Abstract
In the field of tissue engineering, there is a need for advancement beyond conventional scaffolds and preformed hydrogels. Injectable hydrogels have gained wider admiration among researchers as they can be used in minimally invasive surgical procedures. Injectable gels completely fill the defect area and have good permeability and hence are promising biomaterials. The technique can be effectively applied to deliver a wide range of bioactive agents, such as drugs, proteins, growth factors, and even living cells. Hyaluronic acid is a promising candidate for the tissue engineering field because of its unique physicochemical and biological properties. Thus, this review provides an overview of various methods of chemical and physical crosslinking using different linkers that have been investigated to develop the mechanical properties, biodegradation, and biocompatibility of hyaluronic acid as an injectable hydrogel in cell scaffolds, drug delivery systems, and wound healing applications.
Keywords: Hyaluronic acid, crosslinking method, tissue engineering
Introduction
Hydrogels have several unique characteristic properties, including their similarity to tissue extracellular matrix (ECM), support for cell proliferation and migration, controlled release of drugs or growth factors, minimal mechanical irritation to surrounding tissue, and nutrient diffusion, that support the viability and proliferation of cells.1–3 Injectable hydrogels are promising materials in the field of tissue engineering, as they can target defects in very deep tissues with minimal invasiveness and better abandon edge adjustment.4 Hyaluronic acid (HA) and sodium hyaluronate are widely used to prepare biomaterials for tissue engineering because they yield highly reproducible and affordable biomaterials.5 HA is a naturally occurring glycosaminoglycan (GAG), a polysaccharide of high molecular weight that exhibits interesting viscoelastic properties, excellent biocompatibility, and biodegradability. These properties of HA-derived hydrogels make them ideal biomaterials for tissue engineering.6–8
Injectable hydrogels based on HA are prepared using various physical and chemical crosslinking methods.9,10 Several chemical modifications, aimed at enhancing, modulating, or controlling the therapeutic action of HA, are used to develop new products. These modifications are performed at different sites on HA and produce different results in terms of modification effectiveness and chain length damage.11 Physical crosslink hydrogels, or “smart materials,” so-called because they respond to changes in temperature, pH, or ionic strength, have been extensively examined due to their simple application, and the low toxicity of the crosslinking agents to tissues.2
This review focuses on crosslinking method–based hydrogels for tissue engineering applications. First, HA properties, including chemical structure, mechanical properties, biodegradation, and biocompatibility, are summarized. Second, the main functional groups of HA that are widely used to modify the structure and crosslinking agents are indicated. Based on these factors, we then highlight further crosslinking methods for hydrogel preparation, including Schiff-base reaction, thiol-modified HA crosslinking, Diels–Alder click crosslinking, enzyme crosslinking, thermo-responsive crosslinking, ionic crosslinking, and photo-crosslinking. Finally, the applications of HA-based hydrogels are discussed in detail.
Modification of HA
Hyaluronic acid
HA, also referred to as hyaluronan, is a naturally occurring non-sulfate linear polysaccharide composed of repeating disaccharide units of d-glucuronic acid and N-acetyl-d-glucosamine linked by β-1-3 and β-1-4 glycosidic bonds.7 HA occurs with different molecular weights: high molecular weight (HMWHA) is greater than 1 × 106 Da, low molecular weight (LMWHA) is 0.8 to 8 × 105 Da, and oligo-HA is <6 × 103 Da.12 HA is a primary component of the ECM of human connective tissues.13 It is an important structural element in the skin and participates in a number of cell surface receptor interactions; it has immunosuppressive and antiangiogenic activity, and is present in brain tissue, hyaline cartilage, and synovial joint fluid.14 The pKa of HA at pH = 7 is 3–4, and the carboxylic groups being ionized, the HA molecule is a polyanion associated with cations. Due to its strong hydrophilic character and its high molecular weight in biological tissues that can absorb a large amount of water, up to 1000 times its solid volume, HA exhibits important structural and functional roles in the body.15 In fact, because of its important characteristics of biocompatibility and biodegradability, HA has found numerous applications in biomedical and pharmaceutical applications.15,16 Clinically, HA is used in soft tissue replacement and augmentation, as well as in surgical procedures and diagnostics.17 However, HA is highly soluble and often exhibits very poor mechanical properties with rapid degradation behavior in vivo. Thus, HA has been chemically and crosslinker-modified to improve its properties, including mechanical properties, viscosity, solubility, degradation, and biologic properties. HA derivatives have been created and utilized in scaffolds for tissue engineering, in soft tissue surgery such as vocal fold augmentation, drug delivery, intracellular delivery of small interfering RNA (siRNA), wound healing, and as a device in several surgical procedures.6,15,18
Chemical modifications and crosslinking of HA
The fabrication of HA materials has been achieved using a variety of chemical modifications to provide mechanically and chemically robust materials. These HA derivatives have physicochemical properties that may be significantly different from the native polymer, but most derivatives maintain the biocompatibility and biodegradability of native HA. The most common modification of HA is crosslinking to form a hydrogel.19 However, the resilience of HA hydrogels relies on their ability to resist degradation by hyaluronidases and reactive oxygen and nitrogen species, thus limiting their efficient usage. To overcome problems with HA degradation, functional groups on HA have been exploited in the preparation of HA-based materials. The chemical structure of HA highlighting the three most commonly used sites of covalent modification, the carboxylic groups, hydroxyl group, and –NHCOCH3 group,20,21 is shown in Figure 1.
Figure 1.
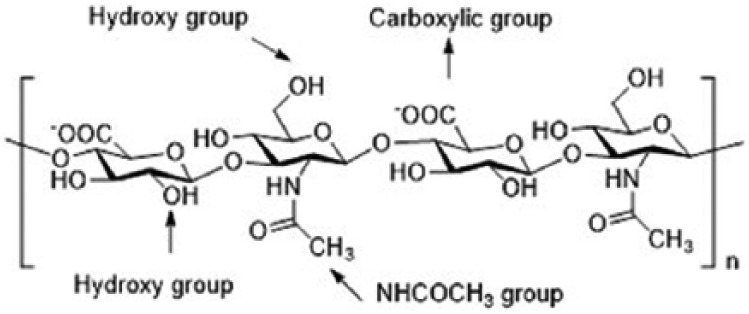
Hyaluronic acid structure showing the disaccharide repeat units and sites for chemical modification.
Modifications of the –COOH group
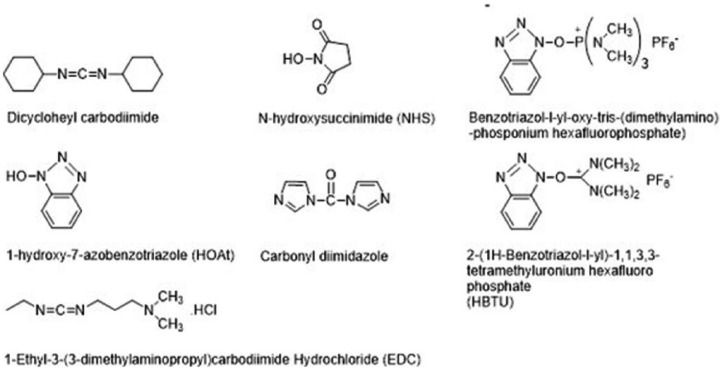
There are many reagents that condense carboxyl and amino groups to form amide bonds. For the crosslinking hydrogel, the most commonly used agents are carbodiimides, carbonyldiimidazole, and others20 (Figure 2). A feature common to the mechanism of action of all these reagents is the initial activation of the carboxyl group.22 The formation of an amide bond or an ester bond is facilitated by these reagents in two process steps. In the first step, the reagent forms a reactive adduct with the carboxyl group. During the subsequent reaction, nucleophilic attack at the activated species eliminates the activating moiety, resulting in the formation of a bond that does not involve the incorporation of the crosslinking agent. The amino group or alcohol group is implied as the nucleophile in these reactions.23,24
Figure 2.
Coupling reagents for the activation of hyaluronic acid (HA)-COOH.
