Abstract
Background/Objectives
This study evaluated nutrition after oesophago-gastric resection and the influence of home jejunostomy feeding in the six months after surgery.
Subjects/Methods
Data on nutritional intake and physiologic measures were collected as part of a randomised trial with measurements taken before and up to six months after surgery.
Results
41 participants (32 oesophagectomy, 9 total gastrectomy) received home jejunostomy feeding (n=18) or usual care without feeding (n=23). At hospital discharge, oral intakes were adequate for energy and protein in 9% and 6% respectively. By three and six months, these values had increased to 61% & 55%, 94% & 77% respectively. Six participants (26%) who received usual care required rescue feeding. Six weeks after hospital discharge, energy intakes were met in those who received jejunal feeding due to the contribution of enteral nutrition. Jejunal feeding did not affect oral intake, being similar in both groups (fed: 77% estimated need, usual care: 79%). At three months, inadequate micronutrient intakes were seen in over one third. Compared to baseline values, six weeks after surgery, weight loss exceeding 5% was seen in 5/18 (28%) who received feeding, 14/17 (82%) who received usual care and 5/6 (83%) of those who required rescue feeding, p=0.002. Weight loss averaged 4.1% (fed), 10.4% (usual care) and 9.2% (rescue fed), p=0.004. These trends persisted out to six months.
Conclusions
Supplementary jejunostomy feeding made an important contribution to meeting nutrition after oesophago-gastric resection. Importantly, oral nutritional intake was not compromised dispelling the assertion that jejunal feeding deincentivises patients from eating.
Keywords: enteral nutrition, jejunostomy, nutritional intake, oesophagectomy
Introduction
Nutritional status is often compromised in the early months following oesophagectomy or total gastrectomy for cancer, with findings from a recent systematic review of cohort studies suggesting a deterioration in body mass index in the region of 8% – 10% during the first six months after surgery.1 The reasons for this are multi-factorial, relating to altered anatomy, gastrointestinal symptoms2, and changes in nutrient absorption3
There is only limited information in the literature regarding assessing nutritional intake in the short and long term following oesophago-gastric resection. Ryan et al.4 noted at hospital discharge that nutritional intake after oesophagectomy did not meet requirements, with patients falling short of 30% of their daily energy and 35% of their daily protein requirements. Haverkort et al.5 identified that at six months after surgery, 23% of participants still did not meet their energy requirements and 9% did not meet their protein requirements. The authors also noted that many patients had an inadequate intake of vitamins and trace elements, more evident at six compared to twelve months after surgery. Ludwig et al.6 in the only study reporting longer follow up, at a mean of 34 months, found that still 22% of patients had an inadequate energy intake.
Randomised controlled trials of the role of nutritional support in meeting nutritional requirements or preventing deterioration in nutritional status, outside of the immediate post-operative period are lacking. Although Hyltander et al.7 showed no significant benefit, in terms of energy and protein intake or change in nutrition status, when giving supplementary enteral or parenteral nutrition in 80 subjects following curative upper gastrointestinal surgery, the amount of supplementary nutrition was relatively small (approximately 120 kcal daily for three months), thus limiting the chances of a beneficial effect for feeding being identified.
The aim of this paper was to report in detail the nutrient intakes of participants enrolled in a pilot and feasibility study of home enteral feeding, via a jejunostomy tube after oesophagectomy or total gastrectomy for cancer8,9. In particular, we aimed to determine (1) nutrient intake in the first six months following surgery, (2) the contribution of dietary and supplementary jejunostomy feeding to meeting estimated nutritional requirements, (3) whether the provision of supplementary jejunostomy feeding affected oral nutritional intake, and (4) the effect of supplementary jejunostomy feeding on nutritional status.
Methods
The sample was drawn from those participating in a prospective randomised controlled trial8,9, conducted at University Hospitals of Leicester NHS Trust. Approval was obtained from the Nottingham 2 Local Research and Ethics committee (protocol #11/EM/0383). The trial was registered with the UK Clinical Research Network (UKCRN 12447 / 13361). Each participant provided written informed consent. Recruitment commenced in July 2012 and closed in March 2014. Participant follow up was completed in September 2014.
Inclusion criteria were adult patients with a diagnosis of oesophago-gastric cancer considered suitable for potentially curative surgical resection. The only specific exclusion criteria were patients in whom, home enteral feeding was deemed inappropriate by either the patient or the managing healthcare team.
All participants received standardised post-operative care while in hospital, consisting of feeds, via the jejunostomy, placed at time of surgery. Tube insertion, commencement of feeds and subsequent increase in rate and volume followed an agreed care pathway8. In all participants, continuous jejunostomy feeds of Nutrison Energy Multifibre (Nutricia) were reduced to supplementary overnight feeds (10 – 15 hours duration) when oral intake recommenced (at approximately post-operative day seven) and continued until the morning of the day of hospital discharge.8 Dietary advice, including food fortification and the use of prescribable nutritional supplements, with supporting written information, was provided to all patients prior to discharge.
Intervention (home jejunostomy feeding) and usual care groups
Participants randomised to the intervention arm received a planned programme of home jejunostomy feeding following hospital discharge. The goal of feeding was to provide at least 50% of estimated energy and protein requirements via overnight jejunostomy feeds for a minimum of six weeks. Participants randomised to the usual care arm were discharged from hospital with a feeding jejunostomy in place. The tube was flushed with 10 ml sterile water daily to prevent tube blockage, but it was not used unless rescue feeding was indicated. The indications for rescue feeding were weight loss of greater than 5% from baseline level, reduced physical functional status or an estimated oral calorie intake less than one third of predicted requirement.8,9 These assessments and the decision to restart jejunostomy feeding were undertaken by the community dietitian or the hospital clinical team, who were independent of the research team.
A small number of patients who had experienced post-operative complications (principally anastomotic leak) were discharged home from hospital on planned total jejunostomy feeding to allow recovery from the complications. These participants were not allowed oral fluids during this time.
Outcome measures
There were five data collection time points: (i) prior to surgery, (ii) at the time of hospital discharge, (iii) six weeks after hospital discharge, (iv) three months after surgery, and (v) six months after surgery. All assessments were undertaken by the Research Dietitian (MB).
