Abstract
The anterior pituitary gland is comprised of specialized cell-types that produce and secrete polypeptide hormones in response to hypothalamic input and feedback from target organs. These specialized cells arise from stem cells that express SOX2 and the pituitary transcription factor PROP1, which is necessary to establish the stem cell pool and promote an epithelial to mesenchymal-like transition, releasing progenitors from the niche. The adult anterior pituitary responds to physiological challenge by mobilizing the SOX2-expressing progenitor pool and producing additional hormone-producing cells. Knowledge of the role of signaling pathways and extracellular matrix components in these processes may lead to improvements in the efficiency of differentiation of embryonic stem cells or induced pluripotent stem cells into hormone producing cells in vitro. Advances in our basic understanding of pituitary stem cell regulation and differentiation may lead to improved diagnosis and treatment for patients with hypopituitarism.
Keywords: Pituitary, Stem Cells, SOX2, PROP1, EMT, Extracellular Matrix
Introduction
The ability of the pituitary gland to increase hormone production in times of demand had long suggested the existence of postnatal pituitary stem cells, and interest in pituitary stem cell biology has intensified since 2008 when SOX2-expressing cells in adult pituitary glands were first shown to possess stem cell capacity to self-renew and differentiate into all of the major hormone-producing cell types of the pituitary gland in vitro (1). Since then, several major advances have taken place, and these have been the focus of recent review articles (2–6). In vitro differentiation of both human and mouse embryonic stem (ES) cells into pituitary hormone producing cells in culture has been successfully developed and improved upon in recent years, shedding light on mechanisms which could stimulate differentiation of pituitary stem cells in vivo (7–10). The composition of the stem cell niche is being investigated, and an epithelial to mesenchymal (EMT-like process was shown to drive migration of progenitors out of the niche (11). Pathways regulating cell turnover are being identified (12), and the limitations of self-renewal over the life of the animal are emerging (13). In this review, after an overview of pituitary development, we concentrate on the most recent advances regarding the role of the transcription factor PROP1 in establishing stem cell pools and driving the EM-like transition, the role of signaling pathways, and the potential role of mesenchymal stem cells. We also cover the challenges of stem cell therapeutics and unanswered questions that may be the focus of future studies. The role of cancer stem cells in pituitary adenomas are the subject of a separate review in this issue (J.P. Martinez-Barbera and colleagues, this Issue).
Pituitary Organogenesis: Formation of the Mature Gland from Multiple Embryonic Origins
Contributions of Surface Ectoderm and Neural Ectoderm
The pituitary gland is an endocrine organ found only in vertebrates, and aspects of its development and the nature of the specialized hormone-producing cell types are evolutionarily conserved across vertebrate species (14). Thus, studies in birds, amphibians, fish, and mammals have informed our understanding of cell specification and pituitary development (15–18). For example, the roles of FGF, BMP, SHH and WNT signaling pathways in pituitary development have been established in multiple species. Fate mapping studies in several different species have revealed that the mature pituitary gland is composed of cells that originate from the surface (oral) ectoderm, the neural ectoderm, and the cranial mesenchyme. The oral ectoderm forms the anterior and intermediate lobes, and the neural ectoderm forms the posterior lobe, while the cranial mesenchyme forms vasculature and connective tissue within and surrounding the mature gland.
Craniofacial placodes are specific regions of the non-neural surface ectoderm that thicken in relation to the adjacent ectoderm, and will give rise to the pituitary gland as well as several other craniofacial structures. The pituitary, lens, olfactory, otic, trigeminal and epibranchial placodes (in mammals) arise from the preplacodal region, which is a region of the surface ectoderm adjacent to the neural ectoderm (Figure 1). The early placodes utilize common signaling pathways and genetic networks as they form the initial placode stages, before diverging and activating unique placode-specific programs to form the distinct tissue systems (19–21). Thus, these placode expression studies provide candidate genes for regulation of pituitary development, and some of the mechanisms that underlie stem cell regulation in other craniofacial placodes may apply to the pituitary gland.
Figure 1. The Preplacodal Region is the Interface Between the Neural and Surface Ectoderms.
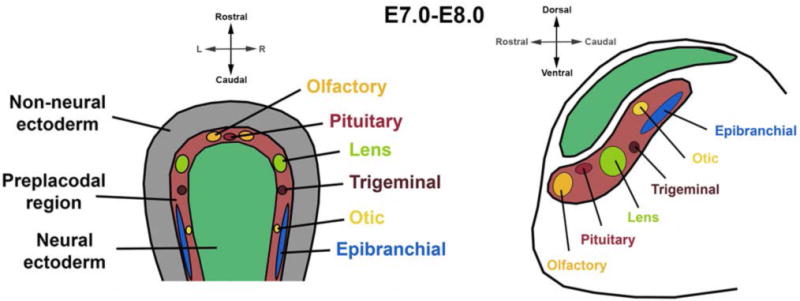
Interaction between the surface and neural ectoderm gives rise to the preplacodal region between E7.0–E8.0 in mouse (~E15–19 in humans, ~E8.5–9.0 in rats), from which multiple craniofacial tissues are ultimately derived.
The pituitary (or adenohypophyseal) placode is a thickening of the oral ectoderm at the roof of the mouth that invaginates to produce Rathke’s pouch (reviewed in (18)) (Figure 2). It is in juxtaposition to the neural ectoderm of the ventral diencephalon, which evaginates to form the infundibulum (pituitary stalk) and posterior lobe (neurohypophysis or pars nervosa) (22). The infundibular region of the ventral diencephalon expresses the morphogenetic proteins BMP4, FGF8, and FGF10, which act as a signaling center, or pituitary organizer, for the induction and proliferation of Rathke’s pouch (reviewed in(23)). SHH is initially expressed in the ventral midline throughout the neural tube; however, in the infundibular region of the ventral diencephalon, SHH expression is inhibited by BMP signaling and by TBX2 and TBX3. Persistent SHH expression in the infundibular region results in loss of the pituitary organizer and reduction or loss of Rathke’s pouch (24,25). The pituitary organizer, especially FGF signaling, also acts as a chemoattractant for axons of oxytocin and vasopressin expressing neurons located in the paraventricular nucleus of the hypothalamus (26). The progenitor cells within the infundibular region give rise to the pituicytes, the glial-like cells of the posterior lobe, and Notch signaling through Hes1 and Hes5 is necessary for promoting pituicyte fate specification (27). The posterior lobe and pituitary stalk are continuous with the median eminence of the hypothalamus, which releases hypothalamic hormones into the hypophyseal portal system and regulates anterior pituitary hormone secretion.
Figure 2. The Pituitary Gland Develops from Distinct Embryonic Origins.
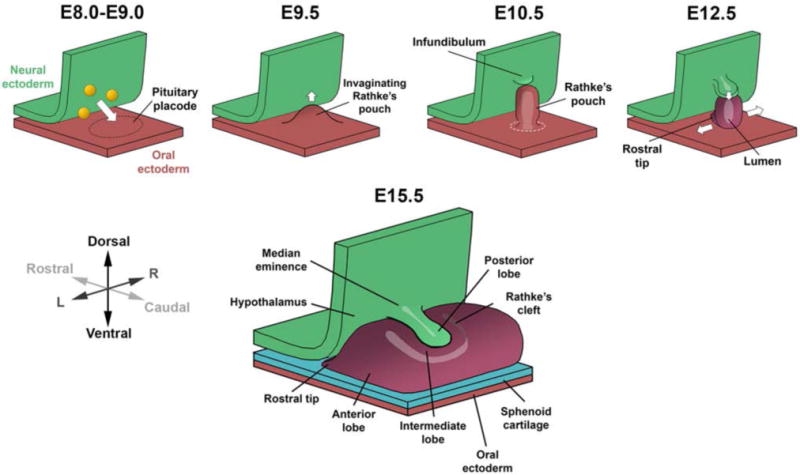
Pituitary development in the mouse begins from E8.0, when the neural ectoderm adjacent to the pituitary placode produces signaling factors (yellow) to initiate the invagination of Rathke’s pouch. The pouch will begin to pinch off from the oral ectoderm at E10.5 and completely separate by E12.5. Rathke’s pouch gives rise to the anterior lobe, which continues to expand ventrally and laterally. The infundibulum evaginates downwards from the neural ectoderm and forms the posterior lobe.
FGF signaling from the pituitary organizer promotes the survival of the pituitary progenitor cells in Rathke’s pouch (28–30). The area of highest proliferation in Rathke’s pouch is at the dorsal aspect, closest to the infundibular signaling center. This region of Rathke’s pouch contains the SOX2-expressing stem cells at E12.5 and E14.5 in mouse development. As the Rathke’s pouch continues to invaginate upwards, the base of the pouch will close off and completely separate from the oral ectoderm, followed by the condensation of the sphenoid cartilage between the two ectodermal structures. The dorsal (top) side of the pouch forms the intermediate lobe while the ventral (bottom) side forms the anterior lobe. The residue of Rathke’s cleft defines the separation between the lobes in rodents, and it can appear as a lumen in histological sections due to the shrinkage of the tissue during processing. In humans and some other species, the intermediate lobe is not distinct, but residual cleft tissue can be found. This tissue is thought to be the stem cell niche and is referred to as the marginal zone. There is an EMT-like transition, including a reduction in E-cadherin expression at the ventral aspect of the Rathke’s cleft, as anterior lobe progenitor cells begin to differentiate and delaminate ventrally and migrate laterally from the lumen, causing the expansion of the parenchyma of the anterior lobe (11,31,32).
