Abstract
Loss of heterozygosity at the human glucocorticoid receptor (hGRα) gene (NR3C1) locus has been found in pituitary adenomas of patients with Cushing disease (CD); rare cases of NR3C1 mutations have also been described in the gemline or somatic state in CD. We describe a pediatric patient with CD with clinical evidence of partial glucocorticoid resistance (GR) because of relative absence of stigmata of Cushing syndrome (CS), who did not have any mutations in the hGRα gene (NR3C1). A 14-year-old boy with slow growth and hypertension but no other signs of CS (i.e. no weight gain, no striae, no facial plethora) was admitted for evaluation of CD. Pituitary MRI revealed a 3 x 4 millmeter hypoenhancing lesion in the right side of the pituitary gland anteriorly (microadenoma). Urinary free cortisol levels (UFC) were consistently 2–3-fold the upper normal range (100–129 mcg/24 hours. A graded dexamethasone suppression test indicated that the patient had partial GR. Histology confirmed an ACTH-producing pituitary adenoma, characterized by proliferation of anterior lobe cells of similar morphology with reticulin breakdown and strong diffuse ACTH staining. However, in contrast to a series of 5 ACTH producing pituitary adenomas characterized by hGRα patchy positive staining in both cytoplasm and nuclei of tumor cells, no GR staining was detected in our patient’s microadenoma. We hypothesized that a NR3C1 mutation was present in the patient’s peripheral DNA but sequencing studies of the entire coding region of the gene produced normal results. We present the case of a pediatric patient with little evidence of CS, despite the unequivocal presence of an aggressive ACTH-producing tumor and high UFCs. This patient did not have peripheral DNA NR3C1 coding sequence mutations; possible explanations for this phenotype include somatic mosaicism for NR3C1 mutations or a mutation in another molecule that participated in hGRα-signaling.
Keywords: Pituitary tumors, Cushing disease, cortisol resistance
Introduction
Primary glucocorticoid resistance (GR) is a rare inherited disorder characterized by hypercortisolism without features of Cushing syndrome (CS) [1]. The syndrome is characterized by increased secretion of cortisol, resistance to suppression by dexamethasone and other glucocorticoids, and the absence of the clinical stigmata of CS. Compensatory increases in corticotrophin releasing hormone (CRH), adrenocorticotrophin (ACTH) and cortisol production are also accompanied by increased secretion of glucocorticoid precursors with mineralocorticoid activity and increased adrenal androgens [2]. The increased cortisol concentrations appear to compensate adequately for the reduced sensitivity of the glucocorticoid receptor to cortisol. Patients with GR do not show signs or symptoms of overt clinical hypo- or hyper-cortisolism, with the exception of fatigue, which has been the presenting sign in some patients [3].
The molecular basis of GR is not always understood. Studies addressing the mechanism of resistance have shown that the human glucocorticoid receptor (hGR), the product of the NR3C1 gene, is the primary target of genetic alterations leading to resistance. The hGR is a transcription factor and a member of the nuclear receptor family. It has been localized to chromosome 5q31, contains a total of 9 exons, and is approximately 80 kb in size. Recently, it has been shown that two previously unidentified exons exist (which are called exon 1A and B) upstream from the originally defined exon 1 (which is now named exon 1C) [4]. There are two naturally occurring isoforms of the hGR gene, hGRα that is functional, and hGRβ that has no hormone-binding ability. A third hGR mRNA has a higher expression level than the other two previously discovered messengers, is 7.0 kb in length and contains exons 1–8 and the entire exon 9, containing exon 9α, the ‘J region’, and exon 9β [5]. It is thought that this mRNA is translated into hGRα. In addition, hGR mRNA may contain five different versions of exon 1. Through the usage of the three different promoters, three different exons 1 can be transcribed (1A, B and C), and alternative splicing of exon 1A can result in three different versions of this exon (1A1, 1A2, 1A3) [4]. Upon glucocorticoid binding, the hGRα dimerizes and translocates to the nucleus, where it exerts its transcriptional activity by binding to its cognate genomic DNA sequences, such as the glucocorticoid response element.
The vast majority of glucocorticoid-resistant cells express low levels or defective forms of the hGR protein [6]. Mutations of hGRα have been described that impair one or more of the mechanisms of hGRα function, including affinity for glucocorticoids [7], affinity for DNA [8], number [9], and translocation to the nucleus [10]. Inactivating mutations within the ligand-binding and DNA-binding domains, as well as deletions at the exon boundaries of the hGRα gene, have been described in kindreds and sporadic cases [11]. In addition, five polymorphisms in the hGR gene have been identified that are not associated with a reduced sensitivity to glucocorticoids, which may be useful in analyzing whether loss of (part of) the hGR gene plays a role in glucocorticoid-resistant malignancies [12]. However, in many patients with clinical biochemical evidence of GR, no abnormalities in the hGRα gene have been observed [13].
