Abstract
The morbidity due to Ascaris lumbricoides and Trichuris trichiura is caused by infections of moderate and heavy intensity while hookworm infections of all intensities are recognized to cause morbidity.
This study aims to evaluate the effect of repeated round of preventive chemotherapy on the proportion of Soil Transmitted Helminth (STH) infections causing morbidity. We identified studies from 17 countries, reporting changes in the proportion of STH infection causing morbidity between baseline and follow up.
In the studies identified, the average proportion of individuals with STH infections of moderate and heavy intensity was of 14% at baseline and was on average reduced to 2% by the intervention (i.e. 85% reduction). There was an average reduction of 73% after the first year of treatment, which reached almost 80% after 5 years and over 95% in 10 years of deworming interventions.
The reduction in hookworm prevalence was 57% after 12 months reaching 78% after 5 years.
We consider the results presented in this study especially useful for decision makers as it demonstrates the effectiveness of Preventive Chemotherapy in reducing STH prevalence and morbidity. We encourage the implementation of deworming programs to achieve the goal, set for 2020, to eliminate STH morbidity in children.
Keywords: Soil Transmitted Helminthiasis, Preventive Chemotherapy, deworming, effectiveness, morbidity
Background
Soil-transmitted helminths (STH) infections are among the most widespread parasitic infections worldwide1 and are classified as neglected tropical diseases. It is estimated that Ascaris lumbricoides, Trichuris trichiura and hookworms (Ancylostoma duodenale and Necator americanus) affect 819 million, 464 million, and 439 million people respectively among the poorest and most deprived populations.2
STH infections are most common in areas where sanitation is poor. Infections occur with the ingestion of parasite eggs (A.lumbricoides, T. trichiura and Ancylostoma duodenale) from soil contaminated with human faeces or, in the case of hookworm, by penetration of the skin by larvae in the soil.3
The higher the number of worms infecting an individual, the more severe is the morbidity caused by the infection.1 The number of worms infecting an individual can be appraised by counting the number of parasite eggs present in a gram of faeces (epg). WHO established epg thresholds for each STH parasite allowing the classification of STH infections within three categories: light, moderate and heavy intensity based on the use of Kato-Katz laboratory technique.1
STH morbidity consists in malabsorption of micronutrients and blood loss resulting in impaired growth, delayed cognitive development and anaemia, among others.1 STH in a small percentage of infected individuals cause mortality,4 this number, however, is not negligible in light of the very high distribution of the infection in tropical and subtropical countries.2
For A. lumbricoides and T. trichiura, infections of moderate and heavy intensity (MHI) are considered the main cause of morbidity while for hookworm any intensity of infection is considered to cause morbidity.5 Global morbidity and mortality due to STH expressed in disability-adjusted life years (DALYs) infections has been estimated at: 1.2-10.5 million DALYs for A. lumbricoides, 1.8-22.2 million DALYs for T. trichiura, and 1.6-6.4 million DALYs for hookworm.6
Children and women of childbearing age suffer from the greatest burden of disease related to STH infection because of their particular need for micronutrients. The World Health Organization (WHO) recommends to control STH morbidity through periodic anthelminthic treatment of at-risk populations living in endemic areas. This approach is referred to as preventive chemotherapy (PC) and targets preschool-age children, school-age children, and women of childbearing age.6,7 Treatment, single-dose of albendazole (400 mg) or mebendazole (500 mg), is recommended once a year when the prevalence of any STH infection among school age children is at least 20% and twice a year when it exceeds 50%.1
The global coverage with preventive chemotherapy of at least 75% of school-age children at risk of morbidity from STH and schistosomiasis is the goal set for 2020. In 2015, over 55% of the children in need of treatment have been reached with PC programmes.8 Significant improvements in sanitation and in health education would be required for the elimination of STH infections. Without these changes in environmental and behavioural factors, dewormed individuals living in highly contaminated environment, are likely to be re-infected within a few months of being treated. PC is therefore recommended to be repeated periodically.
