Abstract
Study design
Validation study using data from a multicenter, randomized, clinical trial (RCT).
Objectives
To evaluate the reliability, validity, responsiveness, and minimal important change (MIC) of the Dutch version of the Oxford Elbow Score (OES) and the Quick Disabilities of the Arm, Shoulder, and Hand (Quick-DASH) in patients with a simple elbow dislocation.
Background
Patient-reported outcome measures are increasingly important for assessing outcome following elbow injuries, both in daily practice and in clinical research. However measurement properties of the OES and Quick-DASH in these patients are not fully known.
Methods
OES and Quick-DASH were completed four times until one year after trauma. Mayo Elbow Performance Index, pain (VAS), Short Form-36, and EuroQol-5D were completed for comparison. Data of a multicenter RCT (n = 100) were used. Internal consistency was determined using Cronbach’s alpha. Construct and longitudinal validity were assessed by determining hypothesized strength of correlation between scores or changes in scores, respectively, of (sub)scales. Finally, floor and ceiling effects, MIC, and smallest detectable change (SDC) were determined.
Results
OES and Quick-DASH demonstrated adequate internal consistency (Cronbach α, 0.882 and 0.886, respectively). Construct validity and longitudinal validity of both scales were supported by >75% correctly hypothesized correlations. MIC and SDC were 8.2 and 12.0 point for OES, respectively. For Quick-DASH, these values were 11.7 and 25.0, respectively.
Conclusions
OES and Quick-DASH are reliable, valid, and responsive instruments for evaluating elbow-related quality of life. The anchor-based MIC was 8.2 points for OES and 11.7 for Quick-DASH.
Introduction
Musculoskeletal elbow injuries may influence health and quality of life [1–3]. Physicians have traditionally been focused on objective parameters such as radiographic healing or range of motion when evaluating recovery following elbow injuries. However, patients’ own appreciation of recovery may differ from the judgment of the treating physician [4–6]. Patient-reported outcome measures (PROMs) are increasingly important for assessing outcome following elbow injuries, both in daily practice and in clinical research [7]. A multitude of such questionnaires is available for monitoring outcome over time. Region-specific questionnaires provide insight in pain and functional problems caused by specific injuries or injuries at a specific anatomic region. Generic quality of life questionnaires like the Short Form-36 (SF-36) and EuroQoL-5D (EQ-5D), on the other hand, enable comparison across populations with different injuries. Instruments should only be used if proven reliable and valid.
The best elbow-specific questionnaire currently is the Oxford Elbow Score (OES). This originally English patient-reported questionnaire measures injury-related quality of life in patients following surgery of the elbow joint [8–10]. The OES was translated into Dutch according to the guideline for Cross Cultural Adaptation of Self-Report Measures and validated for its reliability, validity, and responsiveness [11–14]. Limitations of a pilot validation study, in which the OES was compared with the DASH, were a small sample size and heterogenic population consisting of operatively and non-operatively treated patients. The OES has been shown valid and reliable for the assessment of outcome in patients with surgically treated chronic elbow pathologies [15]. However, measurement properties for patients with acute elbow injuries where full recovery is to be expected are not available.
The most often used questionnaire for upper extremity injuries is the Disability of the Arm, Shoulder, and Hand (DASH). It was designed to describe disability experienced by patients with any musculoskeletal condition of the upper extremity and to monitor change in symptoms and upper limb function over time [16]. The DASH questionnaire has been validated in patients with upper extremity musculoskeletal disorders such as rheumatoid arthritis and shoulder impingement syndrome [17–19]. The Quick-DASH is a shortened version of the DASH [20].
Measurement properties of the OES and Quick-DASH in patients with a simple elbow injury are not fully known. The Minimal Important Change (MIC), which is an important input parameter for sample size calculations in clinical studies, is not available for these scores.
The aim of the current study was to evaluate the reliability, validity, responsiveness, and minimal important change of the OES and the Quick-DASH in adult patients with a non-operatively treated simple elbow dislocation. The Mayo Elbow Performance Index, two general health-related quality of life instruments and subscales (i.e., Short Form-36 and EuroQoL-5D), and pain measured with a Visual Analog Scale were used for comparison.
Materials and methods
Study data
Data of a multicenter randomized clinical trial comparing early functional treatment with plaster immobilization in patients after a simple elbow dislocation (FuncSiE-trial) were used. The trial is registered at the Netherlands Trial Register (NTR2025). The results of this study and the study protocol are published elsewhere [21, 22]. The study was approved by the Medical Research Ethics Committees or Local Ethics Boards of all participating centers. The study was approved by the Medical Research Ethics Committees of Erasmus MC (registration number MEC-2009-239) and Local Ethics Boards of all participating centers (i.e. Red Cross Hospital (Beverwijk), Bronovo Hospital (The Hague), Westfriesgasthuis (Hoorn), Reinier de Graaf Gasthuis (Delft), Slotervaart Hospital (Amsterdam), Onze Lieve Vrouwe Gasthuis (Amsterdam), Medical Center Haaglanden (The Hague), Zaans Medical Center (Zaandam), Academic Medical Center (Amsterdam), Deventer Hospital (Deventer), Maasstad Hospital (Rotterdam), Leiden University Medical Center (Leiden), Hospital Rivierenland (Tiel), Elkerliek Hospital (Helmond), Flevo Hospital (Almere), Medical Center Alkmaar (Alkmaar), Groene Hart Hospital (Gouda), Haga Hospital (The Hague), Diakonessenhuis (Utrecht), Amphia Hospital (Breda), Admiraal de Ruyter Hospital (Goes)).
Patients
Patients were recruited from August 25, 2009 until September 18, 2012. Inclusion criteria were 1) age of 18 years or older; 2) a simple elbow dislocation with successful close reduction; and 3) written informed consent. Exclusion criteria were 1) polytraumatized patients; 2) recurrent or open dislocation; 3) additional traumatic injuries of the affected arm; 4) surgical intervention; 5) impaired elbow function prior to trauma (i.e., stiff or painful elbow or neurological disorder); 6) previous operations or fractures involving the elbow; and 7) expected problems with completing follow-up (e.g., insufficient comprehension of the Dutch language). Baseline characteristics were gender, age, affected side, and hand dominance. Patients completed a set of questionnaires during outpatient visits at six weeks and at three, six, and 12 months after randomization.
Questionnaires
The OES is a 12-item, three domain (elbow function, pain and social-psychological; 4 items each) questionnaire, reflecting injury-related quality of life. Each domain is transformed into a 100-point metric scale with higher score representing better outcome [15]. The same accounts for the total score. The original version was validated against the DASH [9, 15]. They showed a generally better performance for the OES than for the DASH in patients with elbow pathologies. The OES is available in several languages, and all validation studies to date were done in comparison with the DASH [23, 24]. The OES was translated from English into Dutch in compliance with translation guidelines [10, 12–14]. A pilot validation study was done in comparison with the DASH and confirmed sufficient reliability and validity in a heterogeneous group of patients with elbow pathologies [13]. Permission for the use of the OES for this study was obtained from Oxford and Isis Outcomes, part of Isis Innovation Limited (http://www.isis-innovation.com/).