Contributions of the Notochord and Mesenchyme
Studies of pituitary development have focused on the nature of the signaling between the ventral diencephalon and Rathke’s pouch. However, both the notochord and the mesenchyme that surrounds the developing pituitary gland are additional sources of important signaling molecules, which have the potential to influence development of stem cell pools and stimulation of differentiation. The tip of the notochord, which is a critical signaling center, terminates on the caudal side of Rathke’s pouch (33). In chick explant studies the notochord can induce the invagination of surface ectoderm, producing Rathke’s pouch-like structures (34). The BMP inhibitors CHORDIN and NOGGIN are expressed in the notochord and prechordal plate and are likely critical factors for regulating the induction of Rathke’s pouch. In support of this idea, Chrd−/−; Nog+/− mouse embryos fail to express Nkx2.1 in the ventral diencephalon, which results in the loss of the pituitary organizer and Rathke’s pouch (35). The mesenchyme on the rostral side of Rathke’s pouch is derived from neural crest, while definitive mesoderm contributes to the tissue caudal to the pouch (36,37). The cranial mesenchyme has a role in supporting cell specification. It provides a permissive signal for corticotrope differentiation in the chick (34). The head mesenchyme expresses Foxd1, and Foxd1−/− mouse embryos have reduced LHβ expression (38). Thus, signaling from the notochord and mesenchyme plays a role pituitary development. Additional studies are needed to define the cellular targets of these signaling pathways and the detailed molecular mechanisms whereby ectoderm and mesenchyme regulate pituitary stem cell behavior and differentiation.
Both the neural crest and definitive mesoderm derived head mesenchyme make direct contribution to the vasculature of the developing pituitary. Pituitary angiogenesis begins as the vessel network from the surrounding tissues enters between Rathke’s pouch and the ventral diencephalon at E10.5 in the mouse (Figure 3). The infundibulum is vascularized first, as seen by PECAM staining at E11.5, followed by the anterior lobe at E13.5 (39).
Figure 3. Vascularization of the Developing Pituitary Gland.
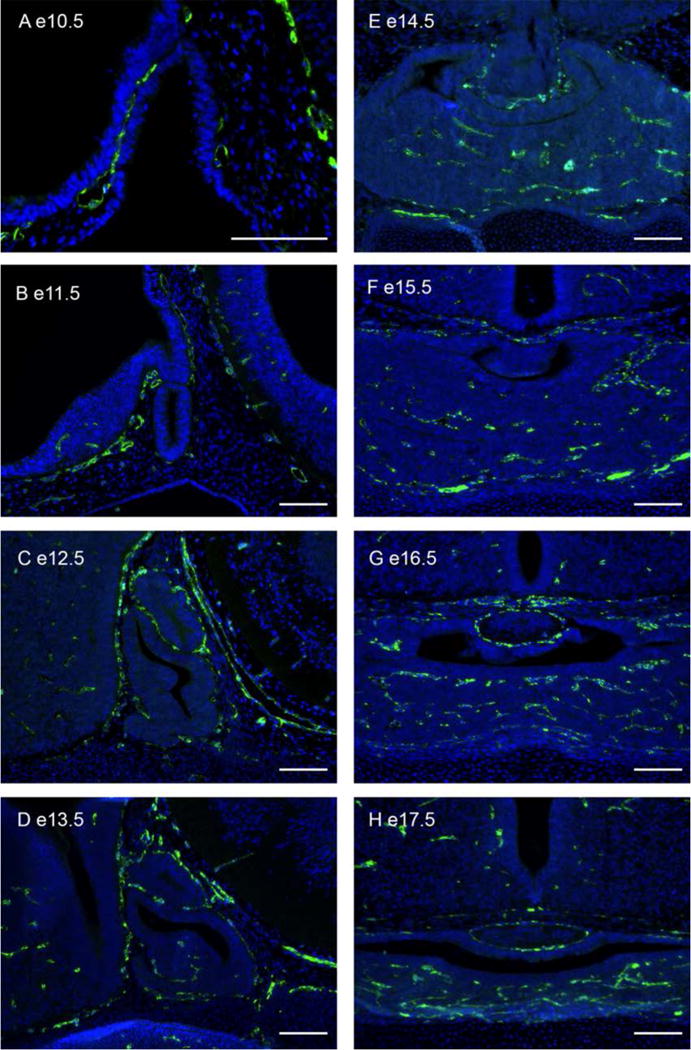
(A–H) Immunostaining for CD-31 (PECAM, green) and counterstained with DAPI (blue). Endothelial cells surround the infundibulum at E11.5 and invade the anterior lobe beginning at E13.5. Scale bars 100 μm.
The neural crest produces pituitary pericytes, contractile cells that wrap around endothelial cells controlling blood flow (37,40). Endothelial cells in the head are derived from the definitive mesoderm, and the endothelial precursors migrate throughout the head, penetrating regions where the head mesenchyme is primarily neural crest derived (41,42). The head mesenchyme that invades the pituitary anterior lobe from the rostral side is, therefore, a mix of neural crest and definitive mesoderm derived progenitor cells that form both the pericytes and endothelial cells of the pituitary vasculature. It expresses genes associated with stem cells, including Prrx1, Prrx2, and Nestin (43). Some of this head mesenchyme is likely to maintain stem cell potential in the mature pituitary gland. Recent studies suggest that pituitary adenomas contain a stem cell population, known as mesenchymal stem cells or mesenchymal stromal cells (MSCs), which can produce mesenchymal derivatives, as opposed to the hormone producing cell types generated from pituitary sphere forming colonies (44,45).
MSCs are defined by their ability to differentiate into adipocytes, osteoblasts, and chondrocytes when cultured in vitro (46). They were originally isolated from bone marrow based on their osteogenic, instead of hematopoietic, potential (47), but have been isolated from most tissues, including umbilical cord blood, adipose tissue, dental pulp, and breast milk (48–52). MSCs typically represent a heterogeneous population, and recent results demonstrate that both neural crest and mesodermal lineages produce the mixed MSC population derived from bone marrow (53,54). Both neural crest derived and bone marrow derived MSCs have the capacity to form adipocytes, chondrocytes, and osteoblasts in culture, as well as ectodermal lineages, including neurons (55). The isolation of MSCs from pituitary adenomas suggests the presence of a mesenchymal cell population in pituitary neoplasms that likely originates from the neural crest derived pericytes, a hypothesis that awaits confirmation using mouse lineage tracing studies.
Mechanisms of endothelial and vascular contribution to cellular differentiation in other endocrine organs may serve as a paradigm for understanding pituitary development and developing treatments. For example, endothelial cells migrate into the pancreas to form the vasculature in response to angiogenic factors produced by the developing islet, and the endothelial cells produce factors that stimulate subsequent islet cell differentiation (56). It is not yet clear whether the invasion of the vasculature has a direct role in stimulating pituitary differentiation like it does in the pancreas, and future studies may focus on identifying the mechanisms that regulate normal vascularization and to deciphering the influence of vascularization on pituitary differentiation.
Pituitary Stem Cells: Postnatal Pituitary Cell Turnover and Responses to Hormone Demand
The ability and requirement of the postnatal pituitary gland to increase hormone production in response to physiological and pathological stimuli, and the natural turnover of cells, had historically been suggestive of the existence of postnatal pituitary stem cells. The rodent pituitary gland increases in size during the postnatal period, and once the animal reaches adulthood, the rate of cell proliferation is quite low; about 0.2% of the cells are in S phase (57). The natural cell turnover of a pituitary cell is estimated to be 60–70 days (58), but the pituitary does not decrease in size over time, meaning that cells must be replenished somehow. The pituitary gland has to increase hormone production under normal physiological events such as puberty and pregnancy, as well as under extreme conditions such as end organ disease, surgical adrenalectomy and gonadectomy, or pharmacological ablation of the thyroid gland.
During pregnancy and lactation in both humans and rats, there is an increase in the size of the pituitary gland and the proportion of lactotropes in the pituitary (59–61), and it was long thought that new lactotropes are generated in order to match the need for more prolactin. However, cell lineage tracing studies in transgenic mice have shown that enlargement of the organ mostly results from a ~20% increase (hypertrophy) in the volume of prolactin producing cells, and that there is no increase in the proportion of lactotropes during pregnancy in mice (62). Other specialized pituitary cell types also undergo hypertrophy under increased hormone demand: corticotrope volume increases 2.6-fold after adrenalectomy (63), gonadotropes increase dramatically in surface area after gonadectomy (64), and thyrotropes do so following pharmacological ablation of the thyroid (65).
At the same time, a significant portion of postnatal proliferating anterior pituitary cells are hormone-positive, suggesting that some differentiated cells have re-entered the cell cycle (66,67). At postnatal day 8–10 the majority of POU1F1 expressing cells are labeled with Ki67, indicative of active progression through the cell cycle (68,69). It is not known what triggers differentiated cells to re-enter the cell cycle, but the hypothalamic releasing hormones GHRH, TRH and GNRH are essential for expansion of the somatotropes, thyrotropes and gonadotropes in the postnatal period, respectively (70–72). Therefore, hypertrophy and proliferation of existing differentiated endocrine cells contribute to pituitary homeostasis and plasticity together with the activity of pituitary stem cells.