Pediatric pituitary adenomas are relatively rare and account for only 2.7 % of supratentorial tumors in children [14]. The majority of pituitary tumors is benign but remains clinically significant because of tumor compression syndrome and hormone overproduction. Although the largest proportion of pituitary adenomas occur sporadically, about 5% of pituitary adenomas occur in a familial setting, including multiple endocrine neoplasia type 1, Carney complex, and familial isolated pituitary adenomas [15]. The interest and significance of understanding pituitary tumorigenesis is increasing, particularly after some recent reports on the prevalence of clinically apparent pituitary adenomas being 3–5 times higher than previously thought [16]. Apart from tumor suppression genes, genetic defects or changes in protein expression in signal transduction pathways (PKA, PKC, PI3K), cell cycle regulation (cyclin D), growth factors and their receptors (FGF, FGFR), chromosomal aneuploidity (PTTG), oncogene activation (Ras), have all been linked to pituitary tumor formation [17].
Although rare cases of NR3C1 mutations have been described in the germline or somatic state in patients with sporadic generalized GR who, years later, developed pituitary Cushing disease (CD) [18], thus far there are no reports of simultaneous pituitary adenoma in conjunction with GR in children. We report a unique pediatric patient with CD, clinical evidence of GR and absence of stigmata of CS, who had pituitary microadenoma without GR staining but did not have peripheral DNA NR3C1 coding sequence mutations.
Subject and Methods
Protocol
For the purposes of this study, the patient and his family members were enrolled in protocol 97-CH-0076 for the genetic investigation of pituitary tumors; the patient and his family signed the appropriate consent and assent forms. The study and all forms have been approved by the institutional review board of the National Institute of Child Health and Development.
Patient
The index subject was a 14-year-old male who was admitted for evaluation and treatment for suspected CS because he was found to have elevated serum cortisol and urinary-free cortisol (UFC) levels on multiple occasions. These were obtained in the course of investigation for short stature.
The patient had a sister with type 1 diabetes mellitus and celiac disease. There were no other known medical problems in the family. His mother had menarche at 12 ½ years of age and his father started puberty around the same time; both parents achieved average adult heights. The patient was in his usual state of good health until his investigation for short stature two years prior to diagnosis. He reported increased fatigue in the past year but he denied fever, polyuria, or polydipsia. He endorsed some mild acne on his face and some mild facial flushing as well over the past year but no other rashes or lesions. He denied any ophthalmologic, ENT, respiratory, cardiovascular, gastrointestinal, or genitourinary complaints, yet did admit to an increase in muscle strains. From a neurologic standpoint he reported headaches approximately once weekly at varied times of the day, which often responded to ibuprofen and rest. There was some associated nausea with the headaches but no emesis. Hematologic, lymphatic, endocrine, immunologic, and psychiatric review of systems was negative. Physical examination failed to show any stigmata of CS. The patient was at the 5th centile for both height and weight. He had Tanner stage IV for pubic hair, and testicular volume approximately 2 mL bilaterally. There were less than 10 comedones on the face and upper back.
Thyroid function tests were normal. Two 24-hour UFCs measurements were 129 and 100 mcg/24 hours, respectively (normal range 4 – 56 mcg/24-hrs). Urine 17-hydroxysteroids were 14.7 and 12.6 mcg/24-hours (3.1±2-0 mg/24 hrs). Table 1 shows the oCRH stimulation test results. Given some concern based on physical examination for GR as opposed to isolated Cushing syndrome with his paucity of physical findings of symptoms with the exception of impaired linear growth, 24-hour cortisol and ACTH levels were measured every 20 minutes. The test was started at 4:00 p.m. and ACTH levels were in the 15–30 pg/ml range (reference values 2–9 pg/ml) with corresponding cortisol levels between 5 and 21.5μg/dl throughout the daytime and evening hours (reference values: midnight 0.8–3; morning 1.5–8 μg/dl). Peaks of ACTH and cortisol were noted three times throughout the day, (corresponding with meals), the first ACTH peak yielded a value of 39.1 pg/ml, followed by 28.5 and 41.4 pg/ml with corresponding cortisol levels of 36.6, 29.7 and 39 μg/dl, with valleys in between those three aforementioned peaks. Further laboratory testing revealed free and total testosterone of 0.6 pg/ml (reference total values for males 13–14 year-old 0.3–13.8 pg/mL) and 24 ng/dL (reference total values for males 13–14 year-old 33–585 ng/dL), a repeat DHEAS of 64 (reference values for Tanner stage IV males 29–412 ug/dL) and a repeat DHEA of 450 ng/dl (reference values for children <18 year-old with Tanner stage IV 10–565 ng/dL). Androstenedione was 75 ng/dL (reference values for children >8 year-old 2–200 ng/dL), estradiol was less than 10 ng/dL (reference male levels 1.5–5.5 ng/dL). Plasma rennin activity was 1.5 ng/ml/hr (reference values 0.6–4.3 ng/ml/hr) and serum aldosterone was 5.2 ng/dL (reference values 7.5±0.80 ng/dL), both performed after the patient had been lying supine overnight. Upright plasma rennin was 5 ng/ml/hr (reference values 2.9–24.0 ng/ml/hr) and serum aldosterone was 38 ng/dL (reference values 4.0–31.0 ng/dL) in the standing position. Subsequent a.m. ACTH and serum cortisol levels were 14.6 pg/ml and 12.3 μg/dl respectively and 24-hour UFCs was 286 and 102 mcg/24 hours with 17-hydroxysteroids of 22.2 and 13.6 mcg/24-hours on two successive day’s measurements.