This study aims to evaluate the impact of the WHO recommended interventions on the control of STH morbidity by evaluating the ability of PC to reduce the proportion of individuals suffering from STH-related morbidity (MHI infections with A. lumbricoides and T. trichiura and infections of any intensity with hookworms). We also aimed at evaluating the average reduction obtained in relation to the number of years for which PC interventions had been implemented.
Materials and Methods
A literature search was conducted on:
PubMed database using the following key words: soil-transmitted helminth(s) (including the individual species names of Ascaris lumbricoides or roundworm, Trichuris trichiura or whipworm, and Ancylostoma duodenale, Necator americanus or hookworm); intensity AND/OR; morbidity; preventive chemotherapy; deworming; helminth control; neglected tropical disease control; STH control;
unpublished reports, examining documents available in the archives of the Department of Neglected Tropical Diseases of the World Health Organization in Geneva, Switzerland.
Studies were eligible for inclusion if: i) they were published after year 2000; ii) PC was conducted with albendazole or mebendazole (to any group at risk) iii) at least two rounds of drug administrations were conducted; iv) the Kato-Katz technique was utilized as the diagnostic method to classify the intensity of infection of each parasite v) information on STH proportion of MHI (by species) was reported at baseline and at least at one follow-up time point or vi) information on hookworm prevalence was reported at baseline and at least at one follow-up time point.
For all the studies identified that reported the proportion of MHI for each of the three STH, if the proportion of MHI for one of them was very low at baseline (i.e. inferior to 3%), the changes in MHI for that parasite were excluded from the calculation. The same approach was applied when evaluating the changes in hookworm prevalence where we excluded baseline prevalence values inferior than 5%. This was done because at these low values, even minimal variations on proportion of MHI or prevalence have great impact on the calculation of the mean change (for example a variation of MHI from 1% to 2% would corresponds to 100% increase in morbidity) and the sensitivity of the Kato Katz technique used to diagnosis STH is, in our opinion, insufficient to confidently detect differences in such small changes.9
The proportion of STH infections of MHI can be calculated in two ways: as the proportion of MHI on the total number of individuals investigated or as the proportion of MHI on the total number of infected. We consider that for our purposes, the proportion of MHI should be calculated using the entire population investigated as denominator because, if the proportion of MHI is calculated on positive individuals only, even a significant reduction of the number of MHI could result in a proportional increase when the number of infected individuals is also significantly decreased and used as denominator. For this reason, in studies identified in which the proportion of MHI at baseline and follow-up was calculated on the positive children only,12,13.15.17 we recalculated the proportion of MHI at baseline and follow-up using the total number of individuals surveyed as denominator.
From each publication identified we extracted the following information: region and country of the intervention, the drug used, the frequency of treatment, the at-risk population targeted by the intervention, the year of the baseline and the year of the follow-up survey, the number of individuals in the baseline and follow-up survey (sample size), the mean age of the individuals surveyed, the prevalence of each STH parasite in the baseline and follow up survey, the proportion (on the total sample investigated) of MHI at baseline and follow-up survey, the duration of the PC intervention.
From the dataset obtained:
we calculated the mean proportion of MHI at baseline and after intervention (for any interval and for any STH) and calculated statistical difference using a paired t-test.
for each STH species, we calculated the reduction of the proportion of infection of MHI from baseline at each reported interval;
we plotted in a graph the reduction of the proportion of MHI at different times from the beginning of the control programme for “all the STH” and for A. lumbricoides and T. Trichiura separately, and added a linear model regression line
for hookworm we calculated the reduction in prevalence at each interval reported and we plotted the results, and added a linear model regression line
Results
We identified 911 articles and screened them by reading the abstract and in case of a possible inclusion we obtained the full article and examined it in detail.
Ten publications10–19 were identified according the inclusion criteria: reporting MHI information at baseline and after a number of years of PC from 8 countries in 4 WHO regions (Table 1); Some publications16;18 reported follow-up measurements at different intervals from a single baseline, while others 10,11 reported multiple baseline and multiple follow-up in different areas of the country. In some cases10–13 baseline and follow up data were presented in different publications. In total, 25 changes in MHI between baseline and follow up were analysed. In this group of studies the average size of the population at baseline, was 3472 (range 268-21,432) and the average period between baseline and follow-up after PC was 46 months (range 12-132). In 60% of countries the drug used for PC was albendazole.