Definitive evidence of adult pituitary stem cells came from Vankelecom and colleagues (73), who identified a side population of about 2% of anterior pituitary cells that are resistant to Hoechst 33342 dye uptake, express stem cell factors, and are able to form clonal spheres in vitro, demonstrating proliferative capacity. Nolan and Levy (74) separately identified that there is a nonhormonal pituitary cell population that proliferated and differentiated into new gonadotropes and corticotropes in response to gonadectomy or adrenalectomy, and that the same population gave rise to both (i.e. were multipotent). The identity of postnatal pituitary stem cells was subsequently identified in 2008, when Fauquier et al. showed that SOX2-expressing cells, found in the region lining the Rathke’s cleft (marginal zone) and the parenchyma of the anterior lobe (Figure 4), were the cells able to form clonal spheres in culture, and that these spheres were then able to differentiate into all five hormone producing cell types of the anterior lobe (1).
Figure 4. SOX2-Expressing Pituitary Stem Cells Line the Marginal Zone.
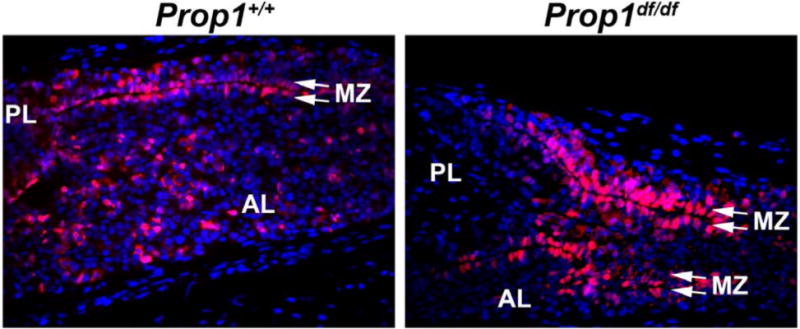
SOX2-expressing cells (red) are found in the postnatal pituitary lining Rathke’s cleft or the marginal zone and in the parenchyma of the anterior lobe. In Prop1df/df mutant mice, SOX2 stem cells cannot undergo an EMT-like process and are retained in the marginal zone, causing it to become convoluted and dysmorphic. AL=anterior lobe, PL=posterior lobe, MZ=marginal zone.
Finally, in vivo evidence to show that SOX2-expressing pituitary cells are postnatal stem cells came from two groups in 2013 (75,76). Firstly, inducible postnatal SOX2-lineage tracing showed that these cells naturally give rise to new hormone cells throughout life (i.e. replenishes cells over time) (75,76). Secondly, it is also these SOX2-expressing cells that proliferate in response to gonadectomy and adrenalectomy to generate new gonadotropes and corticotropes respectively (69,76), finally confirming SOX2-expressing cells as the proliferative non-endocrine cells observed by Nolan & Levy (74). In these cases, the overall number of SOX2-expressing cells does not increase, presumably because the cells are dividing to maintain the size of the stem cell pool and producing daughter cells that progress to differentiation. Together, these studies demonstrated that postnatal pituitary SOX2-expressing cells, meaning these cells can be classed as pituitary stem cells.
Ablation of terminally differentiated somatotropes and lactotropes with an inducible diphtheria toxin paradigm can stimulate regeneration of 40–60% of the lost somatotropes and lactotropes, mainly by differentiation directly from SOX2-expressing progenitors but also from proliferation and transdifferentiation of endocrine cells (13,77,78). In contrast to gonadectomy and adrenalectomy, the ablation of somatotropes and lactotropes caused a doubling of SOX2-expressing cells and clonogenic potential. The basis for this difference is not known, but it could reflect the larger population size of the somatotropes and lactotropes relative to gonadotropes and corticotropes. Taken together, these studies demonstrate that pituitary SOX2-expressing cells are the cells required for both organ maintenance and response to physiological challenge, and thus can be classified as pituitary stem cells.
Other markers expressed in the SOX2 stem cell niche include Sox9, Oct4, Gfra2, Ret, Ly6a (Sca-1), Prop1, Prrx1, Cdh1 (E-cadherin), S100β, β-catenin, neuronatin, neurturin, and Cxadr (Coxsackievirus and adenovirus receptor) (3,73,75,76,79–81). Clonogenic potential has been demonstrated for cells expressing Sox2, Sox9, Gfra2, and S100b, but the remaining markers have not been tested yet. These factors are expressed in various combinations in some SOX2 cells, and the differences between these different subpopulations remain unclear, but it is well established that SOX2-expressing cells are stem cells (75,76).
In mammals, the glial cell-line derived neurotrophic factor (GDNF) family is composed of four different factors that bind to GDNF receptor alpha (GFRα1–4), that act as co-receptors for the tyrosine kinase RET and facilitate ligand binding. Alvarez and colleagues made an important contribution to the field by demonstrating expression of GFRa2 in the adult rodent and human pituitary glands (79). GFRa2 expression is detected in the marginal zone and in cells scattered throughout the anterior lobe, representing 0.9% of the total cells. The GFRa2 positive cells are also positive for the pituitary specific homeodomain transcription factor PROP1. GFPRa2 positive cells are slowly proliferating and able to form spheres in vitro, can generate secondary pituispheres, and differentiate into the five hormone producing pituitary lineages (79).
RET is expressed in ciliated cells on both sides of the marginal zone (5). RET and GFRa1 are co-localized in somatotrophs (82), where RET acts as a dependence receptor (83). The RET dependence pathway is regulated by the transcription factor POU1F1 (PIT 1). In GH cells, RET forms a complex with CASP3 and PKC-delta which leads to increased POU1F1 levels. This, in turn, induces p19Arf (Cdkn2a) transcription, p53 accumulation and apoptosis (12). RET is also expressed in lactotropes, and it may act together with POU1F1 and CASP3 to trigger apoptosis after lactation ceases (84). Interestingly, the RET/POU1F1 apoptotic pathway is active in human somatotrope adenomas, and it is inhibited by GDNF, suggesting the possibility that RET inhibitors may be effective therapeutics for aggressive acromegaly (85).
The mechanism for regulating the choice between pituitary stem cell self-renewal and transition to differentiation is not understood, but Notch signaling is necessary to suppress premature differentiation and to regulate postnatal SOX2 stem cells in vivo (69). In addition, the efficiency of differentiation into hormone producing cells is enhanced by withdrawing FGF and EGF from the media and plating the spheres on an uneven matrigel matrix (1,73), which is comprised of laminin, collagen IV, heparan sulfate proteoglycans and entactin/nidogen. This suggests a supportive role of the extracellular matrix in promoting differentiation (3).
Pituitary stem cells and regenerative capacity declines with age. SOX2 positive cells are more abundant in pituitaries of newborn and early postnatal mice than in adult animals (2-month-old), and aging to 7 months is associated with a substantial decrease in clonogenic potential (73,77,86–88). When 8-month-old mice are subjected to diphtheria toxin inducible ablation, there is no recovery of somatotropes, indicating that the ability to regenerate these cells has been lost (13). The basis for this decline is not known, but expression profiling has revealed an age-related reduction in expression of multiple genes in addition to Sox2, including members of the SHH, WNT, TGFβ and Hippo signaling pathways and numerous chemokines and cytokines. More studies are necessary to determine whether aging ablates the ability to regenerate other pituitary cell types, and to define the critical pathways that recruit stem cells to differentiate in young adult mice.
Epithelial-to-Mesenchymal Transition (EMT) Events in Pituitary Stem Cells
PROP1 is a pituitary-specific transcription factor that is required to activate POU1F1 (89), and the subsequent differentiation of GH, PRL, and TSH cells. Prop1 mutant mice fail to develop the cell types that make GH, TSH and PRL during embryogenesis, which results in hypopituitarism and growth insufficiency, hypothyroidism and infertility (90). PROP1 is the most commonly mutated transcription factor in human patients with congenital hypopituitarism (91), and the patients tend to undergo progressive hormone loss, which can eventually result in life threatening hypocortisolism in the third or fourth decade of life (92). We have recently shown, using Prop1-Cre lineage tracing, that all hormone-producing cells in the anterior and intermediate lobes of the pituitary gland are derived from Prop1-expressing progenitors (93). Thus, PROP1 marks an important progenitor for Rathke’s pouch derivative cells and affects all pituitary cell types in humans.
The progressive hormone loss in patients and the derivation of all hormone producing cells from a PROP1-expressing progenitor in mice suggested the hypothesis that PROP1 deficiency results in depletion of stem cell pools. In support of this idea, we discovered that Prop1 is transiently co-expressed with Sox2 in embryonic pituitary development, and using Prop1 mutant mice we showed that Prop1 is required for initial establishment of adequate stem cell pools, as well as for normal stem cell colony forming behavior (11). Prop1 mutant neonates have more SOX2-positive cells than wild-types, and they remain in the marginal zone (Figure 4). Many of the PROP1-deficient SOX2-expressing cells fail to activate expression of the transitional markers Sox9 and Cyclin E. The Prop1 mutants have substantially reduced ability to form adherent stem cell colonies, and these mutant colonies exhibit abnormal, flat cell morphology. Thus, PROP1 is required to maintain the size and stem cell characteristics of the Sox2-expressing stem cell pool.
