Abstract
Pain is influenced by many factors other than external sources of tissue damage. Among these, the clinician-patient relationship is particularly important for pain diagnosis and treatment. However, the effects of the clinician-patient relationship on pain remain under-examined. We tested the hypothesis that patients who believe they share core beliefs and values with their clinician will report less pain than patients who do not. We also measured feelings of perceived clinician-patient similarity and trust to see if these interpersonal factors influenced pain. We did so by experimentally manipulating perceptions of similarity between participants playing the role of clinicians and participants playing the role of patients in simulated clinical interactions. Participants were placed in two groups based on their responses to a personal beliefs and values questionnaire and painful thermal stimulation was used as an analogue of a painful medical procedure. We found that patients reported feeling more similarity and trust toward their clinician when they were paired with clinicians from their own group. In turn, patients’ positive feelings of similarity and trust toward their clinicians — but not clinicians’ feelings toward patients or whether the clinician and patient were from the same group — predicted lower pain ratings. Finally, the most anxious patients exhibited the strongest relationship between their feelings about their clinicians and their pain report. These findings increase our understanding of context-driven pain modulation and suggest that interventions aimed at increasing patients’ feelings of similarity to and trust in health care providers may help reduce the pain experienced during medical care.
Keywords: clinician-patient, concordance, similarity, trust, pain, health disparities
Introduction
Although pain has historically been conceived of as a stimulus driven, bottom-up process,20 research over the last several decades has demonstrated that pain is highly subjective and modifiable by many factors other than external sources of tissue damage.12, 32, 35, 65 However, the potential pain modulating effects of one of the most proximate contexts to pain diagnosis and treatment, the clinician-patient relationship, remain under-examined. The effects of the clinician-patient relationship have typically been studied in the context of peripheral health outcomes such as patient satisfaction,61, 71 yet a growing body of work has now demonstrated associations between the clinician-patient relationship and biologically-based outcome variables such as blood pressure and blood glucose.63 Several studies have even demonstrated a reduction in disease-related pain with physician training aimed at improving the clinician-patient relationship.14, 31 Within the pain literature, elements of the clinician-patient relationship including interpersonal factors such as communication,50 social support,11, 56 and expectations of pain reduction5, 25 have been found to modulate pain. However, no study to date has tested the hypothesis that mere perceptions of the clinician-patient relationship may modulate the pain experienced during medical care, which is the central hypothesis we tested in the present study.
We focused on perceptions of the clinician-patient relationship related to shared sociocultural group membership, referred to here as group concordance, which has frequently been found to impact patient satisfaction. Patients have been found to report higher levels of satisfaction with clinicians who share their race,16, 39 gender,54 and language,19, 40 than with clinicians who do not. Group concordance is thought to positively impact social interactions by increasing feelings of self-similarity and trust between ingroup members.67 Furthermore, there is evidence that increased feelings of similarity and trust between ingroup members may contribute to the positive effects of clinician-patient group concordance on health outcomes. For example, Street and colleagues64 found that minority patients felt more personally similar to own-race physicians, and that greater feelings of personal similarity to physicians predicted higher levels of patient satisfaction, physician trust, and intent to adhere to treatment recommendations.
The potential of clinician-patient group concordance and feelings of trust and similarity to modulate pain perception has not been directly tested. Low levels of clinician similarity and trust reported by minority patients with other-race/ethnicity clinicians have been hypothesized to contribute to the higher levels of pain reported by minority compared to majority patients,23 suggesting a potential link between clinician-patient group concordance and patients’ perceptions of pain.
The influence of group concordance on interpersonal and intergroup behavior is difficult to study experimentally because participants cannot be randomly assigned to real-world sociocultural groups. Therefore, social psychologists have experimentally studied the behavioral consequences of group concordance by using an arbitrary criterion to create novel groups in the lab, an approach called the minimal group paradigm.66 A large body of literature has demonstrated that participants will favor their ingroup in the minimal group paradigm, and exhibit other behaviors typical of real-world intergroup situations;15 however, the minimal group paradigm has not been used to study the effects of group concordance on the clinician-patient relationship or health outcomes such as pain.
In the present study, we tested the hypothesis that clinician-patient group concordance and perceptions of clinician-patient similarity and trust would influence the pain perceived during medical care. We did so by experimentally manipulating feelings of similarity between participants playing the roles of clinicians and patients in simulated clinical interactions using a modified version of the minimal group paradigm.66 The traditional minimal group paradigm creates artificial sociocultural groups based purely on an arbitrary criterion (e.g., shirt color). In contrast, our modified version of the minimal group paradigm involved the creation of groups based on responses to a questionnaire assessing core personal beliefs and values related to religion, politics, and gender. We chose to form groups using personal beliefs and values because patients’ perceptions of personal belief and value similarity to their physicians have been found to predict health outcomes such as patient satisfaction.64 We predicted lower pain ratings when “patients” were paired with group concordant “clinicians.” We also predicted that higher “patient” ratings of similarity and trust toward “clinicians” would be associated with lower pain ratings.
