Summary
Dendritic cells (DCs) are a diverse subset of innate immune cells that are key regulators of the host response to human immunodeficiency virus-1 (HIV-1) infection. HIV-1 directly and indirectly modulates DC function to hinder the formation of effective antiviral immunity and fuel immune activation. This review focuses upon the differential dysregulation of myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) at various stages of HIV-1 infection providing insights into pathogenesis. HIV-1 evades innate immune sensing by mDCs resulting in suboptimal maturation, lending to poor generation of antiviral adaptive responses and contributing to T-regulatory cell (Treg) development. Dependent upon the stage of HIV-1 infection, mDC function is altered in response to Toll-like receptor ligands, which further hinders adaptive immunity and limits feasibility of therapeutic vaccine strategies. pDC interactions with HIV-1 are pleotropic, modulating immune responses on an axis between immunostimulatory and immunosuppressive. pDCs promote immune activation through an altered phenotype of persistent type I interferon secretion and weak antigen presentation capacity. Conversely, HIV-1 stimulates secretion of indolemine 2,3 dioxygenase (IDO) by pDCs resulting in Treg induction. An improved understanding of the roles and underlying mechanisms of DC dysfunction will be valuable to the development of therapeutics to enhance HIV-specific adaptive responses and to dampen immune activation.
Keywords: HIV-1, myeloid dendritic cell, plasmacytoid dendritic cell, indolemine 2, 3 dioxygenase (IDO), immune activation, T-regulatory cell
Introduction
Dendritic cells (DCs) are a diverse subset of innate immune cells that are key regulators of the host response to human immunodeficiency virus-1 (HIV-1) infection. They orchestrate immune responses, serving as critical links between innate and adaptive immunity. DCs are among the first cells to encounter HIV-1 at mucosal sites, where they are co-opted by HIV-1 to facilitate transmission (1). Once infection is established, HIV-1 directly and indirectly modulates DC function to hinder the formation of effective adaptive immunity and fuel immune activation. This review focuses upon aspects of DC dysregulation during various stages of HIV-1 infection that may impact the course of disease. An improved understanding of the roles and underlying mechanisms of DC dysfunction on the immunopathogenesis of HIV-1 infection may be vital to the design of therapeutics that aim to enhance HIV-specific adaptive responses and/or dampen immune activation.
Myeloid DCs and HIV-1
CD11c+ myeloid DC (mDCs) and CD123+ plasmacytoid DC (pDCs) comprise the two major subsets of DCs and are differentially influenced by HIV-1 infection. mDCs are further classified into various subtypes including mDCs in blood and lymph nodes, monocyte-derived DCs (moDCs), and dermal DCs and Langerhan’s cells (LCs), which are present in the epidermal layer of mucosa and skin. mDCs are potent antigen-presenting cells (APCs) that specialize in directing T-cell responses. They are powerful stimulators of CD4+ and CD8+ T cells, and they are required to prime naive T cells in the formation of antigen-specific responses (2). mDCs richly express pattern recognition receptors (PRRs) including Toll-like receptors (TLRs 2–6, TLR8), C-type lectins, Nod-like receptors (NLRs), DNA sensors, and the viral RNA sensors melanoma differentiation-associated gene 5 and retinoic acid inducible gene I, allowing them to respond to a wide variety of pathogens and danger signals (3, 4). Upon encountering a pathogen, mDCs must undergo maturation to optimize their antigen presentation capacity and facilitate homing to lymphoid tissue. Mature mDCs upregulate surface major histocompatibility complex (MHC) and costimulatory molecules and secrete an array of cytokines or trigger signaling pathways tailored to the nature of the stimulus to regulate adaptive immunity, with results ranging from powerful antiviral responses to tolerance. Depending on the stimulus, activated mDCs secrete interleukin-12 (IL-12), an important regulatory cytokine that plays a critical role in skewing T-helper-1 (Th1) responses and activation of natural killer (NK) cells to generate antiviral immune responses (5–7). Alternatively, the absence of maturation contributes to compromised immunity (8).
mDCs express the HIV receptors/co-receptors CD4, CCR5, and CXCR4; however, viral entry largely occurs via endocytosis following binding to c-type-lectins (DC-SIGN, Langerin, CLEC4A) (3). Although efficient uptake by HIV-1 occurs by mDCs, they are largely restrictive to productive infection (9–12). Despite minimal infection, accumulating evidence suggests that mDC function is strongly impacted during HIV-1 infection. mDCs throughout HIV infection are decreased in frequency and display altered responsiveness to various stimuli (10, 13–24). Dissimilar to infection with other viruses, mDCs fail to adequately sense HIV-1 as a form of immune evasion, which blunts their maturation and ability to mount innate antiviral and adaptive HIV-specific responses and may also contribute to the formation of T-regulatory (Treg) cells (10, 16, 17, 19, 25, 26). Furthermore, we describe in detail how mDCs from HIV-infected individuals are variably responsive to other traditional mDC stimuli, including TLR ligands, which may further contribute to pathogenesis and limit feasibility of therapeutic vaccine strategies (10, 13, 15–20, 24, 27–29).
HIV-1 evasion of recognition and infection by mDCs
Multiple studies have confirmed the inability of mDCs to sense HIV-1 in vitro resulting in poor phenotypic activation and T-cell stimulatory capacity (10, 16, 17, 19, 25). In some contrast with in vitro findings, mDCs isolated from both blood and lymphoid tissue during acute and chronic infection display a level of partial activation, but are not fully mature (10, 24, 26, 30). This discrepancy highlights an important distinction between studies that utilize mDCs from seronegative donors that have been infected with strains of HIV-1 in vitro, as opposed to mDCs isolated from HIV-infected donors. In the latter case, mDCs have not only been exposed to HIV-1 in situ but also to the proinflammatory and immunomodulatory environment present during HIV-1 infection. There are multiple factors that may contribute to this partially activated phenotype mDCs in vivo, including bystander activation of by cytokines secreted by HIV-activated pDCs (25) and other immune cells. These semimature mDCs from HIV-infected individuals have been implicated in the formation of Tregs both ex vivo and within the lymphoid tissue they were isolated from (26). Furthermore, dysregulated crosstalk between NK cells and mDCs further contributes to accumulation of poorly mature mDCs during HIV-1 infection. NK cells isolated during chronic HIV-1 infection possess severely compromised DC editing (31), a process in which NK cells kill immature DC (32). Increased production of IL-10 during HIV-1 infection by immature mDCs confers resistance to NK cell-mediated lysis, resulting in the accumulation of these poorly immunogenic mDCs in the lymph nodes from HIV-infected individuals (30).
Major advances in the field revealing mechanisms by which HIV-1 subverts direct detection by mDCs were recently reviewed in depth by Iwasaki (33) and Luban (34) and are not discussed comprehensively here. Of what is known so far, HIV-1 escapes detection by mDCs at multiple stages including viral uptake, intracellular trafficking, and restriction to productive infection, all of which result in the avoidance of viral nucleic acid coming in contact with PRRs (34). Signaling via PRRs would allow mDCs to become appropriately activated to better direct adaptive immunity, resulting in the qualitative improvement of HIV-specific T-cell responses. One well-established means by which HIV-1 undermines immune recognition is by its lack of the accessory protein, vpx, in its genome. Vpx, which is present in HIV-2 and certain simian immunodeficiency virus (SIV) strains, degrades the restriction factor SAMHD1. In the absence of vpx, SAMHD1 inhibits productive HIV-1 infection in mDCs at the level of reverse transcription by depleting deoxynucleoside triphosphates in the cytoplasm (35–38). Vpx virus-like particles (VLPs) or HIV-1 packaged with vpx has been shown to overcome this block to productive infection, resulting in enhanced recognition of HIV-1 by mDCs as evidenced by upregulation of type I interferon (IFN) (39, 40). Upon addition of vpx VLPs, HIV-1 induced maturation of mDC via interaction of newly synthesized viral capsid protein and the host protein cyclophilin A, with subsequent activation of the transcription factor interferon regulatory factor 3 (IRF3) (39). The intracellular PRRs that recognize and trigger this pathway, however, remain to be identified. Nevertheless, vpx promoted transcription and production of type I IFN production and consequent activation of mDC (39, 40). This resulted in mature mDCs that could prime T cells and expand HIV-specific T-cell clones. The physiologic relevance of improved mDC detection of HIV-1 was underscored in a recent study of HIV-2, which encodes vpx and is less pathogenic than HIV-1. mDCs in these individuals were found to be significantly more activated, which may contribute to more effective viral control and slower disease progression seen in HIV-2 (41). Along these lines, during HIV-2 infection there is an increased frequency of polyfunctional HIV-specific T-cell responses compared with HIV-1 infection (42). Moreover, SIVSM, which expresses vpx, is non-pathogenic in Sooty mangabeys. Despite these advances, it is important to point out that these initial studies examining the effects of vpx in enabling HIV-1 recognition were performed in moDCs. It has been more difficult to use vpx containing VLPs or lentiviruses to activate blood mDCs, which have up to 30-fold more SAMHD1 than moDC (Bloch N, O’Brien M, Norton T, Polsky S, Bhardwaj N, & Landau N, unpublished observation).
Other factors contributing to HIV-1 evasion of detection in mDCs include TREX1, an exonuclease that degrades reverse transcribed HIV-1 DNA in the cytoplasm to avoid sensing by PRRs (33, 43, 44), and human TRIM5, a retroviral PRR (and restriction factor) that poorly recognizes the capsid lattice of most strains of HIV-1, but which otherwise could activate TAK1 to stimulate activating pathways in mDCs (34, 45–47). Additional restriction factors, such as APOBEC3G (A3G), are upregulated in mDCs in response to type I IFN and may further limit productive infection in vivo (48). Collectively, this improved knowledge of how HIV-1 avoids innate sensing by mDCs promotes the advancement of therapeutics for both preventive and therapeutic purposes. It may be necessary to target multiple restriction factors in order to enable HIV-1 recognition by blood and tissue mDCs. Further studies examining the levels of restriction factors in various mDC subsets, especially in individuals who are innately more resistant to infection (e.g. HLA B57, B27 expressing subjects) and/or controlling viremia, is warranted.
