Abstract
Introduction
Parkinson’s disease patients with freezing of gait also experience sudden motor blocks (freezing) during other repetitive motor tasks. We assessed the proportion of patients with advanced PD and freezing of gait who also displayed segmental “freezing” in tapping tasks.
Methods
Fifteen Parkinson’s disease patients with freezing of gait were assessed. Freezing of gait was evaluated using a standardized gait trajectory with the usual triggers. Patients performed repetitive tapping movements (as described in the MDS-UPDRS task) with the hands or the feet in the presence or absence of a metronome set to 4 Hz. Movements were recorded with a video motion system. The primary endpoint was the occurrence of segmental freezing in these tapping tasks. The secondary endpoints were (i) the relationship between segmental episodic phenomena and FoG severity, and (ii) the reliability of the measurements.
Results
For the upper limbs, freezing was observed more frequently with a metronome (21% of trials) than without a metronome (5%). For the lower limbs, the incidence of freezing was higher than for the upper limbs, and was again observed more frequently in the presence of an auditory cue (47%) than in its absence (14%).
Conclusion
Although freezing of the lower limbs was easily assessed during an MDS-UPDRS task with a metronome, it was not correlated with the severity of freezing of gait (as evaluated during a standardized gait trajectory). Only this latter was a reliable measurement in patients with advanced Parkinson’s disease.
Introduction
Freezing of gait (FoG) has been defined as the “absence or marked reduction of forward progression of the feet despite the intention to walk” [1]. FoG is frequently reported in Parkinson’s disease (PD) [2], and has been identified as an independent risk factor for falls [3, 4] and impaired quality of life [5]. The “gold standard” for FoG measurement is a clinical evaluation of video recordings of ambulating patients by one, two or three raters (who ideally should be experts in the assessment of FoG) [6]. One of the main problems in FoG measurement (as highlighted by Snijders et al. [7]) is that an examination in a lab environment or the physician’s office can temporarily suppress a patient’s FoG. This failure to elicit the FoG phenomenon poses a problem for physicians, who need to base their evaluation on observations in the consulting room; this is why researchers have looked for more easily detectable equivalents of FoG or festination in effectors other than the lower limbs.
Finger tapping may be an equivalent of FoG on upper limbs. Indeed, finger tapping provokes “manual motor blocks” which are correlated with patients’ gait impairments. These motor blocks also called segmental “freezing episodes” may occur in repetitive upper and lower limb movements [8]. These blocks are commonly assessed by using items 3.4 and 3.8 of the Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS) [9]). It has been reported that these episodes of upper limb freezing are correlated with FoG scores, independently of disease severity or cognitive impairment [10–12]. Since the initial reports, many studies have used finger-tapping tasks to assess equivalents of FoG in the upper limbs [13–17]. Equivalents of FoG in the lower limbs are currently being used to study the physiopathology of FoG in neuroimaging experiments (e.g. functional MRI) because studying gait directly is not possible inside the bore of an MRI machine [18]. Numerous studies having mainly evaluated the occurrence of segmental (hand or foot) freezing during various protocols (for a review, see [19]), including either unilateral hand or finger tapping without cues, with cues [13, 20–22], during bilateral hand or finger tapping[13–16, 23–25]. Studies on lower limbs also used various protocols including mainly bilateral tapping or pedaling, associated with cues [15] or without [26–28] or with virtual reality protocols [23, 25–27].
The use of external cue can be very useful to trigger freezing [23, 26, 29, 30]. We previously showed that the use of an external cue at an appropriate frequency was associated with an elevated occurrence of episodic rhythmic disorders (at around 4 Hz) [30]. However, those segmental freezing phenomena have not been studied in patients with advanced PD who suffer from residual off-FoG, despite optimized dopatherapy. We therefore sought to determine whether these freezing phenomena were also relevant in this particular patient population.
The primary objective of the present study of advanced PD patients presenting FoG was to determine the occurrence of episodic segmental freezing phenomena on upper and lower limbs during commonly used tests (items 3.4 and 3.8 of the MDS-UPDRS [9]) in the presence and absence of a metronome set to 4 Hz. The secondary objective was to test the reliability of these phenomena between 2 sessions and their relationship with the severity of FoG.