Lastly, we tested whether “patient” participants’ psychological characteristics moderated the impact of the “clinician-patient” relationship on pain perception. We focused on the “patient’s” level of anxiety and fear of pain, because the influence of the clinician-patient relationship on health outcomes has frequently been characterized as a placebo effect8, 21, 31 and there is evidence that both anxiety and fear of pain influence the placebo response. Specifically, there is some evidence that placebos are most effective for highly anxious patients,58, 59 placebo responses are particularly high in clinical trials for anxiety disorders,53 and that placebos may act by decreasing anticipatory anxiety.9, 45 In contrast, fear of pain has been associated with a decrease in placebo response.44, 45 Therefore, we predicted that the clinician-patient relationship might have the greatest impact on pain for the most anxious “patients,” and the least impact for “patients” most fearful of pain.
Methods
Participants
Participants were a total of 80 (40 male) individuals (80% Non-Hispanic White) age 19–54 years old (M = 26.19, SD = 9.43). Participants reported moderate socioeconomic status (M = 33.55, SD = 12.32), on a scale from 8 (lowest SES) to 66 (highest SES) (Barratt, 2006). Participants reported no current or recent (past 6 months) neurological or psychiatric diagnosis and reported no current use of psychoactive or pain medications. Participants also reported no pain-related medical conditions (e.g., fibromyalgia), no reason to believe they would be especially sensitive or insensitive to contact heat, and did not report currently experiencing an unusual amount of pain. Participants were recruited from the Sona paid subject pool at the University of Colorado Boulder, which included members of the university and surrounding community. Only those participants in the Sona database who preliminarily met inclusion criteria were contacted. No participants were excluded from the study after screening other than those individuals who, upon screening, provided different responses that made them now ineligible (e.g., being above the study inclusion age after initially reporting being below it). The study was approved by the University of Colorado Boulder institutional review board (IRB). Written informed consent was obtained from all participants.
Clinician-Patient Trait Measures
At home prior to coming to the lab, participants completed online questionnaires via a cloud based web survey tool, Qualtrics (Qualtrics Labs, Inc.). Questionnaires assessed trait level measures of participant anxiety and fear of pain, as we hypothesized that these factors might moderate the relationship between the simulated clinical interaction and pain perception. Questionnaires included form Y of the State-Trait Anxiety Inventory (STAI)62 trait subscale and the Fear of Pain Questionnaire-III (FPQ-III).47 The STAI-Trait subscale consisted of 20 items which ask participants to rate how they generally feel on a 4-point Likert scale (1 = Not at all, 4 = Very much so). The items were summed to create a trait anxiety score, with higher numbers representing higher levels of trait anxiety. The STAI-Trait has been demonstrated to have good internal consistency and test-retest reliability, with a Cronbach’s α of .97 in a previous validation study.49 The FPQ-III asks participants to rate their fear associated with 30 different painful events (1 = Not at all, 3 to 5 = Extreme), with items grouped into the subscales Severe Pain, Minor Pain, and Medical Pain. Responses to the FPQ-III were summed to create a total Fear of Pain Index, with higher scores corresponding to greater fear of pain. The FPQ-III has been found to have good internal consistency and test-retest reliability in nonclinical samples,52, 57 with an acceptable Cronbach’s α of .935 reported in a previous study of healthy individuals.18
Group creation and manipulation check
We manipulated feelings of interpersonal similarity between participants playing the role of clinician and patient by creating artificial sociocultural groups based on participants’ core beliefs and values, a modification of the minimal group paradigm.68 Participants’ beliefs and values were assessed using the Personal Beliefs and Values Questionnaire (PBVQ), a composite measure created for this study that included questions about religious beliefs and values from the Duke University Religion Index,36 endorsement of politically polarized beliefs and values used in a previous study,43 and gender role beliefs and values from the World Values Survey Wave 5.4 Participants completed the PBVQ online via Qualtrics prior to arriving at the lab for the study session. The average time between completion of the online forms and completion of the experimental session was approximately 1 week.
Each experimental session contained four same-gender participants, as previous studies have demonstrated an effect of subject-experimenter gender concordance on pain report.3, 41 After being shown a copy of the PBVQ as a reminder of the questions, participants were told by the experimenter that “We’re going to use your answers to that questionnaire to divide you into two groups. For confidentiality reasons we’re going to use color labels of green and yellow to assign the groups, but you can assume those in your color group have more similar values to yours than those in the other group,” thus avoiding deception. Participants were indeed assigned to either the “green group” or the “yellow group” based on the correlations of their responses to the PBVQ (highest correlations = same group) and given group color-coded garments to wear during the study. However, because participants were not recruited for the study based on information about their personal beliefs and values, the values held by individuals participating in the study on a given day varied randomly. Therefore, the grouping procedure did not systematically result in a high degree of belief and value similarity between participants in the same group or any consistent association between group identity (green or yellow) and a particular belief or value orientation (e.g., conservative or liberal). Therefore, any consistent effects of the group manipulation were due to the assumption of shared beliefs and values derived from the group assignment – analogous to the likely effects of real-world shared group membership in typically brief clinical interactions – rather than on rapport established between individuals with similar personal beliefs and values.
In order to check the efficacy of the group manipulation, participants completed a three-item Group Identification Questionnaire at the end of the study, modified from the Collective Identification Scale.70 The Group Identification Questionnaire asked participants 3 items regarding their group membership: “Belonging to a member of the green/yellow group is an important part of my identity,” “I am proud to be a member of the green/yellow group,” and “I value being a member of the green/yellow group.” Each item was on a 6-point Likert scale (1 = Strongly disagree, 6 = Strongly agree).