Altered mDC function during acute HIV-1 infection
Acute infection
While it is well established that HIV-1 fails to directly trigger activation by mDCs as a form of immune evasion, mDC responses to traditional DC stimuli, including TLR ligands, are variably altered during different phases of infection. Acute HIV-1 infection (AHIV) is classified into Fiebig stages I–VI, which are characterized by sequential increases in viral RNA, p24 protein, and appearance of HIV-specific antibodies by enzyme-linked immunosorbent assay and Western blot (49). The eclipse phase, where no virus is detectable, is followed by viral ramp-up (VR) during Fiebig stage I. Viral load peaks in Fiebig stages II and III subsequently decrease and then plateau to the viral set point in Fiebig stages IV–VI (49). During VR and up through peak viremia, there is an overwhelming surge in levels of antiviral and proinflammatory cytokines (50). Prior to any elevation in systemic cytokine levels during the eclipse phase, a rise of antiviral acute phase reactants including serum amyloid A and virus inhibitory peptide, a proteolytic fragment of alpha-1-antitrypsin, is observed (51). Then during the early stages of VR there are transient increases in IL-15, type I IFN, and IFN-inducible protein-10 (IP-10), followed by a second wave of IL-6, IL-8, IL-18, IL-10, and type I IFN, and finally later elevations in IL-12p70, IL-4, IL-5, and IL-22 (50). Despite this evidence of innate immune activation, the formation of adaptive responses is delayed (52). Evidence exists that mDC function is altered during these earliest stages of AHIV infection. Indeed, it is becoming apparent that HIV-1 interactions with the innate immune system are key factors in determining subsequent responses. Frleta et al. (16) found that mDCs isolated directly from subjects during early AHIV (Fiebig stages I and II) were compromised in their ability to produce cytokines, including IL-12 and tumor necrosis factor α (TNFα), in response to CLO97 (TLR7/8 agonist). Using an in vitro system to study the of effect circulating plasma factors from AHIV on mDC function, HIV-1 plasma early during Fiebig stages I and II was found to strongly suppresses mDC cytokine secretion in response to TLR stimulation [polyinosinic-polycytidylic acid [poly(I:C)] (TLR3), lipopolysaccharide (LPS) (TLR4), R848(TLR7/8), and peptidoglycan (TLR2)] including IL-12p70, TNFα, and IL-6 (16). Furthermore, AHIV plasma during these early stages impairs mDC ability to activate NK cells and prime Th1 responses from naive CD4+ T cells (16). When mDCs are exposed to plasma from individual donors at successive time points during AHIV, cytokine secretion is maximally suppressed during the VR very early in Fiebig stage I, following which certain donor plasma continues to exhibit suppressive effects, while other donors resemble control plasma at later stages of acute infection (16). Notably, perturbation in mDC cytokine secretion by AHIV plasma was observed even before the observed systemic cytokine elevations. Also, no inhibition of cytokine secretion occurred upon mDC exposure to plasma from acute hepatitis B or C infection, implying that this finding is not generalizable to all acute viral infections (16). Despite the association with VR, inhibition of cytokine production is not directly mediated by the virus as determined following mDC exposure to both laboratory strains and founder strains of HIV-1 in the presence of control donor plasma (16). Therefore, non-viral components of the circulating extracellular milieu are detrimental to mDC function very early during acute infection, hampering the formation of innate and adaptive immune responses at this critical phase. Nevertheless, the cytokine storm that is detected during acute infection comprising immune cytokines and chemokines including type I IFN, IL-15, IL-18, TNF, and IP-10 suggest there is eventual systemic activation of the innate immune system (50), which may be sufficient to account for the induction of detectable and potent antiviral CD8+ T-cell activity (53).
Other studies that have examined ex vivo mDC function during primary infection failed to demonstrate inhibition of cytokine secretion upon TLR ligation (10, 14). Sabado et al. (10) studied highly purified mDCs from AHIV, ranging from Fiebig stages I–VI, most of which were later stages of AHIV (Fiebig stages V and VI). Unlike mDCs from VR, mDCs during the later stages of AHIV (Fiebig stages V and VI) were actually hyperresponsive to stimulation with R848 (TLR 7/8 agonist) producing significantly higher levels of IL-12p70, IP-10, macrophage inflammatory protein-1 α (MIP-1α), MIP-1β, RANTES (regulated upon activation, normal T-cell expressed, and presumably secreted), and TNF compared with controls (10). In this study, cytokine secretion by mDCs isolated from very early stages of AHIV (Fiebig stages I and II) was only performed in two donors, but was not found to be different from controls. In keeping with the findings by Sabado et al. (10), Chang et al. (14) reported that mDCs from individuals during AHIV (Fiebig stages not defined) secreted higher levels of TNFα upon stimulation with CLO97 (TLR8 agonist), albeit a non-significant difference. Ex vivo T-cell stimulatory capacity by mDCs was preserved during AHIV and was higher by mDCs from Fiebig stages III–VI compared with controls, but difference this did not reach statistical significance (10). Large-scale transcriptional analysis of mDCs from AHIV revealed distinct patterns of expression compared with controls, with significant upregulation of 292 genes including those involved with transcription/translation, HIV infection, immune response, apoptosis, cell-cycle, and intracellular trafficking, suggesting global activation (10). It is likely that the variability in these studies during AHIV is largely due to the differences in the stage of acute infection focused upon. Moreover, discrepancies in certain findings may reflect key differences in experimental design, particularly the presence or absence of the circulating immunomodulatory environment within the in vitro culture system. The study by Frleta et al. (16) highlights the impact of the extracellular milieu in suppressing mDC function in situ to hinder their responsiveness to a variety of stimuli. Taken together, the data suggest that there is strong inhibition of mDC function very early during AHIV that impairs the formation of adaptive responses at this critical time, which transitions to mDC hyperreactivity during the later phases of AHIV, possibly contributing to (or being a consequence of) generalized immune activation that ensues.
Chronic infection
The majority of studies evaluating mDCs in the setting of chronic infection have demonstrated varying degrees of impaired cytokine secretion, T-cell stimulation, and NK cell activation in response to TLR ligands and other stimuli (13, 15, 16, 19, 24, 54). Among the notable defects observed in mDC function during chronic HIV-1 infection is impaired secretion of IL-12 (13, 15, 19). IL-12 is a key regulatory cytokine that plays a central role in both skewing of naive CD4+ T cells toward the Th1 pathway and the activation of NK cells to generate antiviral immune responses (5–7). Using a system designed to recapitulate the circulating immunomodulatory environment during HIV-1 infection through exposure of mDCs to plasma from chronically infected individuals, our group led by Miller et al. (19) found that plasma from untreated, viremic donors profoundly inhibits secretion of bioactive IL-12 (IL-12p70) by mDCs upon poly(I:C) (TLR3) and R848 (TLR 7/8) stimulation. To a lesser extent, HIV-1 plasma suppresses production of other cytokines by mDCs, including TNFα and IL-6. In keeping with the lower IL-12p70 secretion observed by mDCs, they exhibit impaired skewing of Th1 responses from naive CD4+ T cells. When mDCs are exposed to plasma from donors receiving suppressive ART, cytokine secretion is partially alleviated, but without direct correlation to plasma donor CD4+ T-cell count or viral load level (19). Along these lines, no direct viral mediated suppression of mDC cytokine secretion occurs upon mDC exposure to patient-derived strains of HIV-1 (cultured from untreated HIV-infected patients) in control plasma, nor does the removal of HIV-1 from plasma from viremic donors mitigate suppression of cytokine secretion upon TLR stimulation (19). This lack of direct inhibition by HIV-1 upon TLR stimulation during chronic infection is consistent with the findings of Frleta et al. (16) during AHIV using laboratory and founder strains of HIV-1. These data suggest that dysregulation in cytokine secretion during chronic infection results from non-viral components of the immunomodulatory extracellular milieu related to the host response to HIV-1. Due to the central role IL-12 plays in antiviral immunity, defects in expression by mDCs may alter the formation of immune responses not only to HIV-1 but to other pathogens, vaccines, and immunotherapies. Multiple studies have demonstrated the importance of IL-12 in the formation of effective HIV-specific adaptive responses. IL-12 enhances ex vivo HIV-specific CD4+ and CD8+ T-cell responses (55–57) and inhibits CD4+ T-cell apoptosis from HIV-infected donors (58). Administration of IL-12 during SIV infection in non-human primate (NHP) partially restores SIV-specific cytotoxic T-lymphocyte function and increases NK cell frequency and lytic function (59, 60). When IL-12 was administered prior to SIV challenge, lower viral load set point and delayed disease progression is achieved (61).
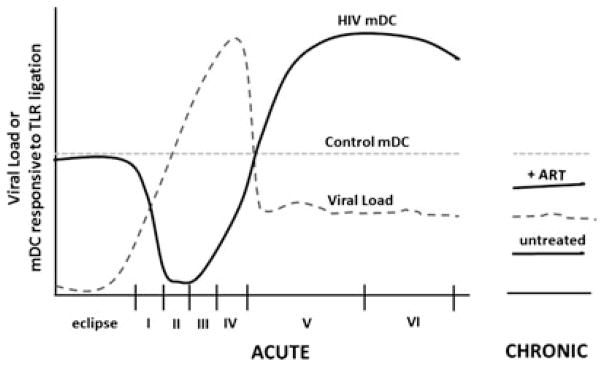
Throughout all stages of HIV-1 infection, mDC responses to stimulation are altered. In AHIV, very early during VR, mDCs are impaired in their ability to secrete key cytokines, including IL-12, resulting in poor stimulation of NK cells and adaptive antiviral response. This quickly transitions to mDC hyperreactivity later during AHIV, followed again by mDC suppression of cytokine secretion during chronic infection that is partially mitigated by suppressive ART (Fig. 1). Given that the restorative impact by ART on mDC cytokine secretion is only partial, an understanding of the underlying mechanisms of this inhibition may pave the way for novel therapies and vaccine adjuvants to overcome mDC dysfunction and enhance antiviral immunity throughout the course of HIV-1 infection.
Fig. 1. mDC function at various stages of HIV-1 infection.
mDC function is suppressed during viral ramp-up (Fiebig stages I/II) and then transitions to hyperresponsiveness to Toll-like receptor (TLR) ligation in the later Fiebig stages of AHI. During chronic infection, mDC function is again suppressed upon TLR ligation, but this is partially mitigated by suppressive ART.
Mechanisms of mDC dysregulation during HIV-1 infection
Direct virus-mediated effects
It remains controversial whether HIV-1 directly inhibits mDC response to traditional stimuli, such as TLR ligands. Several studies have implicated a role of direct mDC modulation by HIV-1 in response to TLR ligands via crosstalk with the C-type lectin pathway (27, 29, 62). In these studies, mDCs were exposed to laboratory strains of HIV-1 (62), an agonistic antibody to DC-SIGN (27), and purified gp120 (29) followed by LPS stimulation. In addition, the accessory protein Vpr has been described as an inhibitor of IL-12 secretion by mDCs following LPS or CD40L stimulation (18). In these studies and others, suppression of mDC function by HIV-1/gp120 was found to be mediated by increased levels of IL-10 (18, 20, 62), an immunoregulatory cytokine that inhibits mDC maturation and induces a tolerogenic phenotype (30, 63–65). In addition, the presence of IL-10 results in upregulation of programmed cell death protein ligand 1 (PD-L1) (30). When PD-L1 binds to its ligand, PD-1, on T cells, it serves to negatively regulate immune responses (66).
Blanchet et al. (67) described a novel mechanism of HIV-1 suppression of mDC function via shutdown of autophagy. Through activation of mammalian target of rapamycin by HIV-1 Env, autophagy was negatively related in mDCs as evidenced by downregulation of the autophagosome-associated protein LC-3. Inhibition of autophagy by HIV-1 in mDCs was found to decrease TNFα secretion in response to LPS (67).
These findings of direct virus-mediated inhibition of mDC function were challenged in recent studies by Miller et al. (16) and Frleta et al. (19) in our group. In these studies, exposure of mDCs to a range of doses of HIV-1, including laboratory strains, founder strains, and virus cultured directly from CD4+ T cells from untreated, viremic HIV-infected individuals, did not result in inhibition of mDC cytokine secretion upon TLR stimulation [poly(I:C)]. In keeping with this finding, the plasma from HIV-infected individuals did not stimulate the production of IL-10 by mDCs (16, 19). It is possible that differences in experimental design may have contributed to the different findings in these studies including genetic variations in the viruses and different mDC stimuli used. Although these HIV-1 strains did not directly inhibit mDC function, recent studies by Parrish et al. (68) have indicated that the source of HIV-1 can nevertheless impact host innate immune responses. Molecular clones of transmitted founder viruses, which express more envelopes per particle, were relatively more resistant to type I IFN and more efficiently captured and transferred to CD4+ T cells by mDCs. Therefore, while the innate responses of mDCs might not be modulated, HIV-1 usurps other characteristics of mDCs to augment and spread infection.