Expression of the angiogenic factor VEGFA coincides with penetration of blood vessels into the anterior lobe (forming the secondary capillary plexus) and connection of the secondary plexus to the hypophyseal portal system. VEGFA is co-expressed with the transcription factor PROP1 at E14.5 (Figure 5), but the relationship between these two factors is not known. Prop1 mutant pituitaries express VEGFA (Figure 6), but they have inadequate vascularization (68), and it is not clear what inhibits this process in Prop1 mutants. During normal pituitary organogenesis an EMT-like process is initiated. There is a reduction in E-cadherin expression at the ventral aspect of Rathke’s cleft, and progenitor cells switch from tightly packed, polarized, planar shape to a rounded cell morphology, and then these cells migrate into the parenchyma of the organ where they commence differentiation (31,32,94). This process fails in Prop1 mutant mice, resulting in a highly dysmorphic and hypoplastic anterior pituitary. Gene expression profiling of adherent stem cell colonies revealed that many genes in the Notch, TGFβ, WNT and SHH signaling pathways were down-regulated in Prop1 mutants. In addition, Prop1 mutant colonies have up-regulated epithelial cell markers like E-cadherin, keratins and claudins, and down-regulation of mesenchymal cell markers like metalloproteinases and the EMT inducer Zeb2 (11). Studies in intact animals confirmed that Prop1 is required for activation of genes like Zeb2 and Slug (32,95). This is consistent with a requirement for Prop1 to trigger EMT, in addition to initially establishing an adequately sized stem cell pool.
Figure 5. PROP1 and VEGFA are Co-Expressed in Pituitary Stem Cells.
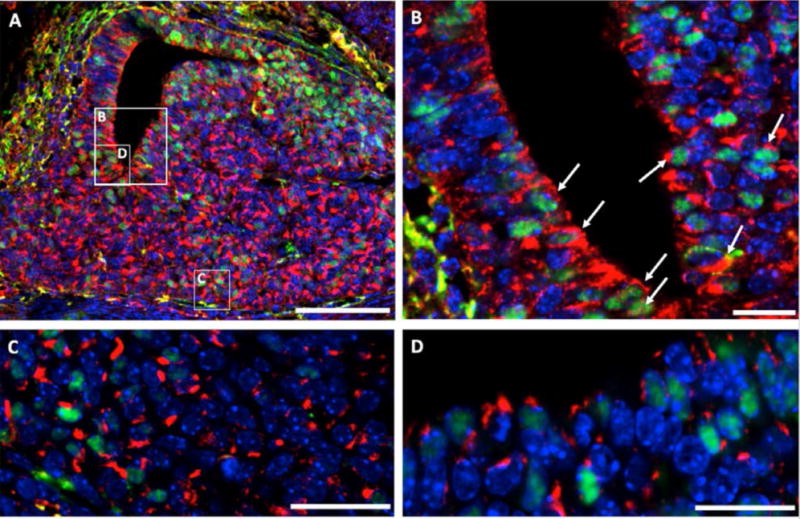
Double immunofluorescence reveals co-staining of PROP1 (green) and VEGFA (red) at E14.5 around the residue of Rathke’s cleft (B and D) and in the anterior lobe (C). Cell nuclei were stained with DAPI (blue). The white box indicates where higher magnification photo (B) was taken. Scale bars 100 μm (A) or 20 μm (B, C and D).
Figure 6. VEGFA Expression is Maintained in Prop1 Mutant Pituitaries.
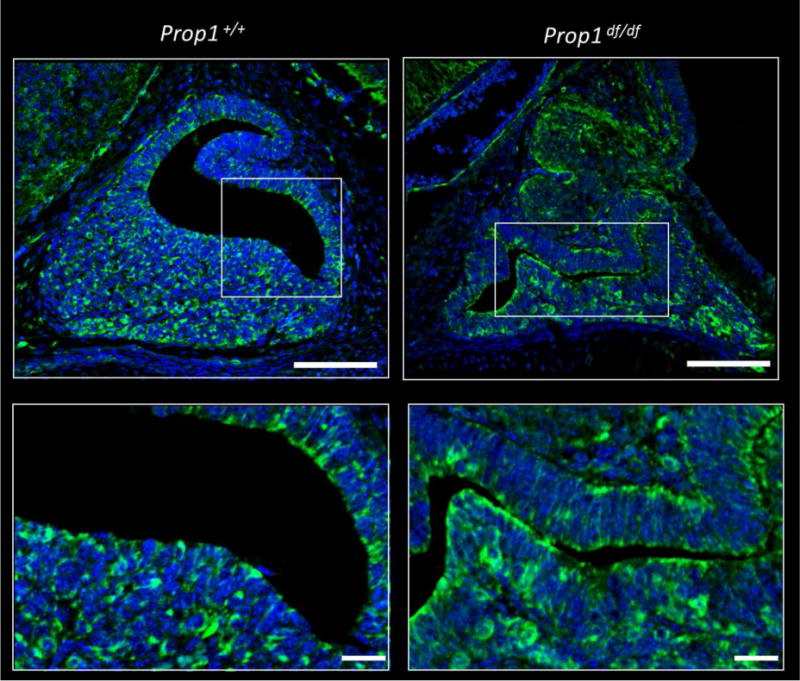
Immunohistochemical staining for VEGFA was performed on pituitary sections from E16.5 Prop1+/+ and Prop1df/df mice (green). Cell nuclei were stained with DAPI (blue). White boxes indicate where higher magnification photos were taken. Upper panel: Scale bars 100 μm. Lower panel: scale bars 20 μm.
Several groups have conducted gene expression profiling to identify candidate genes for regulating the stem cell niche and cell migration. Gene expression profiling yielded numerous markers that might be involved in regulation of the stem cells, including many signaling molecules and genes involved in EMT (88,96,97). Kato and colleagues hypothesized that genes regulating neural stem cells may also be involved in pituitary gland stem cell regulation (3). They developed a model for EMT in which the cells in the niche are expressing SOX2, Cxadr, E-cadherin and TGFβ receptors. TGFβ signaling triggers cells to leave the niche and migrate, and they express Cxadr and vimentin, and have silenced SOX2 expression. This induces expression of Snail1, Slug, Twist and Zeb genes, and these, in turn, drive increased expression of matrix metalloproteinases and chemokines like Cxcl12, and suppress expression of E-cadherin. The chemokines are proposed to orient the direction of migration. Functional studies will help clarify the requirements for individual genes in regulating migration and EMT.
Cell Signaling Pathways Regulating Pituitary Stem Cells
Studies elucidating the specific mechanisms that regulate pituitary stem cell self-renewal, proliferation, and differentiation are ongoing. In light of their expression of SOX2, adult pituitary stem cells are likely to resemble embryonic SOX2-expressing cells and be regulated by similar signaling factors, such as BMPs, FGFs, SHH, Notch, and Wnt. Currently, only the Wnt, Notch, and SHH signaling pathways have been directly implicated in adult pituitary stem cells (86,98,99).
Transmembrane Notch receptors and their cognate ligands are expressed in the developing pituitary gland, with their expression reflecting the location of progenitor cells, suggesting that they play a role in their regulation (100,101). Studies have shown that Notch signaling is required to maintain the progenitor state and inhibit specification of certain pituitary endocrine lineages (32,69,98,101–108), akin to the role of Notch signaling in pancreatic endocrine differentiation (109). In the juvenile postnatal pituitary, conditional deletion of Notch2 from early pituitary development causes a reduction in postnatal SOX2- and SOX9-progenitor cells and a reduction in cell proliferation (98). Similarly, conditional deletion of Rbpjk in Prop1-lineage cells causes a reduction and continued depletion of SOX2-expressing cells, and a loss of their sphere-forming capacity (69). This indicates that Notch signaling is likely involved in the maintenance of self-renewal and the pluripotent state in pituitary stem cells.
Many Wnt pathway molecules are expressed in the embryonic pituitary, and attenuation of Wnt signaling leads to reduced proliferation and reduced POU1F1 expression (89,110,111). Therefore, Wnt signaling stimulates endocrine specification during pituitary development (reviewed in (112)). However, this does not appear to be the case in adult pituitary stem cells. Over-activation of Wnt signaling in embryonic HESX1-expressing pituitary cells, encompassing most anterior pituitary cells, causes hyperplasia and formation of tumors resembling human adamaninomatous craniopharyngioma (86). Human adamaninomatous craniopharyngiomas often have increased expression of β-catenin (113). Furthermore, postnatal induction of Wnt signaling in adult SOX2-expressing cells also causes the same adamaninomatous craniopharyngioma tumor formation, indicating that pituitary stem cells respond to Wnt signaling. However, the tumors are hormone-negative, and in fact, the affected SOX2-cells do not themselves form the tumors, and instead send out signals to induce the transformation of neighboring cells. Pituitary craniopharyngiomas and cancer stem cells are further detailed in an accompanying review in this Issue by J.P. Martinez-Barbera and colleagues.
SHH signaling also regulates pituitary stem cells. Inducible deletion of the SHH receptor PTCH in adult mouse pituitary causes over-activated SHH signaling, increased proliferation of SOX2- and SOX9-expressing cells, and increased expression of ACTH, GH, and PRL (114). This study also found high SHH and GLI1 expression in ACTH-, GH-, and PRL-expressing human pituitary adenomas, suggesting that SHH activity could be involved in stimulating pituitary stem cells into tumorigenesis.