Table 1.
CRH stimulation test
| Preoperative | Postoperative* | |||
|---|---|---|---|---|
| Time (minutes) | Cortisol (μg/dl) | ACTH (pg/ml) | Cortisol (μg/dl) | ACTH (pg/ml) |
| −15 | <1 | 6.1 | ||
| −5 | 33.1 | 14.8 | ||
| 0 | 37 | 43.9 | <1 | 6.2 |
| +5 | <1 | 6.4 | ||
| +15 | 48.8 | 69.5 | <1 | 8.2 |
| +30 | 46.9 | 41.9 | 1.6 | 8.5 |
| +45 | 46.3 | 31.3 | ||
| +60 | 3.5 | 10.8 | ||
| +90 | 3.4 | 10.1 | ||
| +120 | 3.6 | 11.3 |
Before the second graded dexamethasone suppression test
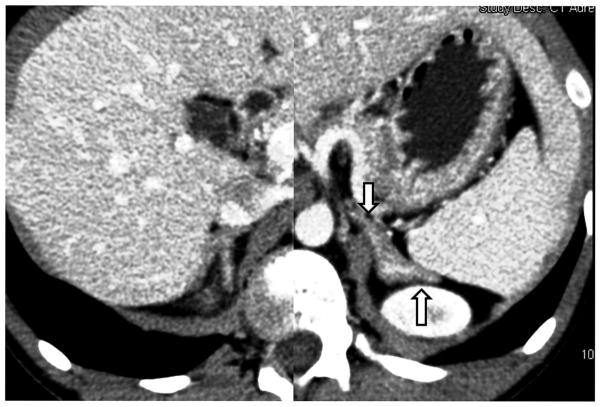
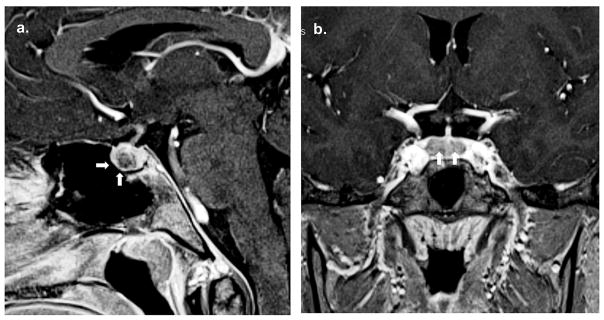
The patient’s bone age was 14 years. Computed tomography (CT) of the adrenal glands showed bilaterally enlarged adrenal glands with slight prominence of the left adrenal gland (Figure 1A). Pituitary MRI revealed a 3 x 4 millimeter hypoenhancing lesion in the right side of the pituitary gland anteriorly (Figure 1B). The rest of the pituitary showed slightly heterogenous enhancement (Figure 1C). The impression was that this was suspicious for a microadenoma, which was thought to be supported by the elevated UFC levels as well as the loss of diurnal variation of ACTH and cortisol. Both testes appeared normal on ultrasound with the left testis 1.1x2.3x1 cm and the right testis 1.3x2.3x1 cm.
Figure 1.
CT of the adrenal glands shows mildly hyperplastic adrenal glands with prominence of the left adrenal gland (arrows).
The patient underwent a graded dexamethasone suppression test (Table 2). The decision was made that based on the MRI findings of the pituitary adenoma as well as the loss of diurnal variation of ACTH and cortisol, and elevated UFC levels, to proceed with excision of the pituitary microadenoma. The biopsy showed a pituitary adenoma, characterized by proliferation of anterior lobe cells of similar morphology with reticulin breakdown and strong diffuse ACTH staining (Figure 2). Postoperative cortisol and ACTH serum levels are presented in Table 3. Based on the results of a postoperative graded dexamethasone suppression test (Table 2), it was thought that at doses of 0.5 and 1 mg of dexamethasone, he did not completely suppress his ACTH production and that probably 2 mg was the dose that corresponded with true suppression of ACTH. This seemed to indicate that his GR likely required an 8-fold increase in serum cortisol with regard to the general population with a normally functioning hGR. Results of a postoperative CRH testing are shown in Table 1. Postoperative MRI of the pituitary, revealed a small focus of non-enhancement measuring 3x1.5 millimeters in the anterior, inferior part of the right medial pituitary gland, thought to possibly represent a residual adenoma.
Table 2.