Apoptotic microparticles
Apoptotic microparticles (MPs) are circulating small membranous fragments released from apoptotic cells into the plasma during AHIV (69). Recently, these apoptotic MPs have been implicated in the suppression of mDC function during early AHIV (16). Apoptotic MPs, which become elevated during VR (Fiebig stages I and II), were isolated by Frleta et al. (16) from plasma during AHIV and found to impair mDC cytokine secretion upon poly(I:C) stimulation, including IL-12p70, TNFα, and IL-6. When compared to the cytokine suppression by whole plasma from the same AHIV donors, apoptotic MPs generally exhibited less inhibition. Moreover, removal of apoptotic MPs via ultracentrifugation from the plasma of chronically infected viremic donors did not alleviate the inhibition of cytokine secretion by HIV-1 plasma (19). Thus, it appears that apoptotic MPs contribute to HIV-1 plasma-mediated inhibition of mDCs, but other factors are additionally involved. These apoptotic MPs during AHIV were found to be qualitatively different from MPs isolated from control donors which did not inhibit mDC function (16). Generally, MPs from control donors are mostly platelet derived (CD41high), whereas MPs in AHIV are low for CD41, indicating that they are derived from non-platelet sources. However, in addition to being CD41low, there was no particular lineage marker that was elevated on AHIV MPs, indicating they were likely derived from a mix of peripheral blood mononuclear cells (PBMCs) that undergo apoptosis during AHIV. Mass spectrometry analysis revealed that CD44 binds to apoptotic MPs from AHIV. CD44 is a cell surface proteoglycan that mediates immune cell binding to extracellular matrix (70, 71), and is upregulated on mDCs during AHIV (16). CD44 blockade relieved suppression of mDC cytokine secretion by apoptotic MPs from AHIV, implicating a role for CD44 in MP-mediated mDC inhibition (16). Although CD44 had been implicated as a phagocytic receptor for clearance of apoptotic neutrophils in lung inflammation, this is the first time it has been implicated on mDCs as a major uptake or binding receptor for apoptotic MPs. As CD44 is prevalent on many cells, we believe that DC-restricted isoforms and/or signaling pathways confer recognition activity to mDCs. The ligand for CD44 on apoptotic microparticles remains unknown, but is clearly not one of the conventional ligands osteopontin or hyaluronan.
Products of microbial translocation
The depletion of mucosal immunity in the gut during HIV-1 infection gives rise to increased levels of circulating products of microbial translocation, including LPS, which contribute to immune activation and disease progression (72–75). Elevated levels of LPS (TLR4 agonist) and other immunomodulatory products may have an impact on chronic innate and adaptive immune activation and subsequent exhaustion throughout infection. In mDCs, LPS signaling has been shown to lead to decreases in cytokine secretion, including IL-12, upon poly(I: C) (TLR3) stimulation via an IL-10-dependent mechanism (76). Miller et al. (19) investigated whether low-level circulating LPS was responsible for suppression of cytokine secretion by mDCs upon exposure to plasma from viremic, untreated, HIV-infected donors. LPS at physiologic levels found during untreated HIV-1 infection was highly suppressive to mDC cytokine secretion when added to control donor plasma followed by poly(I:C) stimulation, and this effect was reversed with the addition of the LPS inhibitor polymyxin B. Interestingly, when polymyxin B was added to mDC cultures in the presence of untreated HIV-1 donor plasma, it did not reverse the suppressive effect of HIV-1 plasma on mDC cytokine secretion (19). Therefore, if elevated levels of LPS during HIV-1 infection play a role in decreased mDC responsiveness to subsequent TLR ligation, there are other overriding suppressive factors in HIV-1 plasma resulting in the lack of reversal with polymyxin B.
Taken together, the existing data suggest that a multifactorial etiology of mDC dysfunction during HIV-1 infection in response to TLR ligands and other stimuli. HIV-1 may directly mediate inhibition of mDC function via induction of IL-10 and/or disruption of autophagy. Furthermore, disturbances in plasma composition that exist during HIV-1 infection contribute to altered mDC function, including apoptotic MPs and possibly LPS. Several other factors that are elevated during the course of infection could also play a role, including but not limited to circulating immune complexes, acute phase reactants such as activated complement, or proinflammatory cytokines leading to exhaustion (51, 77). A more complete understanding of how HIV-1 infection impairs mDC function to various stimuli will have important implications for the design of adjuvants, both for therapeutic HIV-1 vaccines and preventive vaccines against other pathogens for use in HIV-infected individuals.
Strategies to overcome mDC dysfunction during HIV-1 infection
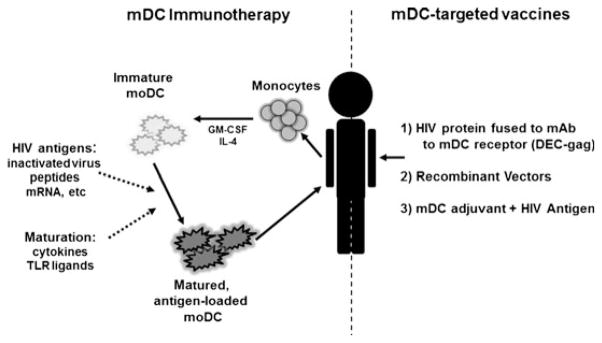
Therapeutic vaccines and immunotherapies for HIV-1 attempt to address the shortcomings of ART and may ultimately be an important component of eradication efforts. Recent studies have demonstrated that curative strategies for HIV-1 will likely require a multi-pronged approach, with one element focusing upon the enhancement of cytotoxic T-lymphocyte (CTL) function to improve killing of infected cells following the disruption of latency (78). mDCs are key players in vaccine strategies directed at the enhancement of cell-mediated immunity, and therefore, mDC dysfunction during HIV-1 infection may represent a significant obstacle toward successful therapeutic vaccination. There are multiple possible approaches to overcoming mDC dysfunction to improve vaccine-induced immune responses during HIV-1 infection. Augmentation of mDC function can be carried out ex vivo, followed by reintroduction of these modified cells to the patient as a form of adoptive immunotherapy. Alternatively, mDC function can be rescued in situ by mDC-targeting formulations, which are capable of combating the suppressive milieu present during HIV-1 infection (Fig. 2).
Fig. 2. Strategies to overcome myeloid DC (mDC) dysfunction during HIV-1 infection.
Augmentation of mDC function can be carried out ex vivo through activation and antigen loading, followed by reintroduction of these modified cells to the patient as a form of adoptive immunotherapy (left). Alternatively, mDC function can be rescued in situ by mDC-targeting formulations that are capable of combating the suppressive milieu present during HIV-1 infection (right). Strategies include targeting mDCs by coupling HIV-protein to monoclonal antibodies toward endocytic receptors on mDCs, utilization of recombinant vectors that naturally target mDCs, or HIV-1 antigens co-formulated with mDC-activating adjuvants.
The goal of mDC immunotherapy is to enhance mDC function during HIV-1 infection via ex vivo maturation and antigen loading of mDCs to bypass some of the mechanisms that HIV-1 employs in vivo to compromise their function and impair effective formation of T-cell responses. Ex vivo generation of large numbers of autologous moDCs can be carried out using HIV-infected donors’ monocytes following culture in the presence of granulocyte-macrophage colony-stimulating factor and IL-4 (11). Once these cells have been removed from the circulating microenvironment and differentiated and stimulated ex vivo, they are competent APCs with the ability to stimulate robust T-cell proliferation and antigen-specific CTLs (11). Antigen presentation is most effectively achieved by mature moDCs compared with immature moDCs, which lack high expression of costimulatory molecules and induce tolerogenic rather than immunogenic responses (79). However, depending on the ex vivo stimulus provided, certain functional defects may persist in moDCs generated from HIV-infected individuals, including decreased secretion of IL-12 (13), which may limit their success in immunotherapies.
Several trials of mDC immunotherapy in humans have demonstrated that immunization with autologous moDCs expressing HIV-1 antigens is safe and immunogenic during HIV-1 infection (80–91). In these studies, the source of HIV-1 antigens loaded onto moDCs has ranged from inactivated whole virus (83, 85, 86), HIV-1 peptides (82, 90), HIV-1 mRNA (87–89, 91), and recombinant pox vectors (80). Among the most impressive results achieved to date in both NHP and humans one involves the use of whole inactivated autologous virus (83, 85, 86, 92). HIV-1 can be efficiently inactivated with aldrithiol-2 (AT-2, 2,2′-dithiodipyridine, aldrithiol-2), which covalently modifies and functionally inactivates key S-H- containing internal viral proteins (e.g. nucleocapsid proteins) that are required for infectivity, while the envelope glycoproteins remain unaffected (93). AT-2 treatment arrests viral replication before reverse transcription, but maintains the conformational integrity of the envelope glycoproteins, so they may bind and fuse with target cells similar to infectious virus (93). Preclinical work by our group and others has demonstrated that AT-2-inactivated HIV-pulsed moDCs are effective immunogens in vitro (94–97). Presentation of CD8+ T-cell epitopes are present on MHC class I molecules following processing via the classical endosomal pathway (94). Both infectious and AT-2 inactivated HIV-pulsed moDCs can prime polyfunctional HIV-specific responses from naive CD4+ and CD8+ T cells (97). In vitro priming efficiencies are greater with AT-2-inactivated versus infectious HIV-1 and are specific to gag with the highest frequency and less so to env and pol (97). Following immunization of chronically infected rhesus macaques (SIVmac251) with moDCs pulsed with AT-2-inactivated SIV, anti-SIV adaptive immunity was enhanced along with >1000-fold reduction in plasma viral load compared with control animals who received unpulsed moDCs (92). Viremia was controlled for up to 240 days after completion of vaccination and was correlated with increased number of SIV-specific IFNγ-producing T-cell responses, cytolytic activity, and reduced destruction of the lymphoid follicular DC network. Supporting these findings, immunization of untreated, viremic HIV-infected individuals with moDCs pulsed with autologous AT-2-inactivated HIV-1 resulted in median reductions in viral load of 80% over the first 112 days post vaccination (83). This response was sustained over 1 year in 44% of vaccinated patients, and suppression was greater than 90% in eight of these patients. Viral suppression correlated with the presence of HIV-1 specific CD4+ T cells secreting IL-2 or IFNγ and gag-specific CD8+ T cells expressing perforin. More recent trials have utilized autologous moDCs pulsed with heat-inactivated HIV-1 with some success, albeit more modest than the findings with AT-2 inactivated virus (85, 86). Viremic, untreated patients that received moDCs pulsed with heat-inactivated HIV-1 had small yet significant decreases in plasma viral loads at 24 weeks compared with unpulsed moDCs (85). Furthermore, the decrease in plasma viral load appeared to be inversely related to the number of HIV-specific T-cell responses. When this same vaccine was administered to ART-treated HIV-infected patients, a substantial decrease (>1 log) in viral load set point following ART interruption was achieved more frequently in patients that received the moDC-HIV vaccine compared to moDCs alone at both 12 and 24 weeks following vaccination (86). Decreases in viral load were associated with an increase in HIV-specific T-cell responses in the moDC-HIV arm. These studies provide proof-of-concept that augmentation of mDC function can lead to suppression of virus replication and support the use of adoptive mDC therapy to bypass defects in immune stimulation.
Other trials that have utilized non-autologous sources of HIV-antigens have been immunogenic, but overall less successful in terms of virologic control. Canarypox vectors have been studied in vitro and administered in human therapeutic trials loaded onto moDCs (80, 98). Preclinical studies by our group revealed that human moDCs infected with recombinant canarypox-HIV efficiently expanded both CD4+ and CD8+ T cells, including CTLs from HIV-infected donors that were cytolytic, and secreted IFNγ and β-chemokines (98). A phase I/II trial that compared vaccination of moDCs infected with canarypox-HIV versus canarypox-HIV alone in patients suppressed on ART found that a greater number of patients in the moDC group had a VL set point below 5000 c/ml during analytic treatment interruption (31% versus 0%), but this result was transient and did not affect the mean viral load setpoint during interruption of ART (80). Similarly, recent trials that have administered moDC electroporated with mRNA encoding HIV-1 genes resulted in the generation of vaccine-specific adaptive responses (87–89, 91). In two of these trials, interruption of ART was performed with partial viral control in one study (91) but no impact on viral set point in a somewhat larger study (87).
Although it appears that there may be an advantage to whole, inactivated, autologous virus as an immunogen for moDC immunotherapy, its use is fraught with the most practical limitations. It requires propagation of large quantities of virus from donor cells, which is particularly difficult in patients receiving ART, and subsequent inactivation with stringent testing to confirm lack of residual infectivity. It is possible that inactivated autologous virus may confer advantages over other immunogens due to presentation of relevant, donor-specific epitopes and/or differences in antigen processing, though it can be argued that some of these autologous epitopes may have already undergone escape at the time of vaccination.
