Abstract
Background
Elevated uric acid is a prevalent condition with controversial health consequences. Observational studies disagree with regard to the relationship of uric acid with mortality, and with factors modifying this relationship.
Objective
We examined the association of serum uric acid with mortality in 15,083 participants in the Scottish Heart Health Extended Cohort (SHHEC) Study.
Methods
Serum uric acid measured at study enrollment. Death was ascertained using both the Scottish death register and record linkage.
Results
During a median follow-up of 23 years, there were 3,980 deaths. In Cox proportional hazards models with sexes combined, those in the highest fifth of uric acid had significantly greater mortality (HR 1.18, 95% CI: 1.06, 1.31) compared with the second fifth, after adjustment for traditional cardiovascular risk factors. This relationship was modified by sex (P-interaction = 0.002) with adjusted HRs of 1.69 (95% CI: 1.40, 2.04) and 0.99 (95% CI: 0.86, 1.14) in women and men, respectively. Compared with the second fifth, the highest fifth of uric acid was most associated with kidney-related death (HR: 2.08, 95% CI: 1.31, 3.32).
Conclusion
Elevated uric acid is associated with earlier mortality, especially in women. Future studies should evaluate mechanisms for these interactions and explore the strong association with renal-related mortality.
Keywords: Scottish Heart Health Extended Cohort (SHHEC), uric acid, hyperuricemia, mortality, cohort, cardiovascular disease, kidney disease, cancer
Introduction
Elevated serum uric acid is a highly prevalent condition. Over 38 million adults meet criteria for hyperuricemia (>416 μmol/L in men and >357 μmol/L in women) in the US alone [1]. Nevertheless, the implications of an elevated uric acid are incompletely elucidated. Uric acid has been viewed as a potent antioxidant [2] with protective effects toward inflammation and circulating free radicals [3]. However, multiple observational studies have demonstrated that elevations in uric acid are associated with excess mortality [4–8]. Such an association has been inconsistently reported, however [9–11]. Furthermore, whether demographic, lifestyle, or mortality-related risk factors modify the relationship between uric acid and mortality is an active area of controversy [12]. Likewise there is no consensus on the causes of death most associated with hyperuricemia.
The purpose of this study was to explore the relationship between uric acid and mortality, utilizing the large size and long-term follow-up of the Scottish Heart Health Extended Cohort (SHHEC) study. We evaluate whether the relationship between uric acid and mortality is modified by demographic, lifestyle, and risk factors related to mortality. Moreover, we examine whether specific causes of death are responsible for the observed association between uric acid and mortality.
Materials/methods
Study Population
The SHHEC study is a population-based, prospective cohort, which includes both The Scottish Heart Health Study [13–15] and the Scottish Multinational MONItoring of Trends and Determinants in CArdiovascular Disease (MONICA) Project, sharing a common protocol approved by ethics committees from multiple countries [14–16]. Men and women aged 40–59 were recruited in 1984–1987 across 25 districts of Scotland for participation in The Scottish Heart Health Study [13–15]. The Scottish MONICA Project recruited adults from Edinburgh in 1986 and north Glasgow in 1986, 1989, 1992, and 1995 [15,16]. Age ranges differed between studies, but overall were 25–76 years [14,15]. Participants completed health questionnaires, a physical examination, and a venous blood draw at study entry and gave written consent to being followed-up through their medical records. They were flagged on the National Health Service Register and matched with the Scottish record linkage system both for deaths and hospital admissions [17].
Serum Uric Acid
Serum uric acid was measured using a uricase-peroxidase enzymatic method on a Cobas Bio centrifugal analyzer standardized in a national (Wellcome) scheme. The inter-batch coefficient of variation (not including later MONICA surveys) was 3.6% and intra-batch was 0.9% [18]. Serum was separated within two hours and stored at +4°C before transfer within five days to Dundee where it was analyzed for uric acid on arrival [14]. Hyperuricemia was defined as a uric acid concentration >416 (7 mg/dL) μmol/L in men and >357 μmol/L (6 mg/dL) in women [19].
Primary & Secondary Outcomes
The primary outcome in this study was mortality. Death was ascertained using death registrations and the national record linkage database through 2009 [15,17]. Examination of cause-specific death was accomplished using the following International Classification of Diseases (ICD) codes listed among causes of death on participants’ death certificates: cardiovascular death (ICD9 codes 390–459, ICD10 codes I00-I99), cancer-related death (ICD9 codes 140–239; ICD10 codes C00–C97, D00–D49), or renal-related death (ICD9 codes 580–586; ICD10 codes N00–N19). Cardiovascular-related deaths were also examined in greater detail, looking specifically at congestive heart failure (ICD9 code 428; ICD10 code I50), coronary heart disease (ICD9 codes 410–414, ICD10 codes I20–I25), cerebrovascular disease (ICD9 codes 430–438, ICD10 codes I60–I69), peripheral vascular disease (ICD9 code 443, 250.7; ICD10 code E10.5, E11.5, E12.5, E13.5, E14.5, I73), and thromboembolic disease (ICD9 code 444; ICD10 code I74) mortality. The above causes of death were not mutually exclusive. We also examined primary causes of death (these were mutually exclusive) in the following categories: acute coronary heart disease (ICD9 codes 410–412, ICD10 codes I20.0–120.1, I21–24), chronic coronary heart disease (ICD9 codes 413–4, ICD10 codes I20.8–20.9, I25), acute stroke (ICD9 codes 435, ICD10 codes I63–64), chronic cerebrovascular disease (ICD9 code 437, ICD10 codes I65-I67), other cardiovascular disease (ICD9 codes 390–409, 415–429, 440–459, ICD10 codes I00–15, I26–52, I70–99), respiratory disease (ICD9 codes 460–519, ICD10 codes J00-J99), cancer (ICD 9 codes 140–239; ICD 10 codes C00–C97, D00–D49), and all other causes of death.
Risk Factors Related to Mortality
Study personnel were trained in data collection, following standardized protocols described elsewhere [13,14]. Age, sex, blood pressure medication use, diabetes mellitus status, smoking status, number of cigarettes per day, and daily alcohol consumption were self-reported. Systolic and diastolic blood pressures were based on an average of 2 measurements; body mass index (BMI) was assessed during the physical examination at baseline. Total cholesterol and high density lipoprotein cholesterol (HDLc) were measured in serum specimens. The Scottish Index of Multiple Deprivation (SIMD), derived from postcode variables, was used to indicate social status, with higher values indicating greater deprivation [15,20]. Estimated glomerular filtration rate (eGFR) based on measured serum creatinine was calculated with The Chronic Kidney Disease Epidemiology Collaboration equation [21].
