Abstract
Enzyme prodrug therapy (EPT) enables localized conversion of inert prodrugs to active drugs by enzymes. Performance of EPT necessitates that the enzyme remains active throughout the time frame of the envisioned therapeutic application. β-glucuronidase is an enzyme with historically validated performance in EPT, however it retains its activity in biomaterials for an insufficiently long period of time, typically not exceeding 7 d. Herein, the encapsulation of β-glucuronidase in liposomal subcompartments within poly(vinyl alcohol) electrospun fibers is reported, leading to the assembly of biocatalytically active materials with activity of the enzyme sustained over at least seven weeks. It is further shown that liposomes provide the highly beneficial stabilization of the enzyme when incubated in cell culture media. The assembled biocatalytic materials successfully produce antiproliferative drugs (SN-38) using externally administered prodrugs (SN-38-glucuronide) and effectively suppress cell proliferation, with envisioned utility in the design of cardiovascular grafts.
Keywords: liposomes, polymer fibers, electrospinning, enzyme prodrug therapy, β-glucuronidase
Implantable biomaterials designed for controlled drug release have entered the mainstream of medicinal and pharmaceutical sciences. Indeed, delivery of hormonal contraceptives can be sustained for years using implantable matrices comprised of organic biodegradable polymers.[1,2] Hydrogel biomaterials with embedded growth factors that aid in wound healing are highly warranted in the treatment of diabetic ulcers.[3,4] Specific delivery of anti-proliferative drugs from cardiovascular stents can prevent vascular smooth muscle cell proliferation and reduce the incidence of thrombosis and restenosis.[5–7] These designs are highly successful in their own right but are not without limitations. Specifically, using implantable biomaterials, it is still challenging to control drug dosing after implantation, to stop drug elution on demand, or to control the release of multiple drugs from the same implant. These opportunities are highly warranted in drug delivery, and designs of biomaterials enabling such drug dosing would open up broader opportunities in biomedicine.
Recently, we introduced an approach to accomplish advanced drug delivery design through engineering biomaterials equipped with the tools of enzyme prodrug therapy (EPT) to enable localized conversion of inert inactive prodrugs by enzymes.[8–11] Key to success of EPT is localization of the enzyme at the nominated target, which can be accomplished using antibody-enzyme conjugates (antibody-directed enzyme prodrug therapy, ADEPT).[12] Alternatively, enzymes can be expressed by cells upon their transfection, specifically using viral vectors. This mode of EPT is often termed gene-directed enzyme prodrug therapy (GDEPT) or “suicide gene the rapy”.[13] The cells are transfected to produce enzymes that are un-natural to the mammalian cells, such as herpes simplex virus kinase. This kinase is particularly active in phosphorylating acyclovir, ganciclovir and their close analogues leading to pronounced toxicity of the otherwise benign nucleosides to the cells expressing these genes. An elegant strategy to suicide gene therapy is being developed by Kasahara et al., whereby viral vectors are not only used to transfect the cells but also to achieve viral proliferation and infection of neighbor cells – leading to enhanced distribution of the viral vector, enhanced expression of the transgene, and thereby increased therapeutic benefits.[14]
In our hands, substrate mediated enzyme prodrug therapy (SMEPT) proved to be highly enabling in the design of functional biomaterials. Specifically, we designed hydrogel biomaterials that contained the tools of biocatalysis, that is the β-glucuronidase enzyme (β-glu).[8,9,11,15] The same enzyme was capable of converting multiple prodrugs, as long as each of these was based on the same protecting trigger, glucuronic acid. Taking advantage of this, we achieved conversion of two or more fluorogenic (imaging) molecules and drugs. The same biomaterial was capable of generating two or more drugs – simply instructed by administration of the proper prodrug. When administered in sequence, biomaterials successfully produced anti-inflammatory drugs followed by cytotoxic drugs at the nominated time points – with relevance to the treatment of atherosclerosis.[9] When prodrugs were administered simultaneously, biomaterials were equipped with EPT mediated combination therapy – a highly prized therapeutic opportunity for treatments of cancer and infectious diseases.[9] We have subsequently adapted SMEPT for applications within the multilayered polyelectrolyte surface coatings.[16] Assembly of these thin films is solution-based and can therefore be performed on biomaterials with no restriction on the surface geometry, topography or materials porosity, such as wire meshes used in the production of balloons for cardiovascular angioplasty. Equipped with the tools of EPT, the multilayered coatings successfully mediated localized production of therapeutic drugs in static[16] and under flow[17] conditions. Most importantly, the therapeutic effect (cell killing) was the highest on the biocatalytic coatings and was significantly lower “downstream” on coatings with no immobilized enzyme, i.e. cell killing through the bystander effect. Tools of EPT were also engineered into implantable biomaterials by Wang[18] and Yang,[19] specifically towards cardiovascular applications. Enzymes were immobilized on the surface of vascular stents, and local release of nitric oxide (NO), a key signaling molecule in the cardiovascular physiology, was achieved upon administration of NO prodrugs. Taken together, the prior reports presented above illustrate that tools of EPT engineered into implantable biomaterials significantly enable implants in terms of their functionality, specifically with regards to the broad opportunities associated with the localized synthesis of therapeutic agents for delivery to the cells and tissues in the immediate vicinity.