It remains unclear which vaccine-induced responses represent the best measures of viral control in these trials. When virologic responses have been attained, a correlation with increased HIV-specific adaptive immune responses has been observed (83, 85, 86). However, the generation of these responses has not been sufficient for improved viral control in certain trials (80, 82, 88). It may relate simply to differences in the magnitude of HIV-specific responses, which can be difficult to compare between trials. Furthermore, more subtle differences in the quality (e.g. polyfunctionality, cytotoxicity, TCR avidity) and specificity of the vaccine-induced T-cell responses must be teased out as well as better characterization of their impact on innate responses, including NK cell activity. Despite the modest success of these approaches, there is room for improvement on measures of both efficacy and feasibility. There are obvious practical constraints when considering the use of mDC immunotherapy on a larger scale. Measures for improved efficacy exist on multiple levels including optimization of the in vitro mDC maturation stimulus to induce maximal costimulation and IL-12 secretion, the dose and form of antigen, the number of mDCs to inject, and frequency and route of immunization. In addition, moDCs should be not be derived in autologous plasma, as is often performed, but rather in seronegative clinical grade plasma or serum to avoid potential moDC suppression in vitro (19).
The use of animal models has shed light on further constraints of mDC immunotherapies by highlighting the critical role of endogenous mDCs during these interventions (99). In mice that were immunized with bone marrow-derived DCs, it was shown that the injected DCs indirectly prime naive CD8+ T cells in vivo by transferring antigen to endogenous CD11c+ DCs. In these studies, mice that had undergone selective reduction of CD11c + DCs were injected with DCs that had been pulsed with either peptide [lymphocytic choriomeningitis virus (LCMV)] or protein (ovalbumin). CD8+ T-cell priming by both peptide and whole protein-loaded DC vaccines was reduced in mice that were depleted of endogenous CD11c+ DCs. Therefore, during DC immunotherapy, there is a partial reliance upon endogenous DCs to uptake antigen from injected DCs to prime CD8+ T cells, presumably via cross-presentation (99). This may explain some of the limited success of mDC immunotherapy for HIV infection, especially given that endogenous mDC function is compromised. It is possible that more successful mDC immunotherapy for HIV-1 will require the simultaneous activation of endogenous DCs with a systemic adjuvant to become effective APCs upon encountering injected mDCs carrying HIV-1 antigens. Determining the appropriate systemic adjuvant to activate endogenous mDCs is complicated by the presence of the immunosuppressive factors that are present in situ. Adjuvants that are tested in seronegative donors may not have comparable effects in HIV-infected subjects and must be evaluated in this context to determine their potency. Immunization of patients suppressed on ART is likely preferable due to partial alleviation in impaired mDC response to adjuvants, such as TLR ligands (19).
Given limitations concerning feasibility and efficacy of mDC immunotherapies for HIV-1, interventions that directly target mDCs in situ are ultimately preferable. Multiple strategies are under investigation and include targeting mDCs by coupling HIV-protein to monoclonal antibodies toward endocytic receptors on mDCs, utilization of recombinant vectors that naturally target mDCs, or HIV-1 antigens co-formulated with mDC-activating adjuvants.
One way to target antigens for delivery to mDCs is to couple immunogens to antibodies toward endocytic receptors on mDCs. This approach has been explored to enhance the immunogenicity of protein-based vaccines for HIV-1. Preclinical work has been undertaken to investigate the use of targeting antibodies toward numerous receptors on mDCs including DEC-205, Clec9, DCIR, and Langerin (100–102). DEC-gag, a vaccine comprised of the HIV p24 gag protein fused with a monoclonal antibody toward CD205/DEC205, a c-type lectin highly expressed on mDCs (100, 102) is the furthest along of these works in clinical development. Targeting proteins to DEC205, a specialized receptor for cross-presentation, enhances CTL responses which are generally poorly elicited by protein-based vaccines (100). The use of DEC-gag has been studied in mice and NHP, and trials are now ongoing in healthy volunteers (100, 102–105). These trials have administered DEC-gag with an adjuvant, poly(I:C) (dsRNA, TLR3 agonist), as a means to activate endogenous mDCs at the time of targeted antigen delivery. Mice immunized with DEC-gag with poly(I:C) demonstrated increases in CD8+ T-cell responses and improved protection against viral challenge following a DNA boost (102). Through adoptive transfer of CD4+ helper T cells generated from DEC-gag immunized wildtype mice to CD40−/− mice, it was determined that the mobilization of gag-specific CD8+ T cells to the viral challenge site was driven by CD40-expressing mDCs (102). In NHP, HIV gag p24 protein alone versus DEC-gag was given with or without PolyICLC [stabilized form of poly(I:C)] (105). Both vaccines elicited CD4+ T-cell responses, but DEC-gag improved CD8+ T-cell responses. These responses were contingent upon the addition of PolyICLC, and were further boosted with New York vaccinia virus-HIV gag/pol/nef. The findings of these studies were confirmed in humanized mouse models (103), and trials of DEC-gag with PolyICLC are now ongoing in healthy volunteers with induction of both T and B-cell responses (104). The mDC CD141+ (BDCA-3) subset may be an important cell to target, as it expresses both TLR3 and TLR8 in addition to clec9 (DNGR1), which has been shown to be critical for cross-presentation (106, 107). Thus, this subset not only produces large amounts of type I IFN but also efficiently cross-primes CD8+ T cells. Future studies targeting clec9 may actually direct antigen to the most desirable mDC subset in vivo for generation of antiviral immunity.
HIV-1 therapeutic vaccine trials in humans have most extensively utilized recombinant vectors, with most studies focusing on the use of Pox vectors, primarily canarypox (ALVAC) (108–112) and modified vaccinia Ankara (113–115), and less so with Adenovirus vectors (116). While the majority of these have proven to be immunogenic, viral control has been minimal and/or transient (108, 110, 112, 115, 116). ALVAC-HIV (combined with recombinant gp120) was also used in the partially successful preventive HIV-1 vaccine trial, RV144 (117). Although direct comparison of these trials is complicated by many factors, the greater success by ALVAC-HIV in the preventive setting highlights the potential hindrance of the immunosuppressive environment and compromised immunity during established infection. New recombinant vectors that are able to simultaneously target mDCs in situ to deliver HIV-1 antigens while providing a strong adjuvant effect to offset immunosuppressive factors are currently under preclinical development. Taking advantage of our recent knowledge regarding mDC restriction of HIV-1 infection, lentiviral (LV) vaccine vectors have been engineered to package vpx to counteract the restriction factor SAMHD1 to achieve productive infection of mDCs (118). mDCs infected with vpx-packaging LV vectors are transduced at a significantly higher frequency (60-fold higher). These vpx-LV vectors have been further modified to express CD40L, an immunostimulatory protein, which results in optimal moDC maturation and secretion of an array of cytokines, including IL-12p70. Notably, CTL responses from antigen-specific clones are enhanced four-fold upon the addition of CD40L (118). These vectors represent a novel vaccine platform that relieves mDC restriction to LV infection to efficiently deliver both antigen and activation signals to mDCs.
Highly attenuated, non-replicative Listeria monocytogenes (Lm) vectors expressing full length HIV-1 gag (Lm-gag) have been studied as a means to combat suppressive factors present during HIV-1 infection (119). Lm is a unique intracellular bacterium that naturally infects and potently activates mDCs, resulting in the generation of both CD4+ and CD8+ T-cell immunity, resulting in lifelong protection following a single exposure (120). Lm provides activation signals via a multitude of membrane-bound and cytoplasmic PRRs, including TLRs, NLRs, and Rig-I-like receptors (121–127). In vitro infection of mDCs with Lm-gag rescues the defect in secretion of IL-12p70 and other pro-inflammatory cytokines observed upon TLR stimulation in the setting of HIV-1 infection, which restores mDC capacity to skew Th1 responses from naive CD4+ T cells (119). Lm-gag-infected mDCs are able to expand polyfunctional gag-specific CD4+ and CD8+ T-cell responses from HIV-infected donors. Therefore, attenuated, recombinant Lm vectors may represent an effective strategy to deliver HIV-1 antigens while optimally activating mDCs in situ. Moreover, a better understanding of the mechanisms by which Lm counters mDC suppressive factors during HIV-1 infection may lead to the development of novel adjuvants for HIV-1 vaccines and immunotherapies.
The co-formulation of HIV-1 antigens with mDC adjuvants represents a practical approach to HIV-1 therapeutic vaccines. The emerging field of nanotechnology has led to the development of the novel nanoparticle-based vaccine DermaVir. Dermavir is comprised of plasmid DNA encoding 15 HIV-1 antigens that self-assemble into VLPs (128). The specialized topical delivery mode of Dermavir improves upon standard DNA vaccines, which do not target mDCs and have been poorly immunogenic (129–131). To provide both access and danger signals to epidermal LCs, skin is first abraded with a device to produce reproducible scarification prior to topical application (128). In a dose escalation study to evaluate safety and immunogenicity in HIV-infected patients receiving ART, Dermavir induced the potent expansion of HIV-specific (Gag, Tat, Rev) memory T cells at 4 weeks post vaccination that largely waned at 1 year follow-up (132). No interruption of ART was performed in this study, so the impact on viral control remains to be seen.
Although this review focuses on DC dysfunction during established HIV-1 infection and potential therapeutic interventions, DCs also have an important impact on B-cell function relevant to preventive HIV vaccine design. To generate long-lived antibody responses from memory B cells, follicular DCs play a key role in promoting the development of IL-21-producing T-follicular helper cells in vivo (133–135). There is also evidence that mDC dysregulation during HIV-1 infection contributes to B-cell dysregulation. mDC upregulation of B-lymphocyte stimulator (BlyS) has been correlated with uncontrolled B-cell proliferation and hypergammaglobulinemia in acute and chronic HIV-1 infection compared with aviremic non-progressors (136). These findings further assert that harnessing mDC function may improve the efficacy of preventive vaccines for a variety of pathogens for use in HIV-infected individuals.
pDCs and HIV-1 infection
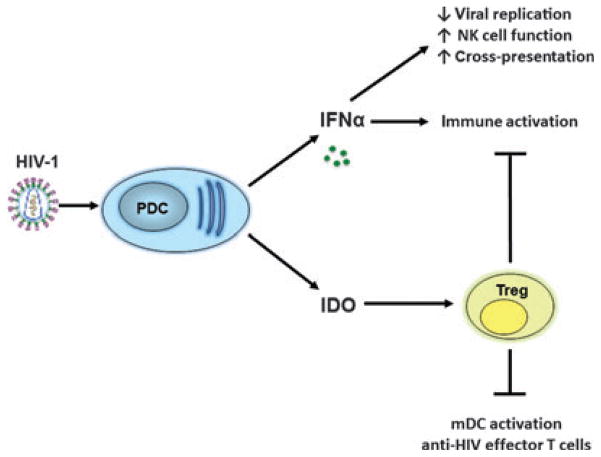
pDCs are the most potent producers of type I IFNs (IFN α/β) to generate innate antiviral immune responses. They were first recognized as a DC subset by Liu and Siegal (137) and independently by a student in our laboratory (138, 139). While Liu and Siegal (137) identified the major type I-IFN producing cells in blood as pDCs, O’Doherty (138, 139) identified pDCs based on key difference between these cells and their conterpart mDCs in blood. pDCs possess fewer and distinct TLRs from mDC, expressing only TLR7 and TLR9 to recognize single-stranded RNA and double-stranded DNA viruses, respectively (3). In addition to secretion of type I IFNs upon stimulation, pDCs also have the ability to upregulate surface MHC and costimulatory molecules and secrete pro-inflammatory cytokines, including TNFα and IL-6. Similar to mDCs, pDCs largely restrict productive infection with HIV-1 (10). The mechanisms of this restriction are not well defined, but may be due to expression of factors such as SAMHD1 (Bloch et al., unpublished observation), IFN-induced tetherin (140, 141), and likely others. Unlike mDCs, pDCs are able to adequately sense HIV-1 resulting in robust IFNα secretion. Although type I IFNs secreted by pDCs contribute to innate antiviral functions by inhibiting HIV-1 replication, dysregulation of these cells during HIV-1 infection may be involved in immunopathogenesis by contributing dichotomously to both immune activation and immunosuppression (Fig. 3).