Nutritional assessment
Weight was measured in kilograms to the nearest 0.1 Kg using calibrated (SECA®) stand on scales with subjects wearing light clothing. Weight in health and at diagnosis were taken from medical records where available or recall weight was used, converted from imperial measurements where required. Height was taken from pre-surgical assessment records, measured using a SECA® height measure in participants with footwear removed. Body mass index (BMI Kg/m2) and percentage weight change (previous weight − current weight / previous weight x 100) were calculated. Anthropometric measures were performed on the left arm in all participants. This was to avoid the confounding effect of the thoracotomy wound on the right side for those individuals undergoing oesophagectomy. Measurements were standardised and recognised techniques were followed10. Triceps skinfold thickness was measured using calibrated Harpenden® callipers and the mean of three attempts was recorded. Mid arm circumference was performed and mid arm muscle circumference was calculated. Hand grip dynamometry was taken on the left side, in the standing position using a calibrated Takei® measure and the mean of three results recorded11. The Malnutrition Universal Screening Tool was derived from this information and calculated for each trial participant.12
Nutritional intake assessment
Subjects were instructed to record all food and fluid intake for three days (two week days and one weekend day) to assess oral diet and fluid intake prior to each study data collection time point. Types of food, brand names and quantities consumed were clarified by the Research Dietitian at the time of visit, using visual aids if required. Each record was analysed using a computerised nutrition package (Dietplan 6®) using data from the UK Nutrient Database (replaced by the food integrated dataset in 201513 with additional food tables imported where appropriate (Nutricia®). Where food labels and packages were available and there was no corresponding item on the database, these items were manually added. Details of oral nutritional supplements and type and amount of jejunostomy feeds were also captured for the same time period to estimate total nutritional intake.
For those receiving jejunostomy feeds, the mean intake provided was estimated for the same period as dietary intake using prospective fluid balance charts during hospital stay or retrospective reports post discharge from hospital.
Estimation and adequacy of requirements
Daily energy requirements were estimated using the Henry Equation14 adjusted for a physical activity level. A factor of 1.3 was used for visit two, whilst in-hospital, and a factor of 1.5 at other time points to reflect increased physical activity levels post-discharge from hospital15. Protein requirements were based on standardised amounts of 1.25g/kg/day16. Both energy and protein needs were based on weight maintenance, adjusted for those individuals with BMI >30 Kg/m2.17
Daily nutrient intakes were considered adequate if mean intake was greater than 90% of estimated requirements for energy and protein, or provided amounts equal to or above estimated average requirement (EAR) for micronutrients18. The UK national dietary intake is indicated for reference19.
Data analysis
Descriptive data was summarised as mean (standard deviation) for the whole group. To consider the impact of jejunostomy feeding on nutritional intake and nutritional status, participants who received home jejunostomy feeds for more than one week were compared to those who did not receive jejunostomy feeds, irrespective of the arm they were randomised to. This per protocol analysis differ from the previously published reports of this clinical trial, which employed an intention to treat analysis8,9. Statistical analysis was performed using SPSS. Comparison between groups was made using repeated measure ANOVA for continuous data and Fisher’s exact test for categorical data. We caution however, that as this was a pilot and feasibility study, this was not a formally powered study, and consequently all formal comparisons are interpreted with caution.
Results
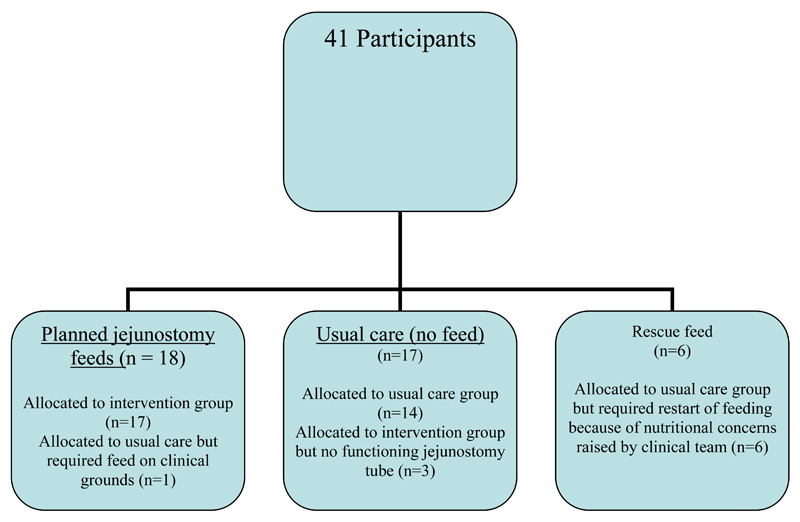
The study population comprised 41 participants (36 males) of mean age 64 years (SD 8), see Table 1. Further clinical details have been previously published9. Data on nutritional intake was available for 35 participants at the time of hospital discharge (two participants were nil by month and four did not complete the food diaries), 38 participants three months after surgery (2 withdrawn and 1 non-completer) and 35 participants six months after surgery (4 withdrawn and 2 non-completers). Changes in nutritional status have been reported for 18 participants who received home jejunostomy feeds (excluding participant withdrawal), the 17 participants who did not receive home jejunostomy feeds and the six participants initially allocated to the usual care arm of the study who restarted “rescue” jejunostomy feeds (Figure 1). For those allocated to planned jejunostomy feeds, the feed provided on average over the course of the six weeks, 63% (SD 15, range 41-91%) and 61% (SD 15, range 44-94%) of predicted energy and protein requirements respectively.
Table 1. Demographic characteristics of study population.