The Hippo signaling pathway is a recently-identified candidate for regulating the pituitary stem cell population. Hippo pathway molecules are expressed during pituitary development, and its effectors YAZ/TAZ are expressed in the postnatal stem cell niche (115), suggesting they play a role there. Accordingly, Hippo pathway factors are up-regulated in the population of stem cells that proliferate in response to somatotrope ablation by diphtheria toxin (13). This is of particular note given that the Hippo pathway is interconnected with EMT, and EMT is a critical step in transitioning stem cells to differentiation (11).
Recent studies suggested that cell adhesion has an important role in the regulation of pituitary stem cell function, similar to its role in intestinal stem cell differentiation (116). In some tissues, like intestines and skin, the stem cell niche participates in ephrin and ephrin receptor signaling. Eph/ephrin signaling is involved in the regulation of many important developmental process, like cell adhesion, migration and morphology. They are expressed in almost all tissues of a developing embryo, and are crucial for maintaining stem cell properties (117).
The role of Ephs in the pituitary is not yet known. The ephrin-A5 and ephrin-B2 are expressed in pituitary organogenesis (118,119). Ephrin-A5 is expressed in the ventral hypothalamus and developing posterior lobe. Ephrin-B2 is co-expressed with SOX2 in Rathke’s pouch, suggesting a role in the formation of the anterior pituitary stem niche. We found altered expression of ephrins and ephrin receptors in adherent stem cell colonies derived from Prop1+/+ and Prop1df/df pituitaries (Table 1). Transcripts for the ligands Epha4 and Efna1 were elevated and the receptors Ephb1 and Ephb2 were substantially reduced in Prop1 mutants. Interestingly, we found PROP1 DNA binding sites in genes of Eph family suggesting the possibility of direct regulation. Taken together, these data support the idea that ephrin signaling is involved in regulation of the pituitary stem cell niche (119), but further studies are needed to demonstrate a functional role.
Table 1. Prop1-Dependent Gene Expression Changes in Adherent Pituitary Stem Cell Colonies.
Selected genes that were up-regulated (green) or down regulated (red) in Prop1 but not Pou1f1 mutant stem cell colonies relative to wild type (p ≤ 0.05, log2FCI ≥1)
| Gene symbol | Gene name |
|---|---|
| Ephrin and Eph Signaling | |
| Epha4 | EPH Receptor A4 |
| Efna1 | Ephrin A1 |
| Ephb1 | EPH Receptor B1 |
| Ephb2 | EPH Receptor B2 |
| Extracellular Matrix (ECM) | |
| Itga8 | Integrin Subunit Alpha 8 |
| Itgb3 | Integrin Subunit Beta 3 |
| Itgb4 | Integrin Subunit Beta 4 |
| Itgb6 | Integrin Subunit Beta 6 |
| Itga1 | Integrin Subuit Alpha 1 |
| Itga4 | Integrin Subuit Alpha 4 |
| Itgal | Integrin Subuit Alpha L |
| Lama4 | Laminin Subunit Alpha 4 |
| Lamb2 | Laminin Subunit Beta 2 |
| Lamc1 | Laminin Subunit Gamma 1 |
| Bgn | Biglycan |
| Ogn | Osteglycin |
Eph/ephrin signaling is involved in cell migration, and a relationship between eph/ephrin signaling and integrin signaling has been postulated. However, the connection between them is uncertain. While a number of studies showed that Eph/ephrin signaling increased integrin cell adhesion, others reported the opposite (120). Integrins are transmembrane proteins that attach cells to components of the Extracellular Matrix (ECM), which provides an environment for maintaining the stem cell niche properties. Major components of ECMs are laminin, collagen, fibronectin and proteoglycan. Several genes from these families were uniquely affected by Prop1 deficiency in stem cell derived colonies (Table 1). Thus, these are candidates for involvement in migration and EMT that fails in Prop1 mutants.
In Vitro Differentiation of Pluripotent Stem Cells into Pituitary Endocrine Cells
The elucidation of the signals active during pituitary development in vivo have contributed to major advances in the efficient differentiation of human and mouse pluripotent stem cells into functional pituitary endocrine cells (7–10), paving the way for translational application in treating hypopituitarism.
Sasai and collagues were the first to demonstrate the rescue of hypopituitarism in mice with differentiated pituitary endocrine cells derived from pluripotent stem cells in vitro (8). They established a 3D culture technique for mouse embryonic stem (ES) cells called serum-free floating culture of embryoid body-like aggregates (SFEB) (8). In these cultures, the ES cells are cultured at high density on low-adhesion plates in defined media containing chemicals modulating SHH signaling, causing them to form aggregates. These aggregates then self-organize into an inner layer resembling hypothalamic neural ectoderm and an outer layer resembling surface ectoderm, recapitulating the inductive process that takes place during normal pituitary development. Endogenous BMP and FGF signals from the inner layer induces the outer layer to invaginate and form a Rathke’s pouch-like structure expressing pituitary-specific transcription factors like Lhx3 and Pitx2. Further modulation of Wnt, Notch, glucocorticoid, and estradiol signaling causes cells in these pouches to differentiate into functional endocrine cells from multiple lineages (8). In a follow up study they successfully adapted the protocol to induce human ES cells to differentiate into pituitary hormone producing cells. Formation of aggregates with inner and outer ectodermal layers required additional inductive factors such as exogenous BMP and Rho kinase inhibitors to form the aggregates with inner and outer ectodermal layers. Exogenous FGF induces the invagination of the Rathke’s pouch-like structures, and manipulation of signaling pathways also stimulates the differentiation of functional pituitary endocrine cells (9).
While the Sasai group performed in vitro pituitary cell differentiation in a 3D culture mimicking the developmental interaction between the neural and oral ectoderms, the Studer group developed a way to differentiate pituitary cells from human ES and induced pluripotent (iPS) cell monolayers (7,10). Timed withdrawal of BMP inhibitors from the culture media of human ES cells caused their progression into an early preplacodal fate (10). Treatment of these preplacodal-like cells with SHH agonists then induced their expression of pituitary-specific markers, and further inhibition of Notch signaling stimulates their differentiation into mature, functional pituitary endocrine cells. Combinations of exogenous BMP and FGF signals also cause the cells to adopt intermediate markers of differentiation seen in the developing pituitary gland (7).
Corticotropes differentiated in vitro from each of these methods were shown to be physiologically functional in vivo when grafted under the kidney capsule of hypophysectomized hypoadrenal mice and rats (7–10). Future studies are necessary to determine if in vitro-derived, hormone secreting cells can be safely re-introduced into human patients to combat hypopituitarism. In recent decades, it has become clear that the safe and efficient translation of laboratory discoveries, notably gene and stem cell therapies, into therapeutic intervention can take a very long time (121). Great progress has been made in the stem cell therapies for spinal cord injury, macular degeneration, diabetes and heart disease, and the use of human iPS cells instead of ES cells has reduced the ethical controversy in investigating stem cell derived therapies in humans (reviewed in (122)). However, quality control for epigenetic regulation and somatic mutation will be required to insure efficacy and safety (123,124). There are also significant concerns about the cost of such treatments for relatively rare disorders, and it may not be realistic for a stem cell therapy to be more cost effective than hormone replacement therapy for CPHD, whether the cause is genetic or not. There is hope that a stem cell therapy would provide a better quality of life, however, if the production of hormones came under the control of physiological feedback loops allowing for better homeostasis. Traumatic head injury is a major cause of hypopituitarism, and recent studies suggest that the hypothalamic inputs to the pituitary are a major source of the dysfunction, which is daunting to repair (125), but there are many genetic and environmental causes of hypopituitarism where pituitary stem cell replacement could be a viable, permanent therapeutic if it were available.
Conclusion and Future Directions
Pituitary progenitor cells were long speculated to exist, and SOX2-expressing pituitary stem cells were formally identified in 2008. A great deal has been learned about pituitary stem cells since then, and in the future it will be important to understand the mechanisms that regulate embryonic progenitor exit from the cell cycle and postnatal cell cycle re-entry, as postnatal pituitary stem cells are likely controlled by similar mechanisms. The roles of ephrin and Hippo signaling, cytokines, chemokines, extracellular matrix, and EMT are exciting and promising areas for future investigation. The ability to monitor differentiation of pluripotent stem cells into hormone producing cells in vitro is an asset for testing the effects of individual genes and prioritizing them for in vivo studies in animals. Ultimately, better understanding of the factors regulating embryonic pituitary stem/progenitor cells may lead to the development of methods to stimulate postnatal pituitary stem cells to safely regenerate in vivo into the specific hormone cell-types required in human patients with different types of hypopituitarism.
Highlights.
Pituitary gland organogenesis involves tissue contributions from oral ectoderm, neural ectoderm, and cranial mesenchyme.
Establishing and maintaining pituitary stem cell pools requires SOX2 and the pituitary transcription factor PROP1.
An epithelial to mesenchymal transition-like process is involved in migration of stem cells from the stem cell niche into the anterior pituitary.
Signaling pathways and matrix components are important in stem cell maintenance and differentiation.