Fig. 3. The contrary roles of plasmacytoid DCs (pDCs) in HIV-1 infection.
HIV-1 stimulates pDCs to produce interferonα, which has antiviral functions, but persistent secretion also contributes to immune activation. HIV-1 induces pDCs to secrete IDO, which directs the formation of Tregs. Tregs have a dual role in dampening both immune activation but also myeloid DC activation and HIV-specific effector T-cell responses.
pDC frequency during HIV-1 infection
Similar to mDCs, circulating pDCs are diminished in number during HIV-1 infection (10, 142–144). Reduced numbers of circulating pDCs reflects trafficking to lymphoid tissues, accelerated death, and possibly reduced differentiation from bone marrow precursors (145–147). The number of circulating pDCs has been positively correlated to CD4+ T-cell count and negatively correlated with viral load and disease progression (142, 144, 148, 149). Sabado et al. (10) examined pDC frequency during primary infection and found a sharp decline as early as Fiebig stages I and II, and numbers remained low throughout all stages of infection, including following treatment with cART. Although numbers of pDCs remain lower throughout infection compared with control subjects, longitudinal analysis revealed that they increase significantly within the initial 200 days postinfection in both treated and untreated patients (10). Studies during chronic infection have demonstrated that the loss of circulating pDCs is partially mitigated by ART, but does not return to levels of controls (142, 144).
Mechanism of pDC activation by HIV-1
Unlike mDCs, pDCs are highly activated by HIV-1 to produce IFNα (25, 150). pDCs secrete high levels of IFNα following exposure to both live and inactivated strains of HIV-1 but not envelope-deficient strains (25). To investigate the mechanism by which HIV-1 enters and activates pDCs to secrete IFNα, Beignon et al. (150) determined whether envelope-receptor interactions trigger signaling that induces pDC activation or alternatively facilitates routing of HIV-1 nucleic acids to TLR-expressing endosomal compartments to stimulate activation. Secretion of IFNα is dependent upon binding of gp160 to CD4 on pDCs, as evidenced by neutralization of gp-120 using monoclonal antibody or blockade of CD4 with anti-CD4 antibodies. However, binding of envelope to CD4 is not sufficient to activate pDCs, as recombinant HIV-1 gp160 fails to induce IFNα secretion (150). The dependence upon CD4 binding to envelope for pDC activation was supported by Haupt et al. (151), who found a correlation with viral affinity to CD4 with the magnitude of IFNα secretion.
It was further established that pDC activation is dependent upon endocytosis and subsequent acidification of endosomal compartments but not viral fusion with the cell membrane (150). Inhibitors of endocytosis including cytochalasin D, dimethyl amiloride, and chlorpromazine inhibit the secretion of IFNα following exposure to HIV-1, along with inhibitors of endosomal acidification including chloroquine, ammonium chloride, quinacrine, and bafilomycin. However, addition of a peptide (C34) that inhibits post-CD4 binding conformational changes in transmembrane gp41 required for viral fusion does not inhibit IFNα secretion by pDC (150).
The contribution of HIV-1 nucleic acids to pDC activation was delineated through delivery of both HIV-1 RNA and DNA via cationic lipids to pDC (150). HIV-1 RNA is able to robustly stimulate pDCs, but linear pNL4-3 DNA delivered to pDCs is unable to stimulate pDCs in comparison with HIV-1NL4-3 virions (wildtype, X4 tropic virus), indicating that HIV-1 DNA (retrotranscripts) is not critical to stimulate pDCs. Furthermore, activation of pDCs by HIV-1 RNA is reversible with RNAse treatment, and recombinant virions deficient in viral RNA do not stimulate pDCs to produce IFNα. This stimulation is TLR-dependent, and using genetic complementation, it has been shown that TLR7 is the likely primary target, not TLR9 (150). This was further supported by short interfering RNA knockdown of TLR7 in a pDC cell line that abrogated the majority of IFNα secretion following HIV-1 exposure (152).
Although pDCs are strongly activated to secrete IFNα in response to HIV-1, they are only partially matured to upregulate costimulatory molecules and secrete proinflammatory cytokines, making them less competent antigen-presenting cells (25, 153). Following activation with HIV-1, pDCs upregulate CD83 and CCR7 and acquire migratory capacity toward CCL19, the ligand of CCR7 (25). However, when compared with other TLR7 agonists including R848 and influenza, HIV-activated pDCs are not completely matured by HIV-1 in terms of CCR7, CD40, and CD86 expression (153). This partially matured phenotype in HIV-exposed pDCs results in low proliferative responses by CD4+ and CD8+ T-cell responses compared with other stimuli including CpGB (TLR9 agonist) and influenza (TLR7 agonist) (153). Similarly, HIV-exposed pDCs secrete proinflammatory cytokines, including TNF and IL-6, but levels tend to be lower than that of other pDC stimuli including influenza (153). Utilizing confocal microscopy, O’Brien et al. (153) revealed that HIV-1 traffics to early endosomes unlike other ligands that traffic to late endosomes/lysosomes, which results in a strong IFNα signal but incomplete maturation and lower secretion of NF-κB-dependent inflammatory cytokines (153). This finding is supported by studies in pDCs, revealing that endosomal trafficking of various stimulating ligands determines whether pDCs become primarily IFN producers versus APCs. Such work has been primarily been carried out using various CpGs in pDCs that traffic differently due to differences in their sequences and/or secondary structures. CpGB remains in a monomeric form and traffics to lysosomes to activate NF-κB-dependent proinflammatory responses. Conversely, CpGA, which forms multimolecular complexes, traffics to and is retained in early endosomes resulting in primarily a strong IFNα signal (154). In summary, HIV-1 stimulates pDCs via viral envelope binding to CD4, followed by receptor-mediated endocytosis, and trafficking of viral RNA to TLR-expressing early endosomal compartments to induce a phenotype characterized by robust type I IFN secretion and weaker antigen presentation capacity.
Role of pDC in chronic immune activation during HIV-1 infection
Progression to acquired immunodeficiency syndrome (AIDS) is strongly associated with persistent generalized immune activation of both HIV-specific and non-specific T cells (155, 156). Chronic immune activation eventually leads to T-cell exhaustion characterized by loss of effector and proliferative T-cell responses, upregulation of inhibitory molecules such as CTLA-4 and PD-1, and apoptosis (66, 157, 158). Immune activation also facilitates HIV-1 infection of CD4+ T cells, especially HIV-specific CD4+ T cells (159–161). Notably, markers of immune activation are now known to provide greater prognostic significance to disease progression than viral load (162–164). Immune activation also contributes to increased risk of non-AIDS conditions such as cardiovascular, liver and kidney diseases, and non-AIDS-defining malignancies (165–169). The pathogenesis of chronic immune activation is complex and incompletely delineated, but triggering of innate immune cells by HIV-1, products of microbial translocation, activated platelets, and other viruses (e.g. herpes viruses) likely play a major role (72, 170, 171). Although type I IFNs possess potent antiviral properties in the appropriate setting, both human and animal studies support a role of chronically elevated IFNα in immune activation and immunopathogenesis of HIV/AIDS (172–176). IFNα levels during HIV-1 infection correlate with disease progression and can lead to apoptosis of uninfected CD4+ T cells through upregulation of TNF-related apoptosis-inducing ligand (173, 176). Furthermore, administration of exogenous IFNα was shown to increase CD8+ T-cell activation in HIV-infected subjects (175). Given that pDCs serve as the most potent producers of IFNα, dysregulation of this cell type may underwrite these processes. Indeed, women have pDCs that produce greater amounts of type I IFN on a per-cell basis and their infection progresses faster than that in men with similar viral loads (177).
Studies of pathogenic versus non-pathogenic SIV infection in NHP models have further supported a role of pDC-associated type I IFN in chronic immune activation. Following infection with SIVSM or SIVAGM, respectively, sooty mangabeys and African green monkeys activate an acute innate immune response including production of type I IFNs and IFN-stimulated genes (ISGs). However, unlike pathogenic SIV infection in rhesus macaques, where the production of type I IFN is sustained, non-pathogenic infection is characterized by resolution of IFN production and associated ISGs, upregulation of defensins, suppressors of the IFN response and factors that dampen T-cell activation (IDO, IL-10, PDL-1, and LAG3)(174, 178). During chronic infection, there is upregulation of the immune dampening genes TGFβ and IL-10, indicating that non-pathogenic infection is characterized by a distinct set of responses contrived to prevent sustained immune activation. In contrast, pathogenic infection is characterized by chronic expression of ISGs, accompanied by enhanced expression of proapoptotic factors, as well as markers of T-cell activation and exhaustion and of inflammation (174, 178). In AIDS-susceptible rhesus macaques, preventing recruitment of pDCs expressing high levels of B7-integrin to the colorectum via in vivo blockade of α4β7 integrin results in dampened immune activation. B7-integrin on circulating pDCs is also upregulated in HIV-infected humans but not in sooty mangabeys, which display much lower levels of immune activation during SIV infection (179). Interestingly, administration of agonist IFN to sooty mangabeys did not worsen immune activation, although it transiently reduced viral loads (180). This observation suggests that sooty mangabeys deploy durable and potent mechanisms to contain immune activation during chronic infection.
Chronic type I IFN production is associated with T-cell exhaustion in addition to apoptosis. T-cell exhaustion is characterized by dysfunctional T cells that express markers of exhaustion including PD-1 and CTLA-4 (66, 157, 158). Recently, PD-1 blockade was shown to suppress hyperimmune activation by durably blocking expression of transcripts associated with type I IFN signaling in blood and colorectal tissue of SIV-infected rhesus macaques (181). Interestingly, this was associated with increased expression of gut-associated junction genes, decreased microbial translocation into the blood, improved immunity to gut-resident and opportunistic pathogenic organisms, and longer survival.
O’Brien et al. (153) described an in vitro phenotype of human pDC hyperreactivity to HIV-1 that may serve as a source of persistent levels of IFNα during HIV-1 infection. As a general rule, pDCs develop tolerance to IFNα production following the initial stimulus with TLR7 or TLR9 agonists, which can be considered a protective mechanism against undesired, persistent immune activation (153, 182, 183). This phenomena is also described for certain viruses that stimulate pDCs via TLR7 including influenza and Sendai viruses (153). In contrast, pDCs are permissive to repeated stimulation with HIV-1 resulting in persistent secretion of IFNα following successive exposures. HIV-activated pDCs constitutively express higher levels of the transcription factor IRF7, which correlates to their ability to secrete secondary IFNα. This expression is IFN-inducible and dependent upon continuous autocrine IFNα/β receptor feedback provided by low levels of type I IFN. Interestingly, pDCs stimulated with R848 (TLR7/8 agonist) sharply upregulate SOCS3, a negative regulator of IFNα when overexpressed, which might contribute to inhibition of continued IFNα production (153). In contrast, AT-2 HIV only slowly upregulated SOCS3 in pDCs and to overall much lower levels than R848. Confocal microscopy revealed that HIV-1 traffics to early endosomes rather than late endosomes favoring continued IFNα secretion, as well as weak maturation, reduced NF-κB-dependent responses, and only a low level of induced T-cell immunity (153). These findings delineate a possible mechanism by which HIV-1 may simultaneous blunt adaptive immune responses to HIV-1 by promoting a partially matured phenotype in pDCs while contributing to immune activation by providing a continuous source of IFNα (153).