The fabrication of hybrid hydrogels from arginine-based poly(ester amide) and HA precursors (Arg-PEA and HA-AEMA) was achieved by photo-crosslinking. Synthesis of HA-AEMA was carried out by dissolving HA in 100 mL 1-ethyl-3-(3-dimethyl aminopropyl)-1-carbodiimide hydrochloride (EDC; 15 mmol); N-hydroxysuccinimide (NHS; 15 mmol) and AEMA (10 mmol) were added to solution to form amide bonds. The product was dialyzed against water and then lyophilized over 3 days. 1H-NMR was used to characterize the chemical structure, using D2O as a solvent.25 HA and sodium alginate (SAL) were used to improve cellular structure and mechanical properties by crosslinking with EDC, as a carboxyl-activating agent, and adipic dihydrazide (ADH) as a crosslinker. The reaction was carried out at pH 4.75 by adding an acetate buffer solution to the HA and SAL solution, and the reaction was maintained for 4 h at room temperature. The hydrogel was purified by several washes with double distilled water to remove residual ADH and EDC.26
Modifications of the –OH group
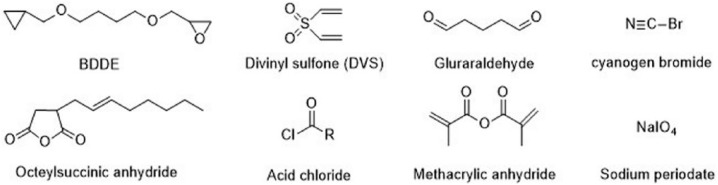
The chemical modification of –OH groups can be divided into four reaction types: ether formation, ester formation, hemiacetal formation, and oxidation. Reagents used in these reactions are summarized in Figure 3.
Figure 3.
Crosslinking agents for modification of –OH groups.
1,2,3,4-diepoxybutane27 (DEB) is a genotoxic bis-electrophile capable of crosslinking cellular biomolecules to form DNA–DNA and DNA–protein crosslinks (DPCs). Over 150 proteins, for example, histones, transcription factors, splicing factors, high mobility group proteins, and tubulins, were found covalently crosslinked to chromosomal DNA in the presence of DEB as characterized by mass spectrometry-based proteomics. Butanediol-diglycidyl ether (BDDE),28 the most commonly used crosslinker in HA hydrogels, can also be used with 1 M NaOH to fabricate a hydrogel. The reaction consists of the epoxide ring opening to form ether bonds with the HA hydroxyl groups.29
HA crosslinking with divinyl sulfone (DVS)30–32 was performed at room temperature in high pH conditions (pH > 8). The advantage of this system is limitation of the degradation of HA in alkaline solutions compared to that observed at high temperature. Ethylene sulfide was also used for ether formation with the addition of dithiothreitol (DTT) in an alkaline solution.33 The presence of graft thiol groups showed that cells were protected from reactive oxygen species because further crosslinking could not occur.
Glutaraldehyde (GTA) is widely used as a crosslinking agent for HA that needs to be initiated in an acidic medium to catalyze the reaction.32,34 In addition, GTA crosslinking is unstable and can be hydrolyzed to recover the starting material in acidic conditions. A further disadvantage of GTA crosslinking is its toxicity and the need to purify the final product.35
Ester formation using octenyl succinic anhydride (OSA) is performed in alkaline conditions (pH ~9) by reacting hydroxyl groups of HA with anhydride to form ester bonds.36 The authors stated that using 50 times more OSA than HA provides 43% of substitution with a fast reaction rate. To graft poly(lactic acid) (PLA) oligomers, HA was converted to acetyltrimethyl ammonium bromide (CTA) salt. Ester formation with acyl chloride-activated carboxylate compounds to form ester bonds was first activated by chloroacylation with thionyl chloride and then reacted with HA at room temperature in organic solvent (dimethyl sulfoxide (DMSO)).37 HA crosslinking with methacrylic anhydride (MA) was performed to obtain methacrylated HA by esterification reaction in alkaline conditions (pH: 8–10).38 The methacrylate groups present on the HA backbone could be further used for photo-crosslinking.
Sodium periodate was used to oxidize the hydroxyl groups of HA to produce dialdehydes, thereby opening the sugar ring. This aldehyde-HA product is the main polymer precursor used for hydrogel fabrication via Schiff-base reactions.39,40
Modifications of the –NHCOCH3 group
The modification reactions of the –NHCOCH3 group include deacetylation, amidation, hemiacetylation, and hemiacetal formation, among others. Amidation methods have been used for deacetylation of the N-acetyl groups of HA that can then react with an acid, and are usually performed using hydrazine sulfate.41
Physical crosslinking of HA
Physical crosslinking can be accomplished using a variety of pH, temperature, ionic strength conditions, and physicochemical interactions, for example, hydrophobic interactions, hydrogen bonding, charge interaction, or stereocomplexation.3 In particular, temperature-responsive hydrogels have been extensively examined for various applications.42 Most temperature-sensitive polymers showed a lower critical solution temperature (LCST) behavior in aqueous solution, but became significantly less water soluble or water insoluble at temperatures above the LCST.43 Common thermogelling polymers that are frequently used to modify HA to prepare thermally sensitive HA hydrogels include poly(N-isopropylacrylamide) (PNIPAM), pluronic acid, methylcellulose, and polyethylene glycol (PEG).44–46
Injectable HA hydrogel
Chemical crosslinking
Schiff-base crosslinking hydrogel
Schiff-base reactions are one of the most widely accepted strategies for the preparation of hydrogels, particularly because of the mild reaction conditions and high reaction rates.47 Schiff bases are typically obtained by facile condensation of an aldehyde or a ketone with primary amines.48 The general formula for Schiff bases is RN = CR′R″ where R, R′, and R″ could be alkyl, aryl, heteroaryl, or cycloalkyl.49 The –C = N– imine bond in Schiff bases plays a unique role in conferring broad-spectrum biologic activities to these compounds.50 Schiff bases also serve as versatile ligands for arranging a variety of metal ions in different coordination geometries and oxidation states.51
The polysaccharide derivatives alginate, dextran, chitosan, and HA are extensively used biopolymers to prepare injectable hydrogels exploiting the Schiff-base reaction.52–55 In the case of polymers with cis-glycols, the introduction of aldehyde functionality by periodate oxidation is an easy approach, particularly for biopolymers.56 Macromolecular dialdehyde derivatives thus formed can react with polymers containing an amino functional group to form crosslinks.
Dialdehyde hyaluronic acid (CHO-HA)
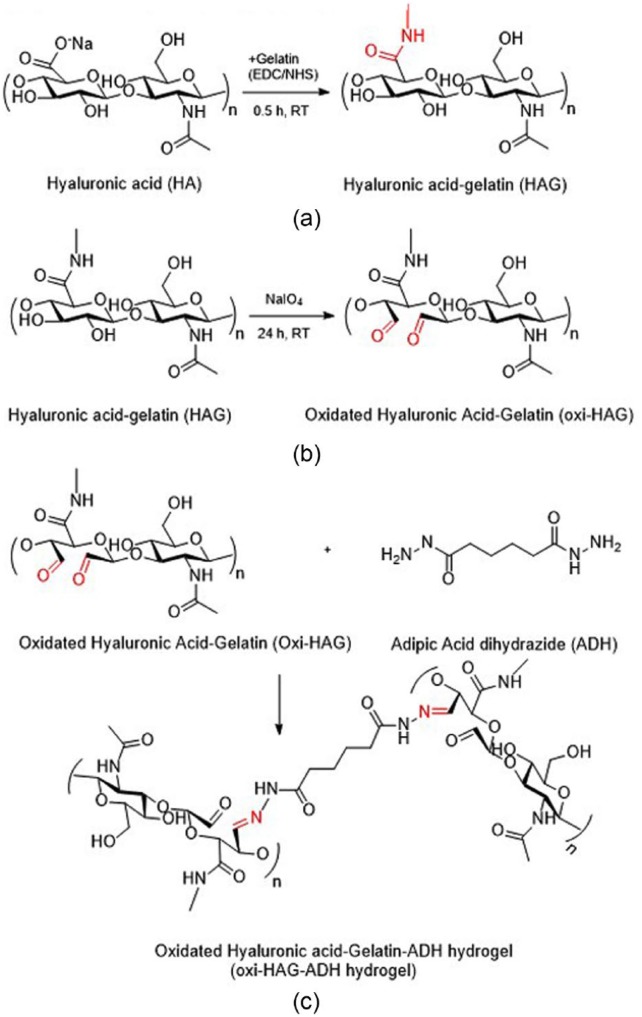
In situ forming amine-modified HA (HA-NH2) and CHO-HA hydrogels were prepared via Schiff-base reaction. HA-NH2 was synthesized by coupling ethylenediamine with EDC and HOBt at pH 6.8 for 24 h. Genipin was used for double crosslinking, resulting in a more compact microstructure, slower mass loss, and higher compressive modulus of hydrogels.57 Injectable HA hydrogel was developed for in vivo bone augmentation by Martínez-Sanz et al. They presented HA modified with 3-amino-1,2-propanediol and subsequently reacted with NaIO4 to provide aldehyde functional groups. Mixing equal volumes of HA-aldehyde derivative and HA-hydrazide derivative formed a hydrazine-crosslinked hydrogel within 30 s. Elastic modulus (G′) of gels was 260 Pa after swelling in phosphate-buffered saline (PBS) for 24 h.58 Chitosan,59 a naturally derived polysaccharide, has been composited with HA to reduce the erosion and degradation rate behaviors of hydrogels. Insulin was entrapped within an N-succinyl-chitosan (SCS) and aldehyde hyaluronic acid (AHA) hydrogel resulting in functional adipose tissue.60 Dexamethasone (Dex) was grafted onto the HA-SCS hydrogel to prepare a bioactive hydrogel.61 This AHA-SCS-Dex hydrogel showed a slightly lower gelation time and weight loss with significantly higher swelling ratio. The number of adipose-derived stem cells (ADSCs) on the surface of AHA-SCS-Dex was greater than that on AHA-SCS hydrogels. These results suggest that AHA-SCS-Dex is more favorable for ADSC attachment due to its higher bioactivity. Glycol chitosan (GC) was used to fabricate a hydrogel with oxidized HA (OHA) as a chondrocyte delivery vehicle for cartilage regeneration.62 A mixing ratio of OHA and GC of 2:1 with concentrations of 3 wt% of OHA25/GC and OHA50/GC hydrogel was considered useful for cell delivery due to the faster gelation time, higher mechanical strength, and retained hydrogel mass of more than 50% of their original weight for 46 days. Injectable OHA-gelation-adipic acid dihydrazide (oxi-HAG-ADH) hydrogel was developed for pulposus regeneration (Figure 4).63 The results showed that oxi-HAG-ADH has higher viscoelastic properties (G′/G″, G* pa) than oxi-HA-ADH due to the immobilization of gelatin.