Statistical Analyses
Our study population was limited to the participants who were not missing a valid uric acid measurement (N = 1,811) and who were not missing risk factors at baseline thought to be related to mortality (N = 1,213). Baseline study population characteristics were summarized via means and proportions across fifths of uric acid. Trends were evaluated across fifths via linear or logistic regression using the median uric acid value in each fifth as an ordinal variable. Trends models were adjusted for age and sex. The distribution of uric acid concentrations by mortality status was compared via two-sample Kolmogorov-Smirnov equality-of-distributions tests, and the medians of the distributions were compared via quantile regression.
Crude cumulative incidence across uric acid fifths was examined via Kaplan-Meier curves with the trend across curves assessed via a logrank test. Nested Cox proportional hazard models were utilized to compare the relationship of uric acid fifths, hyperuricemia (>416 μmol/L in men and >357 μmol/L in women), or uric acid as a continuous variable (per 100 μmol/L) with the risk of mortality. Model 1 was adjusted for age and sex. Model 2 was adjusted for Model 1 covariates as well as systolic blood pressure, diastolic blood pressure, blood pressure-lowering medication use, a systolic blood pressure-blood pressure medication use interaction term, BMI, total cholesterol, HDLc, smoking status, diabetes mellitus, and daily alcohol consumption. Model 3 was adjusted for all the covariates in Model 2 plus SIMD. A restricted cubic spline model with knots at each of the fifth cutpoints was utilized to visualize the continuous relationship between uric acid and the hazard of mortality after adjusting for all Model 3 covariates. Both splines and survival models utilized the second fifth as the reference due to the observation of a mild increase in hazard in the lowest fifth. Trends were evaluated across fifths in Cox proportional hazard models using the median value of uric acid in each fifth as an ordinal variable.
With the second fifth as a reference, we examined the risk of mortality in strata of baseline covariates, namely, age (<40, 40–49, 50–59, and ≥60 years), sex, hypertension status (defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or blood pressure lowering medication use), smoking status (current, yes or no), high total cholesterol (≥ 6.22 mmol/L, yes or no), low HDLc (women <1.295 mmol/L; men <1.036 mmol/L, yes or no), obese (BMI ≥30 or <30 kg/m2), diabetes (yes or no), alcohol use (none or any), and the SIMD (> median value of 22.7 units, yes or no). Each comparison was adjusted for all the Model 3 covariates. Interaction terms were added to Model 3 to determine whether or not the strata were significantly different. We also performed a sensitivity analysis, examining the association between uric acid and mortality by sex-specific strata, generating spline models to visualize these associations.
We characterized the different causes of death, divided into cardiovascular, cancer, and renal causes. Cardiovascular-related deaths were further divided into congestive heart failure, coronary heart disease, cerebrovascular disease, peripheral vascular disease, and thromboembolic mortality. Each of these mortality outcomes was examined using the same exposure variables described previously with adjustment for all Model 3 covariates. In addition we tabulated the primary cause of death by hyperuricemia status and compared the two with Cox proportional hazard models, using model 3 adjustments.
Finally, in a post-hoc sensitivity analysis, we evaluated whether or not adjustment for eGFR significantly altered our findings. This was not included in our initial models due to the high number of missing serum creatinine results (N = 2,472).
Results
The mean uric acid concentration overall (N = 15,083) was 283.5 μmol/L (SD, 75.4) (Table 1), ranging from 37.9 to 782.8 μmol/L. The proportion of the study population with hyperuricemia was 6.7% (N = 1,003), which was 62% men (N = 623). Age, proportion male, BMI, total cholesterol, systolic blood pressure, diastolic blood pressure, proportion blood pressure-lowering medication use, alcohol use, and the SIMD were significantly greater across fifths of uric acid (P for all trends <0.001). HDLc and proportion of cigarette smokers were lower across fifths of uric acid (P for trends both <0.001). There was no trend in number of cigarettes used per day among smokers. Despite demonstrating a significant trend across fifths, the proportion of participants with diabetes was greatest in the lowest and highest fifths of uric acid; although diabetes was rare in this population at baseline.
Table 1.
Baseline Characteristics of Participants by Fifth of Serum Uric Acid
| Serum Uric Acid Level (μmol/L)
|
|||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 15,083) |
37.88 – 218.55 (n = 3,017) |
218.56 – 258.97 (n = 3,017) |
259.01 – 296.58 (n = 3,016) |
296.59 – 344.41 (n = 3,017) |
344.43 – 782.75 (n = 3,016) |
P-trend* | |
| Serum Uric Acid, μmol/L (SD)† | 283.5 (75.4) | 186.9 (25.5) | 239.0 (11.5) | 277.3 (10.8) | 318.9 (13.6) | 395.4 (47.4) | – |
| Age, yr (SD) | 48.7 (9.3) | 46.6 (8.7) | 48.5 (9.1) | 49.2 (9.3) | 49.5 (9.6) | 49.5 (9.6) | <0.001 |
| Male, No. (%) | 7,559 (50.1) | 299 (9.9) | 890 (29.5) | 1,676 (55.6) | 2,172 (72.0) | 2,522 (83.6) | <0.001 |
| Body Mass Index, kg/m2 (SD) | 25.8 (4.2) | 24.0 (3.7) | 25.1 (3.9) | 25.8 (3.9) | 26.6 (4.2) | 27.7 (4.5) | <0.001 |
| Total Cholesterol, mmol/L (SD)‡ | 6.3 (1.2) | 6.1 (1.2) | 6.3 (1.3) | 6.3 (1.3) | 6.3 (1.2) | 6.5 (1.2) | <0.001 |
| HDLc, mmol/L (SD)‡ | 1.5 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.5 (0.4) | 1.4 (0.4) | 1.3 (0.4) | <0.001 |
| Systolic Blood Pressure, mmHg (SD) | 131.8 (20.3) | 126.8 (19.3) | 129.8 (20.4) | 132.3 (19.9) | 133.8 (20.5) | 136.4 (20.1) | <0.001 |
| Diastolic Blood Pressure, mmHg (SD) | 81.1 (12.0) | 77.6 (11.3) | 79.5 (11.7) | 81.1 (11.7) | 82.6 (12.0) | 84.7 (12.1) | <0.001 |
| Blood Pressure-Lowering Medication Use, No. (%) | 1,849 (12.3) | 146 (4.8) | 271 (9.0) | 322 (10.7) | 423 (14.0) | 687 (22.8) | <0.001 |
| Cigarette Smokers, No. (%) | 5,778 (38.3) | 1,262 (41.8) | 1,238 (41.0) | 1,221 (40.5) | 1,127 (37.4) | 930 (30.8) | <0.001 |
| Cigarettes per Day among Smokers (SD) | 18.5 (9.1) | 17.5 (8.0) | 18.1 (8.5) | 18.9 (9.2) | 19.0 (9.5) | 19.4 (10.4) | 0.19 |
| Diabetes, No. (%) | 246 (1.6) | 73 (2.4) | 40 (1.3) | 43 (1.4) | 44 (1.5) | 46 (1.5) | <0.001 |
| Alcohol Use (g/d) | 14.1 (20.6) | 6.5 (9.9) | 9.7 (15.4) | 12.8 (17.6) | 17.4 (22.1) | 24.3 (28.3) | <0.001 |
| Scottish Index of Multiple Deprivation | 28.9 (22.2) | 28.5 (22.0) | 28.4 (21.9) | 29.0 (22.3) | 29.2 (22.6) | 29.2 (22.2) | 0.003 |
P-trend evaluated with linear or logistic regression using the median uric acid value for each fifth as an ordinal variable adjusted for age and sex.