Graded dexamethasone suppression test
| Day 1 | Day 2 | Day 6 | Postoperative | |||||
|---|---|---|---|---|---|---|---|---|
| midnight | morning | morning | morning | morning | morning | morning | morning | morning |
| Dexamethasone dose (mg) | Cortisol (μg/dl) | ACTH (pg/ml) | Cortisol (μg/dl)* | ACTH (pg/ml)* | Cortisol (μg/dl)* | ACTH (pg/ml)* | Cortisol (μg/dl) | ACTH (pg/ml) |
| 0.5 | 1.6 | 9.5 | <1 | 5.8 | ||||
| 1 | 4.7 | 15.5 | <1 | 5.8 | ||||
| 2 | <1 | 8.4 | <1 | <5 | ||||
| 4 | <1 | <5 | ||||||
| 8 | 1.5 | 6.8 | <1 | 5.9 | ||||
| 0 | 10.7 | 28.5 | 10.7 | 28.5 |
without exogenous glucocorticoid administration
Figure 2.
a. Pituitary MRI revealed a 3 x 4 millimeter hypoenhancing lesion in the right side of the pituitary gland anteriorly (arrows). b. The rest of the pituitary shows slightly heterogenous enhancement with no other lesions (arrows).
Table 3.
Postoperative hormonal variation
| Day after surgery | Dexamethasone given | Cortisol (μg/dl) | ACTH (pg/ml) | Urinary Cortisol (mcg/24 hrs) |
|---|---|---|---|---|
| 1 | Yes | 4.1 | 7.4 | |
| 2 | Yes | 1.9 | 5.31 | |
| 3* | No | 1.4 | 5.11 | <3 |
| 4* | No | 1.3 | <5 | <3 |
| 5* | No | 1.4 | 5.9 | <3 |
| 6* | No | 1.1 | 5.8 | <3 |
| 7* | No | <1 | 6 | <3 |
| 8+* | No | <1 | 5 to 7 | <3 |
without exogenous glucocorticoid administration
DNA analysis
Genomic DNA was obtained from peripheral blood from the patient and his parents. The entire coding region of the NR3C1 was PCR amplified using Taq DNA polymerase (Invitrogen Life Technologies, Inc., Carlsbad, CA) on GeneAmp PCR System 9700, PerkinElmer Applied Biosystems, Foster City, CA. The primers used for PCR amplification of genomic DNA were designed to encompass the NR3C1 coding exons and the exon-intron boundaries (Table 4). Exon 2 was divided into four parts due to its length. The amplification conditions we used with these primers have been previously described [11, 19]. The PCR products were gel-purified according to the manufacturer recommendations (Qiagen, miElute) and sequenced in an automatic sequencer (3130xl Genetic Analyzer, Applied Biosystems).
Table 4.
Primers used to amplify each exon of the hGRα are shown. Due to the length of exon 2, four primer pairs were designed
| Exon | Primer | Primer sequence |
|---|---|---|
| 2 | GREX2F | 5′-TCAACAGCAGGATCAGAAGC-3′ |
| GREX2R | 5′-ATGTCCATTCTTAAGAAACAGG-3′ | |
| GREX2FI | 5′-AGCTGCCTCTTACTAATCGG-3′ | |
| GREX2RI | 5′-GCTTTAAGTCTGTTTCCCCC-3′ | |
| GREX2FII | 5′-GGGGGAAACAGACTTAAAGC-3′ | |
| GREX2RII | 5′-CCTCATTCGAGTTTCCTTCC-3′ | |
| GREX2FIII | 5′-GGACTGCAAGCCTCTCATTT-3′ | |
| GREX2RIII | 5′-CCTACTTTCAAAAGGCCACTTA-3′ | |
| 3 | GREX3F | 5′-AGTTCACTGTGAGCATTCTG-3′ |
| GREX3R | 5′-CGTGAGAAATAAAACCAAGT-3′ | |
| 4 | GREX4F | 5′-GACCTGTGAAACTTTAATAGTGCC-3′ |
| GREX4R | 5′-AACATTATGCGTATCAAGCA-3′ | |
| 5 | GREX5F | 5′-GAATAAACTGTGTAGCGCAG-3′ |
| GREX5R | 5′-TAGTCCCCAGAACTAAGAGA-3′ | |
| 6 | GREX6F | 5′-GATCTTCTGAAGAGTGTTGC-3′ |
| GREX6R | 5′-GGGAAAATGACACACATACA-3′ | |
| 7 | GREX7F | 5′-CAAAAATGTGCTTTTTGGGGG-3′ |
| GREX7R | 5′-CTAGGCCTTCATATTTCATGC-3′ | |
| 8 | GREX8F | 5′-GACACAGTGAGACCCTATCT-3′ |
| GREX8R | 5′-CACCAACATCCACAAACTGG-3′ | |
| 9 | GREX9F | 5′-GGAATTCCAGTGAGATTGGT-3′ |
| GREX9R | 5′-TATAAACCACATGTAGTGCG-3′ |
Results
Immunohistological findings
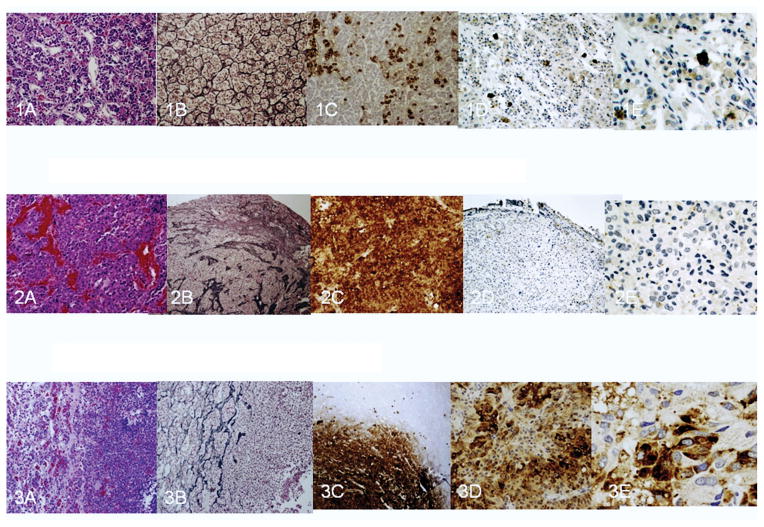
The patient’s pituitary adenoma showed reticulin breakdown and strong diffuse ACTH staining, as expected (Figure 2). A MIB-1 staining revealed a proliferative rate of about 1% and p53 immunostaining was negative. Using hGR antibody (ab52190-100 abcam, lot#481755) hGR was not detected in this patient’s adenoma and preserved border of intact pituitary tissue sections (Figure 2.2 D, E). In contrast to these sections and those of a negative control (unstained sections) normal pituitary tissues examined yielded cytoplasmic immunoreactivity (Figure 2.1 D, E). All sections of a pituitary ACTH producing adenoma tissue obtained from another patient (and also from a series of 5 positive controls) demonstrated both cytoplasmic and nuclear immunolocalization of hGR (Figure 2.3 D, E).