Figure 4.
Synthesis of oxidized hyaluronic acid (HA)-gelatin hydrogels: (a) crosslinking of HA and gelatin by ethyl(dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS), (b) HAG polymer oxidation by sodium periodate, and (c) Oxi-HAG-ADH hydrogel synthesis, with imines bonding oxi-HAG and ADH.63
Limitations of Schiff-base hydrogels for tissue engineering
The advantage of the Schiff-base system is complete avoidance of extraneous toxic crosslinking agents and other triggers that can cause an unwanted tissue response. Although Schiff-base reaction hydrogels are easy to translate to clinical applications because of their simple methodology, one of the limitations of using such injectable hydrogels is their pH sensitivity.64 Schiff-base (imine) linkages are likely to hydrolyze under acidic conditions; therefore, these hydrogels cannot be used for biomedical applications where hydrogel stability is critical even in disease conditions, where the pH is usually slightly acidic.65,66
Thiol-modified HA hydrogel
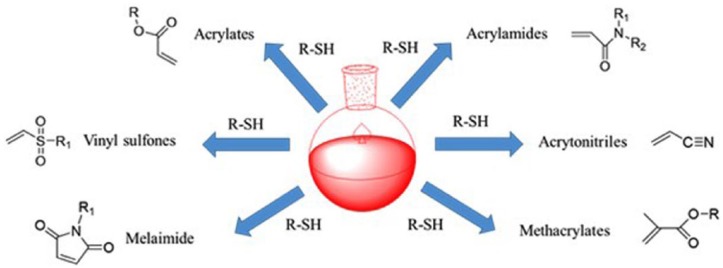
The Michael addition reaction is broadly characterized as the reaction of an enolate-type nucleophile in the presence of a base catalyst to an α,β-unsaturated carbonyl followed by an acid work-up.67 The α,β-unsaturated compound undergoing Michael addition is called the Michael acceptor, the nucleophile the Michael donor, and the product the Michael adduct. The Michael addition reaction is described as a special type of conjugate (1,4) addition, in which the strong nucleophilic attack on the β-carbon of an α,β-unsaturated carbonyl results in the Michael adduct, as in oxa-Michael reactions, aza-Michael reactions,68 and thiol-Michael reactions (Figure 5).69,70
Figure 5.
Commonly utilized vinyl groups in thiol-Michael addition reactions.
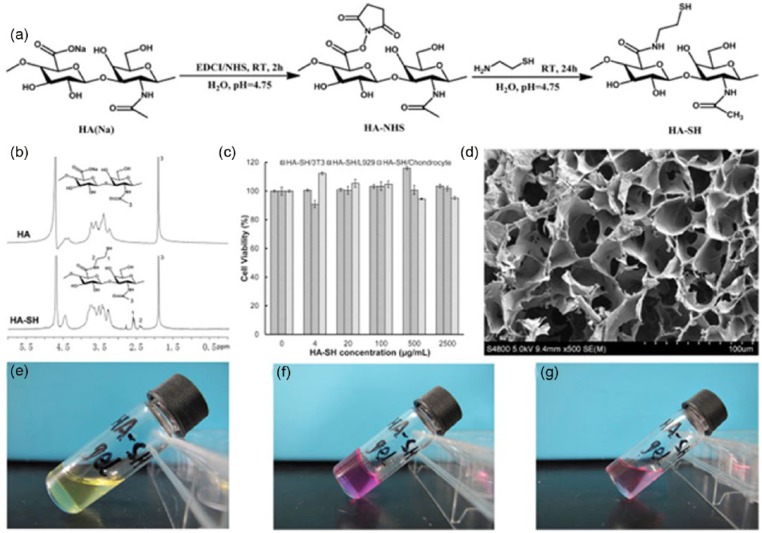
The development of a crosslinking system using oxidized glutathione (GSSG) in conjunction with an existing HA-based hydrogel for ophthalmic applications produced a gel via thiol-disulfide exchange reaction in a gelation time of less than 5 min using 4 mM GSSG, 0.4% w/v thiolated carboxymethyl HA (CMHA-S), and 0.4% Gelin-S.71 A novel self-crosslinking smart hydrogel with in situ gelation properties was prepared from a single component, thiolated HA derivatives (HA-SH), obtained through simple chemical modification with EDC and NHS (Figure 6). The 1H-NMR spectrum was used to confirm the existence of the conjugated thiol groups and the typical spectra of native HA and thiolated HA. It was obvious that new resonant peaks of HA-SH appeared at 2.45 and 2.63 ppm, which represented the methylene protons of –CH2CH2SH and CH2CH2SH in the spectrum of HA-SH polymer, respectively.72
Figure 6.
(a) Synthesis protocol of thiol-modified hyaluronic acid (HA-SH). (b) 1H-NMR (D2O) spectra of HA and HA-SH (Mw = 0.1 MDa, Ds = 37.47%). (c) Biocompatibility of HA-SH polymers (Mw = 0.3 MDa, Ds = 55.44%) for chondrocytes, 3T3 cells and L929 cells after 48 h of co-culture (n = 3). (d) SEM micrograph of HA-SH hydrogel (Mw = 0.3 MDa, Ds = 55.44%, 3.0% (w/v)). (e) Image of HA-SH solution (Mw = 0.3 MDa, Ds = 55.44%, 3.0% (w/v)). (f) Image of HA-SH hydrogel. (g) Image of HA-SH solution derived from the decomposition of HA-SH hydrogel with DTT (100 mM).
Source: Reproduced with permission.72 Copyright 2016, Elsevier B.V.