Biomaterials based on poly(vinyl alcohol) (PVA) have a long and successful history of biomedical applications. PVA hydrogels are biocompatible and are marketed as post-operative tissue sealants[20] and as drug eluting beads for hepatic embolization.[21] They can be formed through chemical or physical stabilization (crosslinking).[22–24] In the latter case, hydrogelation is non-damaging and accommodates incorporation of proteins and live cells into the biomaterial. In our previous work, we observed that β-glu, being a relatively large sized protein, can be incorporated into PVA hydrogels solely through size exclusion effects and does not necessitate chemical immobilization within the hydrogel,[8,9,15] as is the case for a number of other enzymes incorporated into PVA matrices, e.g. for biomass conversion.[25] However, we also observed that β-glu retained its activity for an insufficiently long period of time, typically not exceeding 7 days. In this work, we address significant limitations of SMEPT that were not considered in prior works but are critical to the performance of this platform in biomedical applications. Specifically, performance of EPT necessitates that the enzyme remains active throughout the time frame of the envisioned therapeutic application. For cardiovascular stenting, re-endothelialization typically takes at least 6 weeks[26] – providing an indication of the desired duration of activity of the enzyme for SMEPT, and therefore leaving significant room for improvement in terms of achieving extended lifetime of the enzyme within the biomaterials.
We hypothesize that liposomes, which are vesicular structures formed by the self-assembly of amphiphilic lipids, can provide the sought-after stabilization of the enzyme during manufacturing, storage, and active use of the biomaterial in EPT. Motivated by this, in this work, we use electrospinning to engineer biomaterials based on PVA,[27,28] which can be conveniently performed from aqueous polymer solutions, that is, conditions which are non-denaturing to the enzymatic cargo and facilitate the incorporation of liposomes. PVA fibers have been used as wound dressings[29,30] and, in tubular form, as cardiovascular grafts.[31,32] For the first time, we achieve immobilization of β-glu within the spun fibers through encapsulation in liposomal subcompartments, and illustrate that this strategy provides the highly sought-after stabilization of the protein against proteolytic degradation and loss of activity. In particular, we: i) verify the integrity of the liposomes in electrospun fibers; ii) demonstrate biocatalytic activity of β-glu that is loaded into the liposomes; iii) investigate the long-term stability and functional activity of the enzyme; and iv) demonstrate the local synthesis of anti-proliferative drugs and their therapeutic effects on an in vitro model system. Here, we show that the assembled biomaterials performed well as biocatalytic matrices and mediated the synthesis of anti-proliferative drugs – as warranted in the design of cardiovascular grafts and stents.
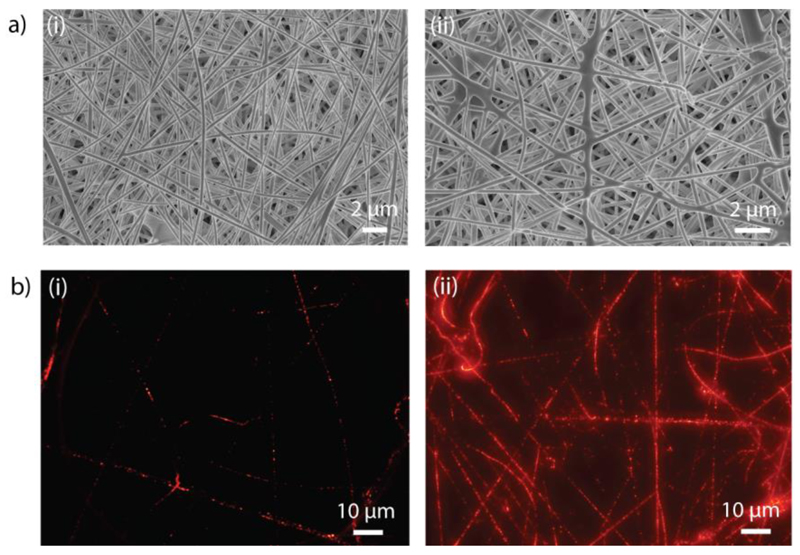
β-glu (0.1 mg mL-1) was incorporated into liposomes composed of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) (DMPC:DPPC = 2:3). Encapsulated enzyme was then mixed into a PVA solution (12 wt% in water), which was processed into non-woven fibers by electrospinning (Scheme 1). We stabilized our liposome-containing fibers using a kosmotropic electrolyte, sodium sulfate (Na2SO4),[15,23,33] and their morphology before and after stabilization was assessed using scanning electron microscopy (SEM). Figure 1a shows morphology of a non-woven mesh as is typical for electrospun fibers and reveals that incorporation of liposomes into the feed polymer solution has minimal detrimental effects on the suitability of PVA solution for electrospinning. Fiber diameter and porosity were characterized by ImageJ analysis and determined to be 307 ± 71 nm and 77 ± 5%, and 350 ± 74 nm and 77 ± 4%, for fibers before and after stabilization, respectively (Table 1).
Scheme 1.
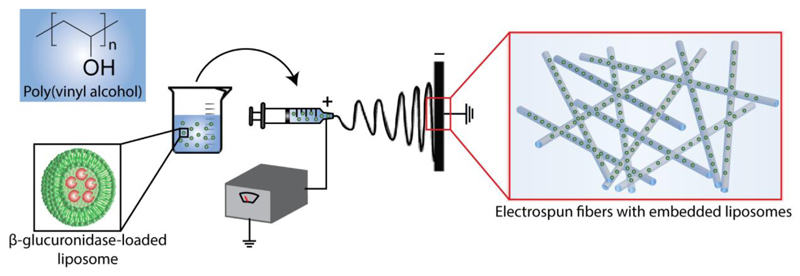
Schematic illustration of assembly of electrospun PVA fibers with embedded liposomes. Liposomes containing enzymes (β-glucuronidase) are mixed with PVA solution and loaded into a syringe equipped with a stainless steel needle. The syringe needle is connected to a high-voltage power supply. The positive voltage is applied to the needle, whereas negative voltage is supplied to the collector. Electrospun fibers with embedded β-glucuronidase-loaded liposomes are deposited on a collector.