Sequencing of the hGRα gene
At least two independent PCR – sequencing experiments of the NR3C1 entire coding region revealed wild type sequence of the patient.
Discussion
We describe a pediatric patient with little evidence of CS, despite the unequivocal presence of an aggressive ACTH-producing tumor and high UFCs. The patient underwent testing to determine the etiology of his elevated cortisol levels and underwent transsphenoidal surgery for resection of a pituitary microadenoma. Postoperatively he had suppressed levels of cortisol and ACTH as would be expected after resection of a pituitary microadenoma but a postoperative graded dexamethasone suppression test indicated that his GR likely required an 8-fold increase in serum cortisol in comparison to the general population with a normally functioning hGR. This patient did not have peripheral DNA NR3C1 coding sequence mutations; possible explanations for this phenotype include somatic mosaicism for NR3C1 mutations or a mutation in another molecule that participated in hGRα-signaling.
Due to the varying degree of end-organ insensitivity to glucocorticoids, the clinical picture of cortisol resistance may vary from fatigue, hirsutism, oligomenorrhea, infertility, obesity, pubertas praecox to severe hypertension with hypokalemic alkalosis [20–21]. Our patient reported increased fatigue in the past year; his only symptom of CD was (mild) short stature. It was his short stature that led to the detection of elevated UFCs and the evaluation and treatment for suspected CD.
It has been suggested that the numerous actions of glucocorticoids are mediated by a set of at least 16 hGR isoforms forming homo- or hetero-dimers consisted of multifunctional domain proteins operating as ligand-dependent transcription factors that interact with many other cell signaling systems, including large and small G proteins [22]. Among the various hGR isoforms a decline in the expression of hGRα may be associated with development of resistance [23]. There is now evidence that serine/arginine-rich SRp40 proteins may influence the alternative splicing of hGR pre-mRNA to regulate the ratio of hGRα to hGRβ, and this effect is cell-dependent [24]. More recently, 15 missense, three nonsense, three frameshift, one splice site, and two alternative spliced mutations have been reported in the hGRα associated with GR as well as 16 polymorphisms [25]. Although the effects of glucocorticoid are mediated through the hGRα isoform, the presence of other 4 isoforms that have altered DNA and ligand-binding domains may influence the response of a particular cell to glucocorticoids [26].
There have been recently identified four novel hGRα transcripts that have various deletions of exon 2 sequences arising from the hGRα 1A and 1B promoters, potentially affecting the hormone sensitivity or resistance [27]. The existence and tissue distribution of a novel splice variant, hGRΔ313-338, has also been reported; it is encoded by exon 2, representing a transcript variant encoding a smaller protein isoform with a predicted 26 residue (78 bp) deletion between the tau 1 domain and the DNA-binding domain encoded by exons 3 and 4 [28]. We therefore examined patient’s DNA for potential mutations in exon 2, coding solely for the first transactivation domain, and exons 3 and 4, coding separately for the two zinc fingers in the DNA-binding domain. No such mutations were found. Similarly, we did not find any known or novel mutation sequencing exons 5 to 9, coding for the C-terminus ligand-binding domain of the receptor. Such a mutant receptor (hGRαF737L) has been recently shown to cause generalized glucocorticoid resistance because of decreased affinity for ligand, marked delay in nuclear translocation and abnormal interaction with the hGR-interacting protein 1 coactivator [29].