The DASH is the most used questionnaire for disorders across the entire upper extremity. Validated versions are available in a multitude of languages, including Dutch. Sufficient validity, reliability, and responsiveness of the DASH has been shown for disorders across the entire upper extremity [25]. The Quick-DASH contains 11 items (scored 1–5) and reflects both function and pain in persons with musculoskeletal disorders of the upper extremity. To be able to calculate a score, at least 10 of the 11 items must be completed. The score is calculated using the formula: ((sum of all item/number of questions answered)-1)*11). The overall score ranges from 0 to 100 points with higher score representing greater disability [18, 25]. Reliability and validity were confirmed for the original version of the Quick-DASH compared with the DASH [20].
The Mayo Elbow Performance Index (MEPI) consists of four domains: pain (one item, maximum score 45 points), range of motion (20 points), stability (one item, 10 points), and function (5 items, 5 points each). Each domain is transformed into a 100-point scale with higher score representing better outcome [26].
A Visual Analog Scale (VAS) was used to measure the level of pain. The ends of the 100-mm horizontal line showed the word descriptors ‘no pain’ at 0 mm and ‘worst pain imaginable’ at 100 mm) [27].
The SF-36 is a validated 36-item health survey. It represent eight health domains (physical functioning (PF; ten items), role limitations due to physical health (RP; four items), bodily pain (BP; two items), and general health perceptions (GH; five items), vitality, energy, or fatigue (VT; four items), social functioning (SF; two items), role limitations due to emotional problems (RE; three items), and general mental health (MH; five items) that are combined into a physical and a mental component summary (PCS and MCS, respectively). The score ranges from 0–100 with higher scores representing higher quality of life. The scores are converted and compared with the norms for the general population of the United States [28]. A validated Dutch version is available [29].
The EQ-5D-3L is a validated instrument for measuring health-related quality of life. The EQ-5D utility score (EQ-US) ranges from 0 to 1 and is determined from five 1-item domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. In addition, the individual’s rating of his/her quality of life state is recorded by means of a standard Visual Analog Scale (EQ-VAS), which ranges from 0 to 100. Higher scores represent better health-related quality of life [30, 31]. A validated Dutch version is available [30].
Statistical analysis
Basic statistics
Analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 21. The receiver operating characteristic (ROC) curve and Youden index were analyzed using MedCalc 14.10.2 software (MedCalc Software, Ostend, Belgium). Data are reported in compliance with the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) guidelines. Since raw data for individual items were analyzed, missing data were not imputed. Descriptive statistics was used in order to describe the main characteristics of the study participants. Measurement properties of the OES and Quick-DASH (sub)scales were determined by comparing these (sub)scales with the VAS (for pain) MEPI, SF-36, and EQ-5D.
Reliability
Internal consistency is a measure of the extent to which items in a (sub)scale are correlated (homogeneous), thus measuring the same concept [4]. For each (sub)scale, correlation between the items was calculated using Cronbach’s alpha. Internal consistency can be considered sufficient if the Cronbach’s alpha value is between 0.70 and 0.95, provided that the scale is unidimensional [4]. The six week data were used, since the largest heterogeneity in the degree of recovery and consequently the largest variability in scores were expected at that time.
Construct validity
Validity is the degree to which a questionnaire measures the construct it is supposed to measure. As there was no gold standard in the current study, the validity of the OES was expressed in terms of the construct validity. Construct validity represents the extent to which scores on a specific questionnaire relate to other measures in a way that is in agreement with prior theoretically derived hypotheses concerning the concepts that are being measured [4]. The six weeks data were used. Construct validity of the OES was assessed by determining the correlation of the OES (sub)scales with (sub)scales of the Quick-DASH, MEPI, SF-36, and EQ-5D. Similar procedures were followed for the Quick-DASH. Since all data deviated from a Normal distribution (i.e., Shapiro-Wilk test had a p<0.05 for each (sub)scale), Spearman’s Rho (rank correlation) coefficients (r) were determined. Strengths of correlation was categorized as high (r>0.6), moderate (0.3<r<0.6), or low (r<0.3) [32]. Construct validity was considered sufficient if at least 75% of the results were in line with the predefined hypotheses in a (sub)sample of at least 50 patients [4]. Predefined hypotheses are shown in S1 Table and were made in consensus between three authors (GITI, DDH, and EMMVL).
Responsiveness
Responsiveness refers to the ability of a questionnaire to detect clinically important changes over time [4]. Longitudinal validity can be considered to be a measure of responsiveness. Longitudinal validity refers to the extent to which change in one measurement instrument relates to corresponding change in a reference measure [33]. Analogous to construct validity, longitudinal validity was assessed by testing predefined hypotheses about expected correlations between changes in OES and Quick-DASH (sub)scales and changes in all other (sub)scales. Change scores were calculated as the difference in score at six weeks (which is the first time all instruments were administered) and the final score at 12 months follow-up. Since all change scores deviated from a Normal deviation, Spearman correlation coefficients were calculated. Predefined hypotheses are shown in S1 Table. Longitudinal validity was considered sufficient if at least 75% of the results were in line with the predefined hypotheses in a (sub)sample of at least 50 patients [4].
The effect size (ES) and standardized response mean (SRM) were determined as measures of the magnitude over time. The ES was calculated by dividing the mean change in score between two time points (i.e., score at 12 months–score at six weeks) by the standard deviation of the first measurement [34]. The SRM was calculated by dividing the mean change in score between two time points (i.e., score at 12 months–score at six weeks) divided by the standard deviation of this change [34]. These effect estimates were interpreted according to Cohen; a value of 0.2–0.4 is considered a small, 0.5–0.7 a moderate, and ≥ 0.8 a large effect [32]. Large effect sizes were expected a priori, since at six weeks patients were expected to have functional limitations, whereas at 12 months full recovery was expected for most patients.
Floor and ceiling effects
Floor and ceiling effects are present if more than 15% of the study population rates the lowest (floor effect) or highest (ceiling effect) possible score on any questionnaire (sub)scale [35]. In the presence of floor and ceiling effects, items might be missing from the upper or lower ends of the scale, reducing content validity. Likewise, patients with the highest or lowest scores cannot be distinguished from one another, indicating limited reliability [4]. Floor and ceiling effect were determined for each follow-up moment separately.
Minimal important change and smallest detectable change
The minimal important change (MIC) is defined as the smallest measurable change in outcome score that is perceived as significant by patients [36]. An anchor-based method was used as this gives a better indication of the importance of the observed change to the patient [4]. In addition to the questionnaires patients were asked to complete a transition item (anchor question) evaluating their perception of change in the general condition of their affected elbow. The question was: How would you judge the condition of your elbow, compared with the last time you completed this questionnaire? The item scored from 1 ‘completely recovered‘ through 2 ‘much better’, 3 ‘slightly better’, 4 ‘no change’, 5 ‘slightly worse’, 6 ‘much worse’, or 7 ‘worse than ever’. The anchor or transition item was judged as adequate if a Spearman’s rank correlation between the anchor and the change score of the questionnaire was > 0.29 [37]. The corresponding change score (score at previous follow-up subtracted from the score at time of completion of the transition item) for patients who answered the transition item as ‘slightly better’ can be considered the MIC [38].
