Abstract
To date, there is no research on voluntary medical male circumcision (VMMC) catchment areas or the relationship between distance to a VMMC facility and attendance at a post-operative follow-up visit. We analyzed data from a randomly selected subset of males self-seeking circumcision at one of 16 participating facilities in Nyanza Province, Kenya between 2008–2010. Among the 1437 participants, 46.7% attended follow-up. The median distance from residence to utilized facility was 2.98km (IQR=1.31–5.38). Nearly all participants (98.8%) lived within 5km from a facility. However, 26.3% visited a facility farther than 5km from their residence. Results stratified by facility type demonstrated that among those utilizing fixed facilities, greater distance was associated with higher odds of follow-up non-attendance (OR5.01–10km vs. 0–1km=1.71, 95% CI: 1.08, 2.70, p=0.02; OR>10km vs. 0–1 km=2.80, 95% CI: 1.26, 6.21, p=0.01), adjusting for age and district of residence. We found 5km marked the threshold distance beyond which follow-up attendance significantly dropped. These results demonstrate distance is an important predictor of attending follow-up, and this relationship appears to be modified by facility type.
Keywords: voluntary medical male circumcision, spatial analysis of catchment, post-operative follow-up, facility attendance, Kenya
INTRODUCTION
Results from three randomized controlled trials in three sub-Saharan African countries demonstrated that male circumcision—the surgical removal of the foreskin from the penis—reduces the incidence of HIV acquisition for males by 60% (1–3). These findings prompted the World Health Organization (WHO) and the Joint United Nations Programme on HIV/AIDS (UNAIDS) to endorse voluntary medical male circumcision (VMMC) as an important HIV prevention strategy in 2007 for communities in which prevalence of HIV is high and that of male circumcision is low (4). Fourteen sub-Saharan African countries with these characteristics are aiming to achieve 80% coverage among men aged 15–49 years by 2016. Although a challenging target, if it is achieved and maintained, an estimated 3.4 million HIV infections would be prevented (5).
By 2012, VMMC scale-up activities were credited with circumcising approximately 1.3 million men in sub-Saharan Africa, representing 6.5% of the 2016 target (5). Among the challenges in scaling-up VMMC are: overcoming cultural barriers (6, 7), effectively utilizing limited human resources, commodity management, and patient adherence to post-operative follow-up (8). This study addresses the last challenge.
In Kenya, standard operating procedures for VMMC recommend that clients attend a follow-up visit seven days post-procedure, during which providers observe the wound, identify and manage adverse events (AEs), and reinforce messages of abstinence during healing and condom use during sexual intercourse once healing is complete (9). In previous studies in sub-Saharan African settings, including Kenya, client-specific barriers to follow-up compliance included perceived lack of an AE and distance to the facility (10–12).
Distance has been identified as a primary barrier to healthcare utilization in sub-Saharan Africa for indications such as HIV and tuberculosis, in which care and treatment requires regular follow-up (13, 14). In rural South Africa, one cross-sectional mapping study found the average straight-line distance traveled to reach primary care—including HIV and other chronic care services—was 4.72 kilometers (km), and that beyond 8 km, facility utilization declined dramatically (15). Similarly, another cross-sectional study in the same rural location found a steep decrease in the odds of accessing anti-retroviral treatment (ART) as distance to the facility increased, with those living 4.78 km from the facility having a 50% lower odds of being on ART than those residing next to the facility (16).
Because VMMC is a one-time procedure with only one recommended follow-up visit, patients may be willing to travel greater distances than they are for ongoing services (17), thereby allowing for a larger catchment area, defined as the geographic area where a facility serves clients effectively. For example, a study of intrauterine device (IUD) uptake in Egypt found that women who lived at least 4 kilometers from a family planning facility compared with those who lived less than 4 kilometers away were equally likely to take up the services in their adjusted model (18). Similarly, therefore, the association between distance and likelihood of follow-up for VMMC may be weaker and the catchment area for VMMC greater compared to that for chronic care or reproductive health services. However, little data exist to evaluate the association between distance and VMMC follow-up.
This study draws on data from an ongoing VMMC program in Kenya, which is one of the countries prioritized by WHO for VMMC scale-up. In Kenya, Nyanza Province—home to the Luo tribe who do not traditionally practice circumcision—has an HIV prevalence of 15.1%, compared with the national prevalence of 5.6% (19). The Ministry of Health began active scale-up of VMMC for HIV prevention in 2008. By 2011, 341,000 men had been circumcised under this initiative, reaching approximately 40% of its 2013 target of 860,000 men. Yet, of these, only 27.5% were found to have attended the recommended follow-up visit scheduled 7–12 days post-procedure (20).
In this study, we (1) defined the catchment area for VMMC facilities, and (2) assessed whether greater distance to facility was associated with lower follow-up attendance. Results from this research will increase understanding of VMMC catchment areas and barriers to follow-up, thus providing guidance to VMMC programs on how to improve uptake and safety of the services.