The median serum uric acid was 276.72 (interquartile range: 229.27, 330.29).
Convert cholesterol to mg/dL by dividing by 0.0259
During a median of 22.7 years of follow-up (range: 3 days to 25 years), there were 3,980 deaths (26.3%). The unadjusted cumulative incidence by fifth of uric acid may be viewed in Supplemental Material, Figure S1. Compared with the second fifth, the upper fifth of uric acid had a hazard ratio of 1.18 (95% CI: 1.06, 1.31; P = 0.003). Furthermore, there was a significant, graded relationship across fifths of baseline uric acid and risk of death even after adjustment (Model 3, P trend =0.008) (Table 2). Hyperuricemia was associated with 39% greater in-study mortality (HR 1.39; 95% CI: 1.24, 1.55; P <0.001). Similarly, as a continuous variable, uric acid (per 100 μmol/L) was significantly associated with a 1.13 times greater risk of mortality even after fully adjusting for covariates (95% CI: 1.07, 1.19; P <0.001).
Table 2.
Hazard Ratios for Mortality According to Fifths of Uric Acid Concentration, Hyperuricemia, and Continuous Uric Acid Concentrations (N=15,083)
| Hazard Ratio (95% CI)
|
|||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Fifths of uric acid, μmol/L | |||
| 37.88 – 218.55 | 1.01 (0.90, 1.13) | 1.04 (0.92, 1.16) | 1.03 (0.92, 1.16) |
| 218.56 – 258.97 | 1 (reference) | 1 (reference) | 1 (reference) |
| 259.01 – 296.58 | 1.03 (0.93, 1.14) | 1.02 (0.92, 1.13) | 1.03 (0.93, 1.14) |
| 296.59 – 344.41 | 1.04 (0.94, 1.16) | 1.03 (0.93, 1.14) | 1.04 (0.93, 1.15) |
| 344.43 – 782.75 | 1.20 (1.08, 1.33) | 1.16 (1.04, 1.29) | 1.18 (1.06, 1.31) |
| P trend across fifths | <0.001 | 0.02 | 0.008 |
| Hyperuricemia | 1.49 (1.34, 1.65) | 1.39 (1.24, 1.55) | 1.39 (1.24, 1.55) |
| P value | <0.001 | <0.001 | <0.001 |
| Uric acid per 100 μmol/L | 1.15 (1.09, 1.20) | 1.12 (1.07, 1.18) | 1.13 (1.07, 1.19) |
| P value | <0.001 | <0.001 | <0.001 |
Model 1: Adjusted for age and sex
Model 2: Model 1 + systolic blood pressure, diastolic blood pressure, blood pressure medication use, systolic blood pressure & medication use interaction, smoking status, number of cigarettes per day among smokers, total cholesterol, high density lipoprotein cholesterol, body mass index, baseline diabetes status, and daily alcohol use
Model 3: Model 2 + Scottish Index of Multiple Deprivation
In a sensitivity analysis, restricting our analysis to participants without hyperuricemia eliminated the association between uric acid and mortality (Supplemental Material, Table S1). Similarly, in a sensitivity analysis using the first rather than the second fifth as a reference (Supplemental Material, Table S2), the upper fifth of uric acid was still associated with mortality risk (HR 1.14, 95% CI: 1.00, 1.29; P = 0.04). When examined in strata by sex, we found that uric acid was a stronger predictor in women versus men (Supplemental Material, Table S3, Table S4, & Figure S2).
Figure 1 demonstrates the fully adjusted hazard ratios for mortality according to uric acid concentration. In general, uric acid concentrations above 380 μmol/L demonstrated a linearly increasing relationship with mortality. This value is similar to the cutpoint for hyperuricemia in women (356.9 μmol/L). There was no evidence of a threshold effect for the risk of death. When examined by sex, we found the association to be stronger among women and significant at lower values of uric acid (Supplemental Material, Figure S2). Comparison of the probability density of uric acid by mortality status (Supplemental Material, Figure S3) supported the above findings, in that the baseline uric acid concentrations were higher in participants that experienced a fatal event versus those that did not experience a fatal event (P-value <0.001). Similarly, the median value of baseline uric acid concentration among mortality cases was 292 μmol/L versus 272 μmol/L among non-cases (P-value <0.001 via quantile regression).
Figure 1.