As an alternative, MIC was also calculated for the total scores by plotting the receiver operating characteristic (ROC) curve of the change in score for patients who scored ‘slightly better’ on the transition item versus patients who scored ‘no change’. The optimal ROC cutoff point (i.e., the associated criterion of the Youden index) reflects the MIC. This MIC is shown with its 95% confidence interval (CI) after bootstrapping (1000 replicates and 900 random-number seeds).
In addition to the MIC, the Smallest Detectable Change (SDC) was determined. SDC is defined as the smallest intra-personal change in score that represents (with p<0.05) a ‘real’ difference above measurement error [7]. As patients were assumed to be stable in the interim period, this was based on the change scores of patients who answered ‘no change’ on the transition item. First, the SEM was calculated by dividing the standard deviation of the mean difference between both measurements (SDchange) by the square root of two [39]. SEM can be considered as a measure of absolute measurement error [4]. For the individual patient, the SDC was calculated as 1.96 x square root of 2 x SEM (herein, SEM = SDchange / square root of 2) [4]. Ideally, for evaluative purposes, the SDC should be smaller than the MIC [4].
Results
One hundred patients were included, of which 48 were treated with early mobilization and 52 with plaster immobilization for three weeks. The median age was 46 year (P25-P75 32–59) and 42 patients were male. The dislocation involved the right arm in 53 patients, and the dominant side was affected in 46 patients. One patient was lost to follow-up and six missed one follow-up visit.
Reliability
The Cronbach’s alpha of OES total scale and all subscales ranged from 0.783 to 0.882. Cronbach’s alpha of the Quick-DASH was 0.886. This represents adequate internal consistency for both (sub)scales (Table 1). Internal consistency was also adequate for SF-36 (sub)scales (Cronbach’s alpha between 0.747 and 0.974), apart for the Bodily Pain (BP) subscale, which had a Cronbach’s alpha of 0.664. Cronbach’s alpha of the EQ-5D US and MEPI did not reach the Cronbach’s alpha threshold value of 0.70, but since these scales are not unidimensional, these values should be interpreted carefully. Internal consistency of the VAS and ED-5D VAS could not be determined, as they consist of one item only.
Table 1. Internal consistency of the instruments used in patients with a simple elbow dislocation.
| (Sub)scale | N | Number of items | Cronbach’s alpha | |
|---|---|---|---|---|
| OES | Total | 99 | 12 | 0.882* |
| Pain | 99 | 4 | 0.783 | |
| Function | 99 | 4 | 0.825 | |
| Social-psychological | 99 | 4 | 0.804 | |
| Quick-DASH | Total | 99 | 11 | 0.886 |
| MEPI | Total | 99 | 6† | 0.383* |
| Function | 99 | 4† | 0.693 | |
| SF-36 | Total | 99 | 35 | 0.880* |
| PF | 99 | 10 | 0.864 | |
| RP | 99 | 4 | 0.792 | |
| BP | 99 | 2 | 0.664 | |
| GH | 99 | 5 | 0.758 | |
| VT | 99 | 4 | 0.747 | |
| SF | 99 | 2 | 0.810 | |
| RE | 99 | 3 | 0.974 | |
| MH | 99 | 5 | 0.788 | |
| SF-36 | PCS | 99 | 21 | 0.834 |
| SF-36 | MCS | 99 | 14 | 0.853 |
| EQ-5D | US | 99 | 5 | 0.528* |
Quick-DASH, Quick disabilities of the arm, shoulder, and hand; PF, physical functioning; RP, role limitations due to physical health; BP, bodily pain; GH, general health perceptions; VT, vitality, energy, or fatigue; SF, social functioning; RE, role limitations due to emotional problems; MH, general mental health; OES, Oxford elbow score; PCS, physical component summary; MCS, mental component summary; SF-36, Short Form-36; EQ-5D, EuroQoL-5D; US, utility score.
*Values should be interpreted carefully, since the total scale is not unidimensional.
†The item related to washing was removed from analysis, as all patients gave the same answer.
Construct validity
Construct validity is shown in Table 2. The Spearman’s rank correlation coefficients of the OES were in line with predefined hypotheses in 35 of the 42 (83%) values, indicating sufficient construct validity. All three OES subscales have sufficient construct validity; 83% (10/12) hypotheses were confirmed. For the Quick-DASH and MEPI, 9 out of 12 correlations (75%) were as hypothesized, also showing sufficient construct validity.
Table 2. Construct validity of the instruments in patients with a simple elbow dislocation.
| (Sub)scale | OES | Quick-DASH | ||||
|---|---|---|---|---|---|---|
| Pain |
Function |
Social- psychological |
Total |
|||
| OES | Pain | [1.00] | 0.44* | 0.56* | 0.76* | -0.56 |
| Function | 0.44 | [1.00] | 0.63* | 0.80* | -0.72 | |
| Social-psychological | 0.56 | 0.63 | [1.00] | 0.91* | -0.66 | |
| Total | 0.76 | 0.80 | 0.91 | [1.00] | -0.75 | |
| Quick-DASH | Total | -0.56 | -0.72 | -0.66 | -0.75 | [1.00] |
| MEPI | Total | 0.51 | 0.47 | 0.49 | 0.55 | -0.64 |
| Pain | VAS | -0.40 | -0.48 | -0.39 | -0.48 | 0.57 |
| SF-36 | PF | 0.27 | 0.52 | 0.38 | 0.44 | -0.65 |
| BP | 0.67 | 0.48 | 0.66 | 0.71 | -0.67 | |
| PCS | 0.44 | 0.53 | 0.58 | 0.61 | -0.70 | |
| MCS | 0.04 | -0.06 | 0.09 | 0.09 | -0.07 | |
| EQ-5D | US | 0.42 | 0.60 | 0.46 | 0.56 | -0.70 |
| VAS | 0.22 | 0.30 | 0.36 | 0.35 | -0.39 | |
Spearman’s rank correlation coefficients are given for all possible combinations; N = 99 for all correlations; r>0.6 indicates high correlation, 0.3<r>0.6 moderate correlation, and r> 0.6 low correlation. Bold and underlined correlations were not hypothesized correctly. Correlations between brackets are self-correlations and are not included in the calculation of the percentage correlations predicted correctly. For the OES, the overall number of correlations is 42 (the 6 correlations given above the self-correlation that are marked with an asterisk are also mentioned in the columns to the left as reversed correlation, and are thus superfluous).
Quick-DASH, Quick disabilities of the arm, shoulder, and hand; BP, bodily pain; MCS, mental component summary; OES, Oxford elbow score; PCS, physical component summary; PF, physical functioning; SF-36, Short Form-36; US, utility score; VAS, visual analog scale.
Responsiveness
Longitudinal validity is shown in Table 3. The calculated Spearman’s rank correlation correlations were in line with predefined hypotheses in 36 out of the 42 (86%) values for the OES and 9 out of 12 (75%) for the Quick-DASH, indicating sufficient longitudinal validity for both instruments. Longitudinal validity was also sufficient for the OES subscales, with 83% (10/12), 75% (9/12), and 100% (12/12) hypotheses predicted correctly for the OES pain, function, and social-psychological subscale, respectively.