Table 1.
Country programmes considered for the calculation of the reduction of the proportion of STH infection of moderate and heavy intensity after preventive chemotherapy
| Country | Year | Drug* | Number of people surveyed at baseline | Individuals with STH infections of moderate high intensity baseline – last follow-up |
Follow up (months) | Age group | PC frequency | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| A. lumbricoides | T trichiura | hookworms | ||||||||
| Myanmar | 2004 | Alb | 1 000 | 18% - 7% | 84 | SAC | 2/year | 10, 11 | ||
| 2013 | ||||||||||
| Bangladesh | 2005 | Alb | 792 | 29.4% - 1.8% | 12.4% - 1.1% | NA | 60 | SAC | 2/year | 12, 13 |
| 2016 | ||||||||||
| Lao | 2008 | Meb | 2 885 | 37.8% - 9.2% | 10.3% - 3.8% | NA | 24 | SAC | 2/year | 14 |
| Tanzania | 2009 | Alb | 1 194 | 23% - 1.4% | 24% - 0.3% | 26% - 0 | 132 | SAC | 1/year | 15 |
| Viet Nam | 2013 | Alb | 389 | 8% - 0 | 2% - 0 | 14% - 0 | 12, 30, 54 | WCBA | 2/year | 16 |
| Cuba | 2014 | Meb | 268 | 6.7% - 1% | NA | NA | 36 | SAC | 2/year | 17 |
| Peru | 2016 | Meb | 816 | 40% - 2.9% | 25.2% - 6.7% | NA | 12, 24 | SAC | 3/year | 18 |
| Kenya | 2016 | Alb | 21 432 | 8.2% - 5.4% | 8.2% - 0.3% | NA | 36 | SAC | 1/year | 19 |
Alb= albendazole; Meb= mebendazole; PC= preventive chemotherapy SAC = School aged children, WCBA=Women of Child bearing age, NA (prevalence of MHI 0-3%)
In addition to the publications reporting change in MHI infections (and therefore also hookworm prevalence), we identified 9 studies20–28 reporting changes in hookworm prevalence data only (i.e. no MHI data) at baseline and after two or more rounds of PC (Table 2). The average time for follow-up assessment of the PC programs in this group was 46.9 months. In total for hookworm prevalence we analysed 37 changes of prevalence at different interval from baseline.
Table 2.
additional studies considered for the calculation of the reduction of hookworm prevalence after preventive chemotherapy
| Country | Year | Drug | Number of people surveyed at baseline | Individuals with hookworm infection baseline – last follow-up |
Follow up (months) | Age group | PC frequency | Ref |
|---|---|---|---|---|---|---|---|---|
| Myanmar | 2004 2013 |
Alb | 1 000 | 6.5%- 0.3% | 84 | SAC | 2/year | 10, 11 |