The ability of the pituitary to self-renew is lost with age in rodents.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (HD30428, HD34283) to SAC, and CONICET (MIPM).
Footnotes
Disclosures: Authors have nothing to disclose.
Contributor Information
Leonard Y. M. Cheung, Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109-5618 USA
Shannon W. Davis, Department of Biological Sciences, University of South Carolina, Columbia, SC 29208-0001, USA
Michelle L. Brinkmeier, Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109-5618 USA
Sally A. Camper, Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109-5618 USA.
María Inés Pérez-Millán, Institute of Biomedical Investgations (UBA-CONICET), University of Buenos Aires, Buenos Aires, Argentina.
References
- 1.Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suga H. Making pituitary hormone-producing cells in a dish [Review] Endocr J. 2016;63:669680. doi: 10.1507/endocrj.EJ16-0232. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida S, Kato T, Kato Y. Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary. Int J Mol Sci. 2016;17:75. doi: 10.3390/ijms17010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida S, Kato T, Kato Y. EMT Involved in Migration of Stem/Progenitor Cells for Pituitary Development and Regeneration. J Clin Med. 2016;5:43. doi: 10.3390/jcm5040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Lavandeira M, Diaz-Rodriguez E, Bahar D, Garcia-Rendueles AR, Rodrigues JS, Dieguez C, Alvarez CV. Pituitary Cell Turnover: From Adult Stem Cell Recruitment through Differentiation to Death. Neuroendocrinology. 2015;101:175–192. doi: 10.1159/000375502. [DOI] [PubMed] [Google Scholar]
- 6.Willems C, Vankelecom H. Pituitary cell differentiation from stem cells and other cells: toward restorative therapy for hypopituitarism? Regen Med. 2014;9:513–534. doi: 10.2217/rme.14.19. [DOI] [PubMed] [Google Scholar]
- 7.Zimmer B, Piao J, Ramnarine K, Tomishima MJ, Tabar V, Studer L. Derivation of Diverse Hormone-Releasing Pituitary Cells from Human Pluripotent Stem Cells. Stem Cell Reports. 2016;6:858–872. doi: 10.1016/j.stemcr.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- 9.Ozone C, Suga H, Eiraku M, Kadoshima T, Yonemura S, Takata N, Oiso Y, Tsuji T, Sasai Y. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun. 2016;7:10351. doi: 10.1038/ncomms10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dincer Z, Piao J, Niu L, Ganat Y, Kriks S, Zimmer B, Shi SH, Tabar V, Studer L. Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Rep. 2013;5:1387–1402. doi: 10.1016/j.celrep.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez Millan MI, Brinkmeier ML, Mortensen AH, Camper SA. PROP1 triggers epithelial-mesenchymal transition-like process in pituitary stem cells. Elife. 2016;5:e14470. doi: 10.7554/eLife.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Rodriguez E, Garcia-Lavandeira M, Perez-Romero S, Senra A, Canibano C, Palmero I, Borrello MG, Dieguez C, Alvarez CV. Direct promoter induction of p19Arf by Pit-1 explains the dependence receptor RET/Pit-1/p53-induced apoptosis in the pituitary somatotroph cells. Oncogene. 2012;31:2824–2835. doi: 10.1038/onc.2011.458. [DOI] [PubMed] [Google Scholar]
- 13.Willems C, Fu Q, Roose H, Mertens F, Cox B, Chen J, Vankelecom H. Regeneration in the Pituitary After Cell-Ablation Injury: Time-Related Aspects and Molecular Analysis. Endocrinology. 2016;157:705–721. doi: 10.1210/en.2015-1741. [DOI] [PubMed] [Google Scholar]
- 14.de Beer GR. The Evolution of the Pituitary. J Exp Sci. 1924;1:271–291. [Google Scholar]
- 15.De Groef B, Grommen SV, Darras VM. The chicken embryo as a model for developmental endocrinology: development of the thyrotropic, corticotropic, and somatotropic axes. Mol Cell Endocrinol. 2008;293:17–24. doi: 10.1016/j.mce.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Pogoda HM, Hammerschmidt M. How to make a teleost adenohypophysis: molecular pathways of pituitary development in zebrafish. Mol Cell Endocrinol. 2009;312:2–13. doi: 10.1016/j.mce.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura K, Kouki T, Kawahara G, Kikuyama S. Hypophyseal development in vertebrates from amphibians to mammals. Gen Comp Endocrinol. 2002;126:130–135. doi: 10.1006/gcen.2002.7784. [DOI] [PubMed] [Google Scholar]
- 18.Rizzoti K. Genetic regulation of murine pituitary development. J Mol Endocrinol. 2015;54:R55–73. doi: 10.1530/JME-14-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Groves AK. The molecular basis of craniofacial placode development. Wiley Interdiscip Rev Dev Biol. 2016;5:363–376. doi: 10.1002/wdev.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCabe KL, Bronner-Fraser M. Molecular and tissue interactions governing induction of cranial ectodermal placodes. Dev Biol. 2009;332:189–195. doi: 10.1016/j.ydbio.2009.05.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunskill EW, Potter AS, Distasio A, Dexheimer P, Plassard A, Aronow BJ, Potter SS. A gene expression atlas of early craniofacial development. Dev Biol. 2014;391:133–146. doi: 10.1016/j.ydbio.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson CA, Placzek M. Development of the medial hypothalamus: forming a functional hypothalamic-neurohypophyseal interface. Curr Top Dev Biol. 2013;106:49–88. doi: 10.1016/B978-0-12-416021-7.00002-X. [DOI] [PubMed] [Google Scholar]
- 23.Davis SW, Ellsworth Bs, Perez Millan MI, Gergics P, Schade V, Foyouzi N, Brinkmeier ML, Mortensen AH, Camper SA. Pituitary gland development and disease: from stem cell to hormone production. Curr Top Dev Biol. 2013;106:1–47. doi: 10.1016/B978-0-12-416021-7.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning L, Ohyama K, Saeger B, Hatano O, Wilson SA, Logan M, Placzek M. Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev Cell. 2006;11:873–885. doi: 10.1016/j.devcel.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Trowe MO, Zhao L, Weiss AC, Christoffels V, Epstein DJ, Kispert A. Inhibition of Sox2-dependent activation of Shh in the ventral diencephalon by Tbx3 is required for formation of the neurohypophysis. Development. 2013;140:2299–2309. doi: 10.1242/dev.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Pogoda HM, Pearson CA, Ohyama K, Lohr H, Hammerschmidt M, Placzek M. Direct and indirect roles of Fgf3 and Fgf10 in innervation and vascularisation of the vertebrate hypothalamic neurohypophysis. Development. 2013;140:1111–1122. doi: 10.1242/dev.080226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goto M, Hojo M, Ando M, Kita A, Kitagawa M, Ohtsuka T, Kageyama R, Miyamoto S. Hes1 and Hes5 are required for differentiation of pituicytes and formation of the neurohypophysis in pituitary development. Brain Res. 2015;1625:206–217. doi: 10.1016/j.brainres.2015.08.045. [DOI] [PubMed] [Google Scholar]
- 28.De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 29.Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- 30.Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- 31.Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- 32.Himes AD, Raetzman LT. Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Dev Biol. 2009;325:151–161. doi: 10.1016/j.ydbio.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Arrones L, Ferran JL, Hidalgo-Sanchez M, Puelles L. Origin and early development of the chicken adenohypophysis. Front Neuroanat. 2015;9:7. doi: 10.3389/fnana.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gleiberman AS, Fedtsova NG, Rosenfeld MG. Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol. 1999;213:340–353. doi: 10.1006/dbio.1999.9386. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- 36.Khonsari RH, Seppala M, Pradel A, Dutel H, Clement G, Lebedev O, Ghafoor S, Rothova M, Tucker A, Maisey JG, Fan CM, Kawasaki M, Ohazama A, Tafforeau P, Franco B, Helms J, Haycraft CJ, David A, Janvier P, Cobourne MT, Sharpe PT. The buccohypophyseal canal is an ancestral vertebrate trait maintained by modulation in sonic hedgehog signaling. BMC Biol. 2013;11:27. doi: 10.1186/1741-7007-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis SW, Mortensen AH, Keisler JL, Zacharias AL, Gage PJ, Yamamura K, Camper SA. beta-catenin is required in the neural crest and mesencephalon for pituitary gland organogenesis. BMC Dev Biol. 2016;16:16. doi: 10.1186/s12861-016-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gumbel JH, Patterson EM, Owusu SA, Kabat BE, Jung DO, Simmons J, Hopkins T, Ellsworth BS. The forkhead transcription factor, Foxd1, is necessary for pituitary luteinizing hormone expression in mice. PLoS One. 2012;7:e52156. doi: 10.1371/journal.pone.0052156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szabo K, Csanyi K. The vascular architecture of the developing pituitary-median eminence complex in the rat. Cell Tissue Res. 1982;224:563–577. doi: 10.1007/BF00213753. [DOI] [PubMed] [Google Scholar]
