccain BB, Stein JE. Contaminant exposure and associated biological effects in juvenile chinook salmon (Oncorhynchus tshawytscha) from urban and nonurban estuaries of puget sound 1993 [Google Scholar]
- Vidal-Dorsch DE, Bay SM, Maruya K, Snyder SA, Trenholm RA, Vanderford BJ. Contaminants of emerging concern in municipal wastewater effluents and marine receiving water. Environ Toxicol Chem. 2012;31:2674–2682. doi: 10.1002/etc.2004. [DOI] [PubMed] [Google Scholar]
- Wallace KB, Starkov AA. Mitochondrial targets of drug toxicity. Annu Rev Pharmacol. 2000;40:353–388. doi: 10.1146/annurev.pharmtox.40.1.353. [DOI] [PubMed] [Google Scholar]
- Weatherly LM, Shim JY, Hashmi HN, Kennedy RH, Hess ST, Gosse JA. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. Journal of Applied Toxicology. 2016;36:777–789. doi: 10.1002/jat.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Qiu WH, Chen JS, Zhan J, Pan CY, Lei XJ, Wu MH. Growth inhibition and coordinated physiological regulation of zebrafish (Danio rerio) embryos upon sublethal exposure to antidepressant amitriptyline. Aquat Toxicol. 2014;151:68–76. doi: 10.1016/j.aquatox.2013.12.029. [DOI] [PubMed] [Google Scholar]
- Yeh A, Kruse SE, Marcinek DJ, Gallagher EP. Effect of omega-3 fatty acid oxidation products on the cellular and mitochondrial toxicity of BDE 47. Toxicol in Vitro. 2015;29:672–680. doi: 10.1016/j.tiv.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BS, Wu RSS, Randall DJ, Lam PKS, Ip YK, Chew SF. Metabolic adjustments in the common carp during prolonged hypoxia. J Fish Biol. 2000;57:1160–1171. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.