| Bangladesh | 2005 2016 |
Alb | 792 | 20.4% - 7.7% | 60 | SAC | 2/year | 12, 13 |
| Lao | 2008 | Meb | 2 885 | 18.7% - 22.23% | 24 | SAC | 2/year | 14 |
| Tanzania | 2009 | Alb | 1 194 | 50.9% - 10.7% | 132 | SAC | 1/year | 15 |
| Viet Nam | 2013 | Alb | 389 | 86% - 12% | 12, 30, 54 | WCBA | 2/year | 16 |
| Cuba | 2014 | Meb | 268 | 44.4% - 18.7% | 36 | SAC | 2/year | 17 |
| Peru | 2016 | Meb | 816 | 4.2% - 3.09% | 12, 24 | SAC | 3/year | 18 |
| Kenya | 2016 | Alb | 21 432 | 14.4% - 2.3% | 36 | SAC | 1/year | 19 |
| Sri Lanka | 2000 | Meb | 177 | 72% - 8.8% | 12, 24, 36, 48, 72 | SAC | 1/year | 20 |
| Oman | 2001 | Alb | 860 | 40% - 0.6% | 12, 24, 36, 48, 60 | SAC | 1/year | 21 |
| Nepal | 2003 | Alb | 711 | 64.7% - 33.9% | 24 | SAC | 2/year | 22 |
| Seychelles | 2003 | Meb | 1058 | 6.3% - 1.5% | 24, 36, 60 | SAC | 2/year | 23 |
| India | 2004 | Alb, DEC | 325 | 5% - 5% | 24 | SAC | 2/year | 24 |
| Cambodia | 2006 | Meb | 50 | 57.8% - 13.5%* | 60 | SAC | 2/year | 25 |
| Ghana | 2006 | Alb | 1010 | 87% - 23% | 12, 24 | SAC | 2/year | 26 |
| Uganda | 2007 | Alb | 4351 | 50.9% - 10.7% | 12, 24 | SAC | 1/year | 27 |
| Zambia | 2013 | Alb, IVR | 490 | 7.8% - 1.9% | 12, 18 | SAC | 1/year | 28 |
Alb= albendazole; Meb= mebendazole; DEC=diethylcarbamazine; IVR= ivermectin, SAC = School aged children PC= preventive chemotherapy, WCBA=Women of Child bearing age
average of 7 different surveys
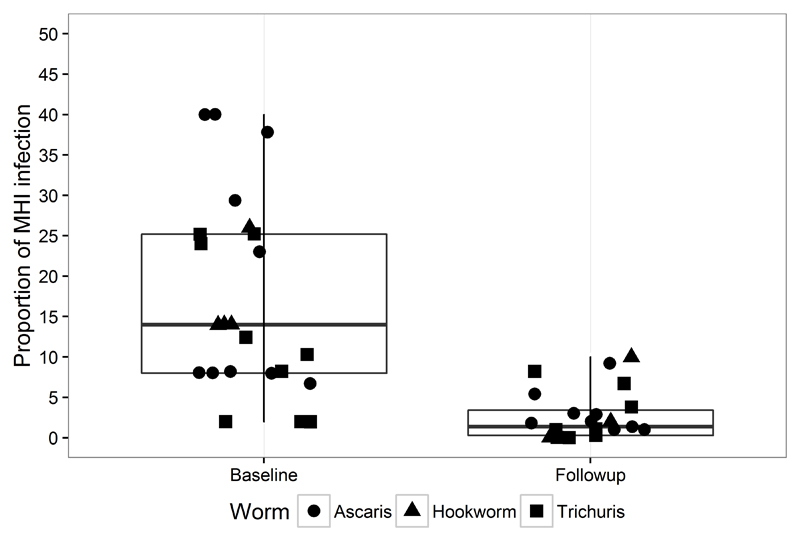
An average of 14% (range in the different surveys 3%- 40%) of the 27 776 individuals participating in the baseline surveys were infected with MHI, after the PC intervention this number was reduced on average to less than 2%, (range in the different surveys 0- 8.2 %) corresponding to a reduction of over 85% (p<0.005) (Figure 1).
Figure 1.
Proportion of STH infections of moderate heavy intensity before and after preventive chemotherapy programme (of any length)
Figure 2 shows the percentage reduction in MHI infections (all STH species included), over time. After the first twelve months of PC implementation, the average proportion of MHI was reduced by 73%, after 60 months MHI infections were on average reduced by 84% and by a 132 months the reduction reaches 95%.
Figure 2.