We hypothesized that freezing equivalents would be more frequent in the presence of a metronome since a metronome could better trigger these segmental freezing episodes [30]. Indeed, freezing episodes during diadochokinetic tasks mainly occur at between 3 and 5 Hz [30]. As a secondary objective, we expected that the reliability of these measurements between 2 sessions would be high. Given that equivalents of freezing have been observed in advanced PD patients in previous literature (see [19]), we also expected that freezing equivalent will be present in all patients presenting actual FoG and highly correlated with its severity as suggested by several authors [10, 29, 31].
Material and methods
Participants
Patients with PD (diagnosed according to UK PDS Brain Bank criteria [32]) were enrolled from the active case file of the Movement Disorders Department at Lille University Medical Center (Lille, France) (Table 1). The patients were selected on the basis of their answer to item 3 of the FoG questionnaire [33]. To check that FoG was indeed not fully controlled by medication, we asked patients to perform 540° turns to the left and to the right (at normal and maximum speeds) when they were in the “on” state (i.e. while taking their usual medications). Only patients with clinically confirmed, treatment-refractory “off” FoG (despite having a stable medication regimen for at least the previous 3 months) were eligible for inclusion.
Table 1. Clinical characteristics of the study population.
| Subject | Age (years) | MOCA (/30) | Gender | BMI (kg/m2) | Disease duration (years) | FOG questionnaire | item 2.13 MDS-UPDRS | item 3.11 MDS-UPDRS | MDS-UPDRS-3 | Hoeh and Yahr 'on drug' |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | 28 | 1 | 27 | 18 | 11 | 2 | 0 | 38 | 2.5 |
| 2 | 72 | 22 | 1 | 22 | 15 | 11 | 2 | 2 | 38 | 2 |
| 3 | 71 | 27 | 2 | 30 | 7 | 12 | 3 | 2 | 46 | 3 |
| 4 | 69 | 19 | 1 | 22 | 15 | 13 | 1 | 1 | 35 | 2 |
| 5 | 72 | 21 | 1 | 22 | 9 | 14 | 3 | 2 | 40 | 2 |
| 6 | 59 | 23 | 1 | 31 | 10 | 16 | 1 | 2 | 42 | 2 |
| 7 | 77 | 25 | 2 | 22 | 4 | 11 | 2 | 2 | 55 | 3 |
| 8 | 65 | 27 | 1 | 25 | 15 | 18 | 0 | 0 | 24 | 2 |
| 9 | 73 | 27 | 1 | 31 | 4 | 12 | 2 | 2 | 42 | 3 |
| 10 | 71 | 21 | 2 | 26 | 17 | 15 | 1 | 0 | 46 | 2 |
| 11 | 53 | 29 | 1 | 26 | 15 | 17 | 1 | 0 | 58 | 2 |
| 12 | 74 | 29 | 1 | 23 | 14 | 15 | 3 | 2 | 41 | 3 |
| 13 | 66 | 25 | 2 | 22 | 13 | 18 | 3 | 3 | 57 | 3.5 |
| 14 | 60 | 20 | 1 | 27 | 12 | 11 | 1 | 1 | 39 | 3 |
| 15 | 58 | 24 | 1 | 24 | 4 | 18 | 3 | 0 | 42 | 4 |
| Median | 69 | 25 | 25 | 13 | 14 | 2 | 2 | 42 | 2.5 | |
| Quartile 1 | 60 | 22 | 22 | 8 | 12 | 1 | 0 | 39 | 2 | |
| Quartile 3 | 72 | 27 | 27 | 15 | 17 | 3 | 2 | 46 | 3 |
The exclusion criteria included the inability to walk alone, the use of deep brain stimulation, the presence of neurological disorders other than PD, and ongoing major depression.
The study was approved by the local institutional review board (CPP Nord-Ouest IV, Lille, France; reference 13/41, n°2013-A00737-38) and promoted by Lille University Medical Center. Signed informed consent was obtained from all patients in accordance with the institutional ethics committee board.
Experimental design
The study comprised a session at baseline and a second session one week later. Two conditions (with and without a metronome) were applied in each session, in random order. All evaluations were made between 9.00 am and 10.30 am in the “on-drug” condition, between 90 and 120 minutes after the usual dopaminergic intake. Indeed, most of them would have been unable to perform the tests in “off-drug” condition and still presented FoG in “on-drug” condition (see inclusion criteria).