The stable thioether sulfone bond is not readily susceptible to hydrolytic degradation and, therefore, has found widespread implementation in applications.73 The resistance to hydrolytic degradation offered by these bonds overcomes problems associated with other carbonyl-conjugated vinyls, such as acrylates and maleimides (MALs) that contain thioether ester and succinimide bonds. Yu and Chau74 reported that generating vinyl sulfone (VS) groups on HA could be achieved in alkaline conditions using a One-step “click” method. The NMR signals of free VS double bonds were at δ 6.3, 6.4, and 6.9 ppm. The degree of modification, defined as the number of VS groups divided by the number of disaccharide repeating units, was calculated from 1H-NMR spectra by comparing the integral signals at δ = 6.9 and δ = 2 (acetyl group of the disaccharide). VS-modified p(HPMAm-lac1-2)-PEG-p(HPMAm-lac1-2) triblock sulfone copolymers and thiol-modified HA were designed to form physically crosslinked hydrogel at 37°C, followed by chemical crosslinking through Michael-type reactions. Hydrogels were fabricated by mixing 15% and 20% of triblock copolymer and thiol-HA at a ratio of 1:1 between VS and thiol groups. In the swelling/degradation study, hydrogel composed of lower thiolation degree HA-SH exhibited shorter degradation times, attributed to the higher polysaccharide content and distance between crosslinks that led to greater water uptake.75
Diels–Alder click crosslink hydrogel
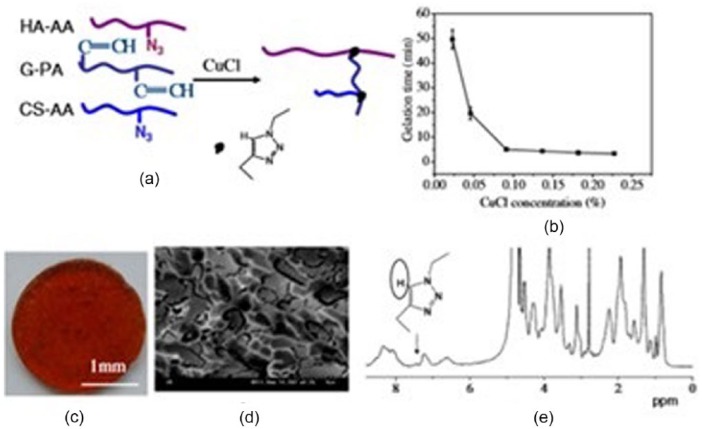
The Diels–Alder reaction (a [4 + 2] cycloaddition) involves the covalent coupling of a conjugated diene with a substituted alkene to form a six-membered ring product in the absence of a catalyst. The electron-withdrawing groups on the alkene and the electron donating groups on the diene are important factors for increasing reaction rate.76 HA, chondroitin sulfate (CS), and gelatin were used to prepare a hydrogel via click chemistry reactions to mimic the natural cartilage ECM. HA and CS were modified with 11-azido-3,6,9-trioxaundecan-1-amine (AA) and gelatin was modified with propiolic acid (PA).77 The N3 groups of HA-AA and CS-AA reacted with the alkynyl groups of G-PA, catalyzed by CuCl, to form a hydrogel confirmed by 1H HR-MAS NMR spectrum of the triazole ring at δ 7.44 ppm. The gelation time decreased from 50 min to 5 min after increasing the CuCl concentration to 0.95 mg/mL (Figure 7). HA-PEG hydrogels were prepared by mixing HA-furan with (MI)2 PEG crosslinkers separately in MES buffer (pH 5.5) via Diels–Alder “click chemistry.” The elastic modulus of the hydrogel increased with rising crosslinker concentration. 1Furan/2MI hydrogels were the strongest with a G′ value of 679 ± 62 Pa. Several years later, HA-furan was modified with tyramine (TA) functional groups. An HA/PEG hydrogel was prepared using enzymatic crosslinking and sequential Diels–Alder click reactions.78 The authors stated that Diels–Alder click reaction crosslinking produced a hydrogel with outstanding shape memory and anti-fatigue properties. HA-furan and PEG-MAL were also used to form hydrogels for ultimate use in regenerative strategies to repair injured spinal cords.79
Figure 7.
(a) Schematic illustration to show formation of a hydrogel through click chemistry. (b) Gelation time of the hyaluronic acid/chondroitin sulfate (HA/CS)/gelatin click hydrogel as a function of CuCl concentration. (c) Macroscopic and (d) microscopic images of the hydrogel taken with a digital camera and by cryo-SEM at −195°C, respectively. (e) 1H HR-MAS NMR spectra of the hydrogel.
Source: Reproduced with permission.77 Copyright 2011 Acta Materialia Inc.
Enzyme crosslink hydrogel
Enzymatic crosslinking is an enzyme-catalyzed oxidation reaction. The increasing interest in enzymatic crosslinked hydrogels has developed due to the mildness of the reaction and its rapid occurrence under physiological conditions.78 However, the poor mechanics and rapid degradation of such hydrogels have limited their application. HA-furan conjugation of TA, HA-furan/TA, was performed by suspension at a concentration of 1.5% w/v in PBS for 24 h at room temperature. After adding MAL-PEG-MAL solution, hydrogen peroxide (H2O2) and horse radish peroxidase (HRP) were added to the solution and hydrogel was obtained due to enzymatic crosslinking reaction.80 HRP and H2O2 were also used to crosslink injectable hydrogels via coupling of TA-modified SAL and HA. Two tyraminated polymer solutions, 10 U HRP, and 1.0% H2O2 were mixed in equal parts to form a composite gel. The swelling ratio and mechanical properties of the composite gel were found to be lower than those of alginate gel, and the protein release was also lower than that of HA gel. However, the metabolic activity of the cells encapsulated in the composite gel significantly increased from days 7 to 17 compared to that of cells in alginate and HA gels. This result indicated that the composite gels were cytocompatible and able to support higher cellular metabolic activity.81 HA was enzymatically crosslinked with silk to form a composited hydrogel that offered a biocompatible, stable, tunable system.82 Later, TA-substituted HA and aqueous silk were combined to yield final HA concentrations of 0, 0.2, 1.05, 2.22, and 5 mg/mL, and a final silk concentration of 20 mg/mL. Crosslinking was initiated by adding 10 U/mL of HRP followed by 0.01% H2O2. Silk–HA hybrid hydrogels completed crosslinking in approximately 20 min and exhibited higher final storage moduli than pure HA in all compositions. Incorporation of silk into HA can offer mechanical integrity and resistance to degradation while HA can provide hydration and biofunctionality.82
Physical crosslinking
Thermo-responsive hydrogel
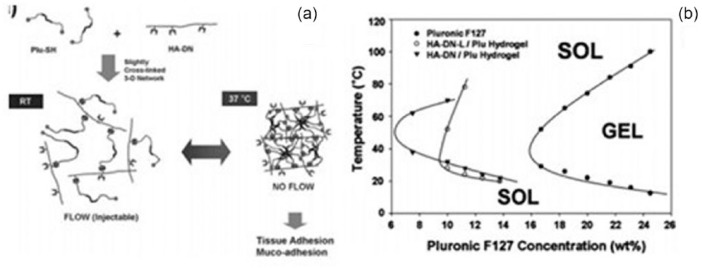
PNIPAM was grafted onto HA to create a thermo-sensitive copolymer hydrogel, AHA-g-PNIPAAm. AHA was synthesized using EDC and HOBt at pH 5. 4,4′-azobis(4-cyanovaleric acid) was used as the initiator to polymerize NIPAAm. To prepare AHA-g-PNIPAAm, PNIPAAm/EDC solution was added to AHA solution at room temperature for 24 h before dialysis (MWCO 25000). The transition from liquid-like behavior to elastic gel-like state occurred at 30°C within 1 min. This behavior is beneficial for cell encapsulation in hydrogel.83 Pluronic-composited HA was used to create a thermo-sensitive hydrogel that was evaluated for its potential as an artificial vitreous substitute.84 HA conjugated with dopamine (HA-DN) was mixed with thiol end-capped Pluronic F127 copolymer (Plu-SH) to obtain crosslinked gels via Michael-type addition reactions, and they exhibited temperature-dependent sol-gel phase transition behaviors (Figure 8).85
Figure 8.
Schematic representation of hyaluronic acid (HA)/pluronic hydrogels. (a) Slightly crosslinked three-dimensional network formation of HA/Pluronic hydrogels and their sol-gel transition behavior. (b) Sol-gel transition curves of HA/Pluronic hydrogels (∇) and HADN-L + Plu-SH hydrogels (○). Pluronic F127 hydrogel (●) was used as control.
Source: Reproduced with permission.85 Copyright 2010, Royal Society of Chemistry.
Hexamethylene diisocyanate (HDI)-pluronic F 127 copolymer was incorporated with HA to improve injectable and thermo-responsive hydrogels for anticancer drug delivery.86 Methyl cellulose was blended with HA of different molecular weights to investigate the thermogelation and biocompatibility for drug delivery or wound healing.87 HA-based nanogels for use as drug carriers were validated in an in vivo study on mice using intravital two-photo laser scanning microscopy.88 Di(ethylene glycol) methacrylate (DEGMA) and oligo(ethylene glycol) methacrylate (OEGMA) were used to synthesize thermo-sensitive HA-p-poly(DEGMA)-co-(OEGMA) via a photo-induced radical thiol-ene reaction starting from HA modified with pentenoate groups. HA-derivative nanogels were created by self-assembly into spherical gel particles at temperatures above 37°C with sizes ranging from 150 to 214 nm.
Ionic crosslink hydrogel
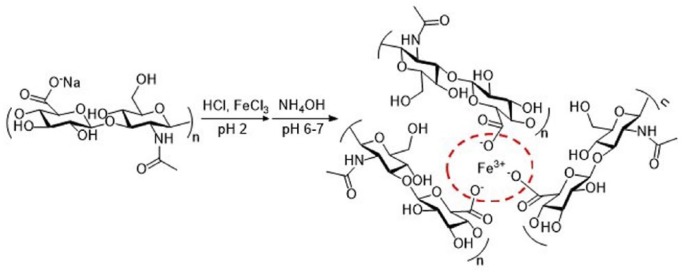
FeCl3 has been used to crosslink with HA for application in medical device materials. FeHA gels were prepared by adding FeCl3 to 0.5% HA at molar ratios [Fe3+]/[COO–] of 0.165, 0.3, and 0.33 to obtain 50%, 90%, and 100%, respectively, crosslinking in FeHA gel. As the crosslinking increased, the contact angle increased from 7.5 to 14, indicating that the surface wetting ability decreases as crosslinking increases89 (Figure 9).
Figure 9.
Reaction scheme and idealized structure of the Fe-hyaluronic acid (FeHA) network.