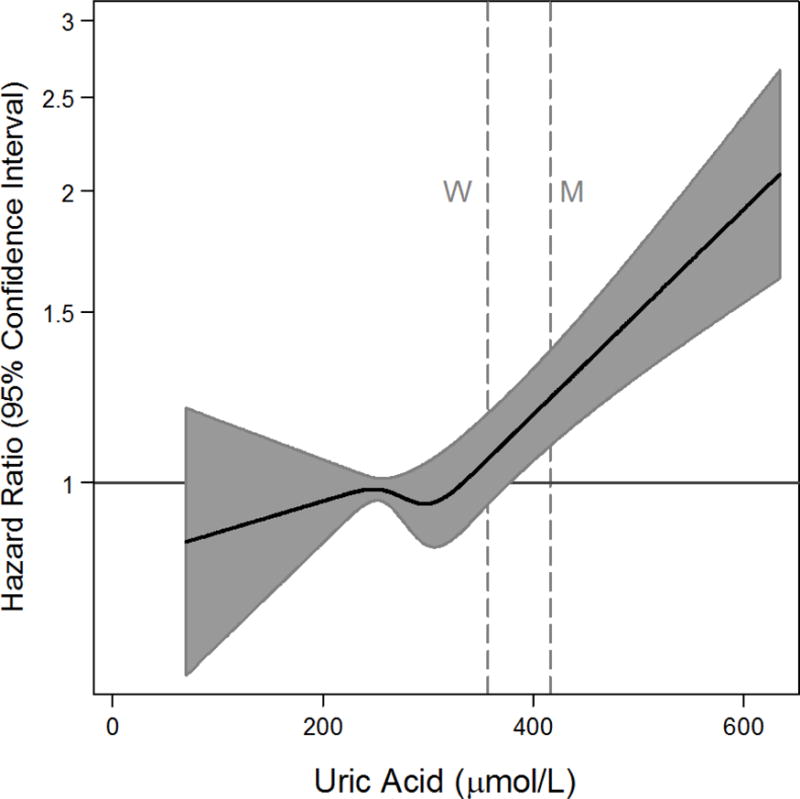
Adjusted hazard ratios (solid line) from restricted cubic spline models for mortality, using overall fifths of baseline uric acid. Gray shading represents the 95% confidence intervals. The models were expressed relative to the 40th percentile with knots specified at the 20th, 40th, 60th, and 80th percentiles and were adjusted for age, sex, systolic blood pressure, diastolic blood pressure, blood pressure medication use, systolic blood pressure & medication use interaction, smoking status, number of cigarettes per day among smokers, total cholesterol, high density lipoprotein cholesterol, body mass index, baseline diabetes status, daily alcohol use, and the Scottish Index of Multiple Deprivation. The plots were truncated at the 0.5th and 99.5th percentiles. The hazard ratios are shown on a natural log scale. Vertical lines depict male (M) and female (F) cutpoints for hyperuricemia.
We compared the hazard of participants with hyperuricemia to participants without hyperuricemia by strata of baseline covariates (Figure 2). While higher uric acid concentrations were not associated with mortality in males, there was a strong association in women (HR 1.68; 95% CI: 1.41, 2.00). These strata were significantly different from each other (P-interaction = 0.03). Similarly, we found evidence of effect modification by strata of HDL cholesterol (P-interaction = 0.02) and diabetes (P-interaction = 0.02).
Figure 2.
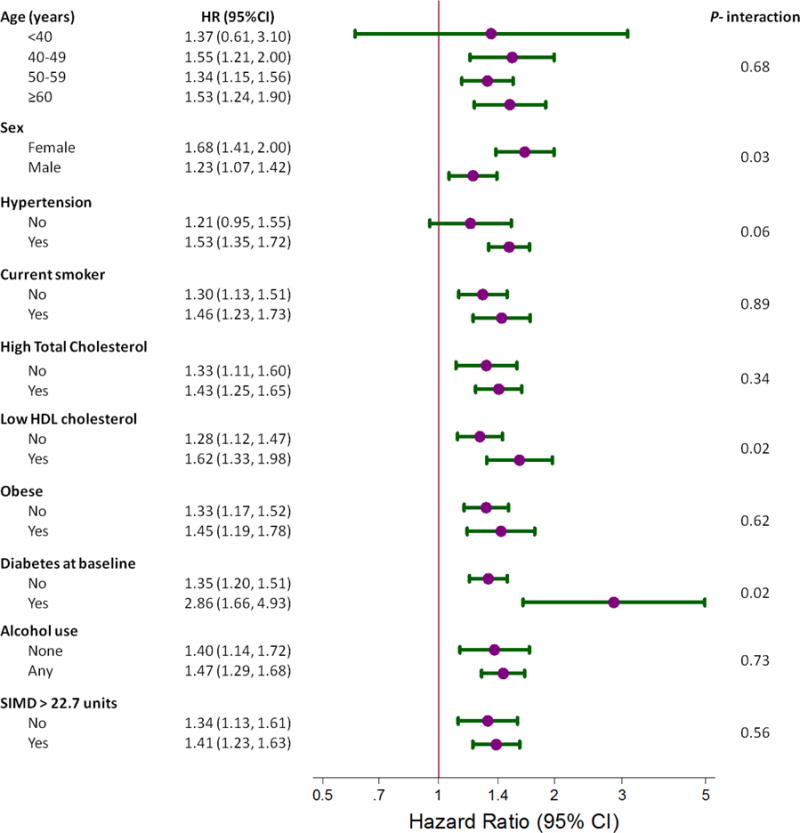
Forest plot portraying the hazard ratio and 95% confidence interval of mortality, comparing participants with hyperuricemia (>416.36 μmol/L in men and >356.88 μmol/L in women) to participants without hyperuricemia. All strata were adjusted for age, sex, systolic blood pressure, diastolic blood pressure, blood pressure medication use, systolic blood pressure & medication use interaction, smoking status, number of cigarettes per day among smokers, total cholesterol, high density lipoprotein cholesterol, body mass index, baseline diabetes status, daily alcohol use, and the Scottish Index of Multiple Deprivation (SIMD). P-values comparing strata were determined using interaction terms.