Clinical evaluations: FoG evaluation
We measured the duration of FoG (and then calculated the percentage time with FoG) during a standardized FoG trajectory comprising gait initiation, turning (360- and 540-degree turns at the preferred speed and at maximum speed), walking through a narrow passage, and dual-tasking (walking while counting backwards in threes) [34]. The trajectories were filmed with a video camera. Offline, two raters (AD and CT) determined the completion time and the time with FoG for each part of the trajectory.
Movement analysis
Kinematic parameters were recorded using a VICON 3D motion analysis system with eight infrared cameras (sampled at 100 Hz). Reflective spheres were placed on the third phalanx of the index for the hand task, and on the calcaneus for the foot task.
Upper and lower limbs tasks (S1 Video)
The hand task: Each hand was tested separately. We demonstrated the task, but did not continue to perform the task while the patient was tested. We instructed the patient to tap the index finger on the thumb (i) as big and as fast as possible (ii) as big as possible in time with an auditory cue (a metronome set to 4 Hz). The tap (index finger on the thumb) had to be in time with the metronome sound. The order of the two conditions was pseudo-random. At least 30 productions on each body side were recorded.
The foot task: The patient sat in a straight-back chair, both feet on the floor. We demonstrated the task, but do not continue to perform the task while the patient is tested. We then instructed the patient to place the foot on the ground and then raise and stomp the foot on the ground (i) as high and as fast as possible, (ii) to stomp the foot on the ground as high as possible in time with the metronome set to 4 Hz. Again, the order of the two conditions was pseudo-random, and at least 30 productions on each body side were recorded.
Data processing
A series of 30 productions were selected for each effector and each condition. For each production, the peak-to-peak interval was determined semi-automatically using a home-made matlab script and corrected manually by the examiner if obviously erroneous (peaks and nadir on sagittal plane were then picked manually). This process yielded the instantaneous frequency and amplitude of each tap.
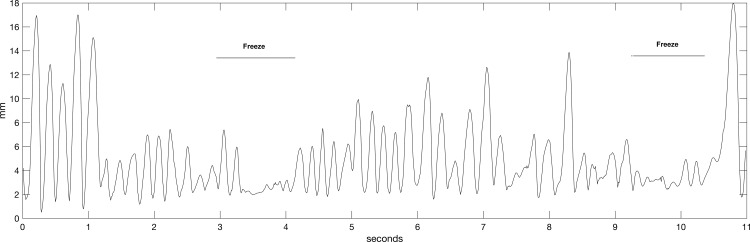
Definition of episodic events (Fig 1)
Fig 1. A patient presented two episodes of freezing while performing the lower-limb tapping task in the presence of auditory cueing.
The position of the right heel marker (in the sagittal plane) is shown here.
Freezing episodes of the hands and feet were defined as involuntary arrests of repetitive movement, i.e. an amplitude less than half the mean amplitude in the test for at least 0.5 seconds (as defined in the literature [11, 17]).
Voluntary stops were excluded by careful examination of the video-taped trials (raters: CT and AD). Depending on the number of episodes of freezing, each trial was further classified as a “freezing trial” or a “no-freezing trial”.
Definition of rhythmicity parameters
The frequency and amplitude of tapping, and the coefficients of variation (CVs) for the amplitude and duration of the tapping (defined as the standard deviation (SD) of the 30 instantaneous amplitudes divided by the mean of the 30 instantaneous amplitudes) were computed.
Statistical analysis
Statistical analysis was performed using SPSS software (version 16.0, IBM Corp., Armonk, NY). The threshold for statistical significance was set to p<0.05.
The primary study criterion was the frequency of episodic events for each condition (the presence or absence of auditory cueing) and each effector (upper limb or lower limb). Categorical data were compared using chi-squared tests. Model assumptions (normality of residuals and homoscedasticity) were checked, and data were rank-transformed if the assumptions were not satisfied. To test our primary objective (occurrence of freezing of effectors in PD freezers and elicitation by auditory cueing), we used a general linear model (analysis of variance with repeated measures) with a between-subject factor (the presence or absence of auditory cueing) and a within-subject factor (the effector). If the interaction was significant, post-hoc comparisons were performed using the Bonferroni correction.