Double-crosslinked composite hydrogels have been reported to display better biocompatibility compared with simple HA hydrogels. Alginate/HA hydrogel mixtures were used for ionic and covalent crosslinking, using CaCl2 and EDC/ADH to crosslink, respectively.90 Fourier transform infrared (FT-IR) results confirmed that there was covalent crosslinking by this method, and the degree of covalent modification was dependent on the concentrations of EDC and CaCl2. Alginate/HA hydrogel with double crosslinks also supported Schwann cell survival and growth. In addition, this hydrogel has a porous microstructure that can be fabricated using a rapid prototyping technique. HA was grafted with polyacrylic acid (PAA) via controlled radical polymerization (CRP) and then crosslinked with CaCl2 to form an insoluble salt of HA-g-PAA with high water content. HA-g-PAA had lower viscosity than pure HA because intra- and/or intermolecular hydrogen-bond formation was prevented by steric hindrance. On the other hand, the degradation rate of HA-g-PAA was much slower than native HA in the presence of HAse.91
Photo-crosslink hydrogel
For cartilage tissue engineering, an injectable hydrogel consisting of methacrylated glycol chitosan (MeGC) and HA was created by photo-crosslinking with a riboflavin photo-initiator. To produce a stable gel for cell encapsulation, at least 40 s of irradiation time under visible light was required. Increasing the radiation time resulted in decreased encapsulated cell viability from 90% to 60%. In addition, it significantly enhanced the compressive modulus of the hydrogel up to 11 or 17 Pa.40 Catechol-functionalized hyaluronic acid (HA-CA) was synthesized by modifying the HA backbone with DN via a carbodiimide coupling reaction using EDC and NHS.92 Gelation of the HA-CA conjugate was induced by crosslinking the conjugate with NaIO4 to the catechol group of HA-CA at pH 8. The generation of o-quinone caused the gel color to turn brown immediately upon gelation. HA-methacrylate (HA-ME) was created using a conventional photo-polymerized crosslinking method. HA-CA hydrogels were found to be less toxic to human adipose-derived stem cells (hADSCs) than HA-ME hydrogels. Visible green light-activated crosslinking systems were presented as a safe alternative to ultraviolet (UV)-photo-crosslinked HA-based hydrogels to overcome the limitation of using UV radiation. Methacrylated HA (HA-MA) hydrogels of varying molecular weight, degree of modification, and concentration exhibited compressive moduli ranging from 3 to 146 kPa for green light crosslinking, which was higher than that achieved using UV photo-crosslinking and showed no cytotoxicity toward human mesenchymal stem cells.93
HA as a biomaterial in tissue engineering
HA-based scaffolds
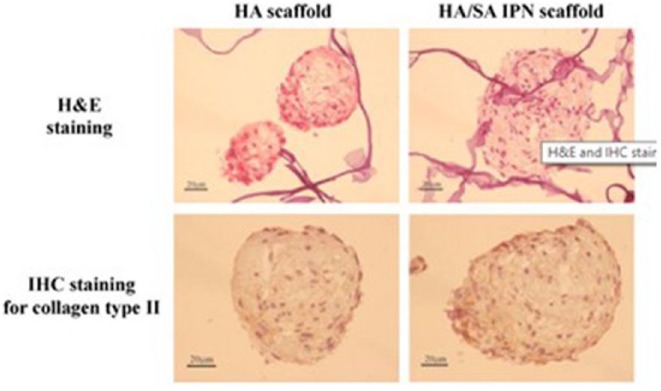
Collagen-CS-HA hybrid hydrogels (CCH) were prepared using genipin as a crosslinking agent at concentrations of 0.6, 0.75 and 1 mM, named CCH-0.6, CCH-0.75, and CCH-1, respectively.94 Histological staining results demonstrated that CCH-0.75 membranes showed GAG secretion, while Col membranes showed no significant GAG secretion until the sixth day. Furthermore, CCH-0.75 could maintain chondrocyte phenotype during culture compared with Col membranes, which could cause de-differentiation. MeGC/HA hydrogels were created to examine the proliferation and differentiation of chondrocytes.40 Almar blue assay showed that MeGC/HA hydrogels could promote cell proliferation better than MeGC hydrogels. After 21 days, positive Safranin-O and Alcian blue staining showed that MeGC/HA hydrogels exhibited greater GAG accumulation in lacunae and ECM surrounding the cell clusters. Son et al.95 developed scaffolds for hADSC delivery based on HA/SAL via an interpenetrating polymeric network (IPN). The production of s-GAC was higher in HA/SAL IPN scaffolds compared with HA scaffolds, as determined by dimethylmethylene blue (DMMB) assay. Reverse transcription polymerase chain reaction (RT-PCR) results corroborated the DMMB information for quantitative s-GAC content. Both aggrecan and collagen type II increased in HA/SAL IPN scaffolds after 14 days in culture. Hematoxylin and eosin (H&E) staining showed that hADSCs had a round shape and even distribution pattern, and secreted ECM was also observed along the cells in both HA and HA/SAL IPN scaffolds. However, immunohistochemistry (IHC) staining for collagen type II revealed that its expression was greater in HA/SAL IPN scaffolds than in HA scaffolds (Figure 10).
Figure 10.
Hematoxylin and eosin (H&E) and immunohistochemistry (IHC) staining of collagen type II in hADSC after 14 days of in vitro culture in hyaluronic acid (HA) and HA/sodium alginate (SAL) interpenetrating polymeric network (IPN) scaffolds. Scale bar = 20 μm.
Source: Reproduced with permission.95 Copyright 2013, Elsevier B.V.
Snyder et al.96 reported that increased mechanical strength was achieved by modifying HA with MA, providing HA-MA reinforcement within fibrin hydrogels, and this was directly correlated with increasing HA-MA concentration. Live/dead staining and metabolic assays confirmed that the crosslinked fibrin/HA-MA hydrogels contributed a suitable three-dimensional (3D) environment for bone marrow stromal cell (BMSC) proliferation. Quantitative polymerase chain reaction (qPCR) of BMSCs incubated in the fibrin/HA-MA hydrogel affirmed reduced expression of collagen type 1 alpha 1 messenger RNA (mRNA) with an increase in Sox9 mRNA expression, especially in the presence of a platelet lysate.
HA in drug delivery systems
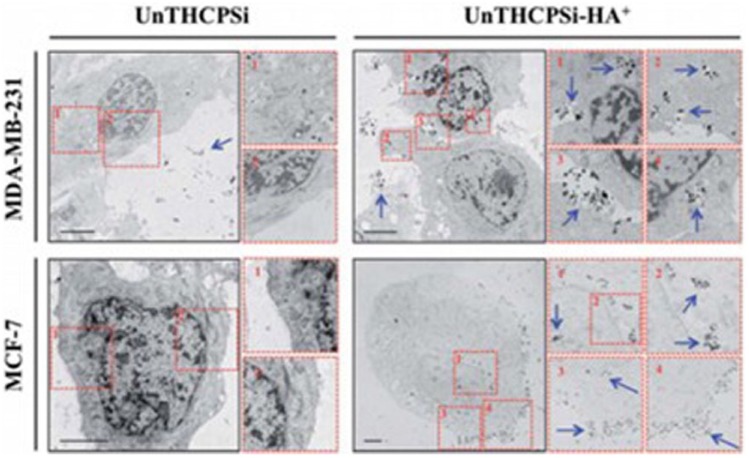
HA and HA-composites, crosslinked to from hydrogel particles, have been reported in the biomedical field as drug and protein carriers. Ilgin et al.97 demonstrated that bare HA, magnetic HA-composites, and modified HA particles can be used as drug carriers. The results showed that the chemically modified HA released much more trimethoprim (TMP) than both bare HA and the magnetic HA-composite. The positive charge of chemically modified HA can interact with amine groups on the drug, thus, much more of this drug can be loaded onto the positively charged HA particle. In contrast, unmodified HA particles (negative charge) can load much more drug than magnetic HA-composite particles because of the lower number of available sites due to occupation by magnetic metal ferrites. This advantage of adsorbing and releasing less amounts of drugs can extend the release time. Later, HA-iron oxide hybrid nanogels were developed for magnetic resonance imaging and drug delivery.98 HA-based anionic and cationic particles were generated as HA-CYs-AMPS and HA-CYs-APTMACl, respectively,99 and naproxen (NN) and TMP were used as model drugs for drug loading and release studies. The results showed that HA-CYs-AMPS releases 92% and 100% of TMP, more than bare HA (25% and 36%) at pH 1.1 and 7.4, respectively. The authors explained that TMP has amine groups that can interact with sulfonyl groups on the modified particles leading to higher drug absorption and release. Positively charged HA-CYs-APTMACl particles also released 78% and 100% of NN at pH 1.1 and 7.4 because the carboxylic groups on NN interacted with the positively charged particles. Cholesteryl group-bearing hyaluronic acid (CHHA) nanogels formed by self-assembly and salt-induced hydrogels were investigated in an in vivo study for recombinant human growth hormone (rhGH) release.100 Two types of CHHA nanogel formulations (suspension formulation and in situ gel formulation) were administered into Sprague Dawley rats. The pharmacokinetic (PK) profile and the PK parameters of gel suspension and in situ gel formulations were similar, and the mean residence time (MRT) of these formulations was 63.1 and 85.0 h, respectively, which was longer than free rhGH at 2 h. Psi-based nanoparticles covalently surface-modified with HA (UTHCPSi-HA+) were developed to target breast cancer tumors.101 The results demonstrated the capability of UTHCPSi-HA+ to bind and consequently target the CD 44 receptor overexpressed on the surface of the MDA-MB-231 and MCF-7 breast cancer cells when compared with non-HA-modified UTHCPSi nanoparticles (Figure 11).