| Enteral feed (n=18) | No feed (n=17) | Rescue feed (n=6) | |
|---|---|---|---|
| Sex | |||
| Male | 16 (89%) | 15 (88%) | 5 (83%) |
| Female | 2 (11%) | 3 (12%) | 1 (17%) |
| Age in years | 63 (7) | 63 (9) | 68 (22) |
| Body mass index at baseline (Kg/m2) | 27.2 (4.9) | 28.7 (4.2) | 26.2 (8.2) |
| BMI at enrolment (Kg/m2) | |||
| 20-25 (%) | 8 (44%) | 4 (24%) | 3 (50%) |
| 25-30 (%) | 6 (33%) | 7 (41%) | 3 (50%) |
| >30 (%) | 4 (22%) | 6 (35%) | 0 (0%) |
| Weight loss at diagnosis (%) | 4.4 (8.1) | 2.7 (9.0) | 3.8 (7.5) |
| MUST score at enrolment12 | |||
| standard risk | 14 (78%) | 13 (76%) | 3 (50%) |
| medium risk | 2 (11%) | 3 (18%) | 1 (17%) |
| high risk | 2 (11%) | 1 (6%) | 2 (33%) |
| aNeoadjuvant chemotherapy | 18 (100%) | 14 (82%) | 6 (100%) |
| Cancer stage | |||
| T stage | 0 (0%) | 1 (6%) | 0 (0%) |
| Tis | 0 (0%) | 0 (0%) | 0 (0%) |
| T1 | 3 (17%) | 4 (24%) | 1 (17%) |
| T2 | 14 (78%) | 12 (71%) | 5 (83%) |
| T3 | 1 (6%) | 0 (0%) | 0 (0%) |
| T4 | |||
| N stage | |||
| N0 | 6 (33%) | 5 (29%) | 1 (17%) |
| N1 | 6 (33%) | 9 (53%) | 5 (83%) |
| N2 | 6 (33%) | 3 (17%) | 0 (0%) |
| N3 | 0 (0%) | 0 (0%) | 0 (0%) |
| Type of surgery | |||
| Transhiatal oesophagectomy | 2 (11%) | 1 (6%) | 0 (0%) |
| Ivor Lewis oesophago-gastrectomy | 13 (72%) | 11 (65%) | 5 (83%) |
| Total gastrectomy | 3 (17%) | 5 (29%) | 1 (17%) |
| bSurgical approach | |||
| Open | 5 (28%) | 6 (35%) | 1 (17%) |
| Hybrid | 13 (72%) | 11 (65%) | 5 (83%) |
Values indicated are mean (standard deviation) for continuous measures, and counts (percentages) for categorical measures
MUST = Malnutition Universal Screening Tool score which categorises risk of malnutrition into standard risk (score of 0), medium risk (score of 1) and high risk (score of 2 or more)12 Tis = in situ carcinoma
No participant received either pre- or post-operative radiotherapy.
Total gastrectomy and transhiatal oesophagectomy were performed through open abdominal incisions. Ivor Lewis oesophago-gastrectomy was performed through a hybrid approach (laparoscopic abdominal and open thoracic access).
Figure 1.
Participant disposition
Jejunostomy complications
As indicated above, all participants received enteral feeding while in hospital. This was discontinued in those allocated to the usual care arm, after discharge from hospital. There were two major (Clavien-Dindo grade 3b or greater20,21) jejunostomy tube feed related complications before hospital discharge. These two participants allocated to the in the control arm, who had undergone total gastrectomy, required laparotomy and small bowel resection for feed related small bowel necrosis. There was no tube associated mortality. There were a number of minor jejunostomy tube complications at the index admission requiring adjustment of the feed type or rate, or tube replacement for blockage, see Table 2. There were no major (Clavien-Dindo grade 3b or greater20,21) tube or feed related complications after discharge from hospital, see Table 2. Ninety-one percent and 71% of participants had a functioning jejunostomy tube still in situ at the time of hospital discharge and six weeks after hospital discharge respectively.
Table 2. Jejunostomy access and feeding complications.
| Minor Jejunostomy complications | In-hospital (n=45) | Out of hospital (n=41) |
|---|---|---|
| Clavien-Dindo grade 3 or 420,21 | 2/45 (4%) | 0/26 (0%) a |
| Feed related small bowel necrosis requiring laparotomy (non-fatal) |
||
| Clavien-Dindo grade 1 or 220,21 | ||
| Diarrhoea (%) | 4/45 (9%) | 7/26 (27%) a |
| Reflux of feed / vomiting (%) | 0/45 (0%) | 2/26 (8%) a |
| Tube displacement or migration (%) | 1/45 (2%) | 1/41 (2%) |
| Inadvertent tube removal (%) | 2/45 (4%) | 8/41 (20%) |
| Leakage around insertion site (%) | 6/45 (13%) | 8/41 (20%) |
| Tube occlusion (%) | 7/45 (16%) | 4/41 (10%) |
| Functional jejunostomy at end of study interval | 41/45 (91%) | 32/45 (71%) |
This includes the 18 participants allocated to the intervention arm who received home jejunostomy feeding as planned and the 6 participants allocated to the usual care arm who required rescue feeding. The remaining patients had the jejunostomy tube left in situ but not utilised. Unless there was a need for ongoing enteral feeding, jejunostomy tubes were removed six weeks after discharge from hospital.
Dietary (oral) nutritional intake
At the time of hospital discharge, oral dietary intake (food and oral fluids, including oral nutritional supplements) was poor and, compared to estimated nutritional requirements, considered adequate for 9% of participants for energy and 6% participants for protein (Table 3). By three months after surgery, these corresponding values were 61% and 55% for energy and protein respectively, and by six months, adequacy of intake was seen for 94% and 77% of participants, for energy and protein respectively. A similar pattern was observed in relation to micronutrient intake. At the time of hospital discharge, compared to estimated average requirement18, inadequate intake was observed in more than 50% of participants for all micronutrients apart from Thiamine (Table 3). At the three month time point, inadequate intake of zinc, magnesium, selenium, vitamins A and C was seen in more than a third of participants. At the six month time point, inadequate intake of all trace elements was observed in some participants with the exception of calcium, thiamine and vitamin B6.
Table 3. Dietary Nutritional Intakes at time of hospital discharge, three and six months after Oesophagectomy and Total Gastrectomy.