The secondary criteria included the reliability of the measurements, and the relationship between episodic events and severity of FoG. For reliability, Cronbach’s alpha was computed. Either Pearson’s or Spearman’s correlation coefficient was calculated as a guide to the relationship between the duration of freezing episodes (in all trials performed) and the corresponding clinical data on FoG.
Results
Occurrence of FoG
Fifteen patients were included in the study. The patients’ demographic and clinical characteristics are summarized in Table 1. The patients had similar levels of motor performance in the two sessions. All 15 patients presented with FoG. The median (interquartile range IQR) time with FoG during the FoG trajectory was 23 (6–51) seconds. The median ratio (IQR) between the time with FoG and the trajectory completion time was 0.35 (0.08–0.56).
Occurrence of freezing episodes for the two effectors
In the hand task, 3 of the 15 patients displayed episodes of freezing in the absence of an auditory cue and 7 displayed episodes in the presence an auditory cue. For the foot task, 9 of the 15 patients displayed episodes of freezing in the absence of an auditory cue and 11 displayed episodes in the presence an auditory cue. We observed an effect of the effector (p = 0.02 in a chi- squared test) but not an effect of cueing (p = 0.2 in a chi- squared test).
For the hand, freezing occurred in 5% of the trials without auditory cueing and in 21% of the trials with auditory cueing. For the foot, freezing occurred in 14% of the trials without auditory cueing and in 47% of the trials with auditory cueing. We observed an effect of auditory cueing (p<0.001) and an effect of the effector (p<0.001).
The mean ± SD duration of episodes of freezing (with pooled data for the two effectors because there was no difference between the hand and the foot) was 0.84 ± 0.45 s without auditory cueing and 1.10 ± 0.60 s with auditory cueing; this difference was not significant).
Rhythmicity parameters (Table 2)
Table 2. Rhythmicity parameters.
| hands | hands metronome | feet | feet metronome | effector | metronome | Interaction | |
|---|---|---|---|---|---|---|---|
| Frequency (mean/SD) Hz | 4.0/0.9 | 4.5/0.9 | 3.1/0.7 | 3.8/0.7 | p<0.001 | p<0.001 | 0.428 |
| Amplitude mm | 25 /13 | 21/12 | 29/21 | 16/14 | p<0.001 | 0.748 | p = 0.001 |
| CV duration | 0.11/0.09 | 0.10/0.08 | 0.07/0.05 | 0.15/0.12 | 0.688 | p<0.001 | p<0.001 |
| CV amplitude | 0.19/0.08 | 0.21/0.12 | 0.22/0.12 | 0.34/0.16 | p<0.001 | p<0.001 | p<0.001 |
For both effectors, the tapping frequency was significantly higher in the presence of auditory cueing (p<0.001). Regardless of the condition, the tapping frequency was lower for the lower limbs than for the upper limbs (p<0.001). The amplitude was lower in the presence of auditory cueing (p<0.001). There was no effect of the effector on amplitude, although a significant cueing x effector interaction was observed (p = 0.001): for the foot task, the amplitude was significantly lower in the presence of auditory cueing. The variability of the amplitude and the duration was higher with auditory cueing (p<0.001), and the effects were more marked for the feet (p<0.001 for the cueing x effector interaction, for both amplitude and duration).
Reliability parameters
When comparing the two sessions, Cronbach’s alpha was 0.75 for the ratio between the time with FoG and the FoG trajectory completion time, 0.72 for time with FoG, and 0.36 for the percentage of trials with FoG.
Correlations between episodic events, impaired rhythm generation, and the severity of FoG
Whatever the effector, neither the mean time spent with freezing during segmental tasks with auditory cueing nor the proportion of trials with FoG was correlated with the time with FoG during FoG trajectory, ratio between the time with FoG and the FoG trajectory completion time or other clinical variables including FoG questionnaire (see Table 1). Time spent with FoG was positively correlated with the MDS-UPDRS item 2.13 and 3.11 scores (Rho = 0.54, p<0.01; Rho = 0.63, p<0.001, respectively) but not with FoG questionnaire.