The purpose of study was described to participants as investigating “the effects of people’s personal beliefs and values on their experience when they get medical care.” In order to assess participant belief in the stated purpose of the study and their perception of the realism of the simulated clinical interaction, we averaged participants’ responses on a 150 point visual analog scale (VAS) to the following three questions to create a Study Belief Index: 1) how much they believed in the stated purpose of the study, 2) how much they believed in the stated basis for group assignment, and 3) how realistic they felt the simulated clinical interaction was.
Clinician-patient role assignment and training
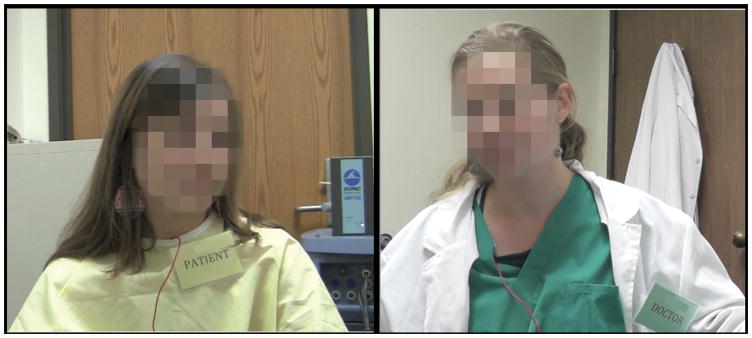
We randomly assigned participants to the role of clinician or patient (one in each group) and participants were given clothing to match their roles: hospital gowns for patients and scrubs and white lab coats for clinicians (Figure 1). Clinicians were trained to introduce themselves and explain the heat stimulation procedure, perform the heat stimulation procedure on the experimenter to allow for assessment and correction of technique, and frequently remind the patient that they could ask to have the heat stimulation stopped at any time if the heat became intolerable. Patient training involved familiarization with the heat task and training and practice in making continuous within-trial and overall post-trial pain intensity ratings. Participants were trained in the simulated clinical interaction in groups of two based on role, not on group assignment. Thus, yellow and green clinicians were trained together and yellow and green patients were trained together.
Figure 1.
Clothing worn by participants playing the role of patient (left) and participants playing the role of clinician (right) during the simulated clinical interactions. Patients wore hospital gowns, while clinicians wore scrubs and medical coats. Color of patients’ hospital gowns and clinicians’ scrubs corresponded to their group (green or yellow).
Simulated clinical interaction
During each experimental session, each patient participated in two simulated clinical interactions, one with a clinician with similar personal beliefs and values (group concordant interaction) and one with a clinician with dissimilar personal beliefs and values (group discordant interaction), with interaction order counterbalanced across participants. The experimenter was then seated at a table behind and partially out of view of the clinician and patient in order to monitor the quality and safety of the heat pain procedure while minimizing the influence on the realism of the simulated clinical interaction. To begin the simulated clinical interaction, the clinician introduced him or herself to the patient and re-explained the thermal stimulation procedure and the fact that it was being used as an analog of a painful medical procedure, answered any questions the participant had, and reminded the participant that they could ask to have the thermal stimulation stopped at any point if the pain became intolerable. During this introductory period and throughout the simulated clinical interaction, the clinician was also free to engage in other kinds of conversation in order to establish rapport. The clinician then performed the thermal stimulation procedure on the patient.
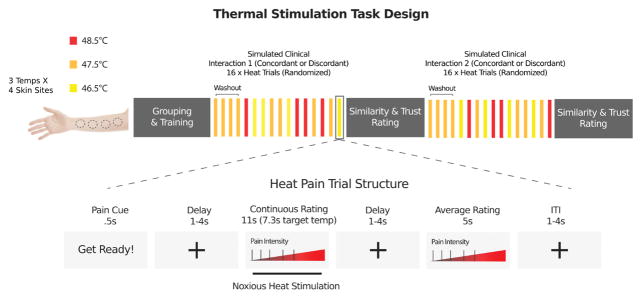
Thermal stimulation was controlled by an E-Prime program and was delivered to four evenly spaced locations on the volar surface of the left forearm of the patient using a 16 mm x 16 mm contact Peltier thermode (Medoc, Inc.). Thermal stimulation was delivered at three target temperatures (46.5, 47.5, 48.5 °C). All heat stimuli were 11 seconds in duration, including 7.3 seconds at the target temperature flanked by 1.85 second ramp periods to get to/from the target temperature to the 32°C baseline. Each heat trial was preceded by a cue that said “Get Ready!” and all parts of the trial were separated by variable delays. See Figure 2 for more details of the trial and task structure. Participants underwent a total of 16 heat trials during each simulated clinical interaction: one trial at each temperature on each of the four skin sites (12 trials), plus a medium heat “washout” stimulus (47.5 °C) delivered to each skin site (4 trials) at the beginning of the thermal stimulation procedure to allow for the initial habituation of the skin site to contact heat.26, 29 Trial order was randomized. Throughout the heat pain procedure, the clinician intermittently reminded the patient that he/she could stop the thermal stimulation at any time if the pain became intolerable or for any other reason.
Figure 2.