METHODS
STUDY POPULATION
Data were drawn from a study conducted by Nyanza Reproductive Health Society and University of Illinois at Chicago (UIC) between 2008 and 2010, which assessed male circumcision scale-up in three districts in Nyanza Province, Kenya. Participants in the evaluation study were recruited upon self-seeking VMMC services at one of 16 participating facilities in Kisumu East, Kisumu West, and Nyando Districts in Nyanza Province, Kenya. Eligible participants were males at least 12 years old at the time of initial visit. After providing parental written informed consent and assent from those less than 18 years of age, a sub-sample of enrolled participants was randomly selected to participate in an active surveillance system, which comprised the study population of this analysis. More details describing the study procedures can be found in a previous publication (21).
ETHICAL CONSIDERATIONS
The original protocol and all source documents were approved by the UIC Institutional Review Board (IRB) and Kenyatta National Hospital Ethics and Research Committee in Nairobi, Kenya. This secondary analysis was approved by the Columbia University Medical Center IRB.
DATA SOURCES
Data sources included (1) demographic information obtained on paper forms by a research assistant during the consultation visit; (2) clinical and procedural information collected by health providers on paper forms during any consultation visit, which included the visit prior to circumcision, the visit in which the circumcision was performed and any post-procedural follow-up visit; and (3) spatial data verifying residence location obtained during home visits 28–45 days post-procedure on a personal digital assistant (PDA) equipped with a global positioning system (GPS). Demographic, clinical, and procedural data, including the date of each visit, were entered into an electronic database by research assistants hired and trained specifically to perform study procedures. GPS data were automatically transferred from the PDA into a dataset and linked to other data using a unique identifier. At the time of the study, there were no national forms associated with VMMC so all forms were developed primarily for the study, but similar forms were subsequently adapted for routine data collection by the Ministry of Health.
MEASURES
Outcome
Post-operative follow-up attendance (yes/no)—defined as attendance any time post-procedure—was the main outcome of interest. Attendance was documented by a clinician or nurse at the facility.
Exposure
The main exposure investigated was distance from residence to facility where VMMC services were performed. Straight-line distance from each participant’s residence to the facility was calculated using ArcGIS version 10.1. Straight-line distance is a measure that Siedner et al. (22) have validated as a proxy for exact route distance and a better measure than self-reported travel distance. Distance was analyzed as a categorical variable because plotting the betas for each distance category demonstrated non-linearity in the log odds of distance. Categories (0–1 km, 1.01–3 km, 3.01–5 km, 5.01–10 km and >10 km) were determined by splitting the data into quartiles and finding distance intervals that roughly fit the quartiles, and then dividing the last interval into two categories to more closely investigate the effects of greater distances. In order to understand the relationship of straight-line distance to other travel-related indicators, comparisons were made between distance category and the number of modes of transportation (foot, bicycle, motorcycle, public vehicle, private vehicle) used to reach the facility, as well as the self-reported time it took to travel to the facility (<15 minutes, 15–30 minutes, 30–60 minutes, and >60 minutes).
Covariates
Factors associated with distance to VMMC facilities and VMMC follow-up attendance are not well established; thus a wide range of potentially confounding variables was considered a priori. These included age in years (continuous variable); district of residence (Kisumu East, Kisumu West, Nyando, and other); highest level of school completed (none, primary, secondary, and post-secondary); employment status (yes/no); employment type (unemployed, self-employed, and salaried); monthly income in Kenyan shillings (none, 1–2,000 ($0.01–$27.82), 2,000–9,999 ($27.83–$139.06), ≥10,000 (≥$139.07); marital status (not-married/married); having a regular live-in partner (yes/no); religion (Catholic, Protestant, and other); district of facility visited (Kisumu East, Kisumu West, and Nyando); reported and/or confirmed HIV status (negative, positive, and unknown); and provider type (clinician/nurse).
Facility type (fixed/mobile) was assessed as a potential confounder and potential effect measure modifier of the relationship between distance to facility and follow-up attendance. Fixed facilities are those that have a permanent structure and staff, while mobile facilities are smaller permanent facilities (usually 1–2 rooms), but have staff who move from one facility to the next.
STATISTICAL ANALYSIS
ArcGIS version 10.1 was used to map the geospatial coordinates of participant residences and VMMC facilities and to calculate the straight-line distance from each residence to the VMMC facility utilized. To determine the average catchment area of VMMC facilities among the study population, pre-set buffers of each distance category were used to create concentric rings around each facility. The percentage of clients living within each buffer was calculated.
Power was calculated post-hoc to be 85%, with the ability to detect a risk ratio (risk of not attending follow-up among those living >10 km from the VMMC facility compared to those living 0–1 km away) of 1.4 based on these data.
Participant descriptive statistics were calculated; bivariate analyses using Wald chi-squared tests were performed to examine the association between participant characteristics and distance, and between participant characteristics and attendance at follow-up. Variables that were associated with both predictor and outcome at p ≤ 0.20 were considered potential confounders of the association between distance and follow-up attendance. They were included in a multiple logistic regression model along with distance, and were removed one-by-one and jointly. Those variables that alone or in combination with other variables changed the beta value for the effect estimate of distance by 10% or more were retained in the model. An interaction term between distance and facility type was introduced to the adjusted regression model to determine if there was significant multiplicative interaction in the full model (p≤ 0.05). These analyses were conducted using SAS version 9.3.