Discussion
The two main findings of the present study are that commonly used tests (items 3.4 and 3.8 of the MDS-UPDRS[9]) can trigger freezing—mainly in the lower limbs. The use of a metronome increased the occurrence of motor blocks by a factor of two or three, although the number of patients who did not present freezing at all during the item 3.8 test was not significantly lower in the presence of auditory cueing. Furthermore, we evaluated the intersession reliability of the “gold standard” clinical test (the FoG trajectory) [36] and the occurrence of freezing during segmental tasks. The intersession reliability was low (Cronbach’s alpha <0.8 in all cases), making these tasks difficult to use as primary criteria in interventional studies. However, the ratio between the time with FoG and the FoG trajectory completion time seems to be the most reliable parameter—in accordance with a literature report [37].
Use of auditory cue to trigger freezing
It has already been reported that the use of a metronome can trigger freezing. In 2006, Moreau et al. [29] showed that patients suffered from FoG more frequently when a metronome was set at 140% of their normal pace. The application of a metronome can be considered as a stressful dual-task condition [38]. One can hypothesize that “mental collapse” leads to freezing; indeed, the stress induced by the metronome and the freezer patients’ difficulties in simultaneously controlling limb movement may trigger FoG. An alternative hypothesis is that the “dual task” increased freezing in our patients because of the latter’s limited ability to distribute attentional resources, due to lack of neural reserve (also called interference hypothesis of freezing [39]). In our study, we didn’t observe any correlation between freezing and the MoCA score that is a very global evaluation of cognition and not specific attentional components that could be more specific of freezing deficits [40]. Furthermore, the potential role of anxiety during the metronome condition should not be overlooked since it is highly prevalent in freezers [41] and can lead to freezing [42]. In the present study, the single imposed frequency (4 Hz, previously demonstrated to trigger freezing [30]) was higher than the patient’s self-generated frequency. Auditory cueing appears to increase the priority given to maintaining frequency because the amplitude decreased for the feet (but not for the hands). The prioritization of frequency might also explain the freezing phenomenon, given that the imposition of low-amplitude movements triggers FoG more easily [43, 44]. However, this was not confirmed in our upper limb task; during the trials without cueing, patients were instruction to perform the movement as big and as fast as possible. This condition was associated with great variability of the spatiotemporal parameters–especially when a higher frequency was imposed. The elevated variability may also have triggered freezing [45]. The instruction given to the patient is a crucial point. Asking to tap “as big/high as possible” can alleviate freezing since freezing is more frequent for low amplitude movements [44], although in the walking task, no instruction about step amplitude was given. This could explain -in part- differences between the 2 tasks.
Occurrence of freezing according to the effectors
Lastly, we were very surprised to observe that hand and foot freezing phenomena occurred less frequently than FoG itself—even under stressed (cued) conditions. Our definition of freezing was based on a decrease in amplitude (i.e. severe hypokinesia) for at least 0.5 s; this might not be totally specific for freezing phenomena. In the absence of PD, freezing episodes of the upper or lower limbs are rarely observed in elderly patients [30]. However, this does not explain the differences observed when comparing upper limb and lower limb effectors. Some researchers have suggested that freezing is a somatotopic task because some patients freeze during activities such as speaking and writing [16]. This hypothesis is strengthened by the fact that all 15 patients presented FoG during an on-medication FoG trajectory but only 11 experienced freezing during a 4 Hz foot-tapping task. In early-stage PD, the production of bimanual movements in time with a metronome was a very sensitive freezing trigger [30]. However, this trigger might be inadequate in advanced PD patients, who present FoG and lower-limb freezing more frequently. It may be that different mechanisms and neural bases are involved. In summary, FoG occurs more frequently than either upper-limb or lower-limb freezing in advanced PD patients.
Limitations
No control group (non-freezer patients) was recorded. Indeed, in studies involving rhythmic tasks of either upper limbs or lower limbs, there is large evidence that freezing is more frequent in patients presenting FoG compared with patients without FoG [8,13,16,21,29–31].