Thermal stimulation task design. During each stimulated clinical interaction thermal stimulation was delivered at three target temperatures (46.5, 47.5, 48.5 °C) to four different skin sites on the forearm. All heat stimuli were 11 seconds in duration, including 7.3 seconds at the target temperature. Each heat trial was preceded by a cue that said “Get Ready!” and all parts of the trial were separated by variable delays. Participants underwent a total of 16 heat trials during each simulated clinical interaction: one trial at each temperature on each of the four skin sites (12 trials), plus a medium heat “washout” stimulus (47.5 °C) delivered to each skin site (4 trials) at the beginning of the thermal stimulation procedure to allow for the initial habituation of the skin site to contact heat. Trial order was randomized.
Pain rating
During each stimulation, the patient continuously rated the intensity of the pain (not heat) that he/she perceived on a 100-point generalized labeled magnitude scale (gLMS)6 using the mouse (0 = No experience, 100 = Strongest imaginable experience). Intermediate labels were placed as follows: 1.4 (Barely Detectable), 6 (Weak), 17 (Moderate), 35 (Strong), 53 (Very Strong), though only labels and not numbers were visible to patients. The general anchors on the scale have been found to allow for effective comparison of sensory and affective experiences across modalities and people, and the labels spacing has been found to provide the scale with ratio properties.6 After each stimulation, patients were also asked to rate the overall pain intensity experienced on the previous trial using the same labeled magnitude scale used for the continuous rating. Overall post-trial pain intensity rating, as well as the peak of the continuous within-trial pain intensity rating, were entered separately as the dependent variable in models testing clinician-patient effects on pain report.
Ratings of the clinician-patient relationship
After each simulated clinical interaction, both clinician and patient completed the following questionnaires about their feelings of similarity and trust toward their interaction partner. Thus, while one clinician and one patient participant were completing a simulated clinical interaction, the other two participants were either completing these questionnaires (for the first interaction), or quietly reading magazines while being monitored by a research assistant to ensure that the participants were not talking with each other or using the Internet. Questionnaire responses were used to assess the influence of perceptions of the clinician-patient relationship on pain rating.
Similarity measures
In the Perceived Similarities Measure (PSM)64 participants rated their perceptions of similarity (10 items) to their interaction partner in terms of their personal beliefs and ethnicity (2 subscales) on a 0 = Strongly disagree to 150 = Strongly agree VAS. Subscale scores were averages of subscale items with higher scores corresponding to more similarity. The PSM has been found to relate to clinician-patient ethnic concordance and predict patient outcome measures.64 In the Similarity Visual Analog Scale (SVAS), a measure created for the present study, participants rated how similar they felt to their interaction partners on eight additional dimensions to those in the PSM including their beliefs and values, interests and hobbies, joys and fears, morals, economic status, position in society, and education (e.g. “How similar do you feel your beliefs and values are to those of the doctor?”), as well as their appearance (“How similar do you feel you look to the doctor?”) on a VAS ranging from 0 = Not at all similar to 150 = Extremely similar. Based on prior work emphasizing the importance of personal rather than ethnic or appearance similarity in the clinician-patient relationship,64 we only use the personal similarity subscales of these measures in our main analyses.
Trust measures
The Trust Visual Analog Scale (TVAS) is a single item measure created for the present study that asked participants to rate how much they trusted the clinician/patient (“I trust the green/yellow clinician/patient”) on scale ranging from 0 = Not at all to 150 = Extremely. The Wake Forest Physician Trust Scale (WFPTS)28 is a clinically validated measure which assesses the patient’s perceptions of the clinician’s behavior and the patient’s trust in the clinician (10 items), which we modified to apply to the simulated clinical interaction context. Patients rated their agreement with each statement from 1 = Strongly agree to 5 = Strongly disagree, and responses were summed with higher values corresponding to less trust. The Physicians Trust In Patients Scale (PTIP)69 is a clinically validated measure used to assess the physician’s trust in patients (10 items), which we also modified to apply to the simulated clinical interaction context. Clinicians rated how confident they were in each statement about the patient’s behavior from 1 = Not confident at all to 5 = Completely confident, and responses were summed with higher numbers corresponding to greater trust.