The proportion of different causes of death among mortality cases may be seen in Table 4. Cardiovascular-related mortality was present in 50.9% of mortality cases and cancer in 40.5%. Of the different diseases evaluated, uric acid (fifths, hyperuricemia, and as a continuous variable) was most associated with deaths where kidney disease was listed among the participants’ cause of death (Table 3). Hyperuricemia (not fifths of uric acid) was associated with mortality related to cardiovascular disease (P <0.001), cancer (P = 0.01), congestive heart failure (P = 0.001) and coronary heart disease (P = 0.04) (Table 3 & Supplemental Material S5). Although hyperuricemia was not significantly associated with mortality related to thromoembolic disease (P = 0.07), both the trend across fifths of uric acid as well as uric acid as a continuous variable (per 100 μmol/L) were significant (P-values of 0.008 and 0.004, respectively). When we examined primary causes of death it was found that hyperuricemia was associated with mortality from chronic cerebrovascular disease and respiratory disease (Supplemental Material, Table S6).
Table 4.
Cause of Death
| N | % of deaths | % of CVD deaths | % of cancer deaths | % of kidney deaths | |
|---|---|---|---|---|---|
| Mortality from any cause | 3,980 | 100.0 | – | – | – |
| CVD mortality | 2,026 | 50.9 | 100.0 | 17.8 | 63.4 |
| CHF mortality | 373 | 9.4 | 18.4 | 1.9 | 19.0 |
| CHD mortality | 1,233 | 31.0 | 60.9 | 8.3 | 31.7 |
| Cerebrovascular mortality | 430 | 10.8 | 21.2 | 2.4 | 8.6 |
| PVD mortality | 161 | 4.1 | 8.0 | 1.1 | 7.7 |
| Thromboembolic mortality | 90 | 2.3 | 4.4 | 2.3 | 3.6 |
| Cancer mortality | 1,613 | 40.5 | 14.2 | 100.0 | 22.6 |
| Kidney mortality | 221 | 5.6 | 6.9 | 3.1 | 100.0 |
| None of the above | 578 | 14.5 | 0.0 | 0.0 | 0.0 |
Abbreviations: CVD, cardiovascular disease; CHF, congestive heart failure; CHD, coronary heart disease; PVD, peripheral vascular disease
Table 3.
Hazard Ratios (95% CI) for Specific Causes of Mortality According to Fifths of Uric Acid Concentration, Hyperuricemia, and Continuous Uric Acid Concentrations (N=15,083)
| Cardiovascular Mortality | Cancer-related Mortality | Kidney-related Mortality | |
|---|---|---|---|
| Fifths of uric acid | |||
| 37.88 – 218.55 | 1.12 (0.95, 1.32) | 0.93 (0.78, 1.10) | 0.64 (0.35, 1.18) |
| 218.56 – 258.97 | 1 (reference) | 1 (reference) | 1 (reference) |
| 259.01 – 296.58 | 0.95 (0.82, 1.10) | 0.99 (0.85, 1.15) | 1.38 (0.87, 2.18) |
| 296.59 – 344.41 | 0.98 (0.84, 1.13) | 0.88 (0.75, 1.03) | 1.35 (0.84, 2.17) |
| 344.43 – 782.75 | 1.14 (0.98, 1.32) | 1.02 (0.86, 1.21) | 2.08 (1.31, 3.32) |
| P trend across fifths | 0.28 | 0.69 | <0.001 |
| Hyperuricemia | 1.40 (1.21, 1.62) | 1.27 (1.05, 1.53) | 3.10 (2.17, 4.43) |
| P value | <0.001 | 0.014 | <0.001 |
| Uric acid per 100 μmol/L | 1.11 (1.04, 1.20) | 1.06 (0.98, 1.15) | 1.92 (1.58, 2.34) |
| P value | 0.003 | 0.17 | <0.001 |
All models adjusted for age, sex, systolic blood pressure, diastolic blood pressure, blood pressure medication use, systolic blood pressure & medication use interaction, smoking status, number of cigarettes per day among smokers, total cholesterol, high density lipoprotein cholesterol, body mass index, baseline diabetes status, daily alcohol use, and the Scottish Index of Multiple Deprivation
There was little effect on our initial findings after including eGFR in our survival models (Supplemental Material, Tables S7–8).
Discussion
This study represents one of the largest and longest prospective cohort studies of the association between uric acid and mortality risk in a general, community-based population. Over a median of 23 years of follow-up, we observed a strong relationship between uric acid and mortality, particularly among participants with hyperuricemia at baseline. Furthermore, we found evidence of effect modification by sex, HDLc concentration, and baseline diabetes status. Uric acid was most predictive of kidney-related death, although hyperuricemia was also significantly associated with cardiovascular disease mortality, congestive heart failure mortality, and cancer mortality. There was also evidence that elevated uric acid was associated with death related to thromboembolic disease.
Uric acid is a product of purine metabolism that is produced by xanthine oxidase as DNA and RNA degrades [22]. Uric acid is subsequently filtered by the glomeruli, reabsorbed in the proximal tubule only to be later secreted by a distal segment of the proximal tubule [23]. Serum concentrations of uric acid are affected by diet [24], metabolism [25], cell turnover [26], and kidney function [27]. There is substantial evidence suggesting that uric acid confers antioxidant capability to humans [28]. In fact it has been hypothesized that humans’ evolved inability to breakdown uric acid carried a survival advantage [3]. Despite evidence supporting a positive role for uric acid, multiple observational studies have demonstrated that uric acid is associated with mortality [4–8,29]. In our study, we observed that uric acid in the upper range (the highest fifth or in persons with hyperuricemia) showed the greatest association with mortality. Whether or not uric acid directly contributes to mortality or is a surrogate of ambient disease is an active area of debate [30].
Uric acid was more strongly associated with mortality in women compared with men. This is consistent with a number of other observational studies that show uric acid measurements in women are more strongly with mortality [31–33], cardiovascular mortality [8,12,31,32,34,35], stroke [32], and cardiovascular events [36]. The exact mechanism of this relationship is unknown. The difference across sexes may be related to estrogen, which alters renal clearance of uric acid [31,37,38]. There is also evidence suggesting that the observed effect modification is mediated by differential medication use between the two sexes [39]. Our observation may also be due to the fact that healthy women have lower physiologic uric acid concentrations than men. Thus for any given uric acid concentration, the disease process contributing to the uric acid elevation is more severe in women compared to men, resulting in a stronger association with mortality.