Ex vivo studies provide support of this phenotype described by O’Brien et al. (153), suggesting that during both acute and chronic HIV-1 infection, pDCs are a persistent source of IFNα production. Sabado et al. (10) described evidence of ongoing IFNα expression by pDC during AHIV. pDCs were purified during early infection, ranging from Fiebig stages I–VI, with the majority of donors in Fiebig stages V and VI. Gene expression profiles indicated the presence of in vivo activation of pDCs during early HIV-1 infection, with HIV-1 donor pDCs expressing significantly higher levels of TLR7 and IRF7 compared with controls. Findings of high basal IFNα expression by pDCs have been shown to persist during chronic infection. pDCs isolated from both blood and lymphoid tissue display increased spontaneous expression of IFNα (147, 172, 184). Similar to the in vitro phenotypic findings of O’Brien et al. (153), these pDCs in the lymph nodes did not upregulate maturation markers compared with controls, suggesting they are activated to produce IFNs but not become optimal APCs.
In addition to heightened IFNα expression at baseline, Sabado et al. (10) demonstrated that isolated pDC from AHIV infection may also be hyperreactive to ex vivo stimuli. pDCs isolated during Fiebig stages V and VI produced significantly higher levels of IFNα in response to in vitro stimulation with AT-2-inactivated HIV-1 compared with controls. Moreover, in response to ex vivo stimulation with R848 (TLR 7/8 agonist), pDCs from HIV-infected donors secreted not only higher levels of IFNα but also a broader array of proinflammatory cytokines and chemokines (IL-6, MIP-1α, MIP-1β, RANTES, and TNFα) compared with control pDCs. Therefore, as early as acute infection, pDCs are hyperreactive indicating a possible role in promoting immune activation. These results were partially discrepant from findings by Huang et al. (185) regarding pDC function in primary HIV-1 infection. In this study, PBMCs from acute and early HIV-infected individuals were stimulated with CLO97 (TLR 7/8 agonist), followed by intracellular cytokine staining to evaluate pDC cytokine secretion (185). Per-cell intensity of TNFα and IL-6 tended to be higher in primary HIV-1 donors compared with controls, in line with the findings by Sabado et al. (10). Conversely, IFNα production by HIV-pDCs was lower than controls in response to stimulation with CLO97. Significant differences in methods between these studies may contribute to the divergent findings including the use of isolated pDCs versus whole PBMCs, the measurement of secreted cytokines versus intracellular cytokine staining, and the use of different TLR7 agonists as pDC stimuli. In other studies ranging from early to chronic infection, pDCs that were stimulated ex vivo with various TLR7 and TLR9 agonists also showed diminished secretion of IFNα and decreased ability to activate NK cells (184, 186, 187). Taken together, the existing data suggest that some degree of pDC exhaustion likely occurs as HIV-1 infection progresses, presumably due to chronic stimulation in situ by HIV-1, products of microbial translocation, and other immunomodulatory factors, which may lead to a decreased responsiveness to exogenous stimuli. Therefore, while it is apparent that pDCs are a persistent source of IFNα in vivo during HIV-1 infection that may contribute to chronic immune activation, over time exhaustion of these cells may occur adding to the multifaceted picture of immune dysfunction during HIV-1 infection. Recent work with LCMV infection in mice supports these observations and implicates a potential therapeutic role for blockade of type I interferon signaling in chronic infection (188, 189). In both studies, blockade of type I IFN signaling diminished immune activation and negative immune regulators, restored architecture of lymphoid tissue, and enhanced viral control and clearance in a CD4+ T-cell-dependent manner. These observations again emphasize the central and multifaceted immunoregulatory contributions of type I IFN in chronic viral infections. Future studies are warranted to determine whether direct IFN blockade or indirect targeting of the type I IFN pathway in pDCs represents a viable treatment strategy to improve immune activation and facilitate viral control.
Roles of pDCs in immunoregulation during HIV-1 infection
Despite their potential role in chronic immune activation, pDCs may also play a contrary role in immunoregulation during HIV-1 infection through the induction of Treg responses. Tregs suppress activation of T cells and DCs and may have both deleterious and beneficial functions during HIV-1 infection. On one hand, they may limit the formation of HIV-specific T-cell responses, but alternatively, they can serve to alleviate chronic immune activation. Which of these roles predominates during the course of HIV-1 infection remains controversial. The frequency of Tregs inversely correlates with SIV-specific CTL responses in NHPs (190) and immune reconstitution in humans (191). When CD4+CD25+ cells from HIV-infected donors have been studied in vitro, they have been shown to hinder the proliferation and cytokine secretion by CD4+ and CD8+ T cells in response to HIV-1 antigens (192). In contrast, low Treg frequency during chronic HIV-1 infection is associated with increased immune activation (193). Studies regarding the identification of Tregs during HIV-1 infection have been complicated by difficulties in definitively identifying this phenotype due to ambiguity in expression markers. Cells that express high CD25 and Foxp3, low CD127, and also express the inhibitory molecules cytotoxic T-lymphocyte antige-4 (CTLA-4) and glucocorticoid-induced TNF receptor have a high probability of being Tregs (194), though not all studies have employed these stringent criteria.
While the exact role Tregs play during the various stages of HIV-1 infection remains to be elucidated, it appears that pDCs are important in generation of these cells during HIV-1 infection. Manches et al. (195) described the development of suppressive Foxp3+CD127loCD25+ cells from naive CD4+ T cells by HIV-exposed pDCs consistent with the phenotype of Tregs. Treg induction relates to the expression of indolemine 2,3 dioxygenase (IDO), an enzyme that is critical in tryptophan catabolism. IDO expression by pDCs occurs via endocytosis of HIV-1 following gp120-CD4 binding, followed by endosomal TLR7 triggering. Using a competitive inhibitor of IDO, 1-methyl tryptophan and short interfering RNA knockdown, it was determined that expression of IDO is primarily required for Treg generation by pDCs. Interestingly, the induction of Tregs cells in this IDO-dependent pathway was independent of type I IFN production or of other molecules expressed by HIV-stimulated pDC including ICOSL. The generation of Tregs following HIV-pDC interaction may help explain the strong negative correlation of viral load and allogeneic T-cell proliferative capacity of pDC isolated from HIV-infected individuals during acute infection (10). It is possible that high levels of circulating virus can negatively impact T-cell stimulatory capacity of pDCs due to induction of Tregs by HIV-exposed pDCs during acute and chronic infection. Indeed, acute SIV infection is associated with rapid development of Tregs and elevated IDO expression (190). The skewing toward Treg responses by HIV-exposed pDCs may be implicated in decreased Th17 responses observed during HIV-1 infection (196), leading to a breakdown of gut integrity and higher levels of bacterial translocation and immune activation (72). In HIV-infected donors, disease progression is associated with elevated Tregs in peripheral blood and gut related to high levels of IDO and reduced Th17 responses (196).
In a later study, Manches et al. (197) demonstrated that TLR-induced IDO expression in pDCs is dependent upon the non-canonical NF-κB pathway and independent of the canonical NF-κB pathway. Upon TLR7 signaling, there is release of TRAF3 to the IRAK1/TRAF6/IRAK4 complex, which allows NIK to activate the non-canonical pathway. These pDC-induced Tregs were able to suppress proliferative CD4+ T-cell responses (197). Furthermore, they were shown to limit the phenotypic maturation of mDCs upon stimulation with LPS and resiquimod (TLR7/8 agonist), which would serve to hinder their antigen presentation capacity. This suppression occurs partially through CTLA-4 engagement. Therefore, in addition to blunting T-cell responses directly during HIV-1 infection, pDC-induced Tregs may also inhibit their induction and effector function by impairing antigen presentation by mDCs (197). This finding highlights another dichotomous role of pDCs during HIV-1 infection, given the previous findings that HIV-exposed pDCs can elicit bystander activation and maturation of mDCs through the secretion of pro-inflammatory cytokines, primarily TNFα, to enhance their antigen presentation capacity (25). Clearly, finding the appropriate balance of all of these factors is crucial, and an improved understanding of the optimal equilibrium of Tregs during HIV-1 infection will be vital to understanding potential beneficial interventions in order to manipulate their function. To better delineate the role of Tregs in the SIV model, attempts to block IDO with the inhibitor 1-methyl tryptophan (1mT) have been undertaken (198, 199). Only one of these studies successfully increased the level of circulating tryptophan in animals following IDO blockade with 1mT, and this resulted in improved viral control in animals receiving ART (198). Future studies that optimize IDO blockade and Treg inhibition during SIV may be illustrative in determining whether therapeutics that target Tregs are of value in improving viral control.
Summary and conclusions
We describe the differential dysregulation of mDCs and pDCs at various stages of HIV-1 infection, providing insight into immunopathogenesis. HIV-1 evades innate immune sensing by mDCs resulting in suboptimal maturation, lending to poor generation of antiviral immunity and contributing to Treg development. In addition to weak recognition of HIV-1, mDCs display perturbed responses to traditional stimuli, such as TLR ligands, which vary depending on the stage of infection. At very early stages of AHIV, mDC secretion of pro-Th1 skewing cytokines is strongly hindered, resulting in inadequate stimulation of NK cells and adaptive antiviral responses. In later stages of AHIV, mDCs conversely display hyperreactivity contributing to overall immune activation, followed by resumption of cytokine restriction during chronic infection that is only partially alleviated by suppressive ART. Evidence suggests that the etiology of mDC dysregulation is multifactorial, implicating a combination of viral and non-viral factors, including yet-to-be identified suppressive plasma factors. Altered mDC function throughout HIV-1 infection may pose a significant obstacle to therapeutic vaccine strategies that aim to enhance HIV-specific adaptive responses. Attempts to overcome mDC suppressive factors with the use of mDC adoptive immunotherapy and mDC-targeting vaccines have had only a modest degree of success. The future and potential success of therapeutic HIV-1 vaccines may lie in our improved ability to activate mDCs in situ to combat suppressive factors. Focus should be placed on further investigation of effective adjuvants and/or vectors in the context of altered mDC function during HIV-1 infection. A more complete understanding of the factors that lead to mDC suppression will have important implications for the design of such adjuvants.
pDC interactions with HIV-1 are pleotropic, modulating immune responses on an axis that fluctuates between immunostimulatory and immunosuppressive. HIV-1 traffics to early endosomal TLR-expressing compartments following CD4 binding on pDC, resulting in robust and persistent secretion of type I IFN, but only weak enhancement of antigen presentation. By serving as the major source of chronic type I IFN secretion during HIV-1 infection, pDCs contribute to immune activation and exhaustion. In contrast, HIV-1 also stimulates secretion of IDO by pDCs that results in the induction of Tregs, which not only serve to blunt anti-HIV immune responses but also may dampen chronic immune activation. Additional studies that directly or indirectly block type I IFN signaling and IDO expression by pDCs in vivo will be helpful to clarify their contribution to both immune activation and viral control. A more refined understanding of the appropriate balance of each of these factors will be vital to the design of therapeutics to enhance HIV-specific adaptive responses and reduce immune activation. Ultimately, adjuvants and therapeutics that are able to target both mDCs and pDCs may promote durable antiviral immunity by simultaneously enhancing adaptive responses while limiting Tregs and/or immune activation and thus facilitating progress toward viral eradication.
Acknowledgments
This work has been supported by National Institutes of Health (K08 AI84578 to E. M., AI044628 and AI081848 to N. B., and U01 A1067854); Bill and Melinda Gates Foundation (Collaboration for AIDS Vaccine Discovery Grant ID: 38645); Center for AIDS Research (P01AI057127); and the New York University Langone Medical Center Grunebaum AIDS Research Fund and Saul Farber Scholar Fund. Possible conflict of interest declared by N. B. for receipt of small royalty (<$2000 annually) for a patent related to DC preparation. There is no conflict of interest for E. M.