Statistical Analysis
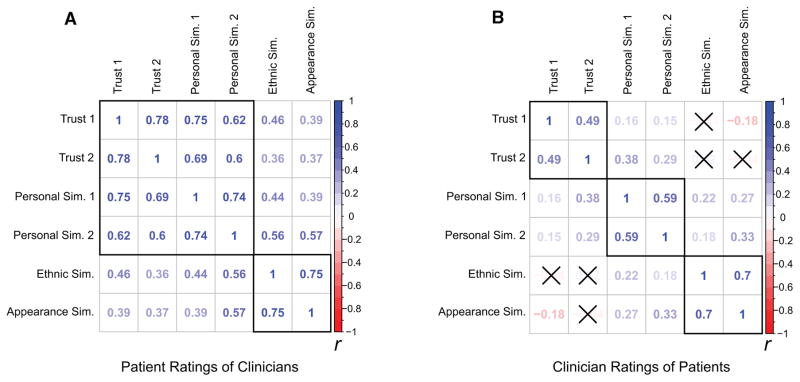
Given the conceptual overlap in many of our clinician-patient rating measures, prior to testing the multiple regression models, we computed Pearson correlations with pairwise deletion among the clinician-patient rating measures. We found that many of the clinician-patient rating measures were strongly and significantly correlated (See Figure 3 for correlation matrices of patient and clinician rating measures). To confirm a problematic level of multicollinearity among potential predictors, we also calculated a variance inflation factor (VIF) for each potential predictor in a preliminary model including all clinician-patient rating measures of interest using the corvif() function in R.72 We found that many clinician-patient rating measures had VIFs over the recommended cutoff of 3 made by Zuur, Ieno, & Elphick.72 Therefore, moderately to strongly correlated measures were rescaled and averaged to create composite ratings. This procedure resulted in a single composite measure of patients’ feelings of similarity and trust toward clinicians combing the WFPTS, TVAS, PSM Personal Similarity subscale, and SVAS Personal Similarity subscale, with higher values reflecting higher levels of trust and similarity. For clinicians’ feelings toward patients, two separate composite measures were created – patient trust (PTIP and TVAS) and patient similarity (PSM Personal Similarity subscale and SVAS Personal Similarity subscale) – as there were low correlations between trust and similarity measures (Figure 3). We chose this method of data reduction because it retained the identity and scale of the original measures and gave equal weights to the combined variables. Thus, we felt this approach was more interpretable than data reduction methods such as principal components analysis (PCA).
Figure 3.
Pairwise Pearson correlation matrices depicting (a) patient ratings of clinicians and (b) clinician ratings of patients. The matrices have black outlines around the measures that were aggregated into composite measures. Colors represent Pearson r values with blue representing positive correlations and red representing negative correlations. A black ‘X’ over a correlation coefficient indicates that it was not significant at the p < .05 level. The labels for the measures are as follows: Trust 1 patients = Wake Forest Physician Trust Scale; Trust 1 clinicians = Physicians Trust In Patients Scale; Trust 2 = Trust Visual Analog Scale; Personal Sim. 1 = Perceived Similarities Measure – Personal Similarity Subscale; Personal Sim. 2 = Similarity Visual Analog Scale – Personal Similarity Subscale; Ethnic Sim. = Perceived Similarities Measure – Ethnic Similarity Subscale; Appearance Sim. = Similarity Visual Analog Scale – Appearance Subscale.
Paired t-tests were used to compare ratings of clinician-patient ratings of similarity and trust between group concordant and group discordant interactions. Due to the multilevel nature of the data, linear mixed-effects regression models were used to test hypothesized predictors of patient pain report. Patient ratings of within-trial and post-trial pain intensity were used as dependent variables in separate models. For each of the pain dependent variables, we created two models: (1) In the first model (referred to as the main model), group concordance, temperature, trial number, and round number were included as fixed factors, and the similarity and trust composite measures were included as covariates. Clinician ID and patient ID were included as two random factors, each with a random intercept. The main model tested the effects of the clinician-patient relationship on pain report. (2) In the second model (referred to as the moderator model), which was partially identical to the first, we also added as covariates patient-level variables related to fear of pain and trait anxiety. The moderator model tested whether any significant relationships between clinician-patient variables and pain report found in the first model were moderated by the patient trait level variables.
All statistical analyses were conducted using R Version 3.2.1.55 Linear mixed-effects models were tested using the lmer function of the lme4 package in R,7 with resulting F-statistic p-values for both fixed and random effects computed using Satterthwaite approximation (lmerTest R package) for degrees of freedom.38 Parametric bootstrapping based on 1000 samples was used to compute 95% confidence intervals for all tests of linear mixed-effects models. Prior to specifying statistical models, the effects of potential outliers were examined using Cook’s Distance (Influence.ME R package51). Examining estimates for the post-trial pain intensity rating dependent variable at the subject level, three potentially influential outlier subjects were identified using a Cook’s D cutoff of 4/n.10 We chose to conduct all subsequent analyses with all subjects retained because we were able to verify that their responses were not due to data collection or measurement error (rather likely reflecting true variation in pain rating in our sample), consistent with recent views on statistical best practices for outlier analysis.72
Next, diagnostic tests of model assumptions revealed some evidence of non-normality in the pain rating dependent variables. Square root transformation of the DVs did not change the significance of the fixed effect predictors of interest. In addition, linear mixed effects models have been shown to be particularly robust against non-normal distributions,2 and there is evidence that the process of subjecting dependent variables to transformations may result in biased parameter estimates in mixed effects models.27 Therefore, we conducted all subsequent analyses with the DVs untransformed. Lastly, we verified we no longer had a problematic level of multicollinearity between predictors in our final models by again computing VIFs for each covariate in the main model and the moderator model. We found that all VIFs were well under the recommended cutoff of 3, confirming that neither of our models had a problem with multicollinearity.
Results
Group Identification, Clinical Simulation and Pain Manipulation Checks
Overall, both patient participants (M = 10.62, SD = 3.54) and clinician participants (M = 9.03, SD = 3.97) reported moderate to strong identification with their assigned group (3 = No identification - 18 = Strongest identification), and average scores on the Study Belief Index (patient participants: M = 75.63, SD = 28.66; clinician participants: M = 84.46, SD = 28.78; 0 = No belief - 150 = Strongest belief) indicated moderate to strong belief in the stated purpose of the study, the stated basis for group assignment, and the realism of the simulated clinical interactions.