RESULTS
CHARACTERISTICS OF THE STUDY POPULATION
Of the 4010 VMMC clients across 16 facilities in Kisumu East, Kisumu West, and Nyando Districts enrolled in the parent study, 1449 were randomly selected to participate in active surveillance. In this sub-sample, 12 were excluded from analysis because neither a nurse nor clinician performed the circumcision. Among these 1437 participants, the median age was 20 years (range 12–76), and nearly all (98.1%) identified as Luo (Table I). Overall, approximately one-third (34.4%) of the study population lived within 1 kilometer of a VMMC facility whether it was the one utilized for circumcision or not; 80.1% lived within 3 km, and 98.8% lived within 5 km of a VMMC facility (data not shown).
Table I.
Characteristics of males who were circumcised and actively followed-up post-circumcision in Nyanza, Kenya between 2008–2010
| Subject Characteristic | Total (%) | Attended follow-up | Did not attend follow-up | Wald χ2 (p-value) | OR (95% CI) |
|---|---|---|---|---|---|
| n=1437a (100) | n (%) | n (%) | |||
| Age (years): median (IQR) | 20 (7) | - | - | 7.03 (<0.01) | 0.98 (0.97, 1.00) |
| Educational background | |||||
| None (ref) | 167 (11.6) | 80 (47.9) | 87 (52.1) | - | - |
| Primary | 709 (49.3) | 316 (44.6) | 393 (55.4) | 1.03 (0.31) | 1.14 (0.82, 1.60) |
| Secondary | 445 (31.0) | 225 (50.6) | 220 (49.4) | 2.57 (0.11) | 0.90 (0.63, 1.28) |
| Postsecondary | 116 (8.1) | 51 (44.0) | 65 (56.0) | 0.57 (0.45) | 1.17 (0.73, 1.89) |
| Employment status | |||||
| Unemployed (ref) | 951 (66.2) | 424 (44.6) | 527 (55.4) | - | - |
| Employed | 486 (33.8) | 248 (51.0) | 238 (49.0) | 5.36 (0.02) | 0.77 (0.62, 0.96) |
| Employment type | |||||
| Unemployed (ref) | 951 (66.2) | 424 (44.6) | 527 (55.4) | - | - |
| Self-employed | 361 (25.1) | 185 (51.3) | 176 (48.8) | 1.13 (0.29) | 0.77 (0.60, 0.98) |
| Salaried | 125 (8.7) | 63 (50.4) | 62 (49.6) | 0.28 (0.60) | 0.79 (0.55, 1.15) |
| Income in Kenyan shillings (USD) | |||||
| None (ref) | 249 (17.3) | 111 (45.1) | 135 (54.9) | - | - |
| 1–2,000 ($0.01–$27.82) | 706 (49.1) | 315 (44.6) | 392 (55.5) | 3.05 (0.08) | 1.02 (0.76, 1.37) |
| 2,001–9,999 ($27.83–$139.06) | 370 (25.8) | 187 (50.1) | 186 (49.9) | 0.60 (0.44) | 0.82 (0.59, 1.13) |
| ≥ 10,000 (≥$139.07) | 112 (7.8) | 59 (53.2) | 52 (46.9) | 1.73 (0.19) | 0.73 (0.46, 1.14) |
| Religion | |||||
| Catholic (ref) | 415 (28.9) | 204 (49.2) | 211 (50.8) | - | - |
| Protestant | 924 (64.3) | 416 (45.0) | 508 (55.0) | <0.01 (0.97) | 0.85 (0.67, 1.01) |
| Other | 98 (6.8) | 52 (53.1) | 46 (46.9) | 1.29 (0.26) | 0.72 (0.48, 1.10) |
| Marital status | |||||
| Not married (ref) | 1109 (77.2) | 513 (46.3) | 596 (53.7) | - | |
| Married | 328 (22.8) | 159 (48.5) | 169 (51.5) | 0.50 (0.48) | 0.92 (0.72, 1.17) |
| Live-in partner | |||||
| No (ref) | 985 (68.6) | 476 (48.3) | 509 (51.7) | - | |
| Yes | 452 (31.5) | 196 (43.4) | 256 (56.6) | 3.06 (0.08) | 1.22 (0.98, 1.53) |
| HIV status | |||||
| Negative (ref) | 517 (36.0) | 244 (47.2) | 273 (52.8) | - | - |
| Positive | 41 (2.9) | 22 (53.7) | 19 (46.3) | 0.64 (0.43) | 0.77 (0.41, 1.46) |
| Unknown | 879 (61.2) | 406 (46.2) | 473 (53.8) | 0.13 (0.72) | 1.04 (0.84, 1.30) |
| District of residence | |||||
| Kisumu East (ref) | 181 (12.6) | 93 (51.4) | 88 (48.6) | - | - |
| Kisumu West | 404 (28.1) | 171 (42.3) | 233 (57.7) | 4.12 (0.04) | 1.44 (1.01, 2.05) |
| Nyando | 766 (53.3) | 366 (47.8) | 400 (52.2) | 0.76 (0.38) | 1.16 (0.84, 1.60) |
| Other | 86 (6.0) | 42 (48.8) | 44 (51.2) | 0.15 (0.70) | 1.11 (0.66, 1.85) |
| Facility type used | |||||
| Fixed (ref) | 690 (48.0) | 362 (52.5) | 328 (47.5) | - | - |
| Mobile | 747 (52.0) | 310 (41.5) | 437 (58.5) | 17.25 (<0.01) | 0.64 (0.52, 0.79) |
| Provider type seena | |||||
| Clinician (ref) | 1010 (70.3) | 490 (48.5) | 520 (51.5) | - | - |