| Nutrient | Oral Dietary Intake Mean (SD) |
UK Populationc (>65yrs) | Estimated Average Requirement d (for >50yr male) | ||
|---|---|---|---|---|---|
| Time of Hospital Discharge (n=35) | 3 Months after surgery (n=38) | 6 Months after surgery (n=35) | |||
| Energy kcal/d | 980 (480) | 1920 (620) | 2250 (540) | 1707 (490) | 2605e |
| [kilojoules/d] | [4100 (2008)] | [8033 (2594)] | [9414 (2259)] | [7140 (2050)] | [10900]e |
| % of estimated requirements | 50 (25) | 84 (24) | 100 (19) | ||
| Adequate intakea | 3 (9%) | 23 (61%) | 32 (94%) | ||
| Protein g/d | 32 (16) | 72 (26) | 83 (24) | 70 (20) | 53.3 |
| % of estimated requirements | 35 (20) | 78 (28) | 93 (26%) | ||
| Adequate intake a | 2 (6%) | 21 (55%) | 27 (77%) | ||
| Calcium mg/d | 591 (283) | 975 (414) | 1037 (317) | 901 (349) | 525 |
| Adequate intake b | 15 (43%) | 33 (87%) | 35 (100%) | ||
| Iron mg/d | 5.1 (4.3) | 9.2 (4.8) | 10.4 (3.1) | 11.2 (7.8) | 6.7 |
| Adequate intake b | 6 (17%) | 26 (68%) | 32 (91%) | ||
| Zinc mg/d | 3.3 (2.9) | 7.8 (3.3) | 9.1 (2.5) | 8.3 (2.6) | 7.3 |
| Adequate intake b | 4 (11%) | 21 (55%) | 24 (69%) | ||
| Magnesium mg/d | 78 (46) | 200 (81) | 240 (66) | 252 (81) | 250 |
| Adequate intake b | 0 (0%) | 12 (32%) | 14 (40%) | ||
| Selenium µg/d | 10.5 (13.7) | 33.5 (19.9) | 48 (27) | 46 (21) | |
| Adequate intake b | 0 (0%) | 1 (3%) | 4 (11%) | ||
| Vitamin A (retinol) µg/d | 206 (220) | 1306 (2588) | 932 (1842) | 846 (1356) | 500 |
| Adequate intake b | 2 (6%) | 19 (50%) | 17 (49%) | ||
| Vitamin C mg/d | 45 (40) | 62 (51) | 92 (126) | 106 (116) | 25 |
| Adequate intake b | 17 (49%) | 23 (61%) | 25 (71%) | ||
| Vitamin E mg/d | 2.5 (2.9) | 6.1 (3.6) | 8.2 (5.1) | 13.1 (24.5) | n/a |
| Adequate intake b | n/a | n/a | n/a | ||
| Thiamine mg/1000kcal | 0.5 (0.3) | 0.7 (0.2) | 0.7 (0.2) | NR | 0.3 |
| [mg/d] | [0.5 (0.4)] | [1.4 (0.4)] | [1.4 (0.4)] | [2.1 (5.2)] | [N/R] |
| Adequate intake b | 24 (69%) | 38 (100%) | 35 (100%) | ||
| Riboflavin mg/d | 1.0 (0.6) | 2.0 (0.9) | 2.0 (0.8) | 2.3 (1.7) | 1.0 |
| Adequate intake b | 15 (43%) | 35 (92%) | 32 (91%) | ||
| Vitamin B6 mg/g protein | 15.1 (9.5) | 21.6 (5.6) | 21.6 (5.6) | N/R | 13 |
| [µg/d] | [0.56 (0.46)] | [1.74 (0.51)] | [1.7 (0.5)] | [2.8 (4.0)] | [N/R] |
| Adequate intake b | 17 (49%) | 38 (100%) | 35 (100%) | ||
| Folate µg/da | 113 (71) | 229 (87) | 228 (86) | 317 (358) | 150 |
| Adequate intake b | 7 (20%) | 33 (87%) | 29 (83%) | ||
The Table shows the contribution from dietary (oral) intake, including oral supplement drinks
Consuming >90% of estimated requirement for energy and protein.
Consuming > Estimated Average Requirement15
UK National Diet and Nutrition Survey 201419
Dietary Reference Values15
Dietary Reference Values for all males for energy16
Contribution of jejunostomy feeding to meeting nutritional requirements following hospital discharge
In-patient jejunostomy feeding was employed up until the time of hospital discharge in 38 participants (93%). In three participants randomised to the usual care group (no home feeding) the jejunostomy feeding was stopped by the healthcare team, because of diarrhoea in two patients, and raised blood glucose level in a third patient.
Overall, 24 participants received jejunostomy feeding at home, 18 as part of a planned programme and six because of the need for rescue feeding (Figure 2). This group received feeding for a mean of 75 days (range 35-172). Average intakes of energy and protein via the jejunostomy feed in the first six weeks were 1410 kcal (SD 270) and 56g (SD 10) daily respectively. Jejunostomy feeding was continued beyond six weeks in 10 of the 18 participants in the intervention group (56%), reducing in quantity as oral intake improved (as assessed by a Dietitian). Nine participants (37%) were still receiving feeding at three months after surgery. None were receiving jejunostomy feed supplementation by six months after surgery. The indications for continued feed were continued poor physical functional status or an estimated oral calorie intake less than one third of predicted requirement. Jejunostomy tubes were usually left in situ beyong the six week intervention time in case rescue feeding was required. In the group that received jejunal feeding in the first six weeks, tubes remained in situ for an average of 105 days (range 53-205) and in the usual care group, they remained in place for an average of 71 days (range 32-155).
Figure 2.
Number of days of administration of home jejunal feeding.
The grey shaded bars indicate participants in the planned jejunal feeding arm. The black shaded bars indicate participants in the usual care arm who required rescue feeding.
Ten participants in the usual care group met the weight loss threshold criteria for recommencing jejunostomy feeding in the first six weeks after hospital discharge, but this was administered either because of a non-functioning jejunostomy tube or participant refusal.
Feed composition
The feed employed was Nutrison Energy Multifibre (Nutricia), which is an isotonic feed containing 153 kcal/100 ml, 6 g protein per 100 ml.22 Calories are delivered 16% by protein, 48% by carbohydrate and 34% by fat. Its mineral content per 100 ml comprises 84 mg calcium, 2.4 mg iron, 1.8 mg zinc, 30 mg magnesium, 8.6 μg selenium, 123 μg vitamin A, 15 mg vitamin C, 1.9 mg vitamin E, 0.23 mg thiamine, 0.24 mg riboflavin, 0.26 mg vitamin B6 and 40 µg folic acid.