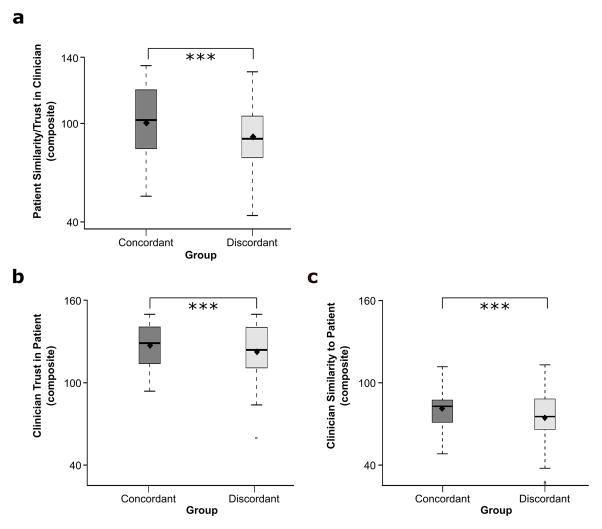
Confirming the effectiveness of our grouping manipulation, patients reported feeling significantly more trust and similarity (single composite measure: 0 = Least, 150 = Most) toward group concordant clinicians (M = 100.29, SD = 21.35) than group discordant clinicians (M = 91.82, SD = 20), t(79) = 40.08, p < .001 (Figure 4a). Clinicians reported feeling significantly greater trust toward group concordant patients (M = 127.10, SD = 16.50) than group discordant patients (M = 122.36, SD = 21.65), t(79) = 57.91, p < .001 (Figure 4b). Clinicians also reported feeling significantly greater similarity toward group concordant patients (M = 89.04, SD = 13.62) than group discordant patients (M = 82.57, SD = 18.21), t(79) = 47.03, p < .001 (Figure 4c; separate composite measures: 0 = Least, 150 = Most). These findings suggest that our experimentally created sociocultural groups increased positive feelings toward group concordant individuals, consistent with the minimal group literature and the effects of real-world sociocultural groups such as ethnicity and gender.15
Figure 4.
Boxplots of average clinician-patient ratings by group concordance. (a) Patients reported feeling more similarity and trust (single composite rating) toward group concordant clinicians than group discordant clinicians. (b) Average clinician trust (composite) ratings of patients by group membership. Clinicians reported trusting group concordant patients more than group discordant patients. (c) Average clinician similarity (composite) ratings to patients by group membership. Clinicians reported feeling more similar to group concordant patients than group discordant patients. Bar lines represent group medians, and black diamonds represent group means. Significance code: *** p < .001.
Next we tested our assumption that the grouping manipulation increased feelings of clinician-patient similarity based on shared group membership rather than any true similarity between patient and clinician related to the correlation of their responses on the PBVQ, the method used to create the groups. To do so, we computed a Pearson correlation coefficient across each clinician-patient pairing between the patients’ composite rating of clinician trust and similarity and the correlation between clinician and patient PBVQ responses. The correlation between these measures was small and nonsignificant, r = .08, p = .50, suggesting that higher feelings of clinician-patient trust and similarity between ingroup members was unlikely to have been driven by actual similarity between patients’ and clinicians’ personal beliefs and values as indexed by the PBVQ.
We also verified the expected effects of temperature and time (trial number and round number) on pain intensity ratings. To simplify the description of our results for the within-trial and post-trial pain rating DVs, we will hereafter refer simply to the pain report outcome variables as “pain intensity.” A similar pattern of results was observed for both post-trial and within-trial pain intensity ratings, with full results visible in Tables 1 and 2. As expected, patients’ ratings of pain intensity increased as the temperature increased (Tables 1–2). Also as expected, patient ratings of pain intensity decreased across trials (Tables 1–2), likely due to heat pain habituation effects observed in other pain studies.26, 29 The trial effect was eliminated when excluding the four initial “washout” trials on each skin site, F(1, 849.60) = .52, p = .47. However, we chose to include washout trials in subsequent analyses, statistically controlling for the effect of trial number, because we believed that the social, psychological, and interpersonal effects related to the clinician-patient interaction of interest in our study might be most pronounced upon the initial exposure to the heat. Finally, there was a significant effect of the round of the simulated clinical interaction on pain rating, with pain intensity decreasing from the first to the second clinical interaction (Tables 1–2). Therefore, the inclusion of clinical interaction round in statistical models controlled for this potential heat habituation effect over time.
Table 1.
Predictors of patient post-trial (average) pain intensity rating
| Fixed Effects Variables | B | SE | β | t | p | 95% CI |
|---|---|---|---|---|---|---|
| Temperature | 11.51 | 0.39 | 0.37 | 29.14 | < .001 *** | [10.61, 12.25] |
| Trial | −0.32 | 0.06 | −0.07 | −5.33 | < .001 *** | [−0.44, −0.20] |
| Round | −2.66 | 0.59 | −0.06 | −4.5 | < .001 *** | [−3.69, −1.59] |
| Group Concordance | 0.18 | 0.66 | 0.004 | 0.28 | 0.78 | [−1.04, 1.49] |
| Patient Similarity/Trust (Composite) | −0.08 | 0.04 | −0.07 | −2.24 | 0.031 * | [−0.16, −0.004] |
| Clinician Trust (Composite) | −0.02 | 0.03 | −0.02 | −0.83 | 0.411 | [−0.09, 0.03] |
| Clinician Similarity (Composite) | 0.04 | 0.03 | 0.03 | 1.18 | 0.241 | [−0.03, 0.10] |
Note.