| Nurse | 427 (29.7) | 182 (42.6) | 245 (57.4) | 4.18 (0.04) | 1.27 (1.01, 1.59) |
| District of facility used | |||||
| Kisumu East (ref) | 187 (13.0) | 94 (50.3) | 93 (49.7) | - | - |
| Kisumu West | 443 (30.8) | 182 (41.1) | 261 (58.9) | 4.49 (0.03) | 1.45 (1.03, 2.04) |
| Nyando | 807 (56.2) | 396 (49.1) | 411 (50.9) | 0.09 (0.77) | 1.05 (0.76, 1.44) |
| Distance from home to utilized facility | |||||
| 0–1 km (ref) | 304 (21.2) | 160 (52.6) | 144 (47.4) | - | - |
| 1.01–3 km | 420 (29.2) | 202 (48.1) | 218 (51.9) | 1.45 (0.23) | 1.20 (0.89, 1.61) |
| 3.01–5 km | 335 (23.3) | 162 (48.4) | 173 (51.6) | 1.16 (0.28) | 1.19 (0.87, 1.62) |
| 5.01–10 km | 274 (19.1) | 111 (40.5) | 163 (59.5) | 8.46 (<0.01) | 1.63 (1.17, 2.27) |
| >10 km | 104 (7.2) | 37 (35.6) | 67 (64.4) | 8.86 (<0.01) | 2.01 (1.27, 3.19) |
| Self-reported travel time (minutes)b | |||||
| Median (Q1, Q3) | 15–30 (15–30, 30–60) | - | - | - | - |
| Number of modes of transport usedb | |||||
| Mean (standard deviation) | 1.28 (0.46) | - | - | - | - |
There were 12 participants excluded from analyses, as they were seen by ‘Other’ provider types including students, laboratory technicians and local anesthesiologists
Not investigated as a covariate; rather this variable used to determine if correlated with distance
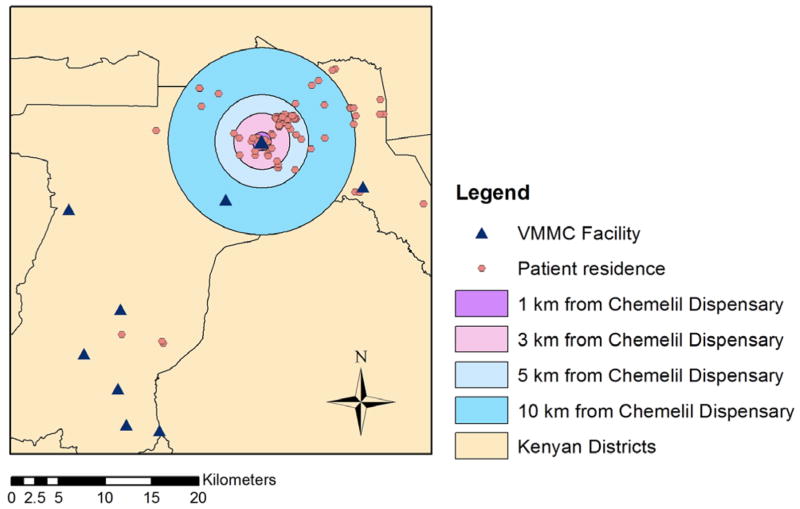
Of all participants, 46.7% attended a post-operative follow-up visit. A similar proportion of participants accessed fixed (48%) and mobile (52%) sites (Table I). The median distance from participant residence to the VMMC facility utilized was 2.98 km, ranging from 0.01 km to 52.67 km (inter-quartile range=1.31–5.38 km). Few participants (5.9%) resided outside the district where the facility was located. Approximately 26.3% of participants (n=378) utilized a facility more than 5 km from their residence. Most participants used one mode of transportation (71.8%) to reach a facility. Figure 1 is an example that illustrates the spatial distribution of VMMC clients who utilized one participating facility, Chemelil Dispensary, where 21.6% traveled more than 5 km to reach this facility, despite living closer to other VMMC facilities.
Figure 1.
VMMC Catchment Area in Nyanza, Kenya Among Participating Males Circumcised at Chemelil Dispensary between 2008 and 2010
BIVARIATE ASSOCIATIONS
A smaller proportion of participants attended follow-up among those who utilized facilities more than 5 km from their residences compared to those who utilized facilities within 1 km from home (χ25.01–10km vs <1km=8.46, p<0.01 and χ2>10km vs <1km =8.86, p<0.01), as shown in Table I. The percentage of those who attended follow-up by distance category was as follows: 0–1 km: 52.6%; 1.01–3 km: 48.1%; 3.01–5 km: 48.4%; 5.01–10 km: 40.5%; >10 km: 35.6%. A smaller proportion of clients attended follow-up among mobile facilities compared to fixed facilities (41.5% versus 52.5%, respectively; χ2=17.25, p<0.01). Other significant bivariate associations with follow-up attendance included age (χ2=7.03, p<0.01), employment status (χ2=5.36, p=0.02), income (χ21–2000 shillings vs none=3.05, p=0.08), live-in partner (χ2=3.06, p=0.08), district of residence (χ2Kisumu West vs East=4.12, p=0.04), and district of facility used (χ2Kisumu West vs East =4.49, p=0.03). The mean number of modes of transportation used was significantly higher among clients who traveled more than 5 km to the facility versus those who traveled 5 km or less (Table II: t=8.59, p<0.001). The median self-reported time to travel to the facility was 15–30 minutes among those who utilized a facility within 5 km of their residence versus 30–60 minutes among those living more than 5 km from the facility and the mean times between these distances proved to be significantly different (t=6.14, p<0.001).