Dietary and total nutritional intake
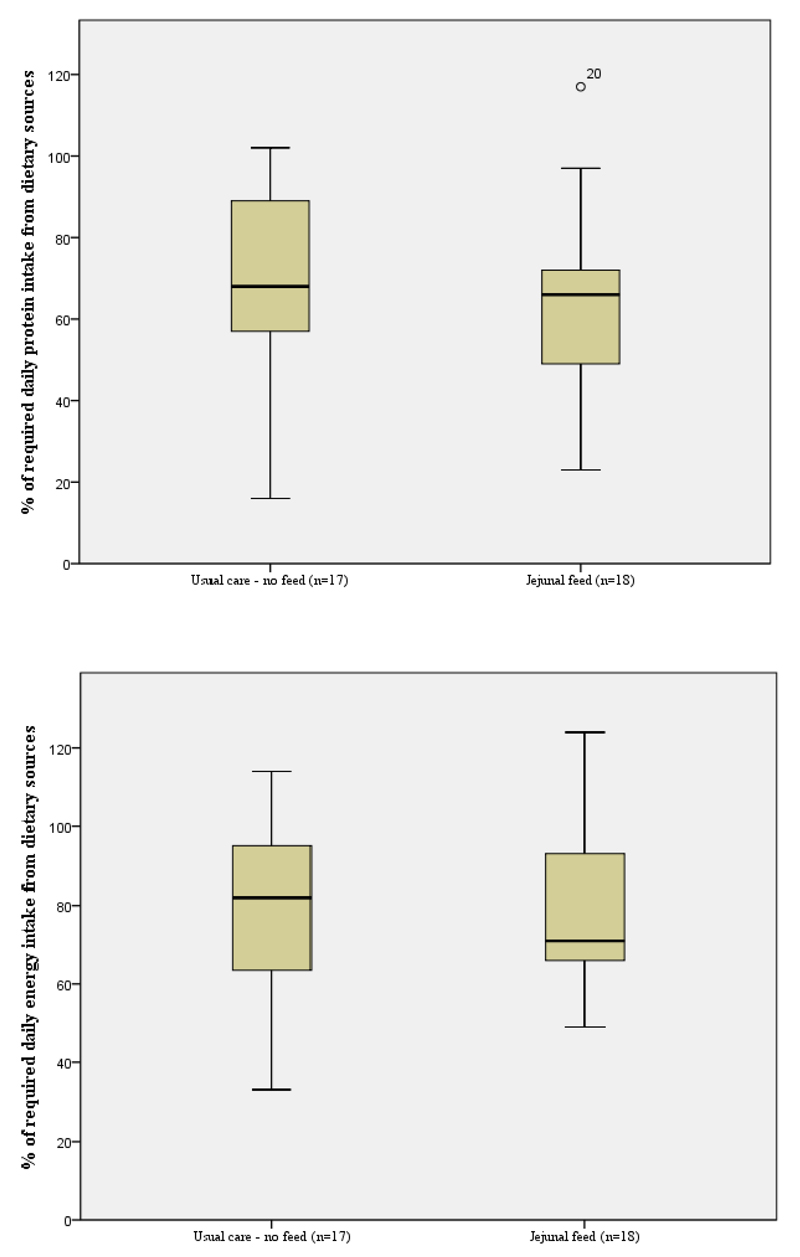
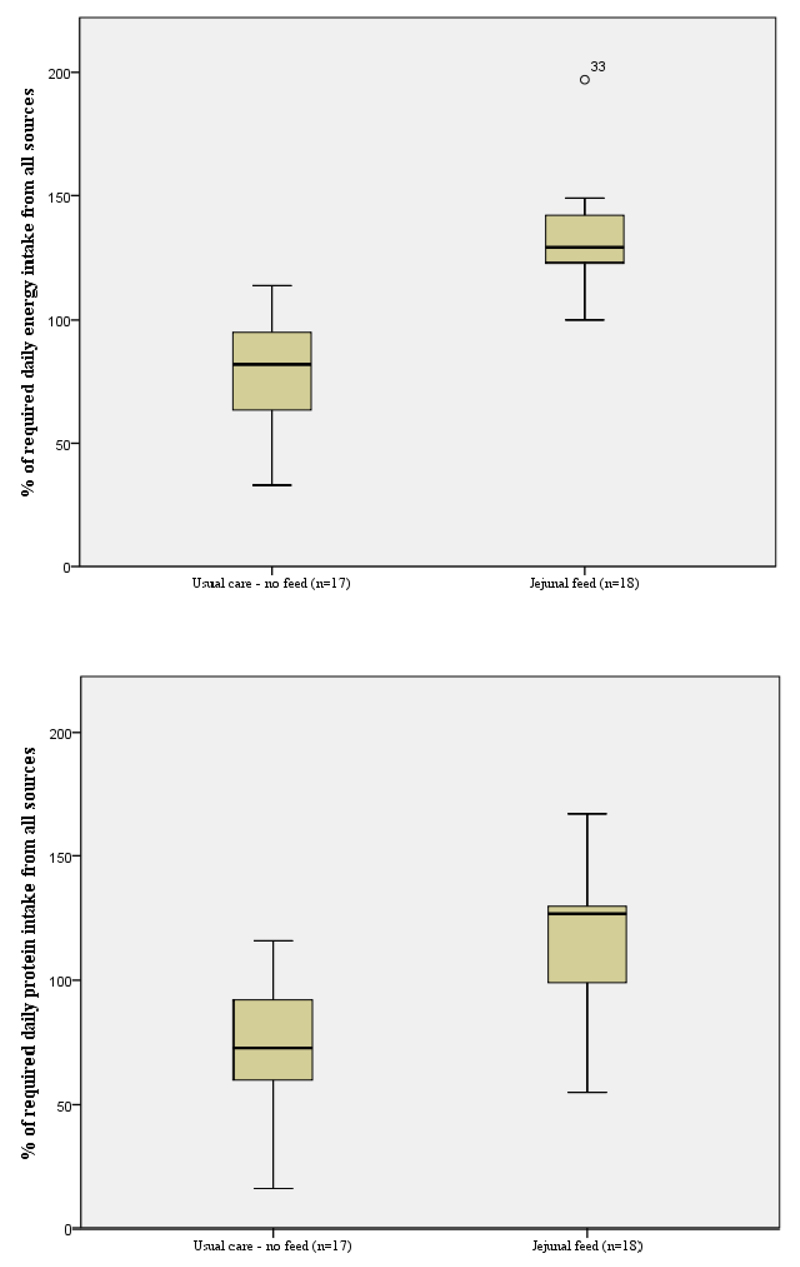
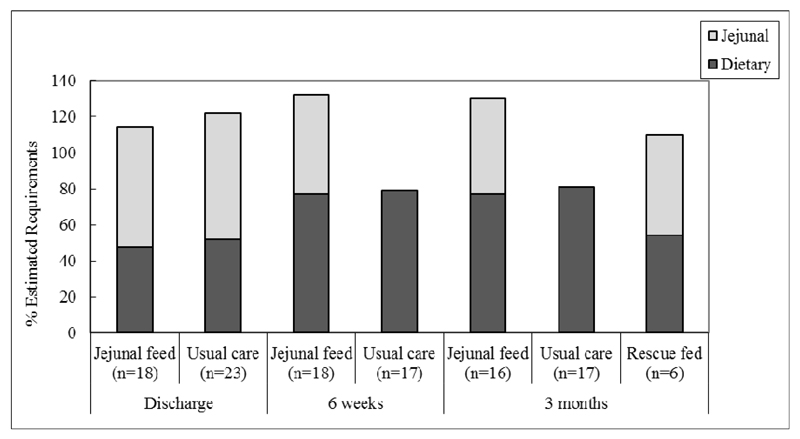
Figures 3 and 4 show the contribution of oral and jejunostomy feeding to meeting energy and protein needs at six weeks after hospital discharge in those who did, and did not, receive supplementary jejunostomy feeds. There was no significant difference in either the dietary energy or protein intake between those who did and did not receive jejunostomy feeding (p=0.80). The mean oral dietary intakes failed to meet 100% of energy or protein requirements in either group. Importantly, planned jejunostomy feeding did not negatively impact the oral intake of those receiving jejunostomy feeds. In the group of participants who received jejunostomy feeds, total nutritional intake (energy and protein) was significantly higher than those participants who did not receive feed (p<0.01), see Figure 5. All participants in the fed group had what would be considered an adequate intake, solely due to the contribution of jejunostomy feeding (Figure 5).