- 40.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 41.Couly G, Coltey P, Eichmann A, Le Douarin NM. The angiogenic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mech Dev. 1995;53:97–112. doi: 10.1016/0925-4773(95)00428-9. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Higuchi M, Kato T, Yoshida S, Ueharu H, Nishimura N, Kato Y. PRRX1- and PRRX2-positive mesenchymal stem/progenitor cells are involved in vasculogenesis during rat embryonic pituitary development. Cell Tissue Res. 2015;361:557–565. doi: 10.1007/s00441-015-2128-5. [DOI] [PubMed] [Google Scholar]
- 44.Orciani M, Davis S, Appolloni G, Lazzarini R, Mattioli-Belmonte M, Ricciuti RA, Boscaro M, Di Primio R, Arnaldi G. Isolation and characterization of progenitor mesenchymal cells in human pituitary tumors. Cancer Gene Ther. 2015;22:9–16. doi: 10.1038/cgt.2014.63. [DOI] [PubMed] [Google Scholar]
- 45.Megnis K, Mandrika I, Petrovska R, Stukens J, Rovite V, Balcere I, Jansone LS, Peculis R, Pirags V, Klovins J. Functional Characteristics of Multipotent Mesenchymal Stromal Cells from Pituitary Adenomas. Stem Cells Int. 2016;2016:11. doi: 10.1155/2016/7103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 47.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 48.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 49.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 51.Sani M, Hosseini SM, Salmannejad M, Aleahmad F, Ebrahimi S, Jahanshahi S, Talaei-Khozani T. Origins of the breast milk-derived cells; an endeavor to find the cell sources. Cell Biol Int. 2015;39:611–618. doi: 10.1002/cbin.10432. [DOI] [PubMed] [Google Scholar]
- 52.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, Choung YH, Kim ES, Yang HC, Choung PH. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–773. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 53.Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, Matsuzaki Y. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun. 2009;379:1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 54.Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Wislet-Gendebien S, Laudet E, Neirinckx V, Alix P, Leprince P, Glejzer A, Poulet C, Hennuy B, Sommer L, Shakhova O, Rogister B. Mesenchymal stem cells and neural crest stem cells from adult bone marrow: characterization of their surprising similarities and differences. Cell Mol Life Sci. 2012;69:2593–2608. doi: 10.1007/s00018-012-0937-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Takahashi Y, Takebe T, Taniguchi H. Engineering pancreatic tissues from stem cells towards therapy. Regenerative Therapy. 2016;3:15–23. doi: 10.1016/j.reth.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gergics P, Christian HC, Choo MS, Ajmal A, Camper SA. Gene expression in mouse thyrotrope adenoma: transcription elongation factor stimulates proliferation. Endocrinology. 2016;157:3631–3646. doi: 10.1210/en.2016-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nolan LA, Kavanagh E, Lightman SL, Levy A. Anterior pituitary cell population control: basal cell turnover and the effects of adrenalectomy and dexamethasone treatment. J Neuroendocrinol. 1998;10:207–215. doi: 10.1046/j.1365-2826.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- 59.Frawley LS, Boockfor FR. Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev. 1991;12:337–355. doi: 10.1210/edrv-12-4-337. [DOI] [PubMed] [Google Scholar]
- 60.Goluboff LG, Ezrin C. Effect of pregnancy on the somatotroph and the prolactin cell of the human adenohypophysis. J Clin Endocrinol Metab. 1969;29:1533–1538. doi: 10.1210/jcem-29-12-1533. [DOI] [PubMed] [Google Scholar]
- 61.Porter TE, Hill JB, Wiles CD, Frawley LS. Is the mammosomatotrope a transitional cell for the functional interconversion of growth hormone- and prolacticsecreting cells? Suggestive evidence from virgin, gestating, and lactating rats. Endocrinology. 1990;127:2789–2794. doi: 10.1210/endo-127-6-2789. [DOI] [PubMed] [Google Scholar]
- 62.Castrique E, Fernandez-Fuente M, Le Tissier P, Herman A, Levy A. Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. J Endocrinol. 2010;205:49–60. doi: 10.1677/JOE-09-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taniguchi Y, Shiino M. Three-dimensional and quantitative observation of the hypertrophy of rat corticotrophs following adrenalectomy. Cell Tissue Res. 1992;267:519–523. doi: 10.1007/BF00319374. [DOI] [PubMed] [Google Scholar]
- 64.Ibrahim SN, Moussa SM, Childs GV. Morphometric studies of rat anterior pituitary cells after gonadectomy: correlation of changes in gonadotropes with the serum levels of gonadotropins. Endocrinology. 1986;119:629–637. doi: 10.1210/endo-119-2-629. [DOI] [PubMed] [Google Scholar]
- 65.Horvath E, Lloyd RV, Kovacs K. Propylthiouracyl-induced hypothyroidism results in reversible transdifferentiation of somatotrophs into thyroidectomy cells. A morphologic study of the rat pituitary including immunoelectron microscopy. Lab Invest. 1990;63:511–520. [PubMed] [Google Scholar]
- 66.Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of pituitary somatotrophs and mammotrophs during late fetal and postnatal periods. Anat Embryol (Berl) 2001;204:469–475. doi: 10.1007/s429-001-8003-x. [DOI] [PubMed] [Google Scholar]
- 67.Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of thyrotrophs in the pars distalis of the rat pituitary gland during the fetal and postnatal period. Anat Embryol (Berl) 2001;203:249–253. doi: 10.1007/s004290100161. [DOI] [PubMed] [Google Scholar]
- 68.Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20:1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- 69.Zhu X, Tollkuhn J, Taylor H, Rosenfeld MG. Notch-Dependent Pituitary SOX2(+) Stem Cells Exhibit a Timed Functional Extinction in Regulation of the Postnatal Gland. Stem Cell Reports. 2015;5:1196–1209. doi: 10.1016/j.stemcr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364:208–213. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- 71.Pask AJ, Kanasaki H, Kaiser UB, Conn PM, Janovick JA, Stockton DW, Hess DL, Justice MJ, Behringer RR. A novel mouse model of hypogonadotrophic hypogonadism: N-ethyl-N-nitrosourea-induced gonadotropin-releasing hormone receptor gene mutation. Mol Endocrinol. 2005;19:972–981. doi: 10.1210/me.2004-0192. [DOI] [PubMed] [Google Scholar]
- 72.Shibusawa N, Yamada M, Hirato J, Monden T, Satoh T, Mori M. Requirement of thyrotropin-releasing hormone for the postnatal functions of pituitary thyrotrophs: ontogeny study of congenital tertiary hypothyroidism in mice. Mol Endocrinol. 2000;14:137–146. doi: 10.1210/mend.14.1.0404. [DOI] [PubMed] [Google Scholar]
- 73.Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology. 2005;146:3985–3998. doi: 10.1210/en.2005-0185. [DOI] [PubMed] [Google Scholar]
- 74.Nolan LA, Levy A. A population of nocluteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J Neuroendocrinol. 2006;18:655–661. doi: 10.1111/j.1365-2826.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- 75.Andoniadou CL, Matsushima D, Mousavy Gharavy SN, Signore M, Mackintosh AI, Schaeffer M, Gaston-Massuet C, Mollard P, Jacques TS, Le Tissier P, Dattani MT, Pevny LH, Martinez-Barbera JP. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13:433–445. doi: 10.1016/j.stem.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Rizzoti K, Akiyama H, Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell. 2013;13:419–432. doi: 10.1016/j.stem.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu Q, Gremeaux L, Luque RM, Liekens D, Chen J, Buch T, Waisman A, Kineman R, Vankelecom H. The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology. 2012;153:3224–3235. doi: 10.1210/en.2012-1152. [DOI] [PubMed] [Google Scholar]
- 78.Fu Q, Vankelecom H. Regenerative capacity of the adult pituitary: multiple mechanisms of lactotrope restoration after transgenic ablation. Stem Cells Dev. 2012;21:3245–3257. doi: 10.1089/scd.2012.0290. [DOI] [PubMed] [Google Scholar]
- 79.Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M, Alvarez CV. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One. 2009;4:e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen M, Kato T, Higuchi M, Yoshida S, Yako H, Kanno N, Kato Y. Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary. Cell Tissue Res. 2013;354:823–836. doi: 10.1007/s00441-013-1713-8. [DOI] [PubMed] [Google Scholar]
- 81.Kanno N, Higuchi M, Yoshida S, Yako H, Chen M, Ueharu H, Nishimura N, Kato T, Kato Y. Expression studies of neuronatin in prenatal and postnatal rat pituitary. Cell Tissue Res. 2016;364:273–288. doi: 10.1007/s00441-015-2325-2. [DOI] [PubMed] [Google Scholar]
- 82.Urbano AG, Suarez-Penaranda JM, Dieguez C, Alvarez CV. GDNF and RET-gene expression in anterior pituitary-cell types. Endocrinology. 2000;141:1893–1896. doi: 10.1210/endo.141.5.7548. [DOI] [PubMed] [Google Scholar]