Table II.
Bivariate associations of characteristics of travel with distance from client residence to utilized facility among male VMMC clients (n=1437)
| Variable | Distance to utilized facility | t-statistic, p-value | |
|---|---|---|---|
|
| |||
| ≤ 5 km n=1059 |
> 5 km n=378 |
||
| Mean number of modes of transport used | 1.22 | 1.47 | 8.59, <0.001 |
| Median self-reported time to reach facility | 15–30 min | 30–60 min | 6.14, <0.001 |
MULTIVARIABLE ASSOCIATIONS
Facility type, age, and district of residence confounded the relationship when included in the model either singly (facility type: χ2=16.98, p<0.001) or together (age: χ2=5.68, p=0.02 and district of residence: χ2=4.78, χ2=0.19) and thus were included in the fully adjusted model (data not shown). No other covariate of interest fulfilled the previously described criteria for confounding. Facility type was also found to be an effect measure modifier based on the significant multiplicative interaction term for one of the distance categories (Wald χ2 = 4.21, p=0.04) in the fully adjusted model (Table III). Stratification by facility type demonstrated that among participants who utilized a fixed facility, those who lived farther from the facility were significantly less likely to attend follow-up compared with the reference distance category (Wald χ25.01–10km vs. 0–1km = 5.30, p=0.02, OR5.01–10km vs. 0–1km=1.71, 95% CI: 1.08, 2.70; Wald χ2>10km vs. 0–1km = 6.40, p=0.01, OR>10km vs. 0–1km =2.80, 95% CI: 1.26, 6.21)). This association was not present among those who visited mobile facilities (Wald χ25.01–10km vs. 0–1km = 3.67, p=0.06; Wald χ2>10km vs. 0–1km = 2.51, p=0.11. ;).
Table III.
Adjusted associations between distance and non-attendance at post-circumcision follow-up visit among all male VMMC clients and results stratified by facility type (n=1437)
| Variable | Fully Adjusted Model: Interaction of distance and facility type, adjusting for confounders | Fully Adjusted Model Stratified by Facility Type | ||||
|---|---|---|---|---|---|---|
| Fixed | | Mobile | |||||
|
| ||||||
| OR (95% CI) | Wald χ2, p-value | OR (95% CI) | Wald χ2, p-value | OR (95% CI) | Wald χ2, p-value | |
| Distance to utilized facility (ref: 0–1 km) | ||||||
| 0–1 km | - | - | - | - | - | - |
| 1.01–3 km | 0.92 (0.61, 1.40) | 0.15, 0.70 | 0.89 (0.58, 1.36) | 0.29, 0.59 | 1.63 (1.04, 2.54)* | 4.61, 0.03 |
| 3.01–5 km | 0.75 (0.45, 1.22) | 1.37, 0.24 | 0.72 (0.44, 1.18) | 1.68, 0.19 | 1.52 (0.98, 2.34) | 3.55, 0.06 |
| 5.01–10 km | 1.70 (1.08, 2.68)* | 5.19, 0.02 | 1.71 (1.08, 2.70)* | 5.30, 0.02 | 1.62 (0.99, 2.66) | 3.67, 0.06 |
| > 10km | 3.24 (1.47, 7.11)* | 8.54, <0.01 | 2.80 (1.26, 6.21)* | 6.40, 0.01 | 1.63 (0.89, 2.97) | 2.51, 0.11 |
| Facility type (ref: fixed) | 1.27 (0.80, 1.99) | 1.04, 0.31 | - | - | - | - |
| Age | 0.98 (0.96, 1.00)* | 6.95, <0.01 | 1.00 (0.97, 1.02) | 0.18, 0.67 | 0.96 (0.94, 0.99)* | 10.61, <0.001 |
| District of residence (ref: Kisumu East) | ||||||
| Kisumu East (ref) | - | - | - | - | - | - |
| Kisumu West | 1.35 (0.94, 1.95) | 2.62, 0.11 | 1.27 (0.77, 2.11) | 0.87, 0.35 | 1.51 (0.89, 2.56) | 2.29, 0.13 |
| Nyando | 1.05 (0.75, 1.48) | 0.08, 0.78 | 0.89 (0.55, 1.44) | 0.24, 0.63 | 1.31 (0.81, 2.13) | 1.19, 0.28 |
| Other | 0.77 (0.45, 1.31) | 0.93, 0.33 | 1.09 (0.30, 3.97) | 0.02, 0.90 | 0.82 (0.44, 1.55) | 0.36, 0.55 |
| Facility type × distance | ||||||
| Facility type × 1.01–3km | 1.76 (0.96, 3.23) | 3.34, 0.07 | - | - | - | - |
| Facility type × 3.01–5km | 1.99 (1.03, 3.82)* | 4.21, 0.04 | - | - | - | - |
| Facility type × 5.01–10km | 0.92 (0.47, 1.80) | 0.05, 0.82 | - | - | - | - |
| Facility type × >10km | 0.53 (0.20, 1.41) | 1.63, 0.20 | - | - | - | - |
Wald χ2 significant (p<0.05)
DISCUSSION
This study assessed whether greater distance from home to VMMC facility was associated with lower follow-up attendance among males who were circumcised in 16 facilities in Nyanza, Kenya. The vast majority of participants (98.8%) lived within 5 km of a VMMC facility; however, 26.3% of participants utilized a VMMC facility that was farther than 5 km from their residence. Among clients attending fixed facilities, the odds of not attending the follow-up visit were 2.80 times greater for those who lived more than 10km from the facility, and 1.71 times greater for those who lived 5.01–10km away compared to those living 1km or less away. There was no significant difference in non-attendance for the smaller distance categories (1.01–3 km and 3.01–5 km) compared to those who utilized a facility within 1 km of their residences. This demonstrates that there is a threshold distance of 5 km, after which VMMC follow-up attendance is significantly less likely in a fixed facility setting. No such relationship was found among those who visited mobile facilities. It is notable, however, that even among those who lived within 1 km from the utilized facility, approximately 47.4% did not attend a follow-up visit. While this analysis demonstrates that distance is an important predictor of post-procedure follow-up for VMMC, a low attendance across distance categories may imply that there are other correlates that also affect attendance.