Table 3. Longitudinal validity of the instruments in patients with a simple elbow dislocation.
| (Sub)scale | OES | Quick-DASH | ||||
|---|---|---|---|---|---|---|
| Pain |
Function |
Social- psychological |
Total |
|||
| OES Pain | Pain | [1.00] | 0.32* | 0.45* | 0.70* | 0.45 |
| Function | Function | 0.32 | [1.00] | 0.53* | 0.76* | -0.68 |
| Social-psychological | Social-psychological | 0.45 | 0.53 | [1.00] | 0.86* | -0.52 |
| Total | Total | 0.70 | 0.76 | 0.86 | [1.00] | -0.68 |
| Quick-DASH | Total | -0.45 | -0.68 | -0.52 | -0.68 | [1.00] |
| MEPI | Total | 0.39 | 0.38 | 0.37 | 0.47 | -0.57 |
| Pain VAS | VAS | -0.37 | -0.32 | -0.32 | -0.41 | 0.37 |
| SF-36 PF | PF | 0.25 | 0.51 | 0.41 | 0.50 | -0.63 |
| BP | BP | 0.41 | 0.24 | 0.38 | 0.40 | -0.41 |
| PCS | PCS | 0.19 | 0.39 | 0.39 | 0.38 | -0.52 |
| MCS | MCS | 0.20 | -0.003 | 0.05 | 0.11 | 0.005 |
| EQ-5D US | US | 0.36 | 0.47 | 0.42 | 0.50 | -0.57 |
| VAS | VAS | 0.14 | 0.35 | 0.25 | 0.29 | -0.18 |
N = 98 patients for all correlations except for the MEPI (N = 96) and EQ-5D VAS (N = 97). Rest of Table caption is identical to Table 2.
The standardized response mean (SRM) and the Effect Size (ES) of the OES and Quick-DASH instruments is shown in Table 4. As expected, the magnitude of change over time was large for the OES (sub)scales (SRM and ES >0.90). For the Quick-DASH, the SRM was large (0.87), but the ES was only moderate (0.73).
Table 4. Responsiveness: Standardized response mean (SRM) and effect size (ES) of the instruments in patients with a simple elbow dislocation.
| (Sub)scale | N | Mean change | SDchange | SRM | SD6 weeks | ES | |
|---|---|---|---|---|---|---|---|
| OES | Total | 98 | 25.2 | 15.6 | 1.61 | 18.5 | 1.36 |
| Pain | 98 | 21.5 | 18.5 | 1.16 | 21.0 | 1.03 | |
| Function | 98 | 18.3 | 18.7 | 0.98 | 19.7 | 0.93 | |
| Social-psychological | 98 | 35.8 | 22.8 | 1.57 | 25.3 | 1.42 | |
| Quick-DASH | Total | 98 | -11.1 | 12.8 | -0.87 | 15.1 | -0.73 |
| MEPI | Total | 96 | 10.0 | 13.9 | 0.72 | 12.8 | 0.78 |
| Pain | VAS | 96 | -0.73 | 1.63 | -0.45 | 1.47 | -0.50 |
| SF-36 | Total | 98 | 7.16 | 10.43 | 0.69 | 9.60 | 0.75 |
| PF | 98 | 3.19 | 7.79 | 0.41 | 7.64 | 0.42 | |
| RP | 98 | 15.32 | 13.10 | 1.17 | 10.96 | 1.40 | |
| BP | 98 | 8.01 | 8.65 | 0.93 | 7.08 | 1.13 | |
| GH | 98 | 0.03 | 7.70 | 0.004 | 8.51 | 0.004 | |
| VT | 98 | 0.71 | 8.93 | 0.08 | 7.57 | 0.09 | |
| SF | 98 | 2.91 | 10.94 | 0.27 | 8.99 | 0.32 | |
| RE | 98 | 1.85 | 9.76 | 0.19 | 8.94 | 0.21 | |
| MH | 98 | -0.45 | 8.61 | -0.05 | 7.62 | -0.06 | |
| SF-36 | PCS | 98 | 9.02 | 8.31 | 1.09 | 7.84 | 1.15 |
| SF-36 | MCS | 98 | -1.87 | 10.08 | -0.19 | 8.15 | -0.23 |
| EQ-5D | US | 98 | 0.05 | 0.10 | 0.50 | 0.09 | 0.51 |
| VAS | 97 | 2.2 | 11.1 | 0.20 | 11.3 | 0.20 |
Change scores were calculated from six weeks to 12 months.
Quick-DASH, Quick disabilities of the arm, shoulder, and hand; PF, physical functioning; RP, role limitations due to physical health; BP, bodily pain; ES, effect size; GH, general health perceptions; VT, vitality, energy, or fatigue; SF, social functioning; RE, role limitations due to emotional problems; MEPI, Mayo elbow performance index; MH, general mental health; OES, Oxford elbow score; PCS, physical component summary; MCS, mental component summary; SD, standard deviation (of mean change and of 6 weeks); SF-36, Short Form-36; SRM, standardized response mean.
Floor and ceiling effects
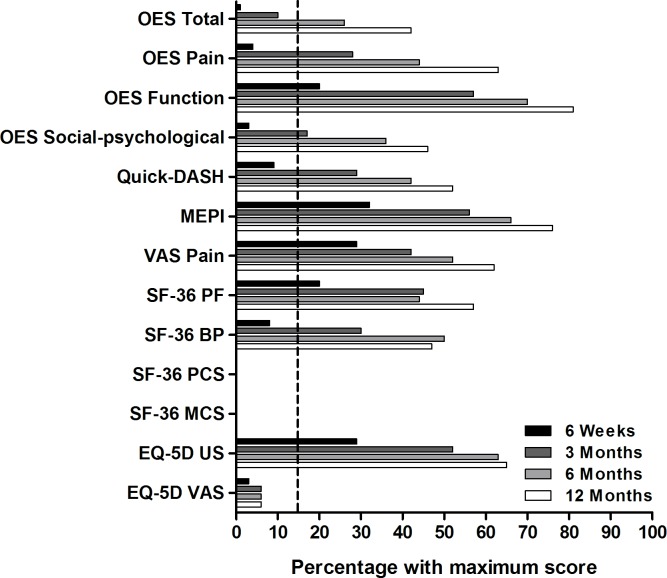
None of the instruments evaluated showed a floor effect. From six weeks onwards the OES function, MEPI, VAS, SF-36 PF, and EQ-5D US demonstrated a ceiling effect (Fig 1); 20%, 32%, 29%, 20%, and 29% of the patients, respectively, reported the maximum score. From three months onwards the OES pain (28%) and social-psychological subscale (17%), Quick-DASH (29%), and SF-36 BP (30%) demonstrated a ceiling effect. The OES as a total scale demonstrated a ceiling effect only from six months onwards, where 26% of the patients reported the maximum score.
Fig 1. Ceiling effects of the instruments used in patients with a simple elbow dislocation.
N = 99 for all (sub)scales at 6 weeks, N = 100 at 3 months (except for the MEPI (N = 99)), N = 97 at 6 months (except for the MEPI (N = 96)), and N = 99 at 12 months (except for the MEPI (N = 97) and EQ-5D VAS (N = 98)). The dotted line represents the acceptable 15% of patients with the maximum score. The SF-36 BP, PF, PCS and MCS did not demonstrate a ceiling effect and are not displayed. None of the (sub)scales demonstrated a floor effect.