Figure 11.
Intracellular uptake and distribution of UnTHCPSi and UnTHCPSi–HA+ nanoparticles. TEM images and the corresponding numerically organized magnifications of ultra-thin sections of MDA-MB-231 and MCF-7 breast cancer cells exposed to UnTHCPSi and UnTHCPSi–HA+ at a concentration of 50 μg/mL, for 6 h at 37°C are shown. The conjugation of HA+ onto the surface of the UnTHCPSi nanoparticles has been shown to significantly enhance the interaction and consequently the uptake of the nanoparticles by both MDA-MB-231 and MCF-7 breast cancer cells. The nanoparticles associated with the cells are highlighted by blue arrows. Scale bars are 5 μm.
Source: Reproduced with permission.101 Copyright 2014, Royal Society of Chemistry.
HA in wound healing
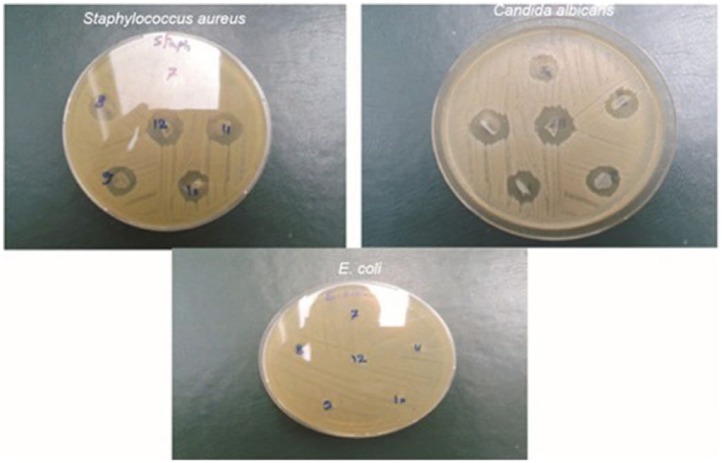
Agarose/HA hydrogels have been prepared to control morphology, thermal stability, and in vivo degradation.102 The weight loss percentages of agarose group at 3.50%, 6.65%, 15.00%, and 23%, corresponded to post-implantation periods of 1, 2, 3, and 4 weeks. In addition, agarose/HA hydrogel at a ratio of 5/5 showed a faster degradation rate than those with 10%, 31%, 66%, and 100% agarose. These results confirmed that agarose/HA hydrogel could be a candidate for scaffolds for wound healing as it increased biologic activity and degradation. Poly(vinyl alcohol) (PVA) membrane hydrogels were created using a freeze–thawing (F-T) method and were tested for their biologic properties (cell viability (%) and antimicrobial activity) and compatibility as wound dressing materials. The in vitro biocompatibility test demonstrated that a high HA content in PVA-HA produced highly viscous media because of the degradation of HA. Thus, it resulted in inhibited migration, movement, and cell viability. In antimicrobial activity tests, PVA-HA without an ampicillin membrane showed effective antimicrobial activity, particularly against Candida albicans because of the presence of HA. On the other hand, PVA-HA exhibited antibacterial activity against Staphylococcus aureus, especially after loading ampicillin, but provided no microbial resistance against Escherichia coli with or without ampicillin103 (Figure 12).
Figure 12.
Representative photographs of antimicrobial activities of PVA-HA membranes showing the appearance of microbial inhibition zones formed against seeded Staphylococcus aureus, Candida albicans, and Escherichia coli.
Source: Reproduced with permission.103 Copyright 2015, Sociedade Brasileira de Quimica.
A new type of hydrogel (HA/poly(vinylphosphonic acid) (PVPA)/CS) was created to be used for skin wound healing applications. Antibacterial tests revealed that this hydrogel has antimicrobial activity against E. coli. and an in vivo study demonstrated that the hydrogel was not rejected by the immune system and could improve the wound healing process.104
Conclusion
This progress review emphasizes the wide range of crosslinking methods available to create various types of hydrogel based on HA. The flexibility of HA to undergo chemical modifications of several different functional groups allows crosslinking of HA to form hydrogels. HA-based hydrogels have been developed to improve properties, such as mechanical properties, biodegradation, antimicrobial activity, and cell biocompatibility, for their use in certain applications, including cell scaffolds, conjugation of bioactive molecules for controlled release, or wound healing. It is likely that the further research will see an expansion in the development of new materials, particularly hydrogels with unique and promising properties for tissue engineering applications.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Nano-material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (2009-0082580), and The Technology Innovation Program (development of disposable diapers based on biomass-oriented biodegradable super absorbent polymers) funded by the Ministry of Trade, Industry, & Energy (MI, Korea; 10050526).
References
- 1. Wang H, Heilshorn SC. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv Mater 2015; 27(25): 3717–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 2011; 32(34): 8771–8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer 2008; 49(8): 1993–2007. [Google Scholar]
- 4. Sivashanmugam A, Arun Kumar R, Vishnu Priya M, et al. An overview of injectable polymeric hydrogels for tissue engineering. Eur Polym J 2015; 72: 543–565. [Google Scholar]
- 5. Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 2011; 23(12): H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering—a review. Carbohydr Polym 2013; 92(2): 1262–1279. [DOI] [PubMed] [Google Scholar]
- 7. Xu X, Jha AK, Harrington DA, et al. Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter 2012; 8(12): 3280–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X, He J, Wang Y, et al. Hyaluronic acid-based scaffold for central neural tissue engineering. Interface Focus 2012; 2(3): 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schanté CE, Zuber G, Herlin C, et al. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr Polym 2011; 85(3): 469–489. [Google Scholar]
- 10. Hemshekhar M, Thushara RM, Chandranayaka S, et al. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int J Biol Macromol 2016; 86: 917–928. [DOI] [PubMed] [Google Scholar]
- 11. Prestwich GD, Marecak DM, Marecek JF, et al. Controlled chemical modification of hyaluronic acid: synthesis, applications, and biodegradation of hydrazide derivatives. J Control Release 1998; 53(1–3): 93–103. [DOI] [PubMed] [Google Scholar]
- 12. Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte Differentiation. PLoS ONE 2011; 6(10): e26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luo Y, Kirker KR, Prestwich GD. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release 2000; 69(1): 169–184. [DOI] [PubMed] [Google Scholar]
- 14. Borzacchiello A, Russo L, Malle BM, et al. Hyaluronic acid based hydrogels for regenerative medicine applications. BioMed Res Int 2015; 2015: 871218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mero A, Campisi M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers 2014; 6(2): 346–369. [Google Scholar]
- 16. Chun C, Lee DY, Kim J-T, et al. Effect of molecular weight of hyaluronic acid (HA) on viscoelasticity and particle texturing feel of HA dermal biphasic fillers. Biomater Res 2016; 20(1): 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Controlled Release 2011; 155: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev 2011; 17: 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seidlits SK, Khaing ZZ, Petersen RR, et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010; 31: 3930–3940. [DOI] [PubMed] [Google Scholar]
- 20. Sakurai K, Ueno Y, Okuyama T. Crosslinked hyaluronic acid and its use. Google Patents, 1986; EP 0161887. A3, Japan.