Studies have found distance to be an important predictor in health-seeking behavior and follow-up attendance, and this study supports these findings (13, 16, 23, 24). Tanser et al. (15) and Conley et al. (13) found 5 kilometers to be a threshold distance for seeking primary care (including HIV care) in rural South Africa, and for follow-up compliance among HIV discordant couples in Kenya, respectively. However, some studies investigating conditions with infrequent or non-chronic follow-up requirements have not found distance to be a significant predictor of uptake or return; rather, factors attributed to utilization and visit compliance included quality of services and insurance type (18, 23, 25, 26). Although transportation allowances are part of some programs in order to improve follow-up attendance, they were not included in this program. Our results show that approximately half of participants returned for follow-up if they lived 5 km or less from the utilized facility, but this proportion dropped to 40.5% among those living 5.01–10 km, and to 35.6% among those living more than 10 km from the VMMC facility. The number of modes of transportation was significantly higher among those traveling more than 5 km compared to those traveling within 5 km, as was the self-reported times it took to travel to the facility. It is likely that both the number of modes of transport and the time to reach the facility are correlated with the cost of transport, as well. Although most national VMMC programs do not require more than the Day 7 follow-up visit, these findings suggest that residing more than 5 km from a facility providing VMMC services likely serves as a deterrent for complying with this follow-up visit.
These findings have implications for defining VMMC catchment areas. Follow-up attendance is crucial in the monitoring of adverse events and the reinforcement of hygiene and abstinence during the healing process. Understanding why or how participants chose a facility may be integral to predicting whether VMMC clients will attend the recommended follow-up. Deciding to visit a more distant facility could be explained by clients being unaware of a nearer option, or by intentional decision-making such as seeking care at a facility with high quality services, longer facility hours, permanent staff presence (i.e. fixed facility), and/or renowned clinicians, as seen in other studies (27, 28). Furthermore, as revealed by qualitative research, circumcision may still hold stigma in Kenya, motivating some clients to purposely choose more distant facilities to avoid recognition by health providers and discovery of their circumcision by community members (6, 7, 29–31). Although qualitative research has investigated how these factors inform choice of VMMC facility, more research must be geared specifically towards factors influencing VMMC follow-up attendance.
Our stratified findings may have various explanations. Herman-Roloff, et al. (32) found that men in Kenya who had an adverse event following male circumcision were more likely to seek care, but not always from the provider directly. Mobile sites—which had lower overall attendance compared with fixed sites in this analysis (41.5% versus 52.5%, respectively)—do not have staff permanently stationed at the facility. Thus, it is possible that clients sought care from a source outside the VMMC team since no one was available to conduct follow-up, or that clients attended follow-up, but a dedicated staff was not available to receive clients and document their attendance. These results could also be explained if we assume that follow-up is more difficult at mobile facilities because it is influenced by factors such as management of teams, outreach events, weather complications, and political events, all of which would have less impact at fixed sites. In contrast to mobile facilities, fixed facilities have a team on site at all times and clients know they can visit the facility whenever it is convenient. Therefore, distance—especially when living more than 5 km from a facility—may play a larger role in attending follow-up when utilizing a fixed facility.