We chose to quantify FoG using time spent with FoG during a standardized trajectory as our main quantification of FOG in our patients rather than other measurement such as FoG questionnaire. Indeed, all our patients presented by definition severe FoG with a high median score (14) with low interquartile interval at the FoG questionnaire (12–17). However, we didn’t find any additional correlation between FoG questionnaire and segmental freezing measurements.
We chose to test unilateral rhythmic movements (items 3.4 and 3.8 of the MDS-UPDRS) since they are used daily by clinicians to evaluate segmental akinesia, hypokinesia or bradykinesia. However, bimanual movement such as antiphase movements [46] or virtual reality [23,47] could have been more efficient to trigger freezing but were less commonly used in clinical evaluations.
Conclusion
In patients with advanced PD and FoG, freezing of the upper and lower limbs can be observed in tapping tasks (especially in the presence of auditory cueing) but with a weak occurrence. The use of a metronome at 4 Hz can be useful to better trigger freezing even in a simple tapping task of the foot or the hand. However the occurrence of segmental freezing was less reliable than the FoG observed during a FoG trajectory and not correlated with FoG measurement. Then, the consideration of segmental freezing as tools or equivalents of FoG for studying freezing of gait should be used cautious since pathophysiology of these phenomena may differ.
Supporting information
(MP4)
Acknowledgments
We thanks Santelys for funding.
Data Availability
All relevant data are in the paper and its Supporting Information files.
Funding Statement
Céline Tard received a grant from Santelys and has received various honoraria for lectures on neuromuscular disorders. Luc Defebvre served on several Scientific Advisory Boards for Abbvie and Zambon. He has received various honoraria from pharmaceutical companies for consultancy and lectures on Parkinson¹s disease at symposia. These companies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10: 734–744. doi: 10.1016/S1474-4422(11)70143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giladi N, Treves TA, Simon ES, Shabtai H, Orlov Y, Kandinov B, et al. Freezing of gait in patients with advanced Parkinson’s disease. J Neural Transm Vienna Austria 1996. 2001;108: 53–61. [DOI] [PubMed] [Google Scholar]
- 3.Latt MD, Lord SR, Morris JGL, Fung VSC. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2009;24: 1280–1289. doi: 10.1002/mds.22561 [DOI] [PubMed] [Google Scholar]
- 4.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75: 116–124. doi: 10.1212/WNL.0b013e3181e7b688 [DOI] [PubMed] [Google Scholar]
- 5.Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov Disord Off J Mov Disord Soc. 2007;22: 2192–2195. doi: 10.1002/mds.21659 [DOI] [PubMed] [Google Scholar]
- 6.Heremans E, Nieuwboer A, Vercruysse S. Freezing of gait in Parkinson’s disease: where are we now? Curr Neurol Neurosci Rep. 2013;13: 350 doi: 10.1007/s11910-013-0350-7 [DOI] [PubMed] [Google Scholar]
- 7.Snijders AH, Nijkrake MJ, Bakker M, Munneke M, Wind C, Bloem BR. Clinimetrics of freezing of gait. Mov Disord Off J Mov Disord Soc. 2008;23 Suppl 2: S468–474. doi: 10.1002/mds.22144 [DOI] [PubMed] [Google Scholar]
- 8.Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur J Neurosci. 2009;29: 1422–1430. doi: 10.1111/j.1460-9568.2009.06681.x [DOI] [PubMed] [Google Scholar]
- 9.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord Off J Mov Disord Soc. 2008;23: 2129–2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 10.Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. Eur J Neurosci. 2009;29: 1422–1430. doi: 10.1111/j.1460-9568.2009.06681.x [DOI] [PubMed] [Google Scholar]
- 11.Vercruysse S, Spildooren J, Heremans E, Vandenbossche J, Levin O, Wenderoth N, et al. Freezing in Parkinson’s disease: a spatiotemporal motor disorder beyond gait. Mov Disord Off J Mov Disord Soc. 2012;27: 254–263. doi: 10.1002/mds.24015 [DOI] [PubMed] [Google Scholar]