Figure 1.
a) Scanning electron microscopy (SEM) images of PVA fibers with embedded liposomes before (i) and after (ii) stabilization through a sodium sulfate (Na2SO4) treatment. Incorporation of liposomes into polymer solution has minimal detrimental effects on the suitability of PVA solution for electrospinning. b) Fluorescence microscopy images of PVA fibers containing 1% v/v (i) or 5% v/v (ii) rhodamine-labeled liposomes. Punctuated fluorescent signals suggest the existence of individual liposomes within the fibers.
Table 1.
Diameter and porosity of PVA fibers with embedded liposomes before and after stabilization through a sodium sulfate (Na2SO4) treatment.
| Diameter (nm) | Porosity (%) | |
|---|---|---|
| Before stabilization | 307.7 ± 71.1 | 77.3 ± 4.6 |
| After stabilization | 350.2 ± 74.1 | 77.0 ± 4.0 |
To confirm the incorporation of liposomes, we visualized the fibers using fluorescence microscopy. For this purpose, liposomes composed of DMPC and DPPC were prepared to contain 1.5 mol% of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rhod-PE), and fibers were produced using PVA solutions with two different concentrations of fluorescently labeled liposomes: 1% v/v (Figure 1bi and Figure S1) and 5% v/v (Figure 1bii). Images show punctuated fluorescent signals (best viewed at low content of liposomes in the fibers, Figure 1bi and Figure S1) as would be expected from labeled liposomes. Increasing the content of liposomes in the polymer solution afforded an expected increase in the abundance of fluorescent subcompartments. Even at the higher concentration of liposomes (5% v/v), fibers reveal non-merged fluorescent signals, suggesting the existence of individual liposomes within the fibers (Figure 1bii).
To verify the integrity of liposomes in the electrospun fibers, we encapsulated a fluorescent probe, carboxyfluorescein (CF, 50 mM), in liposomes, and produced PVA fibers containing CF-loaded liposomes. We incubated stabilized electrospun fibers in phosphate buffered saline (PBS, pH 7.4) for 1 hour at 24 °C or 37 °C and analyzed the fluorescence intensity of supernatants. Liposomes composed of a DMPC:DPPC (2:3) mixture have a phase transition temperature (Tm) of 34 °C, above which the lipids are in a disordered liquid-like phase and the liposomes are expected to have enhanced permeability to solutes. In full accord with this, we detected a 2-fold higher CF released in supernatants at 37 °C as compared to the 24 °C incubation (Figure S2). On the other hand, when CF was not encapsulated in liposomes and was instead directly mixed with PVA for the electrospinning process, the fluorescent molecules were readily detected in the supernatant and the behavior was not dependent on temperature (within the range from 24° C to 37 °C). This provides evidence that the liposomes remained intact within the structure of the electrospun fibers and that cargo release can be mediated via phase transition temperature of the liposomes.
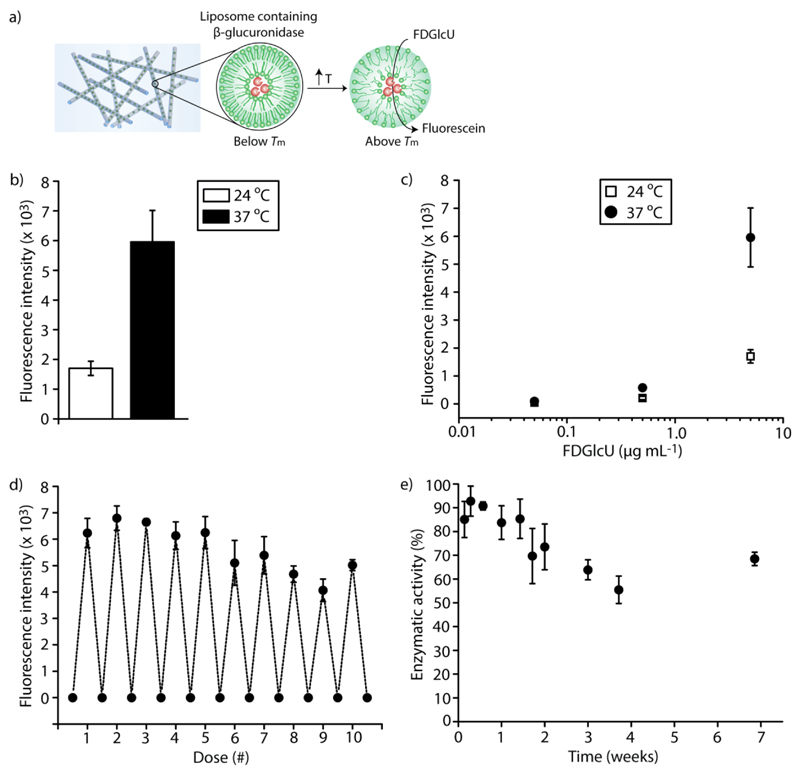
To further validate the integrity of liposomes and to illustrate the functional activity of β-glu within liposomes, we investigated enzymatic conversion of a model fluorogenic substrate, fluorescein di-β-D-glucuronide (FDGlcU), to fluorescein (Figure 2a). This probe is virtually non-fluorescent and exhibits a drastic increase in the quantum yield of fluorescence upon cleavage of the glucuronide bonds by β-glu. PVA fibers containing β-glu-loaded liposomes were incubated with FDGlcU (5 μg mL-1) in PBS for 30 min at 24 °C or 37 °C, and the fluorescence intensity of supernatants was analyzed. Incubation of the fibers with FDGlcU at 24 °C afforded a minor increase in fluorescence, likely due to a restricted access of the substrate into the liposomes, and indicated activity of the enzyme in the fibers (Figure 2b). Increasing the temperature to 37 °C resulted in a 4-fold higher fluorescence signal, indicating significantly increased conversion of the fluorogenic substrate into its product by β-glu due to the enhanced permeability of FDGlcU through the lipid bilayer above the phase transition temperature of the lipids. The presented results further confirmed that the liposomes remained intact within the fibers. By varying the concentration of the administered substrate (0.05, 0.5, or 5 μg mL-1 of FDGlcU), the amount of produced fluorescein can be tuned, providing a convenient tool to externally program the catalytic output of the enzyme containing fibers (Figure 2c).