In order to improve attendance at VMMC follow-up visits and overall quality of VMMC services, program maintenance and expansion must take into account the underlying client population (i.e. demand) and ensure that 1) facilities are accessible, as defined by being within the 5 km catchment area, and that 2) transportation be made available for those who cannot be reached by a dedicated facility. Follow-up rates are likely to be improved if VMMC programs adequately support clients who live farther from a dedicated facility. Strategies could include a free national hotline for VMMC follow-up and enquiries, a dedicated mobile nurse whom clients could contact to conduct at-home check-ins, and/or trained community health workers assigned to specific geographic areas who are responsible for triaging AEs. Mobile technologies could be marshalled to assist lower cadre health workers to handle AEs (e.g., use mobile phones to transmit photos or brief videos to surgical consultants). Such approaches may offer clients the support they need to seek follow-up advice and care despite geographic barriers. More research must be done to determine the most effective and feasible strategy(ies).
There were several limitations to this study that should be considered. Without the availability of properly mapped roads and paths in rural Kenya, straight-line distance was utilized as a proxy for distance traveled from residence to VMMC facility. This method was validated by Siedner et al. (22), who characterized straight-line distance as a less laborious measurement that is highly correlated with GPS-tracked distance in relation to HIV clinic attendance. In this study, straight-line distance was significantly correlated with self-reported travel time. This approach addresses the problem of quantifying distances based on client estimations; however, in a context like Kenya, travel time to the facility may also be affected by variable road conditions and the modes of transport used, neither of which was accounted for in the straight-line distance measurement. Thus, this proxy for distance may be biased without such considerations. Another limitation is that utilization of services, including follow-up compliance, may be affected by healthcare worker attitudes and patient wait time, among other factors; however, these were not accounted for in this analysis. In addition, although the study population was randomly selected from among subjects self-selecting to be circumcised, this group may differ from the general population since they were early adopters of VMMC. Therefore, our results can be generalized to males in Nyanza seeking male circumcision, but not beyond. Furthermore, this study follows a program in one Province with many operating sites and outreaches; the implications of these findings may or may not apply to larger programs with fewer sites. The use of a random sample to create the study population, however, was a strength in the study design, as it increased internal validity.
CONCLUSION
This is the first study investigating the relationship between VMMC follow-up attendance and distance, and we found that greater distance to facility was significantly associated with lower follow-up attendance in services delivered at fixed facilities, but not through mobile services. In fixed facilities, a threshold effect was observed at 5 kilometers, above which follow-up attendance significantly dropped. Spatial analysis revealed that the catchment area for VMMC facilities in which almost all participants resided was 5 kilometers. Therefore, accessibility to VMMC facilities in Kenya seems adequate, but clients do not always visit the closest facility. Although distance may not be the most important predictor in choosing a facility, it is an important predictor of attending follow-up at fixed facilities. These results suggest that distance from the facility should be considered by VMMC programs seeking to optimize program safety through post-operative monitoring and treatment of adverse events.
Acknowledgments
Support for this study was provided by a grant to FHI from the Bill & Melinda Gates Foundation to support the Male Circumcision Consortium (MCC), a partnership between FHI, University of Illinois at Chicago working closely with the Nyanza Reproductive Health Society (NRHS), and Engender Health. The views expressed in this publication do not necessarily reflect those of the Bill & Melinda Gates Foundation or the MCC partners. We express our sincere thanks to those who participated in the study and making this research possible.
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization, UN Joint Programme on HIV/AIDS. New data on male circumcision and HIV prevention: policy and programme implications. 2007. [Google Scholar]
- 5.World Health Organization. Voluntary medical male circumcision for HIV prevention. Geneva: WHO; Jul, 2012. cited January 2014. [Google Scholar]
- 6.Khumalo-Sakutukwa G, Lane T, van-Rooyen H, Chingono A, Humphries H, Timbe A, et al. Understanding and addressing socio-cultural barriers to medical male circumcision in traditionally non-circumcising rural communities in sub-Saharan Africa. Cult Health Sex. 2013;15(9):1085–100. doi: 10.1080/13691058.2013.807519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott BE, Weiss HA, Viljoen J. The acceptability of male circumcision as an HIV intervention among a rural Zulu population, Kwazulu-Natal, South Africa. AIDS Care. 2005;17(3):304–13. doi: 10.1080/09540120412331299744. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Joint Strategic Action Framework to Accelerate the Scale-Up of Voluntary Medical Male Circumcision for HIV Prevention in Eastern and Southern Africa (2012–2016) Geneva: UNAIDS; 2011. [Google Scholar]
- 9.National AIDS and STI Control Programme (NASCOP); USAID, editor. PEPFAR’s Best Practices for Voluntary Medical Male Circumcision Site Operations. PEPFAR; 2012. [Google Scholar]