We also found significant interactions between uric acid and baseline diabetes as well as HDLc. There is mounting evidence that diabetes independently lowers uric acid concentrations over time [40] possibly due to hyperglycemia and hyperinsulinemia [41,42]. As a result, it is possible that the uric acid of those participants with diabetes at baseline would have been higher than measured in absence of diabetes. Thus, hyperuricemia in participants with diabetes is likely more severe than in participants without diabetes. With regard HDLc, prior clinical studies have described an interaction between HDLc and uric acid with respect to hypertension [43]. Hypertension may mediate the interaction observed in our study with respect to mortality.
In our study we found that hyperuricemia was associated with cardiovascular disease-related mortality and more specifically mortality related to congestive heart failure or thromboembolic disease. Uric acid has been observed to be associated with both incident congestive heart failure [44,45] and mortality among persons with congestive heart failure [46,47]. It is thought that uric acid reflects underlying renal failure in congestive heart failure patients [48]. This is supported by our finding that adjustment for eGFR attenuated the association between uric acid and congestive heart failure-related mortality. However, as most fatal events occurred during the latter part of follow-up it is more likely that uric acid would be reflecting early declines in kidney function. We also found evidence that elevated uric acid was associated with mortality related to thromboembolic disease. Several animal models have shown that uric acid can cause activation of proliferative and inflammatory pathways [49] and is strongly associated with the metabolic syndrome [50], a potent risk factor for cardiovascular disease.
Growing evidence suggests that hyperuricemia is associated with cancer mortality [7,51–55] and reduced survival time among cancer patients [56]. However, the observational data is inconsistent [10,11,57,58]. In our study, we only found an association between hyperuricemia (not uric acid in fifths or as a continuous variable) and cancer mortality. This suggests that uric acid is only a meaningful marker of cancer-related mortality risk in the upper range. It is thought that uric acid is a marker of increased cell turnover from the underlying malignant process [26] as well as damaged cells [59]. There is also evidence that it reflects and mediates anti-tumor activity [59,60]. Additional research is needed to further characterize the pathways behind this association.
Despite accounting for only a small proportion of deaths (5.6%), we found that uric acid was highly predictive of kidney-related mortality independent of eGFR. These findings are distinct from other observational studies [61,62]. Although uric acid is highly associated with existing kidney injury [27,29], its relationship with kidney disease is controversial. While some observational studies have shown that uric acid is associated with worsening kidney function [63–69] and incident chronic kidney disease [5,70–73], others have found that these associations were not independent of other factors [29,67]. It is possible that elevations in uric acid result from subclinical declines in kidney function [68], although emerging evidence suggests that it may play a causal role in kidney injury [74–76] via deposition of urate crystals in the interstitium of the renal medulla [77].
This study has a number of important clinical implications. First, it adds substantial weight in support of uric acid as a marker of mortality risk. Further, it helps characterize uric acid-associated mortality, which may be useful for risk stratification in the clinical setting, particularly among patients who are female, who have low HDLc levels, or have a history of diabetes. Finally, our findings provide insight into pathways by which uric acid might be causal (e.g. renal disease) versus a marker of disease (e.g. cancer or cardiovascular disease), which may hold utility were urate-lowering therapy to be used in broader clinical applications such as kidney disease [78].
Strengths of this study include its large dataset, long follow-up using a national system for mortality surveillance, and random selection of subjects from a general population. There are several limitations as well. First, uric acid was only measured once, so we cannot account for changes in uric acid over time. Second, as all covariates were measured at baseline, mediators of the relationship between uric acid and cardiovascular disease cannot be distinguished from confounding factors. Further, factors influencing uric acid concentrations after baseline (for example initiation of urate-lowering therapy) could not be addressed in our analyses. Finally, as with all observational studies, our analyses are subject to residual confounding from inadequate assessment of baseline covariates or from unmeasured variables such as medication use, fasting glucose, or other risk factors for mortality.
Conclusion
In conclusion, we found that uric acid is associated with mortality, especially when mortality was related to kidney disease. Furthermore, we observed that uric acid was more strongly associated with mortality in women compared to men. Future studies are necessary to evaluate the underlying mechanism for this interaction by sex. Moreover, additional research is needed to evaluate the strong association between uric acid and renal-related mortality.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Scottish Heart Health Extended Cohort Study for their important contributions.
SPJ was supported by the NIH/NHLBI T32HL007024 Cardiovascular Epidemiology Training Grant.
Abbreviations
- SHHEC
Scottish Heart Health Extended Cohort Study
- MONICA
Multinational MONItoring of Trends and Determinants in CArdiovascular Disease
- ICD
International Statistical Classification of Diseases
- SIMD
Scottish Index of Multiple Deprivation
- eGFR
estimated glomerular filtration rate
- BMI
body mass index
- HDLc
high density lipoprotein cholesterol
- HR
hazard ratio
- CI
confidence interval
Footnotes
Competing financial interests
The authors have nothing to disclose and no relevant conflicts of interest.