The important finding that most of GR patients are without overt GR-mutations suggests that GR-mutations/polymorphisms are only a rare cause of GR. As determined by extensive single strand conformation polymorphism, however, a polymorphism at the beginning of exon 2 (silent mutation in codon 22/23) was observed in two of five patients without mutations in hGRα gene [30]. In a similar study it was also found that out of 12 GR patients, only 2 presented with a previously unidentified hGRα gene polymorphism (exon 4 codon 477, exon 8 codon 679) [19]. In this study a “common” polymorphism at position 2430, AAT to AAC in exon 9α, not resulting in a change in amino acid expression, was also found in 4 of the 12 patients, a frequency similar to 30% found in healthy controls [29]. In clinical and biochemical evidence of GR with no abnormalities demonstrated in the hGRα gene, other, as yet undefined alterations somewhere in the cascade of events starting with ligand binding to the hGRα protein, and finally resulting in the regulation of the expression of glucocorticoid responsive genes, or postreceptor defects or interactions with other nuclear factors form the pathophysiologic basis of GR in these patients [13]. Therefore, the complexity of glucocorticoid biology lies more in the variety of receptor variants themselves rather than in the ligands to which they bind.
One of the most characteristic biochemical features of corticotroph tumors is their relative resistance to corticosteroid feedback. It has been hypothesized that clonal expansion of a single genetically altered corticothroph cell might occur as a result of diminished negative feedback by cortisol in patients with GR due to a dominant negative hGRα gene mutation [31]. As in our patient, however, no mutations were detected in several ACTH-producing tumors [32] while the ratio hGRα / hGRβ was shown to be relatively constant with an average of a 37-fold overexpression of hGRα [33]. Nontheless, polymorphisms in the hGRα gene might confer a selective advantage to tumorigenesis in corticotropinomas [33].
Our patient had an ACTH-producing pituitary adenoma with reticulin breakdown and strong diffuse ACTH staining. Immunohistochemical staining of a pituitary corticotropinoma in a patient with sporadic generalized GR revealed accumulation of p53 protein, indicating the presence of a putative somatic oncogenic mutation in the p53 gene in the tumor cells [28]. Because investigation of the lymphoblast and skin fibroblast cultures for p53 abnormalities did not show any aberration, a novel de novo germ line heterozygous missense mutation in the hGR gene (isoleucine 559 to asparagine 559) was revealed with strong dominant-negative activity, causing severe sporadic generalized GR, which preceded corticotroph adenoma formation. The later has been attributed to the combined effects of chronic corticotroph hyperstimulation, decreased glucocorticoid negative feedback, and at least one subsequent somatic defect in the control of the cell cycle [28]. The cause of this rare combination of cortisol resistance and adenoma in our patient who did not have peripheral DNA NR3C1 coding sequence mutations awaits elucidation. The promoter regions and the introns of the gene have not yet been studied. Possible explanations for this phenotype include somatic mosaicism for NR3C1 mutations or a mutation in another molecule that participated in hGRα-signaling.
Figure 3.
Normal anterior pituitary (3.1), our patient’s adenoma (3.2), and an ACTH-producing adenoma (one of 5 such adenomas that we tested) (3.3). 3.1. H&E staining of normal anterior pituitary (A) shows small acini surrounded by intact reticulin fiber network (B), scattered ACTH staining; (C), and GR staining (D,E). 10X (A,B,C,D); 20X (E). 3.2. H&E staining of our patient’s pituitary adenoma composed of large tumor cells with abundant cytoplasm with a sinusoidal architecture (A) and with breakdown of reticulin network (B); the tumor cells have abundant cytoplasmic positivity for ACTH (C) and no GR staining (D,E). 10X (A), 4X (B,D), 20X (E). 3.3. ACTH producing pituitary adenoma characterized by proliferation of large adenohypophyseal tumor cells on H&E (A) with reticulin breakdown (B), and difuse ACTH staining (C). GR immunohistochemistry shows patchy positive staining in both cytoplasm and nuclei of tumor cells (D,E).
Acknowledgments
This study was supported by the Intramural Programs of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), and, in part, by a sabbatical to Dr. G. Briassoulis by the University of Crete, Heraklion, Crete, Greece.