Figure 2.
Biocatalytic activity of electrospun PVA fibers containing β-glucuronidase-loaded liposomes. a) Enzymatic activity of β-glucuronidase results in the release of fluorescein (products) from fluorescein di-β-D-glucuronide (FDGlcU, substrates). Liposomes have enhanced permeability to FDGlcU above the phase transition temperature (Tm) of the lipids.
b) Enzymatic conversion of FDGlcU (5 μg mL-1) to fluorescein by PVA fibers containing β-glucuronidase-loaded liposomes at 24 °C or 37 °C (below or above the Tm of the lipids, respectively). c) By varying the concentration of the administered substrate (0.05, 0.5, or 5 μg mL-1 of FDGlcU), the amount of fluorescent product produced by PVA fibers containing β-glucuronidase-loaded liposomes can be controlled. d) The enzymatic catalysis of FDGlcU to fluorescein was repeated over 10 cycles by periodically removing hydrolyzed substrates and adding fresh FDGlcU (5 μg mL-1) to the same PVA fibers containing β-glucuronidase-loaded liposomes. Fluorescein was produced at a similar rate and the enzymatic activity was preserved at a nearly constant level over 7 weeks (e).
To evaluate the stability of the encapsulated enzyme within electrospun fibers and to illustrate the long-term performance of the designed biocatalytic materials, we repeated the enzymatic catalysis over several cycles by periodically removing fluorescent products (fluorescein) and adding fresh FDGlcU (5 μg mL-1) to the same PVA fibers containing β-glu-loaded liposomes. Altogether, 10 rounds of readout were made over a period of 7 weeks (Figure 2d). Fluorescence of the supernatant, as achieved through enzymatic conversion of FDGlcU by the encapsulated enzyme, was only marginally lower at the reading taken on week 7 as compared to the readings during the first days of incubation (Figure 2e), thus illustrating that the enzymatic activity was preserved at a nearly constant level. In other words, encapsulation of β-glu within liposomes as subcompartments within electrospun fibers affords a highly stable preparation of biocatalytically active biomaterials for SMEPT and other biomedical applications with activity of the enzyme sustained over at least 7 weeks. This time frame exceeds that which is quoted to be the typical time required for re-endothelialization of vascular grafts,[26] and the fibers were stable over 14 weeks at physiological conditions (Figure S3).
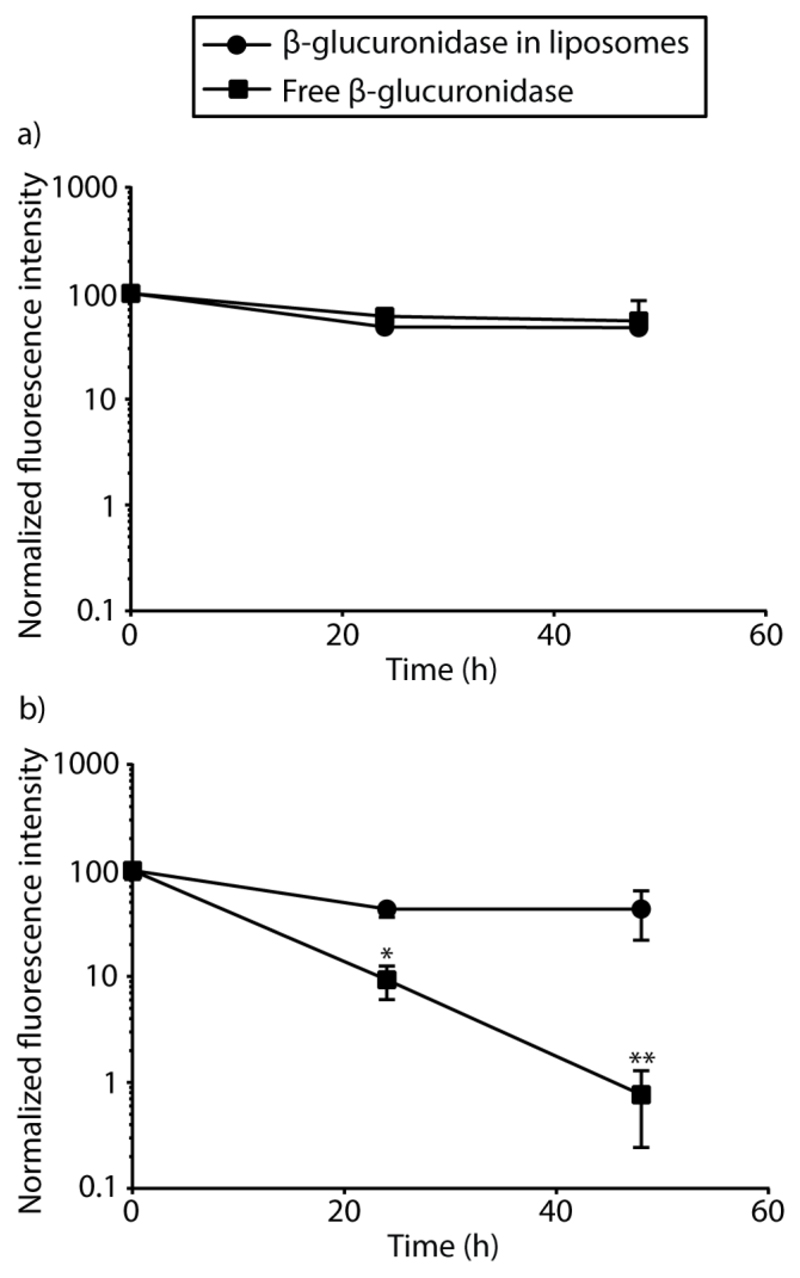
Further tests into stability of the enzyme within liposomal subcompartments in PBS were performed and compared to those in cell culture media. β-glu-loaded liposomes or non-encapsulated β-glu were incubated in PBS or in cell culture media, and the enzymatic activity was monitored over time through conversion of FDGlcU to fluorescein. In PBS, β-glu (encapsulated and non-encapsulated) exhibited only a minor loss of activity over 48 h of incubation (Figure 3a). In contrast, in cell culture media, this enzyme underwent drastic deactivation and over 48 h of incubation, catalytic output of the β-glu in absence of liposomes decreased by over 100-fold (Figure 3b). Liposomes provided stabilization of the enzyme, which exhibited only a minor degree of deactivation in cell culture media. These results further confirm the benefit and necessity of liposomal encapsulation in protecting enzymes and maintaining their functional activity. This result is highly encouraging and together with the data in Figure 2, presents a highly active, durable biomaterial with sustained enzymatic activity.