Figure 3.
Percentage of estimated energy and protein requirements derived from oral dietary sources
Figure 4.
Percentage of estimated energy and protein requirements derived from all sources (oral dietary and jejunostomy feed)
Figure 5.
Mean % contribution to estimated daily requirement from oral dietary sources and jejunal feeding at hospital discharge, six weeks after hospital discharge and three months after surgery
Impact on nutritional status
Weight at six weeks following discharge and three months post operatively was evaluated in 39 participants. Fourteen participants (36%) demonstrated weight maintenance or weight increase between the two visits, while 25 participants demonstrated weight loss. By treatment group, 12 of 16 who received jejunal feeding as planned experienced weight maintenance or increase, compared to one of 17 in the non-fed usual care group and one of six if the rescue fed group, p=0.0001. Energy intakes exceeded estimated requirements at six weeks for 10 of the 14 (71%) who achieved weight stability or gain. On average, participants received 125% (SD 36) of estimated energy requirements. In the group that lost weight, 13 of the 25 (52%) received in excess of estimated energy requirement.
Table 4 shows the change in nutritional status from baseline (pre-operative) levels. Six weeks after hospital discharge, both the degree of weight loss and the deterioration in hand grip dynamometry were significantly better in those that received planned home jejunal feeding compared to those who did not. The favourable effects on weight proved durable and were still evident long after the six week home feeding time.
Table 4. Change in physiologic parameter from baseline (enrolment to study prior to surgery).
| a% Weight change | Degree of weight loss |
Change in MAMC (cm) | Change in TSF (mm) | Change in hand grip strength | |||||
|---|---|---|---|---|---|---|---|---|---|
| None | <5% | 5-10% | >10% | ||||||
| Fed | 6 weeks (n=18) | -4.1 (3.6) | 1 (6%) | 12 (67%) | 4 (22%) | 1 (6%) | -1.6 (1.2) | -0.8 (2.0) | -1.5 (3.2) |
| 3 months (n=16) | -6.6 (5.6) | 1 (6%) | 8 (50%) | 3 (19%) | 4 (25%) | -1.6 (1.3) | -1.2 (2.3) | -2.0 (4.1) | |
| 6 months (n=14) | -8.1 (5.8) | 2 (14%) | 2 (14%) | 4 (29%) | 6 (43%) | -1.5 (1.6) | -2.2 (3.0) | -0.1 (2.9) | |
| Not fed | 6 weeks (n=17) | -10.4 (4.3) | 0 (0%) | 2 (12%) | 7 (41%) | 8 (47%) | -2.3 (2.1) | -1.7 (2.4) | -4.8 (4.7) |
| 3 months (n=17) | -12.2 (4.8) | 0 (0%) | 1 (6%) | 4 (41%) | 12 (71%) | -2.5 (1.9) | -2.3 (2.8) | -4.9 (4.8) | |
| 6 months (n=17) | -13.6 (6.7) | 0 (0%) | 2 (12%) | 2 (12%) | 13 (76%) | -2.4 (2.4) | -3.7 (2.7) | -2.4 (4.5) | |
| Rescue fed | 6 weeks (n=6) | -9.2 (6.4) | 1 (17%) | 0 (0%) | 2 (33%) | 3 (50%) | -1.8 (1.3) | -1.9 (1.3) | -2.6 (4.6) |
| 3 months (n=6) | -8.3 (7.5) | 1 (17%) | 1 (17%) | 1 (17%) | 3 (50%) | -1.7 (1.5) | -1.2 (0.4) | -2.8 (1.9) | |
| 6 months (n=6) | -8.9 (4.8) | 0 (0%) | 1 (17%) | 2 (33%) | 3 (50%) | -2.8 (1.0) | -1.1 (0.5) | -1.8 (1.6) | |
| p-value fed vs non-fed | c0.038 | d0.002 (6 weeks), 0.01 (3 months), 0.17 (6 months) | c0.738 | c0.015 | c0.016 | ||||
Values indicated are means (standard deviation).
Repeated measures ANOVA.
X2 test. Statistical comparisons were not made to the rescue fed group because of the small group size. Time periods relate to 6 weeks after hospital discharge, three months after surgery and six months after surgery
MAMC = mid arm muscle circumference in cm, TSF = triceps skin fold thickness in mm
Indicates percentage change in weight from baseline (study enrolment prior to surgery)
Discussion
This is the first study to prospectively assess the contribution of home jejunostomy feeding to meeting nutritional requirements after oesophagectomy and total gastrectomy, and the only study to report dietary intake three months after surgery in this population.
At the time of hospital discharge, the current study identified adequacy of oral intake in 9% and 6% of participants for energy and protein respectively. These values had climbed to 61% and 55% respectively at three months after surgery, and by six months 94% and 77% had adequate dietary energy and protein intakes. The values observed in the current study are more extreme than those seen by Ryan et al.4 who identified adequacy of oral intake of energy and protein at hospital discharge in 70% and 65% respectively, although the authors did not define adequacy of intake. Haverkort et al.5 who used a similar definition as employed in this study (<90% of recommended daily amount) identified adequacy of intake for energy and protein in 77% and 91%, and 76% and 93% of patients, six and 12 months after oesophagectomy. In the other study assessing nutritional intake, Ludwig et al.6 identified adequacy of dietary intake in 78% of patients an average of three years after surgery, although no definition was given for adequacy. Although there are potential differences in definition and the information is derived from patient recall food diaries, it is evident that the current findings are in agreement with prior studies indicating suboptimal intake of energy and protein for many patients after oesophagectomy.
Three months after surgery, less than two thirds of participants in this study were meeting estimated average intakes18 for Zinc, Magnesium, Selenium, Vitamin A and C. Haverkort et al.5 likewise identified suboptimal intake of multiple micronutrients at both six and 12 month intervals after surgery. In that study, the authors used Recommended Daily Amounts as their defining threshold. In the current study, the threshold employed was estimated average intakes. Both studies used thresholds benchmarked to their index populations. Clinical manifestations of micronutrient insufficiency were not assessed in this study, but it is unlikely that at a follow up interval of six months, any clinical symptoms would have developed. Further, those participants who received jejunostomy feeding would have received micronutrient replacement from this source. Longer term studies are required to assess the risk of micronutrient deficiency in patients who are years out from their surgery.