Reduction in the proportion of STH infections of moderate heavy intensity by length (in months) of preventive chemotherapy programme
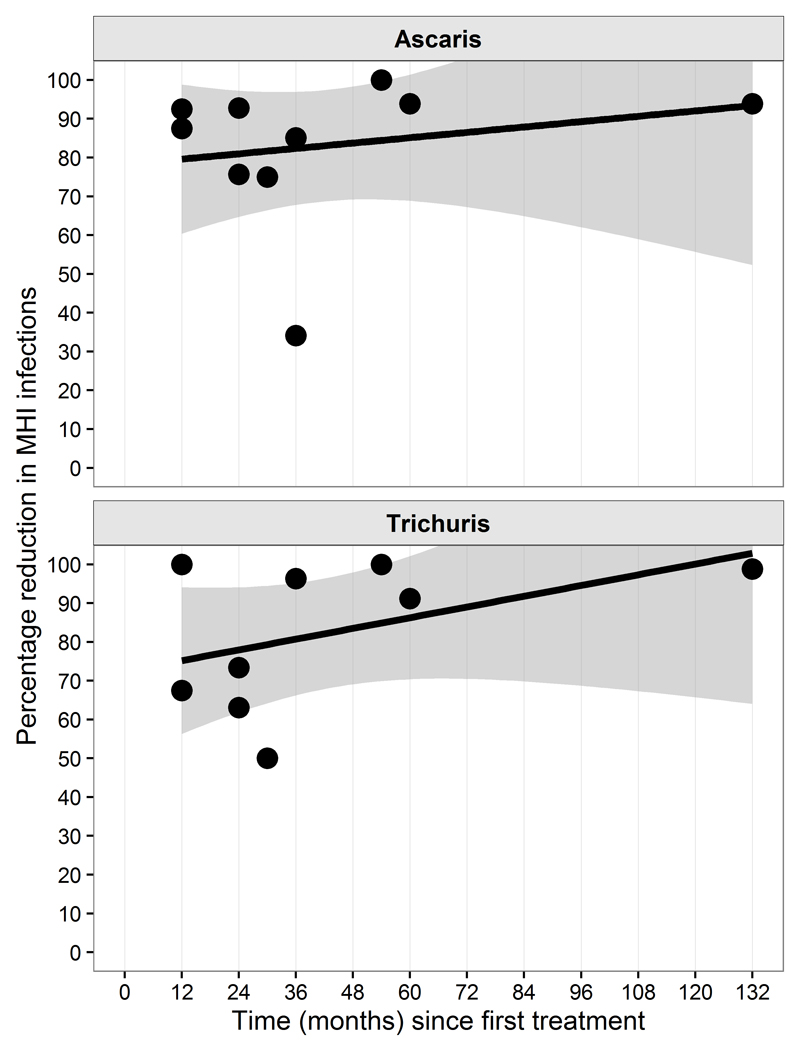
Figure 3 presents the change of proportion of MHI for A. lumbricoides and T. trichiura. After a period of 12 months the reduction of the proportion of infections of MHI was of 80% for A. lumbricoides, and 75% for T. trichiura; after 60 months (5 years) reduction reaches 90% and 100% respectively.
Figure 3.
Reduction in the proportion of infections of moderate heavy intensity by length (in months) of preventive chemotherapy programme. Top panel, A. lumbricoides, bottom panel T. trichiura
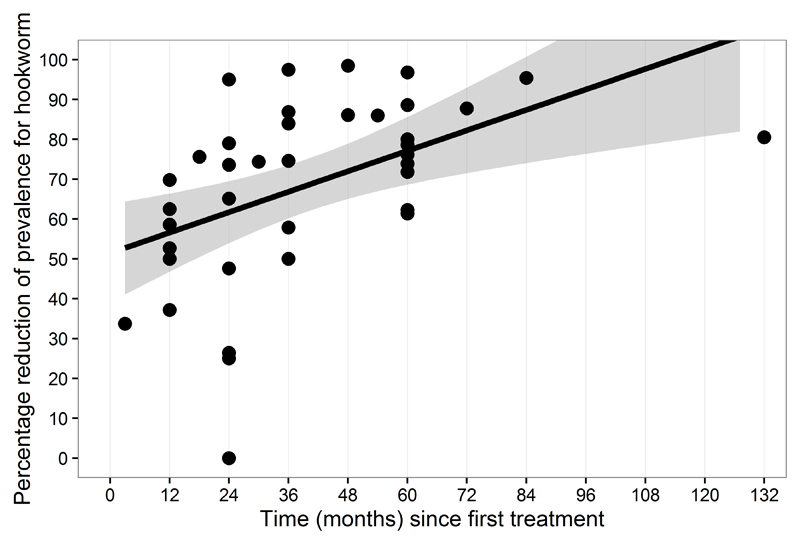
The percentage reduction of the prevalence for hookworm (figure 4) has been of 57% after 12 months reaching 78% after 5 years.