Figure 3.
Effect of liposome encapsulation on the stability of β-glucuronidase. Enzymatic activity was evaluated after incubation of β-glucuronidase-loaded liposomes or non-encapsulated β-glu in PBS (a) or cell culture media (b) for 0, 24, and 48 h through monitoring the conversion of fluorescein di-β-D-glucuronide (FDGlcU) to fluorescein. Liposomes provided stabilization of the enzyme with only a minor degree of deactivation when incubated in cell culture media. *p < 0.05, **p < 0.01.
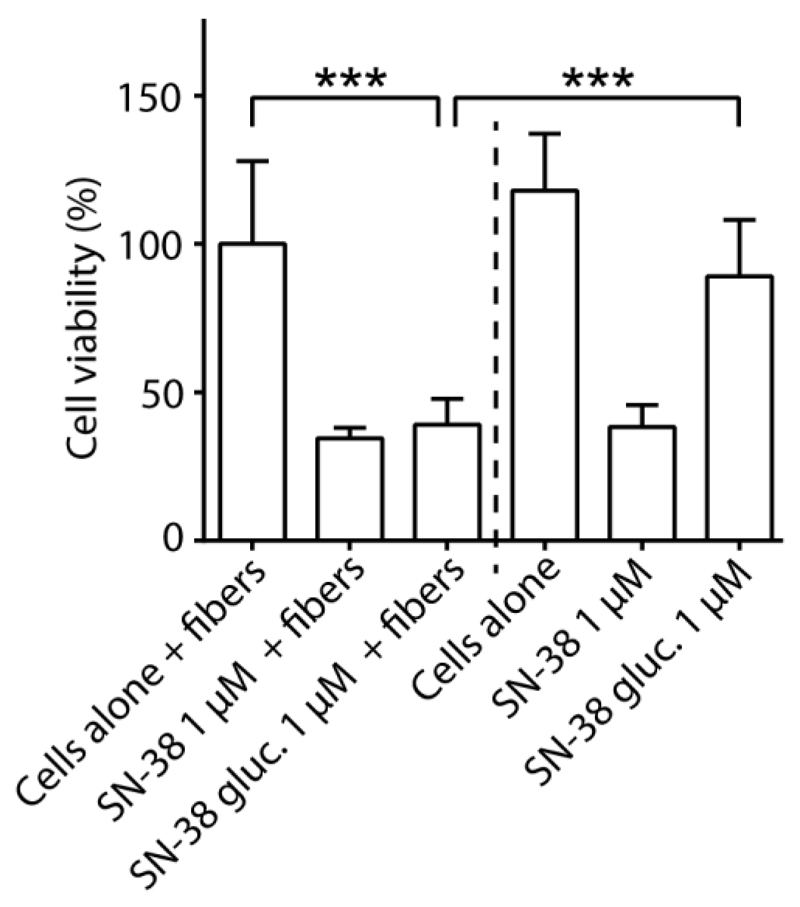
Finally, to illustrate the therapeutic utility of electrospun PVA fibers containing β-glu-loaded liposomes, we engineered these biocatalytic materials to produce an anti-proliferative drug, SN-38, using its benign, externally administered prodrug (SN-38-glucuronide). Human cancer-derived cell lines are fundamental models to test the therapeutic efficacy of anti-proliferative drugs, and in this study, we employed human cervical cancer cells (HeLa) as an in vitro model system. Stabilized PVA fibers containing β-glu-loaded liposomes were immersed and positioned immediately over HeLa cells cultured in 96-well plates – in a setting to investigate drug delivery to surrounding cells and tissues. SN-38-glucuronide (1 μM) was added into the cell culture media and cell metabolic activity was monitored after 48 h treatment. SN-38 (1 μM) was used as a positive control. The presence of spun fibers did not alter metabolic activity of the cells and had no significant effect on the anti-proliferative activity of SN-38 (Figure 4 and Figure S4). With or without the fibers, the presence of SN-38 led to a decrease of metabolic activity of the cells to ~40%. We found that IC50 for SN-38 corresponded to 47 nM (Figure S5). Addition of SN-38-glucuronide in the absence of fibers produced a minor change in the cell viability, most likely due to minor impurities of SN-38 in the sample of its glucuronide derivative. Addition of SN-38-glucuronide to the cell culture in the presence of biocatalytic electrospun fibers resulted in an anti-proliferative effect similar to that of the pristine drug, and metabolic activity of the cells decreased to ~40%.
Figure 4.
Delivery of anti-proliferative drugs (SN-38) via biocatalytic activity of electrospun PVA fibers containing β-glucuronidase-loaded liposomes on externally administered prodrugs (SN-38-glucuronide), and the corresponding viability of HeLa cells after 48 h treatment. Addition of SN-38-glucuronide to the cell culture in the presence of biocatalytic electrospun fibers resulted in an anti-proliferative effect similar to that of the pristine drug. ***p < 0.001. SN-38 gluc. = SN-38-glucuronide.