In this study patient group, supplementary jejunostomy feeding contributed a significant amount of energy and protein to overall nutritional intake. This feeding conferred a nutritional advantage in terms of preserving weight and function assessed by hand grip strength. Six of the 23 participants (26%) in the usual care arm required rescue jejunostomy feeding because of nutritional concerns, so that overall 76% of the study participants received feeding. Accepting that this was a pilot feasibility study and far reaching conclusions cannot be based on the study findings, an argument could be made for routinely offering home jejunostomy feeding to all patients after oesophagectomy.
An unexpected finding from this study was that nutritional status deteriorated in a number of participants even when estimated energy and protein requirements were being met or exceeded. For example at three months post-surgery, 54% of those losing weight were meeting estimated energy requirements. The reasons for this are unclear. It is possible that the food diaries overestimated dietary intake.23,24 It is possible that nutritional requirements in this patient group have been grossly underestimated25. Okamoto et al.26 measured energy expenditure using indirect calorimetry in eight male patients after oesophagectomy receiving parenteral nutrition, and found that although resting energy expenditure was increased initially, by post-operative day 14 values had returned to pre-operative normal levels. Once at home, measured resting energy expenditure was shown to remain stable at three, six and 12 months post oesophagectomy5 and higher than predicted in those after total gastrectomy27. It is possible that some patients experienced malabsorption as this was not routinely assessed3.
A further important finding was that oral energy intake in the first six weeks after surgery was similar between those who did and did not receive jejunostomy supplementation. This provides some evidence to counter the assertion that provision of additional energy through jejunostomy feeds will have a deleterious effect on oral energy intake. This has been an important message that the authors have tried to disseminate to fellow healthcare professionals and patients alike.
In this study, indirect calorimetry was not performed and instead energy requirements were estimated using Henry equations14 adjusted with a physical activity level ranging from 1.3-1.515. The Scientific Advisory Committee on Nutrition have suggested using a physical activity level of 1.49 for less active individuals (based on the 25th percentile of large data sets using healthy volunteers)16. For a post-surgical cancer population, most of whom received perioperative chemotherapy, a physical activity level of 1.49 may not be appropriate. A study assessing physical activity after oesophagectomy indicated a marked impairment at the time of hospital discharge, with a gradual recovery over three to six months28. Haverkort et al.5 reported that normal or minimally restrictive activity level was achieved by 92% after five months. This suggests that total energy expenditure may be reduced after surgery compared to normal individuals while physical activity levels remain low. Further research is needed to evaluate nutritional requirements in this population to inform goals of not only of nutritional interventions but rehabilitation after surgery.
Not all centres routinely place a feeding jejunostomy tube at the time of oesophagectomy. A previous National survey in the United Kingdom identified wide variation in practice. In that audit of over 2000 patients, overall 68% of patients had a feeding jejunostomy placed at the time of surgery.29 Twenty-eight percent had no feeding adjunct and four percent received an alternative adjunct, such as a nasojejunal tube. The audit further highlighted that even within centres practice varied, the proportion of patients undergoing oesophagectomy who received a feeding tube ranging from under 25% to in excess of 75%.29 Some of the reticence about tube placement may stem from the potential complications of the tube and feed. In this study both serious complications occurred in patients who had undergone total gastrectomy. None occurred in patients who had undergone oesophagectomy. As a result of this a subsequent audit of practice,30,31 the in-patient feeding regimen has been amended for patients who undergo total gastrectomy. The feeding rate is not escalated to more than 40 ml/hour until day seven. In that audit, the risk of small bowel complications (necrosis, perforation) requiring laparotomy was 0% (0 out of 285 patients) for oesophagectomy and 8% (6 out of 75 patients) for total gastrectomy. 30,31 All complications developed after discharge from the intensive care unit to the ward, on the seventh post-operative day (range 5-14 days). 30,31 The current study and the literature indicate that the jejunostomy tubes are very safe in the out of hospital setting, with problems being restricted to the tube site (infection, skin excoriation) or feed related (bloating, diarrhoea). The latter can be easily managed by amending the type or rate of feeding.
In an interview study of participants drawn from the same study, we identified a high level of acceptability to patients about home jejunostomy feeding32. All participants and their informal carers reported coping strategies for dealing with feed, jejunal tube and pump site problems. Going forward, this information would be used to amend practice. It was clear from patient reports that overnight feeding caused significant disruption to sleep. A feeding regimen running in the late afternoon and evening might be less disruptive to sleep patterns.
Conclusion
Patients undergoing oesophagectomy or total gastrectomy for cancer are at high risk of compromised oral nutritional intake in the months following surgery. Nutritional advice should consider both macronutrient and micronutrient intake. This study has pointed to the potential role of extended jejunostomy feeding in making a significant contribution to meeting nutritional requirements after surgery and reducing deterioration in body weight.
Acknowledgments
DJ Bowrey has received departmental funding support from Fresenius-Kabi for work unrelated to this submission. Some of the out of hospital costs associated with feeding and associated consumables were defrayed by Nutricia.