References
- 1.Vingerhoeds AC, Thijssen JH, Schwarz F. Spontaneous hypercortisolism without Cushing’s syndrome. J Clin Endocrinol Metab. 1976;435:1128–1133. doi: 10.1210/jcem-43-5-1128. [DOI] [PubMed] [Google Scholar]
- 2.Charmandari E, Kino T, Chrousos GP. Familial/sporadic GR: clinical phenotype and molecular mechanisms. Ann N Y Acad Sci. 2004;1024:168–181. doi: 10.1196/annals.1321.014. [DOI] [PubMed] [Google Scholar]
- 3.Lamberts SW. Glucocorticoid receptors and Cushing’s disease. Mol Cell Endocrinol. 2002;197:69–72. doi: 10.1016/s0303-7207(02)00280-0. [DOI] [PubMed] [Google Scholar]
- 4.Breslin MB, Geng CD, Vedeckis WV. Multiple promoters exist in the human GR gene, one of which is activated by glucocorticoids. Mol Endocrinol. 2001;15:1381–1395. doi: 10.1210/mend.15.8.0696. [DOI] [PubMed] [Google Scholar]
- 5.Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta-isoform: expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- 6.Kino T, Stauber RH, Resau JH, Pavlakis GN, Chrousos GP. Pathologic human GR mutant has a transdominant negative effect on the wild-type GR by inhibiting its translocation into the nucleus: importance of the ligand-binding domain for intracellular GR trafficking. J Clin Endocrinol Metab. 2001;86:5600–5608. doi: 10.1210/jcem.86.11.8017. [DOI] [PubMed] [Google Scholar]
- 7.Chrousos GP, Vingerhoeds A, Brandon D, Eil C, Pugeat M, DeVroede M, Loriaux DL, Lipsett MB. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J Clin Invest. 1982;69:1261–1269. doi: 10.1172/JCI110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nawata H, Sekiya K, Higuchi K, Kato K, Ibayashi H. Decreased deoxyribonucleic acid binding of glucocorticoid-receptor complex in cultured skin fibroblasts from a patient with the GR syndrome. J Clin Endocrinol Metab. 1987;65:219–226. doi: 10.1210/jcem-65-2-219. [DOI] [PubMed] [Google Scholar]
- 9.Iida S, Gomi M, Moriwaki K, Itoh Y, Hirobe K, Matsuzawa Y, Katagiri S, Yonezawa T, Tarui S. Primary cortisol resistance accompanied by a reduction in glucocorticoid receptors in two members of the same family. J Clin Endocrinol Metab. 1985;60:967–971. doi: 10.1210/jcem-60-5-967. [DOI] [PubMed] [Google Scholar]
- 10.Brönnegård M, Werner S, Gustafsson JA. Primary cortisol resistance associated with a thermolabile glucocorticoid receptor in a patient with fatigue as the only symptom. J Clin Invest. 1986;78:1270–1278. doi: 10.1172/JCI112711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charmandari E, Raji A, Kino T, Ichijo T, Tiulpakov A, Zachman K, Chrousos GP. A novel point mutation in the ligand-binding domain (LBD) of the human glucocorticoid receptor (hGR) causing generalized GR: the importance of the C terminus of hGR LBD in conferring transactivational activity. J Clin Endocrinol Metab. 2005;90:3696–3705. doi: 10.1210/jc.2004-1920. [DOI] [PubMed] [Google Scholar]
- 12.Koper JW, Stolk RP, de Lange P, Huizenga NA, Molijn GJ, Pols HA, Grobbee DE, Karl M, de Jong FH, Brinkmann AO, Lamberts SW. Lack of association between five polymorphisms in the human glucocorticoid receptor gene and GR. Hum Genet. 1997;99:663–668. doi: 10.1007/s004390050425. [DOI] [PubMed] [Google Scholar]
- 13.Huizenga NA, de Lange P, Koper JW, de Herder WW, Abs R, Kasteren JH, de Jong FH, Lamberts SW. Five patients with biochemical and/or clinical generalized GR without alterations in the glucocorticoid receptor gene. J Clin Endocrinol Metab. 2000;85:2076–2081. doi: 10.1210/jcem.85.5.6542. [DOI] [PubMed] [Google Scholar]
- 14.Webb C, Prayson RA. Pediatric pituitary adenomas. Arch Pathol Lab Med. 2008;132:77–80. doi: 10.5858/2008-132-77-PPA. [DOI] [PubMed] [Google Scholar]
- 15.Daly AF, Jaffrain-Rea ML, Ciccarelli A, Valdes-Socin H, Rohmer V, Tamburrano G, Borson-Chazot C, Estour B, Ciccarelli E, Brue T, Ferolla P, Emy P, Colao A, De Menis E, Lecomte P, Penfornis F, Delemer B, Bertherat J, Wémeau JL, De Herder W, Archambeaud F, Stevenaert A, Calender A, Murat A, Cavagnini F, Beckers A. Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab. 2006;91:3316–3323. doi: 10.1210/jc.2005-2671. [DOI] [PubMed] [Google Scholar]
- 16.Vandeva S, Tichomirowa MA, Zacharieva S, Daly AF, Beckers A. Genetic Factors in the Development of Pituitary Adenomas. Endocr Dev. 2010;17:121–133. doi: 10.1159/000262534. [DOI] [PubMed] [Google Scholar]