- 83.Canibano C, Rodriguez NL, Saez C, Tovar S, Garcia-Lavandeira M, Borrello MG, Vidal A, Costantini F, Japon M, Dieguez C, Alvarez CV. The dependence receptor Ret induces apoptosis in somatotrophs through a Pit-1/p53 pathway, preventing tumor growth. EMBO J. 2007;26:2015–2028. doi: 10.1038/sj.emboj.7601636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guillou A, Romano N, Bonnefont X, Le Tissier P, Mollard P, Martin AO. Modulation of the tyrosine kinase receptor Ret/glial cell-derived neurotrophic factor (GDNF) signaling: a new player in reproduction induced anterior pituitary plasticity? Endocrinology. 2011;152:515–525. doi: 10.1210/en.2010-0673. [DOI] [PubMed] [Google Scholar]
- 85.Diaz-Rodriguez E, Garcia-Rendueles AR, Ibanez-Costa A, Gutierrez-Pascual E, Garcia-Lavandeira M, Leal A, Japon MA, Soto A, Venegas E, Tinahones FJ, Garcia-Arnes JA, Benito P, Angeles Galvez M, Jimenez-Reina L, Bernabeu I, Dieguez C, Luque RM, Castano JP, Alvarez CV. Somatotropinomas, but not nonfunctioning pituitary adenomas, maintain a functional apoptotic RET/Pit1/ARF/p53 pathway that is blocked by excess GDNF. Endocrinology. 2014;155:4329–4340. doi: 10.1210/en.2014-1034. [DOI] [PubMed] [Google Scholar]
- 86.Gaston-Massuet C, Andoniadou CL, Signore M, Jayakody SA, Charolidi N, Kyeyune R, Vernay B, Jacques TS, Taketo MM, Le Tissier P, Dattani MT, Martinez-Barbera JP. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A. 2011;108:11482–11487. doi: 10.1073/pnas.1101553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gremeaux L, Fu Q, Chen J, Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev. 2012;21:801–813. doi: 10.1089/scd.2011.0496. [DOI] [PubMed] [Google Scholar]
- 88.Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 2009;27:1182–1195. doi: 10.1002/stem.51. [DOI] [PubMed] [Google Scholar]
- 89.Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 90.Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 91.De Rienzo F, Mellone S, Bellone S, Babu D, Fusco I, Prodam F, Petri A, Muniswamy R, De Luca F, Salerno M, Momigliano-Richardi P, Bona G, Giordano M, Italian Study Group on Genetics of C Frequency of genetic defects in combined pituitary hormone deficiency: a systematic review and analysis of a multicentre Italian cohort. Clin Endocrinol (Oxf) 2015;83:849–860. doi: 10.1111/cen.12849. [DOI] [PubMed] [Google Scholar]
- 92.Bottner A, Keller E, Kratzsch J, Stobbe H, Weigel JF, Keller A, Hirsch W, Kiess W, Blum WF, Pfaffle RW. PROP1 mutations cause progressive deterioration of anterior pituitary function including adrenal insufficiency: a longitudinal analysis. J Clin Endocrinol Metab. 2004;89:5256–5265. doi: 10.1210/jc.2004-0661. [DOI] [PubMed] [Google Scholar]
- 93.Davis SW, Keisler JL, Perez-Millan MI, Schade V, Camper SA. All Hormone-Producing Cell Types of the Pituitary Intermediate and Anterior Lobes Derive From Prop1-Expressing Progenitors. Endocrinology. 2016;157:1385–1396. doi: 10.1210/en.2015-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol. 2009;29:1895–1908. doi: 10.1128/MCB.01885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brinkmeier ML, Davis SW, Carninci P, MacDonald JW, Kawai J, Ghosh D, Hayashizaki Y, Lyons RH, Camper SA. Discovery of transcriptional regulators and signaling pathways in the developing pituitary gland by bioinformatic and genomic approaches. Genomics. 2009;93:449–460. doi: 10.1016/j.ygeno.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen J, Crabbe A, Van Duppen V, Vankelecom H. The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol. 2006;20:3293–3307. doi: 10.1210/me.2006-0293. [DOI] [PubMed] [Google Scholar]
- 97.Vankelecom H. Pituitary stem/progenitor cells: embryonic players in the adult gland? Eur J Neurosci. 2010;32:2063–2081. doi: 10.1111/j.1460-9568.2010.07523.x. [DOI] [PubMed] [Google Scholar]
- 98.Nantie LB, Himes AD, Getz DR, Raetzman LT. Notch signaling in postnatal pituitary expansion: proliferation, progenitors, and cell specification. Mol Endocrinol. 2014;28:731–744. doi: 10.1210/me.2013-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leung AW, Kent Morest D, Li JY. Differential BMP signaling controls formation and differentiation of multipotent preplacodal ectoderm progenitors from human embryonic stem cells. Dev Biol. 2013;379:208–220. doi: 10.1016/j.ydbio.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nasonkin IO, Ward RD, Raetzman LT, Seasholtz AF, Saunders TL, Gillespie PJ, Camper SA. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum Mol Genet. 2004;13:2727–2735. doi: 10.1093/hmg/ddh311. [DOI] [PubMed] [Google Scholar]
- 101.Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20:2739–2753. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheung LY, Rizzoti K, Lovell-Badge R, Le Tissier PR. Pituitary phenotypes of mice lacking the notch signalling ligand delta-like 1 homologue. J Neuroendocrinol. 2013;25:391–401. doi: 10.1111/jne.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dutta S, Dietrich JE, Westerfield M, Varga ZM. Notch signaling regulates endocrine cell specification in the zebrafish anterior pituitary. Dev Biol. 2008;319:248–257. doi: 10.1016/j.ydbio.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goldberg LB, Aujla PK, Raetzman LT. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Dev Biol. 2011;358:23–32. doi: 10.1016/j.ydbio.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol Endocrinol. 2007;21:1458–1466. doi: 10.1210/me.2007-0039. [DOI] [PubMed] [Google Scholar]
- 106.Puertas-Avendano RA, Gonzalez-Gomez MJ, Ruvira MD, Ruiz-Hidalgo MJ, Morales-Delgado N, Laborda J, Diaz C, Bello AR. Role of the non-canonical notch ligand delta-like protein 1 in hormone-producing cells of the adult male mouse pituitary. J Neuroendocrinol. 2011;23:849–859. doi: 10.1111/j.1365-2826.2011.02189.x. [DOI] [PubMed] [Google Scholar]
- 107.Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006;20:2898–2908. doi: 10.1210/me.2005-0394. [DOI] [PubMed] [Google Scholar]
- 109.Afelik S, Jensen J. Notch signaling in the pancreas: patterning and cell fate specification. Wiley Interdiscip Rev Dev Biol. 2013;2:531–544. doi: 10.1002/wdev.99. [DOI] [PubMed] [Google Scholar]
- 110.Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Potok MA, Cha KB, Hunt A, Brinkmeier ML, Leitges M, Kispert A, Camper SA. WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn. 2008;237:1006–1020. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chambers TJ, Giles A, Brabant G, Davis JR. Wnt signalling in pituitary development and tumorigenesis. Endocr Relat Cancer. 2013;20:R101–111. doi: 10.1530/ERC-13-0005. [DOI] [PubMed] [Google Scholar]
- 113.Andoniadou CL, Gaston-Massuet C, Reddy R, Schneider RP, Blasco MA, Le Tissier P, Jacques TS, Pevny LH, Dattani MT, Martinez-Barbera JP. Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol. 2012;124:259–271. doi: 10.1007/s00401-012-0957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pyczek J, Buslei R, Schult D, Holsken A, Buchfelder M, Hess I, Hahn H, Uhmann A. Hedgehog signaling activation induces stem cell proliferation and hormone release in the adult pituitary gland. Sci Rep. 2016;6:24928. doi: 10.1038/srep24928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lodge EJ, Russell JP, Patist AL, Francis-West P, Andoniadou CL. Expression Analysis of the Hippo Cascade Indicates a Role in Pituitary Stem Cell Development. Front Physiol. 2016;7:114. doi: 10.3389/fphys.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pitulescu ME, Adams RH. Eph/ephrin molecules–a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 118.Zarbalis K, Wurst W. Expression domains of murine ephrin-A5 in the pituitary and hypothalamus. Mech Dev. 2000;93:165–168. doi: 10.1016/s0925-4773(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 119.Yoshida S, Kato T, Higuchi M, Chen M, Ueharu H, Nishimura N, Kato Y. Localization of juxtacrine factor ephrin-B2 in pituitary stem/progenitor cell niches throughout life. Cell Tissue Res. 2015;359:755–766. doi: 10.1007/s00441-014-2054-y. [DOI] [PubMed] [Google Scholar]
- 120.Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Orkin SH, Reilly P. MEDICINE. Paying for future success in gene therapy. Science. 2016;352:1059–1061. doi: 10.1126/science.aaf4770. [DOI] [PubMed] [Google Scholar]
- 122.Ilic D, Ogilvie C. Human Embryonic Stem Cells-What Have We Done? What Are We Doing? Where Are We Going? Stem Cells. 2016 doi: 10.1002/stem.2450. [DOI] [PubMed] [Google Scholar]
- 123.Ji P, Manupipatpong S, Xie N, Li Y. Induced Pluripotent Stem Cells: Generation Strategy and Epigenetic Mystery behind Reprogramming. Stem Cells Int. 2016;2016:8415010. doi: 10.1155/2016/8415010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Osterstock G, El Yandouzi T, Romano N, Carmignac D, Langlet F, Coutry N, Guillou A, Schaeffer M, Chauvet N, Vanacker C, Galibert E, Dehouck B, Robinson IC, Prevot V, Mollard P, Plesnila N, Mery PF. Sustained alterations of hypothalamic tanycytes during posttraumatic hypopituitarism in male mice. Endocrinology. 2014;155:1887–1898. doi: 10.1210/en.2013-1336. [DOI] [PubMed] [Google Scholar]