Figure 4.
Reduction in prevalence of hookworm by length (in months) of preventive chemotherapy programme.
In conclusion we observed that PC obtained a significant reduction of the proportion of STH infections causing morbidity already after one year. The successive gains are less intense but the proportion of STH infection causing morbidity continue to decline: after 5 years more than 80% of the individuals originally infected with MHI are cured or with light intensity infections and more than 78% of the infected with hookworm has been cured.
Discussion
This study demonstrates that a large part of the STH morbidity is eliminated after the first year of PC intervention and it is then further reduced year after year. Because the environment where these programmes are taking place is, by definition, highly contaminated, an interruption of the PC programme after the first years would result in rapid re-infection and in the return to the original prevalence and intensity levels, which would result in losing the benefits gained by the PC programme. For this reason, the WHO recommends that PC interventions, once started should, be maintained for at least 5 years.4
We consider that, for three reasons, this approach to evaluate the benefits of preventive chemotherapy for STH is more appropriate than the one based on the evaluation of the nutritional benefits with Randomized Control Trials (RCTs). Firstly, as this method focuses on the individuals suffering from STH infection: the ones infected with MHI (which in our estimations are an average of 15% of the population treated), it avoids the dilution bias:29 i.e. only measuring the mean benefits of the population receiving the intervention will dilute by 6 to 7 times the benefit obtained by the individuals infected at MHI. This dilution effect making impossible to discriminate when the sample size is of limited number; secondly, the evaluation time-frame is more appropriate, on average communities were followed up for more than 3 years with several follow ups reaching 5 years. For logistic and financial difficulties, follow up in RCT are normally of 1 year, meaning the long term benefit of PC is not assessed. Finally, this approach allows an evaluation of the global impact of the STH control programme in term of the number of DALYs saved by PC interventions until now.
This study is based on reports from deworming programmes for which coverage was not always optimal, therefore it may under-evaluate the potential benefits of the intervention. Moreover, follow up surveys were not always implemented on the same patients who participated in the baseline surveys and probably most of the MHI observed at follow up were from “newcomers” in the programme (e.g. younger children entering in school for the first time and not previously treated). Where the same cohort was investigated at baseline and during the follow up,16 the reduction in MHI infection and hookworm prevalence was more pronounced reaching 100% MHI reduction for A. lumbricoides and T. trichiura and 90% of prevalence reduction for hookworms after 5 years.
In a few studies the reduction of the prevalence of infection causing morbidity was substantially lower than the average (figure 2). These outliers were in two cases from Vietnam (T. trichiura and hookworms) and one case from Kenya (A. lumbricoides). In the two cases from Vietnam this lower than anticipated decline at 30 months was also noted in the original study and attributed to seasonality. During the following data collection at 54 months MHI infections disappeared from the community meaning drug resistance was excluded as explanation. In the case of Kenya the prevalence of MHI infection from 8.2% to 5.4% (i.e. a 34% reduction) is likely due to young children who have never been treated, entering schools and becoming part of the sample at follow up.
Another limitation of this study is the small number of PC programme evaluated: eight programmes reporting the proportion of MHI and additional nine reporting the hookworm prevalence. We are taking this opportunity to emphasize the need for control programmes to periodically collect and disseminate epidemiological data, including the proportion of MHI infections. These data are essential to properly evaluate the gains in reducing morbidity through PC, and to inform other programmes starting similar activities.