- 21. Walimbe T, Panitch A, Sivasankar PM. A review of hyaluronic acid and hyaluronic acid-based hydrogels for vocal fold tissue engineering. J Voice 31: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reddy N, Reddy R, Jiang Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol 33(6): 362–369. [DOI] [PubMed] [Google Scholar]
- 23. Stawikowski M, Fields GB. Introduction to peptide synthesis. In: Coligan JE, Dunn BM, Speicher DW, et al. (eds) Current protocols in protein science. Hoboken, NJ: John Wiley & Sons, 2002; 18.1.1–18.1.9. [Google Scholar]
- 24. Benoiton NL, Begley TP. Peptide synthesis: coupling methods. In: Wiley encyclopedia of chemical biology. Hoboken, NJ: John Wiley & Sons, 2007; Tadhg. P. Begley : 3204. [Google Scholar]
- 25. Wu D-Q, Wu J, Chu C-C. A novel family of biodegradable hybrid hydrogels from arginine-based poly(ester amide) and hyaluronic acid precursors. Soft Matter 2013; 9(15): 3965–3975. [Google Scholar]
- 26. Chen Y-H, Li J, Hao Y-B, et al. Preparation and characterization of composite hydrogels based on crosslinked hyaluronic acid and sodium alginate. J Appl Polym Sci 2015; 132(19): 41898. [Google Scholar]
- 27. Gherezghiher TB, Ming X, Villalta P, et al. 1,2,3,4-Diepoxybutane-induced DNA-protein cross-linking in human fibrosarcoma (HT1080) cells. J Proteome Res 2013; 12(5): 2151–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kenne L, Gohil S, Nilsson EM, et al. Modification and cross-linking parameters in hyaluronic acid hydrogels—definitions and analytical methods. Carbohydr Polym 2013; 91(1): 410–418. [DOI] [PubMed] [Google Scholar]
- 29. Yang B, Guo X, Zang H, et al. Determination of modification degree in BDDE-modified hyaluronic acid hydrogel by SEC/MS. Carbohydr Polym 2015; 131: 233–239. [DOI] [PubMed] [Google Scholar]
- 30. Shimojo AAM, Pires AMB, Lichy R, et al. The performance of crosslinking with divinyl sulfone as controlled by the interplay between the chemical modification and conformation of hyaluronic acid. J Brazil Chem Soc 2015; 26: 506–512. [Google Scholar]
- 31. Ibrahim S, Kang QK, Ramamurthi A. The impact of hyaluronic acid oligomer content on physical, mechanical, and biologic properties of divinyl sulfone-crosslinked hyaluronic acid hydrogels. J Biomed Mater Res A 2010; 94(2): 355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collins MN, Birkinshaw C. Physical properties of crosslinked hyaluronic acid hydrogels. J Mater Sci Mater Med 2008; 19(11): 3335–3343. [DOI] [PubMed] [Google Scholar]
- 33. Serban MA, Yang G, Prestwich GD, et al. Characterization and chondroprotective properties of a hyaluronan thioethyl ether derivative. Biomaterials 2008; 29(10): 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calles JA, Ressia JA, Llabot JM, et al. Hyaluronan–itaconic acid–glutaraldehyde films for biomedical applications: preliminary studies. Sci Pharm 2016; 84(1): 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirk JF, Ritter G, Finger I, et al. Mechanical and biocompatible characterization of a cross-linked collagen-hyaluronic acid wound dressing. Biomatter 2013; 3(4): e25633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eenschooten C, Guillaumie F, Kontogeorgis GM, et al. Preparation and structural characterisation of novel and versatile amphiphilic octenyl succinic anhydride–modified hyaluronic acid derivatives. Carbohydr Polym 2010; 79(3): 597–605. [Google Scholar]
- 37. Pravata L, Braud C, Boustta M, et al. New amphiphilic lactic acid oligomer−hyaluronan conjugates: synthesis and physicochemical characterization. Biomacromolecules 2008; 9(1): 340–348. [DOI] [PubMed] [Google Scholar]
- 38. Tous E, Ifkovits JL, Koomalsingh KJ, et al. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction induced ventricular remodeling. Biomacromolecules 2011; 12(11): 4127–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Su W-Y, Chen Y-C, Lin F-H. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater 2010; 6(8): 3044–3055. [DOI] [PubMed] [Google Scholar]
- 40. Park H, Choi B, Hu J, et al. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater 2013; 9(1): 4779–4786. [DOI] [PubMed] [Google Scholar]
- 41. Zhang W, Mu H, Dong D, et al. Alteration in immune responses toward N-deacetylation of hyaluronic acid. Glycobiology 2014; 24(12): 1334–1342. [DOI] [PubMed] [Google Scholar]
- 42. Matanović MR, Kristl J, Grabnar PA. Thermoresponsive polymers: insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int J Pharm 2014; 472(1–2): 262–275. [DOI] [PubMed] [Google Scholar]
- 43. Kim MR, Park TG. Temperature-responsive and degradable hyaluronic acid/pluronic composite hydrogels for controlled release of human growth hormone. J Control Release 2002; 80(1): 69–77. [DOI] [PubMed] [Google Scholar]
- 44. Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 2006; 27(11): 2370–2379. [DOI] [PubMed] [Google Scholar]
- 45. Fang J-Y, Chen J-P, Leu Y-L, et al. Temperature-sensitive hydrogels composed of chitosan and hyaluronic acid as injectable carriers for drug delivery. Eur J Pharm Biopharm 2008; 68(3): 626–636. [DOI] [PubMed] [Google Scholar]
- 46. Ha DI, Lee SB, Chong MS, et al. Preparation of thermo-responsive and injectable hydrogels based on hyaluronic acid and poly(N-isopropylacrylamide) and their drug release behaviors. Macromol Res 2006; 14(1): 87–93. [Google Scholar]
- 47. Lin C-Y, Peng H-H, Chen M-H, et al. In situ forming hydrogel composed of hyaluronate and polygalacturonic acid for prevention of peridural fibrosis. J Mater Sci Mater Med 2015; 26(4): 168. [DOI] [PubMed] [Google Scholar]
- 48. Wu X, He C, Wu Y, et al. Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials 2016; 75: 148–162. [DOI] [PubMed] [Google Scholar]
- 49. Chu FL, Yaylayan VA. Post-Schiff base chemistry of the Maillard reaction. Ann N Y Acad Sci 2008; 1126(1): 30–37. [DOI] [PubMed] [Google Scholar]
- 50. Cordes EH, Jencks WP. On the mechanism of Schiff base formation and hydrolysis. J Am Chem Soc 1962; 84(5): 832–837. [Google Scholar]
- 51. Adam FA, El-Haty MT, Ibrahem AA. The mechanism of Schiff base formation of some arylidenes-p-aminosalicylic acid. J Chin Chem Soc 1984; 31(4): 345–349. [Google Scholar]
- 52. Gupta B, Tummalapalli M, Deopura BL, et al. Preparation and characterization of in-situ crosslinked pectin–gelatin hydrogels. Carbohydr Polym 2014; 106: 312–318. [DOI] [PubMed] [Google Scholar]
- 53. Balakrishnan B, Joshi N, Jayakrishnan A, et al. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater 2014; 10(8): 3650–3663. [DOI] [PubMed] [Google Scholar]
- 54. Wei Z, Zhao J, Chen YM, et al. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci Rep 2016; 6: 37841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shi J, Guobao W, Chen H, et al. Schiff based injectable hydrogel for in situ pH-triggered delivery of doxorubicin for breast tumor treatment. Polym Chem 2014; 5(21): 6180–6189. [Google Scholar]
- 56. Li Y, Liu C, Tan Y, et al. In situ hydrogel constructed by starch-based nanoparticles via a Schiff base reaction. Carbohydr Polym 2014; 110: 87–94. [DOI] [PubMed] [Google Scholar]
- 57. Tan H, Li H, Rubin JP, et al. Controlled gelation and degradation rates of injectable hyaluronic acid-based hydrogels through a double crosslinking strategy. J Tissue Eng Regen Med 2011; 5(10): 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martínez-Sanz E, Ossipov DA, Hilborn J, et al. Bone reservoir: injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J Control Release 2011; 152(2): 232–240. [DOI] [PubMed] [Google Scholar]
- 59. Tan H, Chu CR, Payne KA, et al. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009; 30(13): 2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tan H, Rubin JP, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for adipose tissue regeneration. Organogenesis 2010; 6(3): 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun J, Xiao C, Tan H, et al. Covalently crosslinked hyaluronic acid-chitosan hydrogel containing dexamethasone as an injectable scaffold for soft tissue engineering. J Appl Polym Sci 2013; 129(2): 682–688. [Google Scholar]
- 62. Kim DY, Park H, Kim SW, et al. Injectable hydrogels prepared from partially oxidized hyaluronate and glycol chitosan for chondrocyte encapsulation. Carbohydr Polym 2017; 157: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 63. Chen Y-C, Su W-Y, Yang S-H, et al. In situ forming hydrogels composed of oxidized high molecular weight hyaluronic acid and gelatin for nucleus pulposus regeneration. Acta Biomater 2013; 9(2): 5181–5193. [DOI] [PubMed] [Google Scholar]