- 12.Ziv I, Avraham M, Dabby R, Zoldan J, Djaldetti R, Melamed E. Early-occurrence of manual motor blocks in Parkinson’s disease: a quantitative assessment. Acta Neurol Scand. 1999;99: 106–111. [DOI] [PubMed] [Google Scholar]
- 13.Stegemöller EL, Simuni T, MacKinnon C. Effect of movement frequency on repetitive finger movements in patients with Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2009;24: 1162–1169. doi: 10.1002/mds.22535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vercruysse S, Spildooren J, Heremans E, Vandenbossche J, Wenderoth N, Swinnen SP, et al. Abnormalities and cue dependence of rhythmical upper-limb movements in Parkinson patients with freezing of gait. Neurorehabil Neural Repair. 2012;26: 636–645. doi: 10.1177/1545968311431964 [DOI] [PubMed] [Google Scholar]
- 15.Vercruysse S, Spildooren J, Heremans E, Vandenbossche J, Levin O, Wenderoth N, et al. Freezing in Parkinson’s disease: A spatiotemporal motor disorder beyond gait. Mov Disord. 2012;27: 254–263. doi: 10.1002/mds.24015 [DOI] [PubMed] [Google Scholar]
- 16.Williams AJ, Peterson DS, Ionno M, Pickett KA, Earhart GM. Upper extremity freezing and dyscoordination in Parkinson’s disease: effects of amplitude and cadence manipulations. Park Dis. 2013;2013: 595378 doi: 10.1155/2013/595378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbe MT, Amarell M, Snijders AH, Florin E, Quatuor E-L, Schönau E, et al. Gait and upper limb variability in Parkinson’s disease patients with and without freezing of gait. J Neurol. 2014;261: 330–342. doi: 10.1007/s00415-013-7199-1 [DOI] [PubMed] [Google Scholar]
- 18.Herman T, Giladi N, Hausdorff JM. Neuroimaging as a window into gait disturbances and freezing of gait in patients with Parkinson’s disease. Curr Neurol Neurosci Rep. 2013;13: 411 doi: 10.1007/s11910-013-0411-y [DOI] [PubMed] [Google Scholar]
- 19.Vercruysse S, Gilat M, Shine JM, Heremans E, Lewis S, Nieuwboer A. Freezing beyond gait in Parkinson’s disease: A review of current neurobehavioral evidence. Neurosci Biobehav Rev. 2014;43C: 213–227. doi: 10.1016/j.neubiorev.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 20.Yahalom G, Simon ES, Thorne R, Peretz C, Giladi N. Hand rhythmic tapping and timing in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10: 143–148. doi: 10.1016/j.parkreldis.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Jones CRG, Claassen DO, Yu M, Spies JR, Malone T, Dirnberger G, et al. Modeling accuracy and variability of motor timing in treated and untreated Parkinson’s disease and healthy controls. Front Integr Neurosci. 2011;5: 81 doi: 10.3389/fnint.2011.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stegemöller EL, Zadikoff C, Rosenow JM, Mackinnon CD. Deep brain stimulation improves movement amplitude but not hastening of repetitive finger movements. Neurosci Lett. 2013;552: 135–139. doi: 10.1016/j.neulet.2013.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naismith SL, Lewis SJG. A novel paradigm for modelling freezing of gait in Parkinson’s disease. J Clin Neurosci Off J Neurosurg Soc Australas. 2010;17: 984–987. doi: 10.1016/j.jocn.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 24.Abe K, Asai Y, Matsuo Y, Nomura T, Sato S, Inoue S, et al. Classifying lower limb dynamics in Parkinson’s disease. Brain Res Bull. 2003;61: 219–226. [DOI] [PubMed] [Google Scholar]
- 25.Shine JM, Matar E, Ward PB, Frank MJ, Moustafa AA, Pearson M, et al. Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain J Neurol. 2013; doi: 10.1093/brain/awt272 [DOI] [PubMed] [Google Scholar]
- 26.Gilat M, Shine JM, Bolitho SJ, Matar E, Kamsma YPT, Naismith SL, et al. Variability of Stepping during a Virtual Reality Paradigm in Parkinson’s Disease Patients with and without Freezing of Gait. PloS One. 2013;8: e66718 doi: 10.1371/journal.pone.0066718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilat M, Bell PT, Ehgoetz Martens KA, Georgiades MJ, Hall JM, Walton CC, et al. Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson’s disease. NeuroImage. 2017;152: 207–220. doi: 10.1016/j.neuroimage.2017.02.073 [DOI] [PubMed] [Google Scholar]