Bibliography
- 1.World Health Organization. Helminth control in school-age children: a guide for managers of control programmes. Second Edition. World Health Organization; Geneva: 2011. [Google Scholar]
- 2.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7(1):1. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandemark LM, Jia TW, Zhou XN. Social science implications for control of helminth infections in Southeast Asia. Adv Parasitol. 2010;73:137–170. doi: 10.1016/S0065-308X(10)73006-2. [DOI] [PubMed] [Google Scholar]
- 4.Martins-Melo FR, Ramos AN, Alencar CH, Lima MS, Heukelbach J. Epidemiology of soil-transmitted helminthiases-related mortality in Brazil. Parasitology. 2017 Jan 20;:1–11. doi: 10.1017/S0031182016002341. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Soil-Transmitted Helminthiases. Eliminating Soil-Transmitted Helminthiases as a Public Health Problem in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020. Geneva: World Health Organization; 2012. [Google Scholar]
- 6.World Health Organization. Preventive chemotherapy in human helminthiasis. World Health Organization; Geneva: 2006. [Google Scholar]
- 7.Gabrielli AF, Montresor A, Chitsulo L, Engels D, Savioli L. Preventive chemotherapy in human helminthiasis: theoretical and operational aspects. Trans R Soc Trop Med Hyg. 2011;105(12):683–693. doi: 10.1016/j.trstmh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Wkly Epidemiol Rec. 2016;91:585–600. [PubMed] [Google Scholar]
- 9.Lamberton PH, Jourdan PM. Human Ascariasis: Diagnostics Update. Curr Trop Med Rep. 2015;2(4):189–200. doi: 10.1007/s40475-015-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montresor A, Zin TT, Padmasiri E, Allen H, Savioli L. Soil-transmitted helminthiasis in Myanmar and approximate costs for countrywide control. Trop Med Int Health. 2004;9(9):1012–1015. doi: 10.1111/j.1365-3156.2004.01297.x. [DOI] [PubMed] [Google Scholar]
- 11.Tun A, Myat SM, Gabrielli AF, Montresor A. Control of soil-transmitted helminthiasis in Myanmar: results of 7 years of deworming. Trop Med Int Health. 2013;18(8):1017–1020. doi: 10.1111/tmi.12130. [DOI] [PubMed] [Google Scholar]
- 12.Directorate General of Health Services, Ministry of Health and Family Welfare, Government of the People’s Republic of Bangladesh. Base Line Survey on Soil transmitted Helminths (STH) among school children under Filariasis Elimination Program, Communicable Disease Control (CDC) 2005 [unpublished document] [Google Scholar]
- 13.Benjamin-Chung J, Nazneen A, Halder AK, Haque R, Siddique A, Uddin MS, Koporc K, Arnold BF, Hubbard AE, Unicomb L, Luby SP, et al. The Interaction of Deworming, Improved Sanitation, and Household Flooring with Soil-Transmitted Helminth Infection in Rural Bangladesh. PLoS Negl Trop Dis. 2015;9(12):e0004256. doi: 10.1371/journal.pntd.0004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phommasack B, Saklokham K, Chanthavisouk C, Nakhonesid-Fish V, Strandgaard H, Montresor A, Shuey DA, Ehrenberg J. Coverage and costs of a school deworming programme in 2007 targeting all primary schools in Lao PDR. Trans R Soc Trop Med Hyg. 2008;102(12):1201–1206. doi: 10.1016/j.trstmh.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopp S, Mohammed KA, Rollinson D, Stothard JR, Khamis IS, Utzinger J, Marti H. Changing patterns of soil-transmitted helminthiases in Zanzibar in the context of national helminth control programs. Am J Trop Med Hyg. 2009;81(6):1071–1078. doi: 10.4269/ajtmh.2009.09-0377. [DOI] [PubMed] [Google Scholar]
- 16.Casey GJ, Montresor A, Cavalli-Sforza LT, Thu H, Phu LB, Tinh TT, Tien NT, Phuc TQ, Biggs BA. Elimination of iron deficiency anemia and soil transmitted helminth infection: evidence from a fifty-four month iron-folic acid and de-worming program. PLoS Negl Trop Dis. 2013;7(4):e2146. doi: 10.1371/journal.pntd.0002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Werff SD, Vereecken K, van der Laan K, Campos Ponce M, Junco Díaz R, Núñez FA, Rojas Rivero L, Bonet Gorbea M, Polman K. Impact of periodic selective mebendazole treatment on soil-transmitted helminth infections in Cuban schoolchildren. Trop Med Int Health. 2014;19(6):706–718. doi: 10.1111/tmi.12290. [DOI] [PubMed] [Google Scholar]