β-glucuronidase is an enzyme with historically validated performance in EPT.[8,9,15] In the current study, external administration of SN-38-glucuronide led to local synthesis of anti-proliferative drugs by the assembled biocatalytic biomaterials. With a supply of administered prodrugs, SN-38 can be delivered, and EPT enables the opportunities to control the drug dosage within the lifetime of the enzyme. Liposome properties can be tuned by varying lipid composition or by adding components to the lipid mixture during liposome preparation.[34,35] Electrospun fibers can be collected onto a rotating mandrel to assemble tubular conduits with utility as vascular grafts, and PVA is among the materials investigated towards these applications.[31,32,36] Commercially successful vascular stents on the market are engineered to continuously release anti-proliferative drugs (e.g. paclitaxel for Vascular Wrap™[6,37]) to minimize thrombosis and restenosis. This knowledge served as an inspiration to engineer PVA-based biomaterials to synthesize anti-proliferative drugs via EPT for vascular applications.
Taken together, our experiments present the design of electrospun fibers based on PVA with engineered tools of EPT with envisioned utility in the design of cardiovascular grafts and wound dressings. Incorporation of the enzyme into electrospun fibers within liposomal subcompartments afforded the highly beneficial stabilization of the protein against deactivation that was otherwise observed in cell culture media within 48 h. Thus assembled biocatalytic biomaterials successfully produced anti-proliferative drugs, as is achieved by the current most successful cardiovascular stents on the market, and effectively suppressed proliferation of cells. We are now investigating the utility of these biomaterials in diverse biomedical settings.
Experimental Section
PVA Fibers Containing β-glucuronidase-loaded Liposomes
PVA (Mw 85,000-124,000 Da, Sigma-Aldrich) was dissolved in distilled water under constant stirring at 90 °C to generate a 12 wt% solution. Prior to spinning, 10 mL of PVA solution was supplemented with 500 μL of β-glu-loaded liposomes (0.1 mg mL-1 β-glu, 5 mg mL-1 lipid). Polymer solutions were processed by electrospinning on a custom-built device as previously established.[38] Briefly, the device consisted of two high voltage sources (ETPS Ltd), one syringe pump (NE-1010 High Pressure Syringe Pump, World Precision Instruments), providing a constant polymer flow through a needle (blunt needle, 18G Terumo), and a planar stainless steel collector. All equipment was placed in an electrically grounded Faraday cage (Phoenix Mechano Ltd). The positive voltage was applied to the needle, whereas negative voltage was supplied to the collector. High voltage was controlled by a custom-made software based on LabVIEW (Empa, Swiss Federal Laboratories for Materials Science and Technology, Switzerland) via a LabJack interface (RS Components). Fibers were spun at 10/-5kV, 20 μL min-1 flow rate, and a distance of 15 cm.
Biocatalytic Activity of PVA Fibers Containing β-glucuronidase-loaded Liposomes
Electrospun PVA fibers containing β-glu-loaded liposomes were incubated in 0.5 M Na2SO4 for 1 h, followed by washing via immersion in PBS for 1 h. Stabilized fibers were incubated with 0.05, 0.5, or 5 μg mL-1 of FDGlcU (Molecular Probes) in PBS for 30 min at 24 °C or 37 °C, and fluorescence intensity of products (fluorescein) was monitored using a SpectraMax M5 microplate reader (Molecular Devices) (λex = 495 nm; λem = 520 nm). The enzymatic conversion of FDGlcU to fluorescein was repeated over several cycles by periodically removing fluorescent products (fluorescein) and adding fresh FDGlcU (5 μg mL-1) to the same PVA fibers containing β-glu-loaded liposomes.