- 28.Nantel J, de Solages C, Bronte-Stewart H. Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson’s disease. Gait Posture. 2011;34: 329–333. doi: 10.1016/j.gaitpost.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 29.Moreau C, Defebvre L, Bleuse S, Blatt JL, Duhamel A, Bloem BR, et al. Externally provoked freezing of gait in open runways in advanced Parkinson’s disease results from motor and mental collapse. J Neural Transm Vienna Austria 1996. 2008;115: 1431–1436. doi: 10.1007/s00702-008-0099-3 [DOI] [PubMed] [Google Scholar]
- 30.Delval A, Rambour M, Tard C, Dujardin K, Devos D, Bleuse S, et al. Freezing/festination during motor tasks in early-stage Parkinson’s disease: A prospective study. Mov Disord Off J Mov Disord Soc. 2016;31: 1837–1845. doi: 10.1002/mds.26762 [DOI] [PubMed] [Google Scholar]
- 31.Bronte-Stewart HM, Ding L, Alexander C, Zhou Y, Moore GP. Quantitative digitography (QDG): a sensitive measure of digital motor control in idiopathic Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2000;15: 36–47. [DOI] [PubMed] [Google Scholar]
- 32.Gibb WR. Accuracy in the clinical diagnosis of parkinsonian syndromes. Postgrad Med J. 1988;64: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2009;24: 655–661. doi: 10.1002/mds.21745 [DOI] [PubMed] [Google Scholar]
- 34.Snijders AH, Nijkrake MJ, Bakker M, Munneke M, Wind C, Bloem BR. Clinimetrics of freezing of gait. Mov Disord Off J Mov Disord Soc. 2008;23 Suppl 2: S468–474. doi: 10.1002/mds.22144 [DOI] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53: 695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 36.Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord. 2012;18: 149–154. doi: 10.1016/j.parkreldis.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 37.Morris TR, Cho C, Dilda V, Shine JM, Naismith SL, Lewis SJG, et al. A comparison of clinical and objective measures of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2012; doi: 10.1016/j.parkreldis.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 38.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22: 1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x [DOI] [PubMed] [Google Scholar]
- 39.Lewis SJG, Barker RA. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15: 333–338. doi: 10.1016/j.parkreldis.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 40.Tard C, Delval A, Duhamel A, Moreau C, Devos D, Defebvre L, et al. Specific Attentional Disorders and Freezing of Gait in Parkinson’s Disease. J Park Dis. 2015; doi: 10.3233/JPD-140498 [DOI] [PubMed] [Google Scholar]
- 41.Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. 2014;71: 884–890. doi: 10.1001/jamaneurol.2014.753 [DOI] [PubMed] [Google Scholar]
- 42.Ehgoetz Martens KA, Ellard CG, Almeida QJ. Does Anxiety Cause Freezing of Gait in Parkinson’s Disease? PloS One. 2014;9: e106561 doi: 10.1371/journal.pone.0106561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord Off J Mov Disord Soc. 2006;21: 1419–1424. doi: 10.1002/mds.20998 [DOI] [PubMed] [Google Scholar]
- 44.Heremans E, Nackaerts E, Vervoort G, Vercruysse S, Broeder S, Strouwen C, et al. Amplitude Manipulation Evokes Upper Limb Freezing during Handwriting in Patients with Parkinson’s Disease with Freezing of Gait. PloS One. 2015;10: e0142874 doi: 10.1371/journal.pone.0142874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149: 187–194. doi: 10.1007/s00221-002-1354-8 [DOI] [PubMed] [Google Scholar]
- 46.Almeida QJ, Wishart LR, Lee TD. Bimanual coordination deficits with Parkinson’s disease: the influence of movement speed and external cueing. Mov Disord Off J Mov Disord Soc. 2002;17: 30–37. [DOI] [PubMed] [Google Scholar]
- 47.Matar E, Shine JM, Naismith SL, Lewis SJG. Using virtual reality to explore the role of conflict resolution and environmental salience in Freezing of Gait in Parkinson’s disease. Parkinsonism Relat Disord. 2013; doi: 10.1016/j.parkreldis.2013.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(MP4)
Data Availability Statement
All relevant data are in the paper and its Supporting Information files.