= p < .05
= p < .001.
Table 2.
Predictors of patient within-trial (peak of continuous) pain intensity rating
| Fixed Effects Variables | B | SE | β | t | p | 95% CI |
|---|---|---|---|---|---|---|
| Temperature | 13.8 | 0.45 | 0.39 | 30.91 | < .001 *** | [12.91, 14.64] |
| Trial | −0.42 | 0.07 | −0.08 | −6.1 | < .001 *** | [−0.55, −0.29] |
| Round | −3.97 | 0.66 | −0.08 | −5.99 | < .001 *** | [−5.31, −2.83] |
| Group Concordance | 0.14 | 0.73 | 0.003 | 0.19 | 0.848 | [−1.36, 1.57] |
| Patient Similarity/Trust (Composite) | −0.08 | 0.04 | −0.06 | −2.03 | 0.051† | [−0.16, −0.004] |
| Clinician Trust (Composite) | −0.03 | 0.02 | −0.02 | −1.02 | 0.316 | [−0.09, 0.03] |
| Clinician Similarity (Composite) | 0.02 | 0.03 | 0.02 | 0.7 | 0.485 | [−0.05, 0.09] |
Note.
= p < .10
= p < .001.
Together, these findings suggest that 1) the grouping manipulation was successful, 2) participants believed in the study premise and found the simulated clinical interactions to be realistic, and 3) the thermal stimulation task induced pain as expected, confirming the effectiveness of our study design.
Predictors of Pain Report
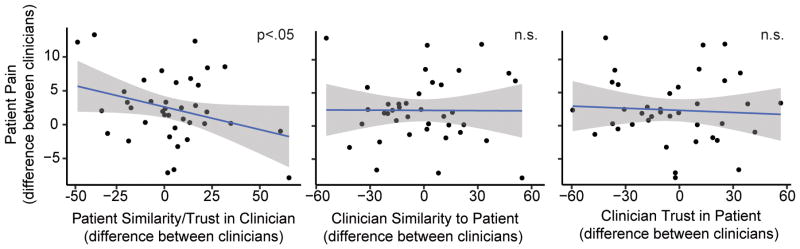
Consistent with our hypothesis, we found that the higher patients rated their feelings of similarity and trust (single composite measure) toward their clinicians, the lower they rated their pain intensity (Figure 5, Tables 1–2). In order to test whether our findings were consistent with previous findings emphasizing the importance of personal rather than appearance similarity,64 we tested a version of the main model with the addition of composite measures of ethnic and appearance similarity for patients and clinicians respectively created from the PSM Ethnic Similarity Subscale and the SVAS Appearance Similarity Subscale. Neither patient reported ethnic and appearance similarity, F(1, 42.12) = .34, p = .56, 95% CI[−.04, .07], nor clinician reported ethnic and appearance similarity, F(1, 32.32) = .62, p = .44, 95% CI[−.06, .03], were significant predictors of post-trial pain intensity. Additionally, neither of the composite measures were significant predictors of within-trial pain intensity rating (patient ethnic/appearance similarity composite: p = .66, clinician ethnic/appearance similarity composite: p = .84). Our findings are consistent with previous findings that personal similarity is more influential than ethnic or appearance similarity in the clinician-patient relationship. Interestingly, neither clinicians’ feelings of similarity nor trust toward their patients (separate composite measures) significantly impacted patients’ pain intensity rating (Figure 5, Tables 1–2 ), suggesting that the patient’s perceptions of the clinician-patient interaction may have more influence on the pain the patient reports during medical care than the clinician’s perceptions of the interaction.
Figure 5.
Scatter plots depicting the effects of feelings of clinician-patient similarity and trust on patient pain intensity. Although statistical models reflect multilevel data, for illustration purposes a single point is plotted for each patient participant. The x-axis represents the difference between patients’ ratings of their two clinicians (left graph) or the difference between clinicians’ ratings of the patient (center and right graphs). The y-axis represents the difference in patients’ average pain intensity ratings between their two clinicians. The higher patients rated their feelings of similarity and trust (single composite measure) toward their clinicians, the lower they rated pain intensity (left graph). In contrast, clinicians’ ratings of patients did not affect patient pain (center and right graphs). Shaded bands are standard error.
Contrary to our hypothesis, group concordance between clinician and patient did not significantly predict pain intensity (Tables 1–2). Even when we removed clinician and patient ratings of similarity and trust from the main model, we still did not find a significant effect of clinician-patient group concordance on either post-trial pain intensity rating, F(1, 1170.5) = 1.60, p = .21, 95% CI[−.39, 1.85], or within-trial pain intensity rating, F(1, 1168.7) = 1.46, p = .23, 95% CI[−.51, 2]. Additionally, controlling for age did not qualitatively change the results of any of the models tested, suggesting that the difference in age between clinician and patient likely did not influence patient pain ratings. Age was therefore not included as a covariate in the models tested. Together, these results suggest that group concordance may indirectly affect pain report by influencing patient feelings of similarity and trust, which are also likely influenced by a number of other aspects of the clinician-patient interaction.