Minimal important change and smallest detectable change
The number of patients per transition item for the different time intervals is shown in S2 Table. Anchor-based MIC and distribution-based SDC values are shown in Table 5. Overall, 57 transition items were reported as ‘slightly better’ and 31 as ‘no change’. The transition item demonstrated adequate correlation (i.e. r > 0.29) with the change scores of the OES total scale, the OES pain and function subscales, and the Quick-DASH. Spearman’s rank correlations with the transition item were below this threshold for the OES psychosocial subscale (r = -0.20) and all other (sub)scales. Therefore the MIC for the these could not be determined reliably.
Table 5. Minimal important change (MIC) and smallest detectable change (SDC) of the instruments in patients with a simple elbow dislocation.
| (Sub)scale | Score range | MIC | SDC | |||||
|---|---|---|---|---|---|---|---|---|
| N | MIC (95% CI) | N | SDchange | SEM | SDC | |||
| OES | Total | 0–100 | 57 | 8.2 (5.7–10.7) | 31 | 6.1 | 4.3 | 12.0 |
| Pain | 0–100 | 57 | 7.3 (3.3–11.4) | 31 | 6.6 | 4.6 | 12.9 | |
| Function | 0–100 | 57 | 5.6 (2.0–9.2) | 31 | 7.2 | 5.1 | 14.1 | |
| Social-psychological | 0–100 | 57 | 11.7 (7.6–15.9) | 31 | 12.8 | 9.0 | 25.0 | |
| Quick-DASH | Total | 0–100 | 57 | 3.5 (1.6–5.5) | 31 | 6.2 | 4.4 | 12.2 |
| MEPI | Total | 0–100 | 56 | N.A. | 30 | 10.5 | 7.4 | 20.6 |
| Pain | VAS | 0–100 | 57 | N.A. | 31 | 0.9 | 0.6 | 1.7 |
| SF-36 | Total | 0–100 | 57 | N.A. | 31 | 8.9 | 6.3 | 17.4 |
| PF | 0–100 | 57 | N.A. | 31 | 4.7 | 3.4 | 9.3 | |
| RP | 0–100 | 57 | N.A. | 31 | 6.8 | 4.8 | 13.4 | |
| BP | 0–100 | 57 | N.A. | 31 | 5.9 | 4.2 | 11.6 | |
| GH | 0–100 | 57 | N.A. | 31 | 5.9 | 4.2 | 11.7 | |
| VT | 0–100 | 57 | N.A. | 31 | 7.9 | 5.6 | 15.6 | |
| SF | 0–100 | 57 | N.A. | 31 | 10.5 | 7.4 | 20.5 | |
| RE | 0–100 | 57 | N.A. | 31 | 10.7 | 7.5 | 20.9 | |
| MH | 0–100 | 57 | N.A. | 31 | 7.3 | 5.1 | 14.3 | |
| SF-36 | PCS | 0–100 | 57 | N.A. | 31 | 5.4 | 3.8 | 10.5 |
| SF-36 | MCS | 0–100 | 57 | N.A. | 31 | 9.6 | 6.8 | 18.8 |
| EQ-5D | US | 0–1 | 57 | N.A. | 31 | 0.1 | 0.04 | 0.1 |
| VAS | 0–100 | 57 | N.A. | 31 | 10.1 | 7.1 | 19.8 | |
Anchor-based and distribution-based methods for Minimal Important Change (MIC) and Smallest Detectable Change (SDC) values, respectively. Change scores were calculated from six weeks to 12 months. MIC is the mean score for patients who reported ‘slightly better’ on the transition item. It is shown with the 95% confidence interval between brackets.
N.A., not applicable, as the correlation with the transition item was <0.29.
MIC, minimal important change; SDchange, standard deviation of the change score of patients that reported ‘no change’ on the transition item; SEM, standard error of measurement; SDC, smallest detectable change.
For the OES, the anchor-based MIC was 8.2 points (95% CI 5.7–10.7) for the total scale, 7.3 (95% CI 3.3–11.4) points for the pain subscale, 5.6 (95% CI 2.0–9.2) points for the function subscale, and 11.7 (95% CI 7.6–15.9) points for the social-psychological subscale (Table 5). The anchor-based MIC for the Quick-DASH change score was 3.5 (95% CI 1.6–5.5) points. The ROC curve analysis produced similar results, with wider confidence intervals. There the MIC was 6.3 (95% CI 4.2–8.3) points for the OES and 4.5 (95% CI 2.3–11.4) for the Quick-DASH.
For each of these four (sub)scales, the MIC was smaller than the SDC values. These SDC was 12.0 (SEM 4.3) for the OES total scale, 12.9 (SEM 4.6) for the OES pain subscale, 14.1 (SEM 5.1) for the OES function subscale, 25.0 (SEM 9.0) for the OES social-physiologic subscale, and 12.2 (SEM 4.4) for the Quick-DASH.
Discussion
This study showed that the OES and Quick-DASH are reliable, valid, and responsive instruments for the evaluation and follow-up of patients after a simple elbow dislocation that was treated non-operatively. The anchor-based MIC was 8.2 points for OES and 3.5 for Quick-DASH.
The reliability of the OES (Cronbach’s alpha 0.882) and Quick-DASH (Cronbach’s alpha 0.886) was comparable with published values [10, 13, 23, 25, 40–44]. The OES has previously been acknowledged as the most reliable questionnaire [8]. The current data confirm that it is at least as good as the DASH. The MEPI demonstrated inadequate internal consistency which had also been shown previously [45].
The OES proved its validity by demonstrating strong correlations with the Quick-DASH and SF-36 BP and PCS. The latter is a novel observation, as no data were available on the correlation between the OES and SF-36 subscales. Correlation with the Quick-DASH and MEPI has been published before for patients who had undergone elbow surgery [9, 15].
There is no available literature concerning the validity of the OES and Quick-DASH in non-operatively treated patients with an elbow dislocation. Construct validity of the (Quick-)DASH has been reported before [40, 46]. The correlation in change scores between the subdomains of the OES and Quick-DASH are comparable with data from Dawson et al. [9]. Change scores of the OES correlated moderately with change scores of the Quick-DASH and MEPI. The moderate correlation of change scores of the OES and MEPI could be explained by the fact that the MEPI demonstrated significant ceiling effects from the first follow-up onwards. The ceiling effect does not allow to detect actual changes over time.
The finding that the standardized response mean (SRM) and effect size (ES) of the OES and Quick-DASH (sub)scales were large (except moderate ES for Quick-DASH) suggests that both instruments display good to excellent ability to detect clinical change over time. Moderate to large ES and large SRM values have been shown before for the (Quick-)DASH or DASH [25, 40, 47–49].
All instruments displayed a ceiling effect. This was as expected, since the type of elbow dislocations studied are relatively mild injuries, with expected full recovery within six months. Full recovery implies the largest score, and hence a ceiling effect. A similar phenomenon was also seen for the DASH in patients treated for a humeral shaft fracture [50]. Patients treated operatively for Dupuytren’s contracture also showed ceiling effect for the DASH from three months after surgery onwards [51]. The expected ceiling effect is not a problem per se, but one should realize that the instruments are not useful for comparing treatment outcome at times where a ceiling effect is observed.