Detailed methods are available in the Supporting Information.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author. The raw data supporting this publication is available online.
Acknowledgements
R.C. acknowledges the support from the Marie Curie International Incoming Fellowship within the 7th Framework Programme under Grant Agreement No. 299936. A.G.G. acknowledges funding from the Swiss National Science Foundation under Grant No. P2BEP3_152091 and P300PB_161072. T.C.C.M. and M.G.O. acknowledge funding from São Paulo Research Foundation, FAPESP, Grant No. 2014/03266-4 and 2015/13290-2, respectively. A.N.Z. acknowledges funding from the European Research Council Consolidator Grant (ERC-2013-CoG 617336 BTVI). M.M.S. acknowledges the support from the ERC Seventh Framework Programme Consolidator grant “Naturale CG” under Grant Agreement No. 616417, the Engineering and Physical Sciences Research Council (EPSRC) grant “Bio-functionalised nanomaterials for ultrasensitive biosensing” (EP/K020641/1), and a Wellcome Trust Senior Investigator Award (098411/Z/12/Z). The authors thank Dr Coline Jumeaux (Imperial College London) for the preparation of rhodamine-labeled liposomes.
Contributor Information
Dr. Rona Chandrawati, Department of Materials, Department of Bioengineering, and Institute of Biomedical Engineering, Imperial College London, London SW7 2AZ, UK
Morten T. J. Olesen, Department of Chemistry and iNANO Interdisciplinary Nanoscience Center, Aarhus University, Aarhus C 8000, Denmark
Thatiane C. C. Marini, Institute of Chemistry, University of Campinas, UNICAMP, Campinas, 13083-970, São Paulo, Brazil
Gurpal Bisra, Department of Materials, Department of Bioengineering, and Institute of Biomedical Engineering, Imperial College London, London SW7 2AZ, UK.
Dr. Anne Géraldine Guex, Department of Materials, Department of Bioengineering, and Institute of Biomedical Engineering, Imperial College London, London SW7 2AZ, UK
Prof. Marcelo G. de Oliveira, Institute of Chemistry, University of Campinas, UNICAMP, Campinas, 13083-970, São Paulo, Brazil
Dr. Alexander N. Zelikin, Department of Chemistry and iNANO Interdisciplinary Nanoscience Center, Aarhus University, Aarhus C 8000, Denmark
Prof. Molly M. Stevens, Department of Materials, Department of Bioengineering, and Institute of Biomedical Engineering, Imperial College London, London SW7 2AZ, UK
References
- [1].Funk S, Miller MM, Mishell DR, Archer DF, Poindexter A, Schmidt J, Zampaglione E, Implanon US Study Group Contraception. 2005;71:319. doi: 10.1016/j.contraception.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [2].Mansour D. J Fam Plann Reprod Health Care. 2010;36:187. doi: 10.1783/147118910793048629. [DOI] [PubMed] [Google Scholar]
- [3].Obara K, Ishihara M, Ishizuka T, Fujita M, Ozeki Y, Maehara T, Saito Y, Yura H, Matsui T, Hattori H, Kikuchi M, et al. Biomaterials. 2003;24:3437. doi: 10.1016/s0142-9612(03)00220-5. [DOI] [PubMed] [Google Scholar]
- [4].Moura LIF, Dias AMA, Carvalho E, de Sousa HC. Acta Biomaterialia. 2013;9:7093. doi: 10.1016/j.actbio.2013.03.033. [DOI] [PubMed] [Google Scholar]
- [5].Puranik AS, Dawson ER, Peppas NA. Int J Pharm. 2013;441:665. doi: 10.1016/j.ijpharm.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kwon H, Park S. Polymers. 2014;6:755. [Google Scholar]
- [7].Im E, Hong MK. Expert Rev Cardiovasc Ther. 2016;14:87. doi: 10.1586/14779072.2016.1112267. [DOI] [PubMed] [Google Scholar]
- [8].Fejerskov B, Zelikin AN. PLOS ONE. 2012;7:e49619. doi: 10.1371/journal.pone.0049619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mendes AC, Zelikin AN. Adv Funct Mater. 2014;24:5202. [Google Scholar]
- [10].Chandrawati R, Chang JYH, Reina-Torres E, Jumeaux C, Sherwood JM, Stamer WD, Zelikin AN, Overby DR, Stevens MM. Adv Mater. 2017;29 doi: 10.1002/adma.201604932. 1604932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fejerskov B, Jarlstad Olesen MT, Zelikin AN. Adv Drug Deliv Rev. 2017 doi: 10.1016/j.addr.2017.04.013. [DOI] [PubMed] [Google Scholar]