- 64. Billman JH, Diesing AC. Reduction of Schiff bases with sodium borohydride. J Org Chem 1957; 22(9): 1068–1070. [Google Scholar]
- 65. Singhal R, Gupta K. A review: tailor-made hydrogel structures (classifications and synthesis parameters). Polym-Plast Technol 2016; 55(1): 54–70. [Google Scholar]
- 66. Rayati S, Bohloulbandi E, Zakavi S. Sodium borohydride reduction of aldehydes catalyzed by an oxovanadium(IV) Schiff base complex encapsulated in the nanocavity of zeolite-Y. Inorg Chem Commun 2015; 54: 38–40. [Google Scholar]
- 67. Nair DP, Podgórski M, Chatani S, et al. The Thiol-Michael addition click reaction: a powerful and widely used tool in materials chemistry. Chem Mater 2014; 26(1): 724–744. [Google Scholar]
- 68. Hoffmann C, Stuparu MC, Daugaard A, et al. Aza-Michael addition reaction: post-polymerization modification and preparation of PEI/PEG-based polyester hydrogels from enzymatically synthesized reactive polymers. J Polym Sci A1 2015; 53(6): 745–749. [Google Scholar]
- 69. Fu Y, Xu K, Zheng X, et al. 3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels. Biomaterials 2012; 33(1): 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu ZQ, Wei Z, Zhu XL, et al. Dextran-based hydrogel formed by thiol-Michael addition reaction for 3D cell encapsulation. Colloid Surface B 2015; 128: 140–148. [DOI] [PubMed] [Google Scholar]
- 71. Zarembinski TI, Doty NJ, Erickson IE, et al. Thiolated hyaluronan-based hydrogels crosslinked using oxidized glutathione: an injectable matrix designed for ophthalmic applications. Acta Biomater 2014; 10(1): 94–103. [DOI] [PubMed] [Google Scholar]
- 72. Bian S, He M, Sui J, et al. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloid Surface B 2016; 140: 392–402. [DOI] [PubMed] [Google Scholar]
- 73. Li F, Ba Q, Niu S, et al. In-situ forming biodegradable glycol chitosan-based hydrogels: synthesis, characterization, and chondrocyte culture. Mater Sci Eng C 2012; 32(7): 2017–2025. [DOI] [PubMed] [Google Scholar]
- 74. Yu Y, Chau Y. One-step “click” method for generating vinyl sulfone groups on hydroxyl-containing water-soluble polymers. Biomacromolecules 2012; 13(3): 937–942. [DOI] [PubMed] [Google Scholar]
- 75. Dubbini A, Censi R, Butini ME, et al. Injectable hyaluronic acid/PEG-p(HPMAm-lac)-based hydrogels dually cross-linked by thermal gelling and Michael addition. Eur Polym J 2015; 72: 423–437. [Google Scholar]
- 76. Crescenzi V, Cornelio L, Di Meo C, et al. Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules 2007; 8(6): 1844–1850. [DOI] [PubMed] [Google Scholar]
- 77. Hu X, Li D, Zhou F, et al. Biological hydrogel synthesized from hyaluronic acid, gelatin and chondroitin sulfate by click chemistry. Acta Biomater 2011; 7(4): 1618–1626. [DOI] [PubMed] [Google Scholar]
- 78. Yu F, Cao X, Li Y, et al. An injectable hyaluronic acid/PEG hydrogel for cartilage tissue engineering formed by integrating enzymatic crosslinking and Diels-Alder “click chemistry.” Polym Chem 2014; 5(3): 1082–1090. [Google Scholar]
- 79. Nimmo CM, Owen SC, Shoichet MS. Diels−Alder click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules 2011; 12(3): 824–830. [DOI] [PubMed] [Google Scholar]
- 80. Fan L, Ki Hyun B, Motoichi K. Injectable hydrogel systems crosslinked by horseradish peroxidase. Biomed Mater 2016; 11(1): 014101. [DOI] [PubMed] [Google Scholar]
- 81. Ganesh N, Hanna C, Nair SV, et al. Enzymatically cross-linked alginic–hyaluronic acid composite hydrogels as cell delivery vehicles. Int J Biol Macromol 2013; 55: 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Raia NR, Partlow BP, McGill M, et al. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017; 131: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tan H, Ramirez CM, Miljkovic N, et al. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 2009; 30(36): 6844–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin Y-K, Chen K-H, Kuan C-Y. The synthesis and characterization of a thermally responsive hyaluronic acid/Pluronic copolymer and an evaluation of its potential as an artificial vitreous substitute. J Bioact Compat Pol 2013; 28(4): 355–367. [Google Scholar]
- 85. Lee Y, Chung HJ, Yeo S, et al. Thermo-sensitive, injectable, and tissue adhesive sol-gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter 2010; 6(5): 977–983. [Google Scholar]
- 86. Chen Y-Y, Wu H-C, Sun J-S, et al. Injectable and thermoresponsive self-assembled nanocomposite hydrogel for long-term anticancer drug delivery. Langmuir 2013; 29(11): 3721–3729. [DOI] [PubMed] [Google Scholar]
- 87. Mayol L, De Stefano D, De Falco F, et al. Effect of hyaluronic acid on the thermogelation and biocompatibility of its blends with methyl cellulose. Carbohydr Polym 2014; 112: 480–485. [DOI] [PubMed] [Google Scholar]
- 88. Fernandes Stefanello T, Szarpak-Jankowska A, Appaix F, et al. Thermoresponsive hyaluronic acid nanogels as hydrophobic drug carrier to macrophages. Acta Biomater 2014; 10(11): 4750–4758. [DOI] [PubMed] [Google Scholar]
- 89. Vorvolakos K, Isayeva IS, Luu H-MD, et al. Ionically cross-linked hyaluronic acid: wetting, lubrication, and viscoelasticity of a modified adhesion barrier gel. Med Devices 2011; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang M-D, Zhai P, Schreyer DJ, et al. Novel crosslinked alginate/hyaluronic acid hydrogels for nerve tissue engineering. Front Mater Sci 2013; 7(3): 269–284. [Google Scholar]
- 91. Nakagawa Y, Nakasako S, Ohta S, et al. A biocompatible calcium salt of hyaluronic acid grafted with polyacrylic acid. Carbohydr Polym 2015; 117: 43–53. [DOI] [PubMed] [Google Scholar]
- 92. Park H-J, Jin Y, Shin J, et al. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules 2016; 17(6): 1939–1948. [DOI] [PubMed] [Google Scholar]
- 93. Fenn SL, Oldinski RA. Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair. J Biomed Mater Res B 2016; 104(6): 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang L, Li K, Xiao W, et al. Preparation of collagen–chondroitin sulfate–hyaluronic acid hybrid hydrogel scaffolds and cell compatibility in vitro. Carbohydr Polym 2011; 84(1): 118–125. [Google Scholar]
- 95. Son Y-J, Yoon I-S, Sung J-H, et al. Porous hyaluronic acid/sodium alginate composite scaffolds for human adipose-derived stem cells delivery. Int J Biol Macromol 2013; 61: 175–181. [DOI] [PubMed] [Google Scholar]
- 96. Snyder TN, Madhavan K, Intrator M, et al. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng 2014; 8(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ilgin P, Avci G, Silan C, et al. Colloidal drug carries from (sub)micron hyaluronic acid hydrogel particles with tunable properties for biomedical applications. Carbohydr Polym 2010; 82(3): 997–1003. [Google Scholar]
- 98. Zhang Y, Sun Y, Yang X, et al. Injectable in situ forming hybrid iron oxide-hyaluronic acid hydrogel for magnetic resonance imaging and drug delivery. Macromol Biosci 2014; 14(9): 1249–1259. [DOI] [PubMed] [Google Scholar]
- 99. Ekici S, Ilgin P, Butun S, et al. Hyaluronic acid hydrogel particles with tunable charges as potential drug delivery devices. Carbohydr Polym 2011; 84(4): 1306–1313. [Google Scholar]
- 100. Nakai T, Hirakura T, Sakurai Y, et al. Injectable hydrogel for sustained protein release by salt-induced association of hyaluronic acid nanogel. Macromol Biosci 2012; 12(4): 475–483. [DOI] [PubMed] [Google Scholar]
- 101. Almeida PV, Shahbazi M-A, Mäkilä E, et al. Amine-modified hyaluronic acid-functionalized porous silicon nanoparticles for targeting breast cancer tumors. Nanoscale 2014; 6(17): 10377–10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang L-M, Wu C-X, Huang J-Y, et al. Synthesis and characterization of a degradable composite agarose/HA hydrogel. Carbohydr Polym 2012; 88(4): 1445–1452. [Google Scholar]
- 103. Fahmy A, Kamoun EA, El-Eisawy R, et al. Poly(vinyl alcohol)-hyaluronic acid membranes for wound dressing applications: synthesis and in vitro bio-evaluations. J Brazil Chem Soc 2015; 26: 1466–1474. [Google Scholar]
- 104. Hoang Phuc D, Thi Hiep N, Ngoc Phuc Chau D, et al. Fabrication of hyaluronan-poly(vinylphosphonic acid)-chitosan hydrogel for wound healing application. Int J Polym Sci 2016; 2016: 6723716. [Google Scholar]