The interpretability represented by the MIC was 8.2 for the OES total score. The MIC for the OES pain, function and social-psychological subdomains (7.3, 5.6, and 11.7 points, respectively) were lower than for patients who underwent elbow surgery for chronic elbow pathologies (17.41–19.23, 9.23–9.64, and 17.79–18.30 points, respectively) as reported before [9]. Fourteen patients in their study answered ‘slightly better’ on the transition item, which was much lower than the 57 patients in the current study. The difference in population (and recovery pattern) most likely explains the difference in MIC [4]. Patients with chronic pathology like in Dawson’s study have a moderate to poor score at baseline and retain functional limitation after surgery. The patients in the current study with an acute injury started at full loss of function immediately after injury, and the majority showed full recovery at the end. MIC values are known to differ depending on patient population and the type of injury and intervention [9, 37]. Although the MIC for the OES in this study were evaluated in a cohort of patients with a simple elbow dislocation, one may expect that the MIC can be extrapolated to also be useful in the evaluation of other acute elbow injuries where full recovery is to be expected.
The MIC for the Quick-DASH was only 3.5 points. This is hard to believe for a scale that runs from 0 to 100, especially as previously published anchor-based MIC values for the (Quick-)DASH-score ranged from 8 to 19 points [47, 48, 52–55]. Data on patients with an elbow dislocation are not available. The most plausible explanation for this is again the fact that already on the first evaluation (six weeks) the Quick-DASH showed a ceiling effect, which implies that subtle impediments and changes cannot be measured from that point onward. This emphasizes the need for elbow-specific questionnaires like the OES for the less severe types of injuries. The OES also demonstrated a ceiling effect, however not before the six months follow-up moment, at which time patients were recovered to the largest degree.
Ideally, the MIC should be larger than the smallest detectable change (SDC) in order to be able to differentiate between ‘real’ change and change caused by measurement error [4]. For the OES and Quick-DASH the SDC was larger than the MIC. For the Quick-DASH, Polson et al. reported a SDC of 11, which was lower than 19 for the MIC [47]. For the OES, a previous study that also used both anchor- and distribution-based methods for calculating the MIC, also found that SDC values were higher than anchor-based MIC values [9]. Our findings confirm this. It implies that any change score reported by a patient that is larger than the MIC but smaller than the SDC should be interpreted with care; it may represent clinical improvement but can also be due to chance.
The SEM in the current study was calculated with the corresponding change scores of patients that answered ‘no change’ on the transition item as a surrogate for test-retest values. This could have introduced some bias, which might have influenced the SDC value. Future studies should include an adequate test-retest analysis in order to be able to calculate a true SEM. Nevertheless, the anchor-based MIC values are the closest estimate of actual clinical change, therefore the MIC values in current study are of definite value.
This study has some limitations. First, the relatively long time between the follow-up moments hindered an adequate test-retest analysis. Furthermore, it could also have led to recall bias with regard to the transition item. However, the interval for the transition item in the only other study that analyzed the MIC of the OES using an anchor-based approach was at least six months [9]. Secondly, the transition item for the MIC analysis included “completely recovered” was a heterogeneous group. This group included patients who 1) were already completely recovered at the previous follow-up visit; 2) truly experienced no change; or 3) reported complete recovery for the first time but actually improved little/much since the previous follow-up. For future studies the outlying answers (i.e., “completely recovered” and “worse than ever”) should be left out. Finally, there were insufficient data for evaluating whether the MIC values were the same for the consecutive time intervals.
Strengths of this study were its sample size and homogenous patient population. Furthermore, to the best of our knowledge, it is the first study to validate the OES for patients with elbow injuries treated non-operatively. Previous studies focused primarily on operated patients [9, 10, 13, 14, 23].
Conclusion
The OES and Quick-DASH have proven to be reliable, valid, and responsive instruments for evaluating elbow-related quality of life in patients who sustained a simple elbow dislocation. Whereas validity of the OES was known for surgically treated chronic elbow pathologies, this study demonstrated the OES is also valid for acute elbow injuries treated non-operatively. Both instruments are useful for research purposes, and could play an important role in daily practice. The MIC and SDC values facilitate statistical power analysis and sample-size calculations for future clinical studies.
Supporting information
Hypothesized correlations between the instruments for (A) construct validity and (B) Longitudinal validity in patients with a simple elbow dislocation.
Expected strength of correlation or all possible combinations; r>0.6 indicates high correlation, 0.3<r>0.6 moderate correlation, and r> 0.6 low correlation.
Quick-DASH, Quick disabilities of the arm, shoulder, and hand; BP, bodily pain; MCS, mental component summary; OES, Oxford elbow score; PCS, physical component summary; PF, physical functioning; SF-36, Short Form-36; US, utility score; VAS, visual analog scale.
(PDF)
Data are shown as number with valid percentage.
(PDF)
Acknowledgments
The Oxford and Isis Outcomes, part of Isis Innovation Limited, are acknowledged for their kind support. Oxford Elbow Score Isis Innovation Limited, 2008. All rights reserved. The authors, being Professor Ray Fitzpatrick and Dr Jill Dawson, have asserted their moral rights.
Kiran C. Mahabier, Harold Goei, Gerben De Reus, and Liza van Loon (Erasmus MC, Rotterdam, The Netherlands) are acknowledged for their assistance in data collection.
Membership of the FuncSiE Trial Investigators
Roelf S. Breederveld (Department of Surgery, Red Cross Hospital, Beverwijk, The Netherlands); Maarten W.G.A. Bronkhorst (Department of Surgery, Bronovo Hospital, The Hague, The Netherlands); Jeroen De Haan (Department of Surgery, Westfriesgasthuis, Hoorn, The Netherlands), Mark R. De Vries (Department of Surgery, Reinier de Graaf Gasthuis, Delft, The Netherlands); Boudewijn J. Dwars (Department of Surgery, Slotervaart Hospital, Amsterdam, The Netherlands); Robert Haverlag (Department of Surgery, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands); Sven A.G. Meylaerts (Department of Surgery, Medical Center Haaglanden, The Hague, The Netherlands); Jan-Willem R. Mulder (Department of Surgery, Zaans Medical Center, Zaandam, The Netherlands); Peter Patka (Accident and Emergency Department, Erasmus MC, Rotterdam, The Netherlands); Kees J. Ponsen (Trauma Unit, Department of Surgery, Academic Medical Center, Amsterdam, The Netherlands); W. Herbert Roerdink (Department of Surgery, Deventer Hospital, Deventer, The Netherlands); Gert R. Roukema (Department of Surgery, Maasstad Hospital, Rotterdam, The Netherlands); Inger B. Schipper (Department of Trauma Surgery, Leiden University Medical Center, Leiden, The Netherlands); Michel A. Schouten (Department of Surgery, Hospital Rivierenland, Tiel, The Netherlands); Jan Bernard Sintenie (Department of Surgery, Elkerliek Hospital, Helmond, The Netherlands); Senail Sivro (Department of Surgery, Flevo Hospital, Almere, The Netherlands); Johan G.H. Van den Brand (Department of Surgery, Medical Center Alkmaar, Alkmaar, The Netherlands); Frits M. Van der Linden (Department of Surgery, Groene Hart Hospital, Gouda, The Netherlands); Hub G.W.M. Van der Meulen (Department of Surgery, Haga Hospital, The Hague, The Netherlands); Egbert J.M.M. Verleisdonk (Department of Surgery, Diakonessenhuis, Utrecht, The Netherlands); Jos P.A.M. Vroemen (Department of Surgery, Amphia Hospital, Breda, The Netherlands); Marco Waleboer (Department of Surgery, Admiraal de Ruyter Hospital, Goes, The Netherlands); W. Jaap Willems (Department of Orthopaedic Surgery, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands)
Data Availability
Erasmus MC as sponsor is the legal owner of the data of our study. The data protection office at Erasmus MC explained that the Dutch Data Protection Act prohibits us from making the requested dataset publicly available on a website. One of the reasons is that due to the low incidence of the injury studied, full anonymity of study participants cannot be guaranteed. Patients also have not consented to putting data in an openly accessible website. Erasmus MC as well as the authors support data sharing, but due to the legal restrictions mentioned, data can only be made available on request. Requests can be sent to the science office of Erasmus MC (onderzoeksbureauheelkunde@erasmusmc.nl).