- 17.Xekouki P, Azevedo M, Strataksi CA. Anterior pituitary adenomas: inherited syndromes, novel genes and molecular pathways. Expert Rev Endocrinol Metab. 2010;5:697–709. doi: 10.1586/eem.10.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karl M, Lamberts SW, Koper JW, Katz DA, Huizenga NE, Kino T, Haddad BR, Hughes MR, Chrousos GP. Cushing’s disease preceded by generalized glucocorticoid resistance: clinical consequences of a novel, dominant-negative glucocorticoid receptor mutation. Proc Assoc Am Physicians. 1996;108:296–307. [PubMed] [Google Scholar]
- 19.Ruiz M, Lind U, Gåfvels M, Eggertsen G, Carlstedt-Duke J, Nilsson L, Holtmann M, Stierna P, Wikström AC, Werner S. Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance. Clin Endocrinol (Oxf) 2001;55:363–371. doi: 10.1046/j.1365-2265.2001.01323.x. [DOI] [PubMed] [Google Scholar]
- 20.Malchoff CD, Javier EC, Malchoff DM, Martin T, Rogol A, Brandon D, Loriaux DL, Reardon GE. Primary cortisol resistance presenting as isosexual precocity. J Clin Endocrinol Metab. 1990;70:503–507. doi: 10.1210/jcem-70-2-503. [DOI] [PubMed] [Google Scholar]
- 21.Arai K, Chrousos GP. Syndromes of glucocorticoid and mineralocorticoid resistance. Steroids. 1995;60:173–179. doi: 10.1016/0039-128x(94)00007-y. [DOI] [PubMed] [Google Scholar]
- 22.Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann N Y Acad Sci. 2009;1179:153–166. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez-Vega B, Krett N, Rosen ST, Gandhi V. Glucocorticoid receptor transcriptional isoforms and resistance in multiple myeloma cells. Mol Cancer Ther. 2006;5:3062–3070. doi: 10.1158/1535-7163.MCT-06-0344. [DOI] [PubMed] [Google Scholar]
- 24.Yan XB, Tang CH, Huang Y, Fang H, Yu ZQ, Wu LM, Liu RY. Alternative splicing in exon 9 of glucocorticoid receptor pre-mRNA is regulated by SRp40. Mol Biol Rep. 2010;37:1427–1443. doi: 10.1007/s11033-009-9529-z. [DOI] [PubMed] [Google Scholar]
- 25.Bray PJ, Cotton RG. Variations of the human glucocorticoid receptor gene (NR3C1): pathological and in vitro mutations and polymorphisms. Hum Mutat. 2003;21:557–568. doi: 10.1002/humu.10213. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Vega B, Krett N, Rosen ST, Gandhi V. Glucocorticoid receptor transcriptional isoforms and resistance in multiple myeloma cells. Mol Cancer Ther. 2006;5:3062–3070. doi: 10.1158/1535-7163.MCT-06-0344. [DOI] [PubMed] [Google Scholar]
- 27.Geng CD, Pedersen KB, Nunez BS, Vedeckis WV. Human glucocorticoid receptor alpha transcript splice variants with exon 2 deletions: evidence for tissue- and cell type-specific functions. Biochemistry. 2005;44:7395–7405. doi: 10.1021/bi047485e. [DOI] [PubMed] [Google Scholar]
- 28.Turner JD, Schote AB, Keipes M, Muller CP. A new transcript splice variant of the human glucocorticoid receptor: identification and tissue distribution of hGR Delta 313–338, an alternative exon 2 transactivation domain isoform. Ann N Y Acad Sci. 2007;1095:334–341. doi: 10.1196/annals.1397.037. [DOI] [PubMed] [Google Scholar]
- 29.Charmandari E, Kino T, Ichijo T, Jubiz W, Mejia L, Zachman K, Chrousos GP. A novel point mutation in helix 11 of the ligand-binding domain of the human glucocorticoid receptor gene causing generalized GR. J Clin Endocrinol Metab. 2007;92:3986–3990. doi: 10.1210/jc.2006-2830. [DOI] [PubMed] [Google Scholar]
- 30.Koper JW, Stolk RP, de Lange P, Huizenga NA, Molijn GJ, Pols HA, Grobbee DE, Karl M, de Jong FH, Brinkmann AO, Lamberts SW. Lack of association between five polymorphisms in the human glucocorticoid receptor gene and glucocorticoid resistance. Hum Genet. 1997;99:663–668. doi: 10.1007/s004390050425. [DOI] [PubMed] [Google Scholar]
- 31.Karl M, Lamberts SW, Koper JW, Katz DA, Huizenga NE, Kino T, Haddad BR, Hughes MR, Chrousos GP. Cushing’s disease preceded by generalized GR: clinical consequences of a novel, dominant-negative glucocorticoid receptor mutation. Proc Assoc Am Physicians. 1996;108:296–307. [PubMed] [Google Scholar]
- 32.Antonini SR, Latronico AC, Elias LL, Cukiert A, Machado HR, Liberman B, Mendonca BB, Moreira AC, Castro M. Glucocorticoid receptor gene polymorphisms in ACTH-secreting pituitary tumours. Clin Endocrinol (Oxf) 2002;57:657–662. doi: 10.1046/j.1365-2265.2002.01639.x. [DOI] [PubMed] [Google Scholar]
- 33.Dahia PL, Honegger J, Reincke M, Jacobs RA, Mirtella A, Fahlbusch R, Besser GM, Chew SL, Grossman AB. Expression of glucocorticoid receptor gene isoforms in corticotropin-secreting tumors. J Clin Endocrinol Metab. 1997;82:1088–1093. doi: 10.1210/jcem.82.4.3861. [DOI] [PubMed] [Google Scholar]