- 18.Ferruci HR, Razuri H, Casapia M, Rahme E, Silva H, Ault S, Blouin B, Mofid LS, Montresor A, Gyorkos TW. Governance, organization, accountability and sustainability of a region-wide school-based deworming program in Loreto, Peru. Acta Trop. 2016;159:219–226. doi: 10.1016/j.actatropica.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okoyo C, Nikolay B, Kihara J, Simiyu E, Garn JV, Freeman MC, Mwanje MT, Mukoko DA, Brooker SJ, Pullan RL, Njenga SM, et al. Monitoring the impact of a national school based deworming programme on soil-transmitted helminths in Kenya: the first three years, 2012–2014. Parasit Vectors. 2016;9(1):408. doi: 10.1186/s13071-016-1679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail MM. Report from The Ministry of Health of Sri Lanka. 2000. Fifth evaluation of the by annual deworming programme in the plantations (NOV/DEC 2000) [Google Scholar]
- 21.Idris MA, Shaban MA, Fatahallah M. Effective control of hookworm infection in school children from Dhofar, Sultanate of Oman: a four-year experience with albendazole mass chemotherapy. Acta Trop. 2001;80:139–143. doi: 10.1016/s0001-706x(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 22.Bordignon GP, Shakya DR. Controlling diseases due to helminth infection. World Health Organization; Geneva: 2003. A deworming programme in Nepal supported by the World Food Programme. [Google Scholar]
- 23.Shamlaye N. Controlling diseases due to helminth infection. World Health Organization; Geneva: 2003. The Seychelles experience in controlling helminth infections. [Google Scholar]
- 24.Mani T, Rajendran R, Sunish IP, Munirathinam A, Arunachalam N, Satyanarayana K, Dash AP. Effectiveness of two annual, single-dose mass drug administrations of diethylcarbamazine alone or in combination with albendazole on soil-transmitted helminthiasis in filariasis elimination programme. Trop Med Int Health. 2004;9(9):1030–1035. doi: 10.1111/j.1365-3156.2004.01298.x. [DOI] [PubMed] [Google Scholar]
- 25.Sinuon M, Tsuyuoka R, Socheat D, Odermatt P, Ohmae H, Matsuda H, Montresor A, Palmer K. Control of Schistosoma mekongi in Cambodia. Results of eight years of control activities in the two endemic provinces. Trans R Soc Trop Med Hyg. 2006;101:34–9. doi: 10.1016/j.trstmh.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziem JB, Magnussen P, Olsen A, Horton J, Asigri VL, Polderman AM. Impact of repeated mass treatment on human Oesophagostomum and hookworm infections in northern Ghana. Trop Med Int Health. 2006;11:1764–1772. doi: 10.1111/j.1365-3156.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Koukounari A, Kabatereine N, Fleming F, Kazibwe F, Tukahebwa E, Stothard JR, Webster JP, Fenwick A. Parasitological impact of 2-year preventive chemotherapy on schistosomiasis and soil-transmitted helminthiasis in Uganda. BMC medicine. 2007;5(1):1. doi: 10.1186/1741-7015-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halwindi H, Magnussen P, Siziya S, Handema R, Meyrowitsch DW, Olsen A. Impact of community directed treatment on soil transmitted helminth infections in children aged 12 to 59 months in Mazabuka District, Zambia. J Biosoc Sci. 2013 Jan;45(1):95–109. doi: 10.1017/S0021932012000302. [DOI] [PubMed] [Google Scholar]
- 29.Montresor A, Addiss D, Albonico M, Ali SM, Ault SK, Gabrielli A-F, Garba A, Gasimov E, Gyorkos T, Jamsheed MA, Levecke B, et al. Methodological Bias Can Lead the Cochrane Collaboration to Irrelevance in Public Health Decision-Making. PLoS Negl Trop Dis. 2015;9(10):e0004165. doi: 10.1371/journal.pntd.0004165. [DOI] [PMC free article] [PubMed] [Google Scholar]