Funding Statement
This project was supported by a grant from the European Society for Surgery of the Shoulder and the Elbow (www.secec.org; (SECEC/ESSSE grant 2010) to DE. This organization was not involved in the trial design, patient recruitment, data collection, data analysis, data interpretation, publication decisions, or in any aspect pertinent to this study.
References
- 1.Polinder S, Iordens GIT, Panneman MJM, Eygendaal D, Patka P, Den Hartog D, et al. Trends in incidence and costs of injuries to the shoulder, arm and wrist in The Netherlands between 1986 and 2008. BMC Public Health 2013;13: 531 doi: 10.1186/1471-2458-13-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Beeck EF, van Roijen L, Mackenbach JP. Medical costs and economic production losses due to injuries in the Netherlands. J Trauma 1997;42(6): 1116–23. [DOI] [PubMed] [Google Scholar]
- 3.Meerding WJ, Mulder S, van Beeck EF. Incidence and costs of injuries in The Netherlands. Eur J Public Health 2006;16(3): 272–8. doi: 10.1093/eurpub/ckl006 [DOI] [PubMed] [Google Scholar]
- 4.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007;60(1): 34–42. doi: 10.1016/j.jclinepi.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 5.Valderas JM, Kotzeva A, Espallargues M, Guyatt G, Ferrans CE, Halyard MY, et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res 2008;17(2): 179–93. doi: 10.1007/s11136-007-9295-0 [DOI] [PubMed] [Google Scholar]
- 6.Lindenhovius AL, Buijze GA, Kloen P, Ring DC. Correspondence between perceived disability and objective physical impairment after elbow trauma. J Bone Joint Surg Am 2008;90(10): 2090–7. doi: 10.2106/JBJS.G.00793 [DOI] [PubMed] [Google Scholar]
- 7.Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med 2014;48(9): 792–6. doi: 10.1136/bjsports-2012-091704 [DOI] [PubMed] [Google Scholar]
- 8.The B, Reininga IH, El Moumni M, Eygendaal D. Elbow-specific clinical rating systems: extent of established validity, reliability, and responsiveness. J Shoulder Elbow Surg 2013;22(10): 1380–94. doi: 10.1016/j.jse.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 9.Dawson J, Doll H, Boller I, Fitzpatrick R, Little C, Rees J, et al. Comparative responsiveness and minimal change for the Oxford Elbow Score following surgery. Qual Life Res 2008;17(10): 1257–67. doi: 10.1007/s11136-008-9409-3 [DOI] [PubMed] [Google Scholar]
- 10.Dawson J, Doll H, Boller I, Fitzpatrick R, Little C, Rees J, et al. Specificity and responsiveness of patient-reported and clinician-rated outcome measures in the context of elbow surgery, comparing patients with and without rheumatoid arthritis. Orthop Traumatol Surg Res 2012;98(6): 652–8. doi: 10.1016/j.otsr.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 11.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25(24): 3186–91. [DOI] [PubMed] [Google Scholar]
- 12.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 1993;46(12): 1417–32. [DOI] [PubMed] [Google Scholar]
- 13.De Haan J, Goei H, Schep NWL, Tuinebreijer WE, Patka P, Den Hartog D. The reliability, validity and responsiveness of the Dutch version of the Oxford elbow score. J Orthop Surg Res 2011;6: 39 doi: 10.1186/1749-799X-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Haan J, Schep NWL, Tuinebreijer WE, Patka P, Den Hartog D. Rasch analysis of the Dutch version of the Oxford elbow score. Patient Relat Outcome Meas 2011;2: 145–9. doi: 10.2147/PROM.S22257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson J, Doll H, Boller I, Fitzpatrick R, Little C, Rees J, et al. The development and validation of a patient-reported questionnaire to assess outcomes of elbow surgery. J Bone Joint Surg Br 2008;90(4): 466–73. doi: 10.1302/0301-620X.90B4.20290 [DOI] [PubMed] [Google Scholar]
- 16.Slobogean GP, Noonan VK, O'Brien PJ. The reliability and validity of the Disabilities of Arm, Shoulder, and Hand, EuroQol-5D, Health Utilities Index, and Short Form-6D outcome instruments in patients with proximal humeral fractures. J Shoulder Elbow Surg 2010;19(3): 342–8. doi: 10.1016/j.jse.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 17.Wylie JD, Beckmann JT, Granger E, Tashjian RZ. Functional outcomes assessment in shoulder surgery. World J Orthop 2014;5(5): 623–33. doi: 10.5312/wjo.v5.i5.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med 1996;29(6): 602–8. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 19.Veehof MM, Sleegers EJ, van Veldhoven NH, Schuurman AH, van Meeteren NL. Psychometric qualities of the Dutch language version of the Disabilities of the Arm, Shoulder, and Hand questionnaire (DASH-DLV). J Hand Ther 2002;15(4): 347–54. [DOI] [PubMed] [Google Scholar]
- 20.Beaton DE, Wright JG, Katz JN. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am 2005;87(5): 1038–46. doi: 10.2106/JBJS.D.02060 [DOI] [PubMed] [Google Scholar]
- 21.De Haan J, Den Hartog D, Tuinebreijer WE, Iordens GIT, Breederveld RS, Bronkhorst MWGA et al. Functional treatment versus plaster for simple elbow dislocations (FuncSiE): a randomized trial. BMC Musculoskelet Disord 2010;11: 263 doi: 10.1186/1471-2474-11-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iordens GIT, Van Lieshout EMM, Schep NWL, De Haan J, Tuinebreijer WE, Eygendaal D, et al. Early mobilisation versus plaster immobilisation of simple elbow dislocations: results of the FuncSiE multicentre randomised clinical trial. Br J Sports Med 2016. [DOI] [PubMed] [Google Scholar]
- 23.Plaschke HC, Jorgensen A, Thillemann TM, Brorson S, Olsen BS. Validation of the Danish version of the Oxford Elbow Score. Dan Med J 2013;60(10): A4714 [PubMed] [Google Scholar]
- 24.Marquardt J, Schottker-Koniger T, Schafer A. [Validation of the German version of the Oxford Elbow Score: A cross-sectional study]. Orthopade 2016;45(8): 695–700. doi: 10.1007/s00132-016-3292-3 [DOI] [PubMed] [Google Scholar]
- 25.Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther 2001;14(2): 128–46. [PubMed] [Google Scholar]
- 26.Morrey BF, An KN, Chao EYS. Functional evaluation of the elbow In: The Elbow and Its Disorders. 2nd edition Edited by Morrey BF. Philadelphia: WB Saunders; 1993:86–89. [Google Scholar]
- 27.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 1990;13(4): 227–36. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6): 473–83. [PubMed] [Google Scholar]
- 29.Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998;51(11): 1055–68. [DOI] [PubMed] [Google Scholar]
- 30.Lamers LM, Stalmeier PF, McDonnell J, Krabbe PF, van Busschbach JJ. [Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff]. Kwaliteit van leven meten in economische evaluaties: het Nederlands EQ-5D-tarief. Ned Tijdschr Geneeskd 2005;149(28): 1574–8. [PubMed] [Google Scholar]