- 10.Moïsi JC, Nokes DJ, Gatakaa H, Williams TN, Bauni E, Levine OS, et al. Sensitivity of hospital-based surveillance for severe disease: a geographic information system analysis of access to care in Kilifi district, Kenya. Bull World Health. 2011;89(2):102–11. doi: 10.2471/BLT.10.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danso-Appiah A, Stolk WA, Bosompem KM, Otchere J, Looman CW, Habbema JDF, et al. Health seeking behaviour and utilization of health facilities for schistosomiasis-related symptoms in Ghana. PLoS Negl Trop Dis. 2010;4(11):e867. doi: 10.1371/journal.pntd.0000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odeny TA, Bailey RC, Bukusi EA, Simoni JM, Tapia KA, Yuhas K, et al. Text messaging to improve attendance at post-operative clinic visits after adult male circumcision for HIV prevention: a randomized controlled trial. PloS One. 2012;7(9):e43832. doi: 10.1371/journal.pone.0043832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley NJ, Pavlinac PB, Guthrie BL, Mackelprang RD, Muiru AN, Choi RY, et al. Distance from Home to Study Clinic and Risk of Follow-Up Interruption in a Cohort of HIV-1-Discordant Couples in Nairobi, Kenya. PloS One. 2012;7(8):e43138. doi: 10.1371/journal.pone.0043138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Needham DM, Bowman D, Foster SD, Godfrey-Faussett P. Patient care seeking barriers and tuberculosis programme reform: a qualitative study. Health Policy. 2004;67(1):93–106. doi: 10.1016/s0168-8510(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Tanser F, Hosegood V, Benzler J, Solarsh G. New approaches to spatially analyse primary health care usage patterns in rural South Africa. Trop Med Int Health. 2001;6(10):826–38. doi: 10.1046/j.1365-3156.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 16.Cooke GS, Tanser FC, Bärnighausen TW, Newell M-L. Population uptake of antiretroviral treatment through primary care in rural South Africa. BMC Public Health. 2010;10(1):585. doi: 10.1186/1471-2458-10-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chumnijarakij T, Sunyavivat S, Onthuam Y, Udomprasertgul V. Study on the factors associated with contraceptive discontinuations in Bangkok. Contraception. 1984;29(3):241–9. doi: 10.1016/s0010-7824(84)80004-9. [DOI] [PubMed] [Google Scholar]
- 18.Hong R, Montana L, Mishra V. Family planning services quality as a determinant of use of IUD in Egypt. BMC Health Serv Res. 2006;6(1):79. doi: 10.1186/1472-6963-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National AIDS and STI Control Programme (NASCOP), editor Kenya AIDS Indicator Survey 2012: Preliminary Report. Ministry of Health; Kenya: 2013. [Google Scholar]
- 20.Centers for Disease Control and Prevention. Progress in voluntary medical male circumcision service provision-Kenya, 2008–2011. MMWR Morb Mortal Wkly Rep. 2012;61(47):957. [PubMed] [Google Scholar]
- 21.Herman-Roloff A, Bailey RC, Agot K. Factors associated with the early resumption of sexual activity following medical male circumcision in Nyanza Province, Kenya. AIDS Behav. 2012;16(5):1173–81. doi: 10.1007/s10461-011-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siedner MJ, Lankowski A, Tsai AC, Muzoora C, Martin JN, Hunt PW, et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS (London, England) 2013;27(9):1503. doi: 10.1097/QAD.0b013e32835fd873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohela TJ, Campbell OM, Gabrysch S. Distance to care, facility delivery and early neonatal mortality in Malawi and Zambia. PloS One. 2012;7(12):e52110. doi: 10.1371/journal.pone.0052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigogo G, Audi A, Aura B, Aol G, Breiman RF, Feikin DR. Health-seeking patterns among participants of population-based morbidity surveillance in rural western Kenya: implications for calculating disease rates. Int J Infect Dis. 2010;14(11):e967–e73. doi: 10.1016/j.ijid.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Kyei NN, Campbell OM, Gabrysch S. The influence of distance and level of service provision on antenatal care use in rural Zambia. PloS One. 2012;7(10):e46475. doi: 10.1371/journal.pone.0046475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moses RA, Dagrosa LM, Hyams ES, Steinberg PL, Pais VM. Failing to follow up: predicting patients that will “no-show” for medically advised imaging following endourologic stone surgery. Can J Urol. 2013;20(5):6939–43. [PubMed] [Google Scholar]
- 27.Kruk ME, Mbaruku G, Rockers PC, Galea S. User fee exemptions are not enough: out-of-pocket payments for ‘free’delivery services in rural Tanzania. Trop Med Int Health. 2008;13(12):1442–51. doi: 10.1111/j.1365-3156.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- 28.Andaleeb SS. Service quality perceptions and patient satisfaction: a study of hospitals in a developing country. Soc Sci Med. 2001;52(9):1359–70. doi: 10.1016/s0277-9536(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 29.Herman-Roloff A, Otieno N, Agot K, Ndinya-Achola J, Bailey RC. Acceptability of medical male circumcision among uncircumcised men in Kenya one year after the launch of the national male circumcision program. PLoS One. 2011;6(5):e19814. doi: 10.1371/journal.pone.0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey R, Muga R, Poulussen R, Abicht H. The acceptability of male circumcision to reduce HIV infections in Nyanza Province, Kenya. AIDS Care. 2002;14(1):27–40. doi: 10.1080/09540120220097919. [DOI] [PubMed] [Google Scholar]
- 31.Westercamp M, Agot KE, Ndinya-Achola J, Bailey RC. Circumcision preference among women and uncircumcised men prior to scale-up of male circumcision for HIV prevention in Kisumu, Kenya. Aids Care. 2012;24(2):157–66. doi: 10.1080/09540121.2011.597944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman-Roloff A, Bailey RC, Agot K. Factors associated with the safety of voluntary medical male circumcision in Nyanza province, Kenya. Bulletin of the World Health Organization. 2012;90(10):773–81. doi: 10.2471/BLT.12.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]