References
- 1.Juraschek SP, Kovell LC, Miller ER, Gelber AC. Dose-response association of uncontrolled blood pressure and cardiovascular disease risk factors with hyperuricemia and gout. PLoS ONE. 2013;8:e56546. doi: 10.1371/journal.pone.0056546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000;148:131–9. doi: 10.1016/s0021-9150(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 3.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SY, De Vera MA, Choi HK. Gout and mortality. Clin Exp Rheumatol. 2008;26:S115–119. [PubMed] [Google Scholar]
- 5.Tomita M, Mizuno S, Yamanaka H, Hosoda Y, Sakuma K, Matuoka Y, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10:403–9. doi: 10.2188/jea.10.403. [DOI] [PubMed] [Google Scholar]
- 6.Niskanen LK, Laaksonen DE, Nyyssönen K, Alfthan G, Lakka H-M, Lakka TA, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–51. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 7.Levine W, Dyer AR, Shekelle RB, Schoenberger JA, Stamler J. Serum uric acid and 11.5-year mortality of middle-aged women: findings of the Chicago Heart Association Detection Project in Industry. J Clin Epidemiol. 1989;42:257–67. doi: 10.1016/0895-4356(89)90061-9. [DOI] [PubMed] [Google Scholar]
- 8.Freedman DS, Williamson DF, Gunter EW, Byers T. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1995;141:637–44. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 9.Franse LV, Pahor M, Di Bari M, Shorr RI, Wan JY, Somes GW, et al. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP) J Hypertens. 2000;18:1149–54. doi: 10.1097/00004872-200018080-00021. [DOI] [PubMed] [Google Scholar]
- 10.Jee SH, Lee SY, Kim MT. Serum uric acid and risk of death from cancer, cardiovascular disease or all causes in men. Eur J Cardiovasc Prev Rehabil. 2004;11:185–91. doi: 10.1097/01.hjr.0000130222.50258.22. [DOI] [PubMed] [Google Scholar]
- 11.Takkunen H, Reunanen A, Aromaa A, Knekt P. Raised serum urate concentration as risk factor for premature mortality in middle aged men. Br Med J (Clin Res Ed) 1984;288:1161. doi: 10.1136/bmj.288.6424.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndrepepa G, Braun S, King L, Fusaro M, Tada T, Cassese S, et al. Uric acid and prognosis in angiography-proven coronary artery disease. Eur J Clin Invest. 2013;43:256–66. doi: 10.1111/eci.12039. [DOI] [PubMed] [Google Scholar]
- 13.Smith WC, Crombie IK, Tavendale R, Irving JM, Kenicer MB, Tunstall Pedoe H. The Scottish Heart Health Study: objectives and development of methods. Health Bull (Edinb) 1987;45:211–7. [PubMed] [Google Scholar]
- 14.Tunstall-Pedoe H, Woodward M, Tavendale R, A’Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ. 1997;315:722–9. doi: 10.1136/bmj.315.7110.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodward M, Brindle P, Tunstall-Pedoe H, for SIGN group on risk estimation Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC) Heart. 2007;93:172–6. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tunstall-Pedoe H, for the WHO MONICA Project, editor. MONICA monograph and multimedia sourcebook. Geneva: World Health Organization; p. 2003. n.d. [Google Scholar]
- 17.Kendrick S, Clarke J. The Scottish Record Linkage System. Health Bull (Edinb) 1993;51:72–9. [PubMed] [Google Scholar]
- 18.Bais R, White RG, Elston D, Geary TD. Assessment of the Cobas Bio centrifugal analyser. J Automat Chem. 1982;4:21–4. doi: 10.1155/S1463924682000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feig DI, Kang D-H, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scottish Executive SAH. Scottish Index of Multiple Deprivation. 2011 [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorensen LB, Levinson DJ. Origin and extrarenal elimination of uric acid in man. Nephron. 1975;14:7–20. doi: 10.1159/000180432. [DOI] [PubMed] [Google Scholar]
- 23.Rieselbach RE. Renal handling of uric acid. Adv Exp Med Biol. 1977;76B:1–22. doi: 10.1007/978-1-4684-3285-5_1. [DOI] [PubMed] [Google Scholar]
- 24.Choi JWJ, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–16. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 25.Brecher AS, Lehti MD. A hypothesis linking hypoglycemia, hyperuricemia, lactic acidemia, and reduced gluconeogenesis in alcoholics to inactivation of glucose-6-phosphatase activity by acetaldehyde. Alcohol. 1996;13:553–7. doi: 10.1016/s0741-8329(96)00067-5. [DOI] [PubMed] [Google Scholar]
- 26.Ultmann JE. Hyperuricemia in disseminated neoplastic disease other than lymphomas and leukemias. Cancer. 1962;15:122–9. doi: 10.1002/1097-0142(196201/02)15:1<122::aid-cncr2820150117>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Juraschek SP, Kovell LC, Miller ER, 3rd, Gelber AC. Association of kidney disease with prevalent gout in the United States in 1988–1994 and 2007–2010. Semin Arthritis Rheum. 2013 doi: 10.1016/j.semarthrit.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peden DB, Hohman R, Brown ME, Mason RT, Berkebile C, Fales HM, et al. Uric acid is a major antioxidant in human nasal airway secretions. Proc Natl Acad Sci USA. 1990;87:7638–42. doi: 10.1073/pnas.87.19.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeemon P, Prabhakaran D. Does uric acid qualify as an independent risk factor for cardiovascular mortality? Clin Sci. 2013;124:255–7. doi: 10.1042/CS20120524. [DOI] [PubMed] [Google Scholar]
- 31.Ioannou GN, Boyko EJ. Effects of menopause and hormone replacement therapy on the associations of hyperuricemia with mortality. Atherosclerosis. 2013;226:220–7. doi: 10.1016/j.atherosclerosis.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakoda M, Masunari N, Yamada M, Fujiwara S, Suzuki G, Kodama K, et al. Serum uric acid concentration as a risk factor for cardiovascular mortality: a longterm cohort study of atomic bomb survivors. J Rheumatol. 2005;32:906–12. [PubMed] [Google Scholar]
- 33.Chen J-H, Chuang S-Y, Chen H-J, Yeh W-T, Pan W-H. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. 2009;61:225–32. doi: 10.1002/art.24164. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–10. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 35.Reunanen A, Takkunen H, Knekt P, Aromaa A. Hyperuricemia as a risk factor for cardiovascular mortality. Acta Med Scand Suppl. 1982;668:49–59. doi: 10.1111/j.0954-6820.1982.tb08521.x. [DOI] [PubMed] [Google Scholar]
- 36.Høieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–9. doi: 10.1111/j.1523-1755.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973;1:449–51. doi: 10.1136/bmj.1.5851.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999;354:650. doi: 10.1016/S0140-6736(99)92381-4. [DOI] [PubMed] [Google Scholar]
- 39.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 40.Juraschek SP, McAdams-Demarco M, Miller ER, 3rd, Gelber AC, Maynard JW, Pankow JS, et al. Temporal Relationship between Uric Acid and Diabetes in a Community-Based Population. American Journal of Epidemiology. 2014 doi: 10.1093/aje/kwt320. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boner G, Rieselbach RE. The effect of glucose upon reabsorptive transport of urate by the kidney. Adv Exp Med Biol. 1974;41:781–7. doi: 10.1007/978-1-4757-1433-3_55. [DOI] [PubMed] [Google Scholar]