References
- 1.Borrow P. Innate immunity in acute HIV-1 infection. Curr Opin HIV AIDS. 2011;6:353–363. doi: 10.1097/COH.0b013e3283495996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakao Y, et al. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J Immunol. 2005;174:1566–1573. doi: 10.4049/jimmunol.174.3.1566. [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 6.D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf SF, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 8.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granelli-Piperno A, et al. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabado RL, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapp M, Engelmayer J, Larsson M, Granelli-Piperno A, Steinman R, Bhardwaj N. Dendritic cells generated from blood monocytes of HIV-1 patients are not infected and act as competent antigen presenting cells eliciting potent T-cell responses. Immunol Lett. 1999;66:121–128. doi: 10.1016/s0165-2478(98)00169-2. [DOI] [PubMed] [Google Scholar]
- 12.Smed-Sorensen A, et al. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J Virol. 2005;79:8861–8869. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buisson S, Benlahrech A, Gazzard B, Gotch F, Kelleher P, Patterson S. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. J Infect Dis. 2009;199:1862–1871. doi: 10.1086/599122. [DOI] [PubMed] [Google Scholar]
- 14.Chang JJ, et al. Differential regulation of toll-like receptor pathways in acute and chronic HIV-1 infection. AIDS. 2012;26:533–541. doi: 10.1097/QAD.0b013e32834f3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Z, Huang XL, Kalinski P, Young S, Rinaldo CR., Jr Dendritic cell function during chronic hepatitis C virus and human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2007;14:1127–1137. doi: 10.1128/CVI.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frleta D, et al. HIV-1 infection-induced apoptotic microparticles inhibit human DCs via CD44. J Clin Invest. 2012;122:4685–4697. doi: 10.1172/JCI64439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci USA. 2004;101:7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majumder B, et al. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J Virol. 2005;79:7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller EA, et al. Plasma factors during chronic HIV-1 infection impair IL-12 secretion by myeloid dendritic cells via a virus-independent pathway. J Acquir Immune Defic Syndr. 2012;61:535–544. doi: 10.1097/QAI.0b013e31826afbce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smed-Sorensen A, Lore K, Walther-Jallow L, Andersson J, Spetz AL. HIV-1-infected dendritic cells up-regulate cell surface markers but fail to produce IL-12 p70 in response to CD40 ligand stimulation. Blood. 2004;104:2810–2817. doi: 10.1182/blood-2003-07-2314. [DOI] [PubMed] [Google Scholar]
- 21.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–271. [PubMed] [Google Scholar]
- 22.Almeida M, Cordero M, Almeida J, Orfao A. Persistent abnormalities in peripheral blood dendritic cells and monocytes from HIV-1-positive patients after 1 year of antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;41:405–415. doi: 10.1097/01.qai.0000209896.82255.d3. [DOI] [PubMed] [Google Scholar]
- 23.Dillon SM, et al. Blood myeloid dendritic cells from HIV-1-infected individuals display a proapoptotic profile characterized by decreased Bcl-2 levels and by caspase-3+ frequencies that are associated with levels of plasma viremia and T cell activation in an exploratory study. J Virol. 2011;85:397–409. doi: 10.1128/JVI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yonkers NL, Rodriguez B, Asaad R, Lederman MM, Anthony DD. Systemic immune activation in HIV infection is associated with decreased MDC responsiveness to TLR ligand and inability to activate naive CD4 T-cells. PLoS ONE. 2011;6:e23884. doi: 10.1371/journal.pone.0023884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fonteneau JF, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 27.Hodges A, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 28.Lester RT, et al. Toll-like receptor expression and responsiveness are increased in viraemic HIV-1 infection. AIDS. 2008;22:685–694. doi: 10.1097/QAD.0b013e3282f4de35. [DOI] [PubMed] [Google Scholar]
- 29.Shan M, et al. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alter G, et al. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010;120:1905–1913. doi: 10.1172/JCI40913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tasca S, et al. Escape of monocyte-derived dendritic cells of HIV-1 infected individuals from natural killer cell-mediated lysis. AIDS. 2003;17:2291–2298. doi: 10.1097/00002030-200311070-00003. [DOI] [PubMed] [Google Scholar]
- 32.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki A. Innate immune recognition of HIV-1. Immunity. 2012;37:389–398. doi: 10.1016/j.immuni.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luban J. Innate immune sensing of HIV-1 by dendritic cells. Cell Host Microbe. 2012;12:408–418. doi: 10.1016/j.chom.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstone DC, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480:379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 36.Laguette N, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahouassa H, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012;13:223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell RD, Holland PJ, Hollis T, Perrino FW. Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J Biol Chem. 2011;286:43596–43600. doi: 10.1074/jbc.C111.317628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunseri N, O’Brien M, Bhardwaj N, Landau NR. Human immunodeficiency virus type 1 modified to package Simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells. J Virol. 2011;85:6263–6274. doi: 10.1128/JVI.00346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavaleiro R, et al. Monocyte and myeloid dendritic cell activation occurs throughout HIV type 2 infection, an attenuated form of HIV disease. J Infect Dis. 2013;207:1730–1742. doi: 10.1093/infdis/jit085. [DOI] [PubMed] [Google Scholar]
- 42.Duvall MG, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11:1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Battivelli E, Lecossier D, Matsuoka S, Migraine J, Clavel F, Hance AJ. Strain-specific differences in the impact of human TRIM5alpha, different TRIM5alpha alleles, and the inhibition of capsid-cyclophilin A interactions on the infectivity of HIV-1. J Virol. 2010;84:11010–11019. doi: 10.1128/JVI.00758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pertel T, et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472:361–365. doi: 10.1038/nature09976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 48.Trapp S, et al. Double-stranded RNA analog poly (I:C) inhibits human immunodeficiency virus amplification in dendritic cells via type I interferon-mediated activation of APOBEC3G. J Virol. 2009;83:884–895. doi: 10.1128/JVI.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiebig EW, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 50.Stacey AR, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramer HB, et al. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 2010;6:e1000893. doi: 10.1371/journal.ppat.1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turnbull EL, et al. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J Immunol. 2009;182:7131–7145. doi: 10.4049/jimmunol.0803658. [DOI] [PubMed] [Google Scholar]
- 53.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yonkers NL, et al. Accessory cell dependent NK cell mediated PBMC IFN-gamma production is defective in HIV infection. Clin Immunol. 2009;131:288–297. doi: 10.1016/j.clim.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clerici M, et al. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 56.Dybul M, et al. CD40 ligand trimer and IL-12 enhance peripheral blood mononuclear cells and CD4+ T cell proliferation and production of IFN-gamma in response to p24 antigen in HIV-infected individuals: potential contribution of anergy to HIV-specific unresponsiveness. J Immunol. 2000;165:1685–1691. doi: 10.4049/jimmunol.165.3.1685. [DOI] [PubMed] [Google Scholar]
- 57.Landay AL, Clerici M, Hashemi F, Kessler H, Berzofsky JA, Shearer GM. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J Infect Dis. 1996;173:1085–1091. doi: 10.1093/infdis/173.5.1085. [DOI] [PubMed] [Google Scholar]
- 58.Estaquier J, et al. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villinger F, et al. In vitro and in vivo responses to interleukin 12 are maintained until the late SIV infection stage but lost during AIDS. AIDS Res Hum Retroviruses. 2000;16:751–763. doi: 10.1089/088922200308756. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe N, et al. Administration of recombinant human interleukin 12 to chronically SIVmac-infected rhesus monkeys. AIDS Res Hum Retroviruses. 1998;14:393–399. doi: 10.1089/aid.1998.14.393. [DOI] [PubMed] [Google Scholar]
- 61.Ansari AA, et al. Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques. J Virol. 2002;76:1731–1743. doi: 10.1128/JVI.76.4.1731-1743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 63.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–5944. [PubMed] [Google Scholar]
- 64.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 66.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 67.Blanchet FP, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parrish NF, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci USA. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gasper-Smith N, et al. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J Virol. 2008;82:7700–7710. doi: 10.1128/JVI.00605-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haynes BF, Liao HX, Patton KL. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cell. 1991;3:347–350. [PubMed] [Google Scholar]
- 71.Vachon E, et al. CD44 is a phagocytic receptor. Blood. 2006;107:4149–4158. doi: 10.1182/blood-2005-09-3808. [DOI] [PubMed] [Google Scholar]
- 72.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 73.Hunt PW, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang W, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pandrea I, et al. Cutting edge: experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J Immunol. 2008;181:6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bogunovic D, et al. TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer Res. 2011;71:5467–5476. doi: 10.1158/0008-5472.CAN-10-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S, Licorish K. Circulating immune complexes in AIDS. N Engl J Med. 1984;310:1530–1531. doi: 10.1056/NEJM198406073102312. [DOI] [PubMed] [Google Scholar]
- 78.Shan L, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 80.Gandhi RT, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009;27:6088–6094. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia F, et al. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J Infect Dis. 2005;191:1680–1685. doi: 10.1086/429340. [DOI] [PubMed] [Google Scholar]
- 82.Ide F, et al. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: a phase 1 trial. J Med Virol. 2006;78:711–718. doi: 10.1002/jmv.20612. [DOI] [PubMed] [Google Scholar]
- 83.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 84.Garcia F, Routy JP. Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection Workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine. 2011;29:6454–6463. doi: 10.1016/j.vaccine.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 85.Garcia F, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Dis. 2011;203:473–478. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia F, et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Trans Med. 2013;5:166ra2. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]
- 87.Allard SD, et al. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption. Clin Immunol. 2012;142:252–268. doi: 10.1016/j.clim.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Van Gulck E, et al. mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients. AIDS. 2012;26:F1–F12. doi: 10.1097/QAD.0b013e32834f33e8. [DOI] [PubMed] [Google Scholar]
- 89.Routy JP, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010;134:140–147. doi: 10.1016/j.clim.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Connolly NC, Whiteside TL, Wilson C, Kondragunta V, Rinaldo CR, Riddler SA. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin Vaccine Immunol. 2008;15:284–292. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Routy JP, Nicolette C. Arcelis AGS-004 dendritic cell-based immunotherapy for HIV infection. Immunotherapy. 2010;2:467–476. doi: 10.2217/imt.10.28. [DOI] [PubMed] [Google Scholar]
- 92.Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9:27–32. doi: 10.1038/nm806. [DOI] [PubMed] [Google Scholar]
- 93.Rossio JL, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sabado RL, et al. Pathways utilized by dendritic cells for binding, uptake, processing and presentation of antigens derived from HIV-1. Eur J Immunol. 2007;37:1752–1763. doi: 10.1002/eji.200636981. [DOI] [PubMed] [Google Scholar]
- 95.Larsson M, Fonteneau JF, Lirvall M, Haslett P, Lifson JD, Bhardwaj N. Activation of HIV-1 specific CD4 and CD8 T cells by human dendritic cells: roles for cross-presentation and non-infectious HIV-1 virus. AIDS. 2002;16:1319–1329. doi: 10.1097/00002030-200207050-00003. [DOI] [PubMed] [Google Scholar]