- [12].Bagshawe KD. Exp Rev Anticancer Ther. 2006;6:1421. doi: 10.1586/14737140.6.10.1421. [DOI] [PubMed] [Google Scholar]
- [13].Morgan RA. Mol Ther. 2012;20:11. doi: 10.1038/mt.2011.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tai C-K, Wang WJ, Chen TC, Kasahara N. Mol Ther. 2005;12:842. doi: 10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fejerskov B, Jensen BEB, Jensen NBS, Chong S-F, Zelikin AN. ACS Appl Mater Interfaces. 2012;4:4981. doi: 10.1021/am3013467. [DOI] [PubMed] [Google Scholar]
- [16].Andreasen SO, Fejerskov B, Zelikin AN. Nanoscale. 2014;6:4131. doi: 10.1039/c3nr05999e. [DOI] [PubMed] [Google Scholar]
- [17].Fejerskov B, Jensen NBS, Teo BM, Städler B, Zelikin AN. Small. 2014;10:1314. doi: 10.1002/smll.201303101. [DOI] [PubMed] [Google Scholar]
- [18].Wang Z, Lu Y, Qin K, Wu Y, Tian Y, Wang J, Zhang J, Hou J, Cui Y, Wang K, Shen J, et al. J Control Release. 2015;210:179. doi: 10.1016/j.jconrel.2015.05.283. [DOI] [PubMed] [Google Scholar]
- [19].Yang Z, Yang Y, Xiong K, Li X, Qi P, Tu Q, Jing F, Weng Y, Wang J, Huang N. Biomaterials. 2015;63:80. doi: 10.1016/j.biomaterials.2015.06.016. [DOI] [PubMed] [Google Scholar]
- [20].Sakai S, Tsumura M, Inoue M, Koga Y, Fukanob K, Taya M. J Mater Chem B. 2013;1:5067. doi: 10.1039/c3tb20780c. [DOI] [PubMed] [Google Scholar]
- [21].Osuga K, Maeda N, Higashihara H, Hori S, Nakazawa T, Tanaka K, Nakamura M, Kishimoto K, Ono Y, Tomiyama N. Int J Clin Oncol. 2012;17:306. doi: 10.1007/s10147-012-0445-1. [DOI] [PubMed] [Google Scholar]
- [22].Alves M-H, Jensen BEB, Smith AAA, Zelikin AN. Macromol Biosci. 2011;11:1293. doi: 10.1002/mabi.201100145. [DOI] [PubMed] [Google Scholar]
- [23].Jensen BEB, Alves M-H, Fejerskov B, Städler B, Zelikin AN. Soft Matter. 2012;8:4625. [Google Scholar]
- [24].Marcilli RHM, de Oliveira MG. Colloids Surf B Biointerfaces. 2014;116:643. doi: 10.1016/j.colsurfb.2013.10.036. [DOI] [PubMed] [Google Scholar]
- [25].Lozinsky VI, Galaev IY, Plieva FM, Savina IN, Jungvid H, Mattiasson B. Trends Biotechnol. 2003;21:445. doi: 10.1016/j.tibtech.2003.08.002. [DOI] [PubMed] [Google Scholar]
- [26].Rade JJ, Hogue CW. Anesthesiology. 2008;109:573. doi: 10.1097/ALN.0b013e3181870a4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Agarwal S, Wendorff JH, Greiner A. Polymer. 2008;49:5603. [Google Scholar]
- [28].Park J-C, Ito T, Kim K-O, Kim K-W, Kim B-S, Khil M-S, Kim H-Y, Kim I-S. Polym J. 2010;42:273. [Google Scholar]
- [29].Hong KH. Polym Eng Sci. 2007;47:43. [Google Scholar]
- [30].Kang YO, Yoon IS, Lee SY, Kim DD, Lee SJ, Park WH, Hudson SM. J Biomed Mater Res B Appl Biomater. 2010;92B:568. doi: 10.1002/jbm.b.31554. [DOI] [PubMed] [Google Scholar]
- [31].Chaouat M, Le Visage C, Baille WE, Escoubet B, Chaubet F, Mateescu MA, Letourneur D. Adv Funct Mater. 2008;18:2855. [Google Scholar]
- [32].Cutiongco MFA, Anderson DEJ, Hinds MT, Yim EKF. Acta Biomaterialia. 2015;25:97. doi: 10.1016/j.actbio.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fejerskov B, Smith AAA, Jensen BEB, Hussmann T, Zelikin AN. Langmuir. 2013;29:344. doi: 10.1021/la3040903. [DOI] [PubMed] [Google Scholar]
- [34].Chandrawati R, Caruso F. Langmuir. 2012;28 doi: 10.1021/la301958v. 13798. [DOI] [PubMed] [Google Scholar]
- [35].Pattni BS, Chupin VV, Torchilin VP. Chem Rev. 2015;115 doi: 10.1021/acs.chemrev.5b00046. 10938. [DOI] [PubMed] [Google Scholar]
- [36].Cutiongco MFA, Kukumberg M, Peneyra JL, Yeo MS, Yao JY, Rufaihah AJ, Le Visage C, Ho JP, Yim EKF. Front Bioeng Biotechnol. 2016;4:44. doi: 10.3389/fbioe.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mátyás L, Berry M, Menyhei G, Tamás L, Acsády G, Cuypers P, Halmos F, de Vries AC, Forgacs V, Ingenito G, Avelar R. Eur J Vasc Endovasc Surg. 2008;35:715. doi: 10.1016/j.ejvs.2007.11.024. [DOI] [PubMed] [Google Scholar]
- [38].Guex AG, Kocher FM, Fortunato G, Körner E, Hegemann D, Carrel TP, Tevaearai HT, Giraud MN. Acta Biomaterialia. 2012;8:1481. doi: 10.1016/j.actbio.2011.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.