- 42.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–11. [PubMed] [Google Scholar]
- 43.Teng F, Zhu R, Zou C, Xue Y, Yang M, Song H, et al. Interaction between serum uric acid and triglycerides in relation to blood pressure. J Hum Hypertens. 2011;25:686–91. doi: 10.1038/jhh.2010.112. [DOI] [PubMed] [Google Scholar]
- 44.Ekundayo OJ, Dell’Italia LJ, Sanders PW, Arnett D, Aban I, Love TE, et al. Association between hyperuricemia and incident heart failure among older adults: a propensity-matched study. Int J Cardiol. 2010;142:279–87. doi: 10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Lipoprotein components and risk of congestive heart failure in 84,740 men and women in the Apolipoprotein MOrtality RISk study (AMORIS) Eur J Heart Fail. 2009;11:1036–42. doi: 10.1093/eurjhf/hfp129. [DOI] [PubMed] [Google Scholar]
- 46.Strasak AM, Kelleher CC, Brant LJ, Rapp K, Ruttmann E, Concin H, et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. Int J Cardiol. 2008;125:232–9. doi: 10.1016/j.ijcard.2007.11.094. [DOI] [PubMed] [Google Scholar]
- 47.Strasak A, Ruttmann E, Brant L, Kelleher C, Klenk J, Concin H, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clin Chem. 2008;54:273–84. doi: 10.1373/clinchem.2007.094425. [DOI] [PubMed] [Google Scholar]
- 48.Tian Y, Chen Y, Deng B, Liu G, Ji Z-G, Zhao Q-Z, et al. Serum uric acid as an index of impaired renal function in congestive heart failure. J Geriatr Cardiol. 2012;9:137–42. doi: 10.3724/SP.J.1263.2011.11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang D-H, Park S-K, Lee I-K, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–62. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 50.Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442–7. doi: 10.1016/j.amjmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 51.Petersson B, Trell E. Raised serum urate concentration as risk factor for premature mortality in middle aged men: relation to death from cancer. Br Med J (Clin Res Ed) 1983;287:7–9. doi: 10.1136/bmj.287.6384.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammarsten J, Damber J-E, Peeker R, Mellström D, Högstedt B. A higher prediagnostic insulin level is a prospective risk factor for incident prostate cancer. Cancer Epidemiol. 2010;34:574–9. doi: 10.1016/j.canep.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Kolonel LN, Yoshizawa C, Nomura AM, Stemmermann GN. Relationship of serum uric acid to cancer occurrence in a prospective male cohort. Cancer Epidemiol Biomarkers Prev. 1994;3:225–8. [PubMed] [Google Scholar]
- 54.Strasak AM, Rapp K, Hilbe W, Oberaigner W, Ruttmann E, Concin H, et al. The role of serum uric acid as an antioxidant protecting against cancer: prospective study in more than 28 000 older Austrian women. Ann Oncol. 2007;18:1893–7. doi: 10.1093/annonc/mdm338. [DOI] [PubMed] [Google Scholar]
- 55.Strasak AM, Rapp K, Hilbe W, Oberaigner W, Ruttmann E, Concin H, et al. Serum uric acid and risk of cancer mortality in a large prospective male cohort. Cancer Causes Control. 2007;18:1021–9. doi: 10.1007/s10552-007-9043-3. [DOI] [PubMed] [Google Scholar]
- 56.Shin H-S, Lee H-R, Lee D-C, Shim J-Y, Cho K-H, Suh S-Y. Uric acid as a prognostic factor for survival time: a prospective cohort study of terminally ill cancer patients. J Pain Symptom Manage. 2006;31:493–501. doi: 10.1016/j.jpainsymman.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Hiatt RA, Fireman BH. Serum uric acid unrelated to cancer incidence in humans. Cancer Res. 1988;48:2916–8. [PubMed] [Google Scholar]
- 58.Bozkir A, Simşek B, Güngört A, Torun M. Ascorbic acid and uric acid levels in lung cancer patients. J Clin Pharm Ther. 1999;24:43–7. doi: 10.1046/j.1365-2710.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- 59.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 60.Ullrich E, Ménard C, Flament C, Terme M, Mignot G, Bonmort M, et al. Dendritic cells and innate defense against tumor cells. Cytokine Growth Factor Rev. 2008;19:79–92. doi: 10.1016/j.cytogfr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Fessel WJ. Renal outcomes of gout and hyperuricemia. Am J Med. 1979;67:74–82. doi: 10.1016/0002-9343(79)90076-7. [DOI] [PubMed] [Google Scholar]
- 62.Navaneethan SD, Beddhu S. Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transplant. 2009;24:1260–6. doi: 10.1093/ndt/gfn621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691–7. doi: 10.1291/hypres.24.691. [DOI] [PubMed] [Google Scholar]
- 64.Syrjänen J, Mustonen J, Pasternack A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 2000;15:34–42. doi: 10.1093/ndt/15.1.34. [DOI] [PubMed] [Google Scholar]
- 65.Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest. 2001;31:318–21. doi: 10.1046/j.1365-2362.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- 66.Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron. 2001;87:333–9. doi: 10.1159/000045939. [DOI] [PubMed] [Google Scholar]
- 67.Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239–47. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Wang F, Zhang L, Zuo L, Liu L, Wang H. Mortality and renal function decline among a community-based Chinese population with normal or mildly impaired renal function. Nephrol Dial Transplant. 2011;26:2847–52. doi: 10.1093/ndt/gfq816. [DOI] [PubMed] [Google Scholar]
- 69.Liu W-C, Hung C-C, Chen S-C, Yeh S-M, Lin M-Y, Chiu Y-W, et al. Association of hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2012;7:541–8. doi: 10.2215/CJN.09420911. [DOI] [PubMed] [Google Scholar]
- 70.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–50. [PubMed] [Google Scholar]
- 71.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–11. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu C, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–50. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ben-Dov IZ, Kark JD. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: the Jerusalem Lipid Research Clinic cohort study. Nephrol Dial Transplant. 2011;26:2558–66. doi: 10.1093/ndt/gfq740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siu Y-P, Leung K-T, Tong MK-H, Kwan T-H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–9. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Kang D-H, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–97. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 76.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–93. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonick HC, Rubini ME, Gleason IO, Sommers SC. The Renal Lesion in Gout. Ann Intern Med. 1965;62:667–74. doi: 10.7326/0003-4819-62-4-667. [DOI] [PubMed] [Google Scholar]
- 78.Bose B, Badve SV, Hiremath SS, Boudville N, Brown FG, Cass A, et al. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