- 96.Lu W, Andrieu JM. In vitro human immunodeficiency virus eradication by autologous CD8(+) T cells expanded with inactivated-virus-pulsed dendritic cells. J Virol. 2001;75:8949–8956. doi: 10.1128/JVI.75.19.8949-8956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lubong SR, et al. In vitro priming recapitulates in vivo HIV-1 specific T cell responses, revealing rapid loss of virus reactive CD4 T cells in acute HIV-1 infection. PLoS ONE. 2009;4:e4256. doi: 10.1371/journal.pone.0004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Engelmayer J, et al. Mature dendritic cells infected with canarypox virus elicit strong antihuman immunodeficiency virus CD8+ and CD4+ T-cell responses from chronically infected individuals. J Virol. 2001;75:2142–2153. doi: 10.1128/JVI.75.5.2142-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS ONE. 2010;5:e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Idoyaga J, et al. Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci USA. 2011;108:2384–2389. doi: 10.1073/pnas.1019547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Klechevsky E, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nchinda G, Amadu D, Trumpfheller C, Mizenina O, Uberla K, Steinman RM. Dendritic cell targeted HIV gag protein vaccine provides help to a DNA vaccine including mobilization of protective CD8+ T cells. Proc Natl Acad Sci USA. 2010;107:4281–4286. doi: 10.1073/pnas.1000621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheong C, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human antihuman DEC205 monoclonal antibody. Blood. 2010;116:3828–3838. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trumpfheller C, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Int Med. 2012;271:183–192. doi: 10.1111/j.1365-2796.2011.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Flynn BJ, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci USA. 2011;108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jongbloed SL, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Poulin LF, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Angel JB, et al. A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune. AIDS. 2011;25:731–739. doi: 10.1097/QAD.0b013e328344cea5. [DOI] [PubMed] [Google Scholar]
- 109.Autran B, et al. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452) AIDS. 2008;22:1313–1322. doi: 10.1097/QAD.0b013e3282fdce94. [DOI] [PubMed] [Google Scholar]
- 110.Kilby JM, et al. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024) J Infect Dis. 2006;194:1672–1676. doi: 10.1086/509508. [DOI] [PubMed] [Google Scholar]
- 111.Kinloch-de LS, et al. Impact of therapeutic immunization on HIV-1 viremia after discontinuation of antiretroviral therapy initiated during acute infection. J Infect Dis. 2005;192:607–617. doi: 10.1086/432002. [DOI] [PubMed] [Google Scholar]
- 112.Levy Y, et al. Immunological and virological efficacy of a therapeutic immunization combined with interleukin-2 in chronically HIV-1 infected patients. AIDS. 2005;19:279–286. [PubMed] [Google Scholar]
- 113.Dorrell L, et al. Safety and tolerability of recombinant modified vaccinia virus Ankara expressing an HIV-1 gag/multiepitope immunogen (MVA. HIVA) in HIV-1-infected persons receiving combination antiretroviral therapy. Vaccine. 2007;25:3277–3283. doi: 10.1016/j.vaccine.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 114.Dorrell L, et al. Expansion and diversification of virus-specific T cells following immunization of human immunodeficiency virus type 1 (HIV-1)-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 Gag vaccine. J Virol. 2006;80:4705–4716. doi: 10.1128/JVI.80.10.4705-4716.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harrer E, et al. Therapeutic vaccination of HIV-1-infected patients on HAART with a recombinant HIV-1 nef-expressing MVA: safety, immunogenicity and influence on viral load during treatment interruption. Antiviral Ther. 2005;10:285–300. [PubMed] [Google Scholar]
- 116.Schooley RT, et al. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. 2010;202:705–716. doi: 10.1086/655468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 118.Norton EM, Bhardwaj N, Landau N. Dendritic cells transduced with Vpx-packaged LV vector encoding CD40L enhance cytotoxic T cell responses and disrupt HIV-1 latency. Conference on Retroviruses and Opportunistic Infections (CROI); 2013, March 3–7, 2013; Atlanta, GA, USA. Paper #125. [Google Scholar]
- 119.Miller MS, Demmler M, Lauer P, Brockstedt D, Bhardwaj N. Rescue of myeloid dendritic cell dysfunction in the setting of chronic HIV-1 infection with recombinant, Attenuated listeria monocytogenes vectors. Conference on Retroviruses and Opportunistic Infections (CROI); 2013, March 3–7, 2013; Atlanta, GA, USA. Paper # 389. [Google Scholar]
- 120.Mackaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 121.Abdullah Z, et al. RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. EMBO J. 2012;31:4153–4164. doi: 10.1038/emboj.2012.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Corr SC, O’Neill LA. Listeria monocytogenes infection in the face of innate immunity. Cell Microbiol. 2009;11:703–709. doi: 10.1111/j.1462-5822.2009.01294.x. [DOI] [PubMed] [Google Scholar]
- 123.Edelson BT, Unanue ER. Immunity to Listeria infection. Curr Opin Immunol. 2000;12:425–431. doi: 10.1016/s0952-7915(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 124.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 125.Kim S, et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40:1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol. 2010;30:693–702. doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yano T, Kurata S. Induction of autophagy via innate bacterial recognition. Autophagy. 2008;4:958–960. doi: 10.4161/auto.6802. [DOI] [PubMed] [Google Scholar]
- 128.Lori F. DermaVir: a plasmid DNA-based nanomedicine therapeutic vaccine for the treatment of HIV/AIDS. Exp Rev Vaccines. 2011;10:1371–1384. doi: 10.1586/erv.11.118. [DOI] [PubMed] [Google Scholar]
- 129.Gudmundsdotter L, et al. Amplified antigen-specific immune responses in HIV-1 infected individuals in a double blind DNA immunization and therapy interruption trial. Vaccine. 2011;29:5558–5566. doi: 10.1016/j.vaccine.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 130.Rosenberg ES, et al. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS ONE. 2010;5:e10555. doi: 10.1371/journal.pone.0010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilson CC, et al. Clinical phase 1 testing of the safety and immunogenicity of an epitope-based DNA vaccine in human immunodeficiency virus type 1-infected subjects receiving highly active antiretroviral therapy. Clin Vaccine Immunol. 2008;15:986–994. doi: 10.1128/CVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lisziewicz J, et al. Single DermaVir immunization: dose-dependent expansion of precursor/memory T cells against all HIV antigens in HIV-1 infected individuals. PLoS ONE. 2012;7:e35416. doi: 10.1371/journal.pone.0035416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goenka R, et al. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011;187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rankin AL, et al. IL-21 receptor is critical for the development of memory B cell responses. J Immunol. 2011;186:667–674. doi: 10.4049/jimmunol.0903207. [DOI] [PubMed] [Google Scholar]
- 135.Schmitt N, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fontaine J, Chagnon-Choquet J, Valcke HS, Poudrier J, Roger M. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood. 2011;117:145–155. doi: 10.1182/blood-2010-08-301887. [DOI] [PubMed] [Google Scholar]
- 137.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 138.O’Doherty U, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 139.O’Doherty U, et al. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cao W, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 143.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 144.Donaghy H, et al. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 145.Reeves K, Evans T, Gillis J, Rajakumar P, Kuzmichev Y, Johnson P. SIV infection induces increased trafficking of pDC to gut mucosa. Conference on Retroviruses and Opportunistic Infections (CROI); 2011, February 27 March 2, 2011; Boston, MA, USA. [Google Scholar]
- 146.Dillon SM, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lehmann C, et al. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS ONE. 2010;5:e11110. doi: 10.1371/journal.pone.0011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Geng W, et al. Rapid disease progression in HIV-1-infected men who have sex with men is negatively correlated with peripheral plasmacytoid dendritic cell counts at the early stage of primary infection. J Clin Immunol. 2011;31:882–890. doi: 10.1007/s10875-011-9556-0. [DOI] [PubMed] [Google Scholar]
- 149.Lehmann C, et al. Increased interferon alpha expression in circulating plasmacytoid dendritic cells of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2008;48:522–530. doi: 10.1097/QAI.0b013e31817f97cf. [DOI] [PubMed] [Google Scholar]
- 150.Beignon AS, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Haupt S, et al. CD4 binding affinity determines human immunodeficiency virus type 1-induced alpha interferon production in plasmacytoid dendritic cells. J Virol. 2008;82:8900–8905. doi: 10.1128/JVI.00196-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lepelley A, et al. Innate sensing of HIV-infected cells. PLoS Pathog. 2011;7:e1001284. doi: 10.1371/journal.ppat.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O’Brien M, et al. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest. 2011;121:1088–1101. doi: 10.1172/JCI44960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 155.Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 156.Hazenberg MD, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 157.Kaufmann DE, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 158.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 159.Shattock RJ, Rizzardi GP, Hayes P, Griffin GE. Engagement of adhesion molecules (CD18, CD11a, CD45, CD44, and CD58) enhances human immunodeficiency virus type 1 replication in monocytic cells through a tumor necrosis factor-modulated pathway. J Infect Dis. 1996;174:54–62. doi: 10.1093/infdis/174.1.54. [DOI] [PubMed] [Google Scholar]
- 160.Wu L, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 162.Fahey JL, et al. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 163.Salazar-Gonzalez JF, et al. Increased immune activation precedes the inflection point of CD4 T cells and the increased serum virus load in human immunodeficiency virus infection. J Infect Dis. 1998;178:423–430. doi: 10.1086/515629. [DOI] [PubMed] [Google Scholar]
- 164.Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 165.Baker JV, Duprez D. Biomarkers and HIV-associated cardiovascular disease. Curr Opin HIV AIDS. 2010;5:511–516. doi: 10.1097/COH.0b013e32833ed7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lekakis J, Ikonomidis I. Cardiovascular complications of AIDS. Curr Opin Crit Care. 2010;16:408–412. doi: 10.1097/MCC.0b013e32833e10a9. [DOI] [PubMed] [Google Scholar]
- 167.Ho JE, et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS. 2010;24:1897–1905. doi: 10.1097/QAD.0b013e32833bee44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lichtenstein KA, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 169.El-Sadr WM, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 170.Hunt PW, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.O’Brien M, et al. Aspirin attenuates platelet activation and immune activation in HIV-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Herbeuval JP, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci USA. 2006;103:7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.von Sydow M, Sonnerborg A, Gaines H, Strannegard O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7:375–380. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]
- 174.Jacquelin B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Manion M, et al. Interferon-alpha administration enhances CD8+ T cell activation in HIV infection. PLoS ONE. 2012;7:e30306. doi: 10.1371/journal.pone.0030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Herbeuval JP, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–3531. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meier A, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kwa S, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118:2763–2773. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vanderford TH, et al. Treatment of SIV-infected sooty mangabeys with a type-I IFN agonist results in decreased virus replication without inducing hyperimmune activation. Blood. 2012;119:5750–5757. doi: 10.1182/blood-2012-02-411496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dyavar SR, et al. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bjorck P. Dendritic cells exposed to herpes simplex virus in vivo do not produce IFN-alpha after rechallenge with virus in vitro and exhibit decreased T cell alloreactivity. J Immunol. 2004;172:5396–5404. doi: 10.4049/jimmunol.172.9.5396. [DOI] [PubMed] [Google Scholar]
- 183.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 184.Tilton JC, et al. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol. 2008;82:3997–4006. doi: 10.1128/JVI.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huang J, et al. Dendritic cell dysfunction during primary HIV-1 infection. J Infect Dis. 2011;204:1557–1562. doi: 10.1093/infdis/jir616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reitano KN, et al. Defective plasmacytoid dendritic cell-NK cell cross-talk in HIV infection. AIDS Res Hum Retroviruses. 2009;25:1029–1037. doi: 10.1089/aid.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kamga I, et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 188.Wilson EB, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Estes JD, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 191.Horta A, et al. Poor Immune Reconstitution in HIV-Infected Patients Associates with High Percentage of Regulatory CD4(+) T Cells. PLoS ONE. 2013;8:e57336. doi: 10.1371/journal.pone.0057336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kinter AL, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Eggena MP, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 194.Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 195.Manches O, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Favre D, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Trans Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Manches O, Fernandez MV, Plumas J, Chaperot L, Bhardwaj N. Activation of the noncanonical NF-kappaB pathway by HIV controls a dendritic cell immunoregulatory phenotype. Proc Natl Acad Sci USA. 2012;109:14122–14127. doi: 10.1073/pnas.1204032109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Boasso A, et al. Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J Immunol. 2009;182:4313–4320. doi: 10.4049/jimmunol.0803314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dunham RM, et al. Preclinical evaluation of HIV eradication strategies in the simian immunodeficiency virus-infected rhesus macaque: a pilot study testing inhibition of indoleamine 2,3-dioxygenase. AIDS Res Hum Retroviruses. 2013;29:207–214. doi: 10.1089/aid.2012.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]