Footnotes
Conflict of Interest:
This article presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit Programme (Grant Reference Number PB-PG-0610-22480. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author Roles
| Author | Conception and Study Design | Analysis and Interpretation | Drafting the Paper | Critically Reviewed and Approved Submission |
|---|---|---|---|---|
| Melanie Baker | YES | YES | YES | YES |
| Vanessa Halliday | YES | YES | YES | YES |
| David Bowrey | YES | YES | YES | YES |
| Karen Smith | YES | YES | NO | YES |
| Pauline Robinson | YES | NO | NO | YES |
References
- 1.Baker M, Halliday V, Williams R, Bowrey D. A systematic review of the nutritional consequences after oesophagectomy. Clinical Nutrition. 2015 doi: 10.1016/j.clnu.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honda M, Wakita T, Onishi Y, Nunobe S, Hiki N, Miura A, et al. Development and validation of a symptom scale to evaluate post-operative patients with esophageal cancer. J Am Coll Surg. 2014;219:895–903. doi: 10.1016/j.jamcollsurg.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Heneghan HM, Zaborowski A, Fanning M, McHugh A, Doyle S, Moore J, et al. Prospective Study of Malabsorption and Malnutrition After Esophageal and Gastric Cancer Surgery. Annals of Surgery. 2015;262(5):803–808. doi: 10.1097/SLA.0000000000001445. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AM, Rowley SP, Healy LA, Flood PM, Ravi N, Reynolds JV. Post-oesophagectomy early enteral nutrition via a needle catheter jejunostomy: 8-year experience at a specialist unit. Clin Nutr. 2006;25:386–93. doi: 10.1016/j.clnu.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Haverkort EB, Binnekade JM, de Haan RJ, Busch OR, van Berge Henegouwen MI, Gouma DJ. Suboptimal Intake of Nutrients after Esophagectomy with Gastric Tube Reconstruction. J Acad Nutr Diet. 2012;112(7):1081–1087. doi: 10.1016/j.jand.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig DJ, Thirlby RC, Low DE. A prospective evaluation of dietary status and symptoms aftrer near-total esophagectomy without gastric emptying procedure. Am J Surg. 2001;181:454–458. doi: 10.1016/s0002-9610(01)00600-6. [DOI] [PubMed] [Google Scholar]
- 7.Hyltander A, Bosaeus I, Svedlund J, Liedman B, Hugosson I, Wallengren O, et al. Supportive nutrition on recovery of metabolism, nutritional state, health-related quality of life, and exercise capacity after major surgery: A randomized study. Clinical Gastroenterol Hepatol. 2005;3(5):466–474. doi: 10.1016/s1542-3565(05)00151-5. [DOI] [PubMed] [Google Scholar]
- 8.Bowrey DJ, Baker M, Halliday V, Thomas AL, Pulikottil-Jacob R, Smith K, et al. Six weeks of home enteral nutrition versus standard care after esophagectomy or total gastrectomy for cancer: study protocol for a randomized controlled trial. Trials. 2014;15:187. doi: 10.1186/1745-6215-15-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowrey DJ, Baker M, Halliday V, Thomas AL, Pulikottil-Jacob R, Smith K, et al. A randomized controlled trial of six weeks of home enteral nutrition versus standard care after esophagectomy or total gastrectomy for cancer: report on a pilot and feasibility study. Trials. 2015;16:531. doi: 10.1186/s13063-015-1053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norton K, Olds T. Anthropometrica: A text book of body measurement for sports and health courses. University of New South Wales Press; Australia: 2000. [Google Scholar]
- 11.Mathiowetz V. Effects of three trials on grip and pinch strength measurements. Journal of Hand Therapy. 1990;3:195–198. [Google Scholar]
- 12. http://www.bapen.org.uk/screening-and-must/must-calculator.
- 13. [accessed 11 August 2015];Composition of foods UK dataset. found at https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid.
- 14.Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2005;8:1133–1152. doi: 10.1079/phn2005801. [DOI] [PubMed] [Google Scholar]
- 15.Scientific Advisory Committee on Nutrition. Dietary reference values for energy. London: TSO; 2011. [Google Scholar]
- 16.National Institute for Health and Care Excellence. Nutrition Support in Adults: Oral Nutritional Support, Enteral Tube Feeding and Parenteral Nutrition. [accessed 11 August 2015];2006 https://guidance.nice.org.uk/cg32.
- 17.Soulsby, Weekes . In: Estimating nutritional requirements in adults in A pocket guide to clinical nutrition. 4th. Todorovic VE, Micklewright AM, editors. British Dietetic Association London; 2011. [Google Scholar]
- 18.Department of Health. Dietary Reference Values for food energy and nutrients for the United Kingdom. London: HMSO; 1991. [Google Scholar]
- 19.Food Standards Agency. National Diet and Nutrition Survey. 2014 http://www.gov.uk/government/statistics/national-diet-and-nutrition-survery-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-to-2011-and-2009-to-2011-and-2012.
- 20.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. http://nutricia.co.uk/files/uploads/documents/Nutrison_Energy_Multi_Fibre_2012.pdf.
- 23.Poslusna K, Ruprich J, de Vries JHM, Jakubikova M, van’t Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101:S73–S85. doi: 10.1017/S0007114509990602. [DOI] [PubMed] [Google Scholar]
- 24.Mertz W, Tsui JC, Judd JT, Reiser S, Hallfrisch J, Morris ER, et al. What are people really eating? The relation between energy intake derived from estimated diet records and intake determined to maintain body weight. Am Soc Clin Nutr. 1991;54:291–295. doi: 10.1093/ajcn/54.2.291. [DOI] [PubMed] [Google Scholar]
- 25.Murphy SP, Poos MI. Dietary Reference Intakes: summary of applications in dietary assessment. Pub Health Nutr. 2002;5(6A):843–849. doi: 10.1079/PHN2002389. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto H, Sasaki M, Johtatsu T, Kurihara M, Iwakawa H, Akabane M, et al. Resting energy expenditure and nutritional status in patients undergoing transthoracic Oesophagectomy for esophageal cancer. J Clin Biochem Nutr. 2001;49(3):169–173. doi: 10.3164/jcbn.11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamiji M, Troncon L, Suen V, de Oliveira RB. Gastrointestinal transit, appetite and energy balance in gastrectomized patients. Am J Clin Nutr. 2009;89(1):231–239. doi: 10.3945/ajcn.2008.26518. [DOI] [PubMed] [Google Scholar]
- 28.Skipworth RJE, Hendry P, Paterson-Brown S, Fearon K. Objective assessment of physical activity as a measure of functional recovery and quality of life following Oesophago-gastric cancer resection. GUT. 2012;61:A308–A309. [Google Scholar]
- 29.National Oesophago-Gastric Cancer Audit. Third Annual Report. London: 2010. pp. 1–69. Available from: http://www.ic.nhs.uk/webfiles/Services/NCASP/audits_and_reports/NHSICOGC Audit 2010 interactive.pdf. [Google Scholar]
- 30.Al-Taan OS, Nyasvajjala M, Paul M, Sharpe D, Ubhi S, Bowrey D. Differing risk of small bowel necrosis in patients undergoing oesophagectomy and total gastrfectomy with feeding jejunostomy placement. Gut. 2015;64(suppl 1):A514. [Google Scholar]
- 31.Al-Taan OS, Williams RN, Stephenson JA, Baker M, Nyasavajjala SM, Bowrey DJ. Feeding jejunostomy associated small bowel necrosis after elective esophago-gastric resection. J Gastrointest Surg. 2017 doi: 10.1007/s11605-017-3438-6. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Halliday V, Baker M, Thomas AL, Bowrey DJ. Patient and family caregivers’ experiences of living with a jejunostomy feeding tube after surgery for esophagogastric cancer. J Parenter Enteral Nutr. 2017 doi: 10.1177/0148607115604114. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]