- 31.Brooks R. EuroQol: the current state of play. Health Policy 1996;37(1): 53–72. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences Academic Press: New York: (1997); pp 474. [Google Scholar]
- 33.Middel B, van Sonderen E. Statistical significant change versus relevant or important change in (quasi) experimental design: some conceptual and methodological problems in estimating magnitude of intervention-related change in health services research. Int J Integr Care 2002;2: e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angst F, Verra ML, Lehmann S, Aeschlimann A. Responsiveness of five condition-specific and generic outcome assessment instruments for chronic pain. BMC Med Res Methodol 2008;8: 26 doi: 10.1186/1471-2288-8-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995;4(4): 293–307. [DOI] [PubMed] [Google Scholar]
- 36.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10(4): 407–15. [DOI] [PubMed] [Google Scholar]
- 37.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008;61(2): 102–9. doi: 10.1016/j.jclinepi.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 38.De Vet HC, Terwee CB, M L.B., Knol DL. Measurement in Medicine, a Practical Guide. Cambridge University Press; 2011. [Google Scholar]
- 39.De Vet HC, Terwee CB, Knol DL, Bouter LM. When to use agreement versus reliability measures. J Clin Epidemiol 2006;59(10): 1033–9. doi: 10.1016/j.jclinepi.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 40.Fayad F, Lefevre-Colau MM, Gautheron V, Mace Y, Fermanian J, Mayoux-Benhamou A, et al. Reliability, validity and responsiveness of the French version of the questionnaire Quick Disability of the Arm, Shoulder and Hand in shoulder disorders. Man Ther 2009;14(2): 206–12. doi: 10.1016/j.math.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 41.Offenbaecher M, Ewert T, Sangha O, Stucki G. Validation of a German version of the disabilities of arm, shoulder, and hand questionnaire (DASH-G). J Rheumatol 2002;29(2): 401–2. [PubMed] [Google Scholar]
- 42.Padua R, Padua L, Ceccarelli E, Romanini E, Zanoli G, Amadio PC, et al. Italian version of the Disability of the Arm, Shoulder and Hand (DASH) questionnaire. Cross-cultural adaptation and validation. J Hand Surg Br 2003;28(2): 179–86. [DOI] [PubMed] [Google Scholar]
- 43.Atroshi I, Gummesson C, Andersson B, Dahlgren E, Johansson A. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: reliability and validity of the Swedish version evaluated in 176 patients. Acta Orthop Scand 2000;71(6): 613–8. doi: 10.1080/000164700317362262 [DOI] [PubMed] [Google Scholar]
- 44.Lovgren A, Hellstrom K. Reliability and validity of measurement and associations between disability and behavioural factors in patients with Colles' fracture. Physiother Theory Pract 2012;28(3): 188–97. doi: 10.3109/09593985.2011.583174 [DOI] [PubMed] [Google Scholar]
- 45.De Boer YA, Van den Ende CH, Eygendaal D, Jolie IM, Hazes JM, Rozing PM. Clinical reliability and validity of elbow functional assessment in rheumatoid arthritis. J Rheumatol 1999;26(9): 1909–17. [PubMed] [Google Scholar]
- 46.SooHoo NF, McDonald AP, Seiler JG 3rd, McGillivary GR. Evaluation of the construct validity of the DASH questionnaire by correlation to the SF-36. J Hand Surg Am 2002;27(3): 537–41. [DOI] [PubMed] [Google Scholar]
- 47.Polson K, Reid D, McNair PJ, Larmer P. Responsiveness, minimal importance difference and minimal detectable change scores of the shortened disability arm shoulder hand (QuickDASH) questionnaire. Man Ther 2010;15(4): 404–7. doi: 10.1016/j.math.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 48.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord 2003;4: 11 doi: 10.1186/1471-2474-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDermid JC, Khadilkar L, Birmingham TB, Athwal GS. Validity of the QuickDASH in patients with shoulder-related disorders undergoing surgery. J Orthop Sports Phys Ther 2015;45(1): 25–36. doi: 10.2519/jospt.2015.5033 [DOI] [PubMed] [Google Scholar]
- 50.Mahabier KC, Den Hartog D, Theyskens N, Verhofstad MHJ, Van Lieshout EMM, investigators Ht. Reliability, validity, responsiveness, and minimal important change of the DASH and Constant-Murley scores in patients with a humeral shaft fracture. J Shoulder Elbow Surg 2016: in press. [DOI] [PubMed] [Google Scholar]
- 51.Forget NJ, Jerosch-Herold C, Shepstone L, Higgins J. Psychometric evaluation of the Disabilities of the Arm, Shoulder and Hand (DASH) with Dupuytren's contracture: validity evidence using Rasch modeling. BMC Musculoskelet Disord 2014;15: 361 doi: 10.1186/1471-2474-15-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg 2009;18(6): 920–6. doi: 10.1016/j.jse.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 53.Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-rated outcomes instruments. J Hand Surg Am 2013;38(4): 641–9. doi: 10.1016/j.jhsa.2012.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther 2014;44(1): 30–9. doi: 10.2519/jospt.2014.4893 [DOI] [PubMed] [Google Scholar]
- 55.Stepan JG, London DA, Boyer MI, Calfee RP. Accuracy of patient recall of hand and elbow disability on the QuickDASH questionnaire over a two-year period. J Bone Joint Surg Am 2013;95(22): e176 doi: 10.2106/JBJS.L.01485 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hypothesized correlations between the instruments for (A) construct validity and (B) Longitudinal validity in patients with a simple elbow dislocation.
Expected strength of correlation or all possible combinations; r>0.6 indicates high correlation, 0.3<r>0.6 moderate correlation, and r> 0.6 low correlation.
Quick-DASH, Quick disabilities of the arm, shoulder, and hand; BP, bodily pain; MCS, mental component summary; OES, Oxford elbow score; PCS, physical component summary; PF, physical functioning; SF-36, Short Form-36; US, utility score; VAS, visual analog scale.
(PDF)
Data are shown as number with valid percentage.
(PDF)
Data Availability Statement
Erasmus MC as sponsor is the legal owner of the data of our study. The data protection office at Erasmus MC explained that the Dutch Data Protection Act prohibits us from making the requested dataset publicly available on a website. One of the reasons is that due to the low incidence of the injury studied, full anonymity of study participants cannot be guaranteed. Patients also have not consented to putting data in an openly accessible website. Erasmus MC as well as the authors support data sharing, but due to the legal restrictions mentioned, data can only be made available on request. Requests can be sent to the science office of Erasmus MC (onderzoeksbureauheelkunde@erasmusmc.nl).