Moderation by Patient Anxiety
Finally, while patients’ trait anxiety and fear of pain were not significant predictors of pain intensity (all p > .40), we found that patients’ level of trait anxiety significantly moderated the relationship between patients’ ratings of clinicians and patients’ post-trial (but not within-trial) ratings of pain intensity, F(1, 67.91) = 6.32, p = .014, 95% CI[−.02, −.0002]. Patients higher in trait anxiety demonstrated greater reduction in pain intensity as their feelings of similarity and trust to their clinician increased compared to those lower in trait anxiety (Figure 6). In contrast, patients’ level of fear of pain did not moderate the relationship between patients’ ratings of clinicians and patients’ pain intensity rating, F(1, 147.35) = 1.99, p = .16, 95% CI[−.001, .01].
Figure 6.
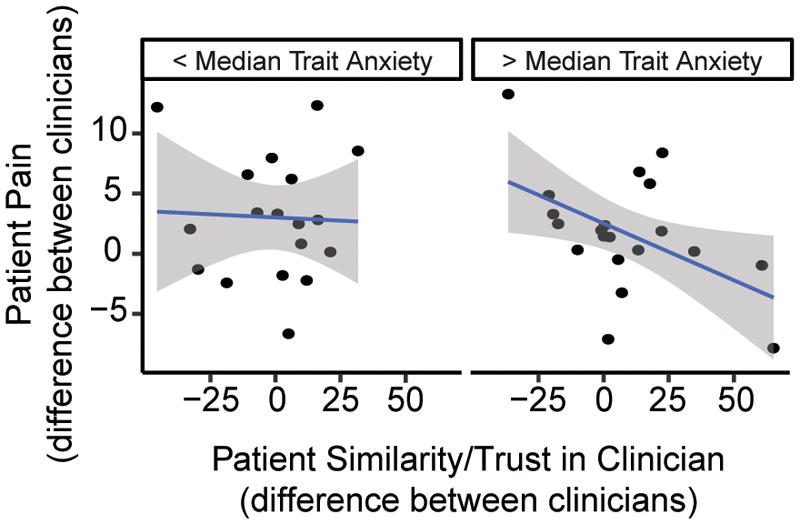
Scatter plots depicting the moderating effect of patients’ trait anxiety on the relationship between patients’ feelings of similarity and trust toward their clinicians and patients’ pain intensity ratings. Although statistical models tested moderation using a continuous measure of trait anxiety, for display purposes we have plotted data from participants with anxiety scores below and above the median trait anxiety score separately. Additionally, although statistical models reflect multilevel data, for illustration purposes a single point is plotted for each patient participant representing the difference between patients’ ratings of their two clinicians on the x-axis and the difference in patients’ average pain intensity ratings between their two clinicians on the y-axis. As patients’ trait anxiety increased they demonstrated a stronger relationship between their ratings of clinicians and their pain intensity. Shaded bands are standard error.
Overall, participants reported a level of trait anxiety (M = 34.45, SD = 8.01) that was similar to norms reported previously for healthy individuals in a comparable age group.34 Participants also reported moderate (M = 72.93, SD = 15.97) fear of pain (30 = No fear of pain, 150 = Extreme fear of pain). Together, these results suggest that positive feelings toward clinicians might be most influential in reducing pain for the most constitutively anxious, similar to effects observed for placebo analgesia.45, 53, 58, 59
Discussion
We tested the hypothesis that perceptions of the clinician-patient relationship — group concordance and feelings of similarity and trust — influence the pain patients perceive during medical care using simulated clinical interactions. We found that participants playing the role of patients who were paired with participants playing the role of clinicians from their own group (group concordant) reported feeling more similarity and trust toward their clinicians than patients paired with clinicians who were from a different group (group discordant). In turn, we found that patients’ positive feelings of similarity and trust toward their clinicians — but not clinicians’ feelings toward patients or whether the clinician and patient were from the same group — predicted lower pain intensity ratings by patients in response to painful thermal stimulation, an analog of a painful medical procedure. Finally, using moderation analysis, we found that the most anxious patients exhibited the strongest relationship between their feelings about their clinicians and their reported pain intensity.
Our findings contribute to our understanding of top-down modulation of pain perception. Although a growing body of work has demonstrated that pain perception can be modulated by social, cultural, and contextual factors,17, 42, 48, 65 evidence that the clinician-patient relationship — one of the social factors most relevant to clinical pain — can impact pain perception remains limited. The few studies that have experimentally demonstrated a link between improvements to the clinician-patient relationship and decreased clinical pain have manipulated the clinician’s behavior, such as enhancing communication and reassurance.14, 31 For example, Kaptchuk et al.31 found that providing placebo treatment for irritable bowel syndrome along with a structured positive patient-provider interaction improved the symptoms of irritable bowel syndrome (including pain) significantly more than placebo treatment with minimal practitioner interaction. Our present findings suggest that simply increasing patients’ feelings of similarity and trust toward their clinician may decrease perceived pain independent of any systematic manipulation of the clinician’s behavior.
Our findings do not fully speak to the psychological and biological mechanisms underlying the effects of patient feelings of clinician similarity and trust on pain perception. However, the placebo analgesia literature suggests that decreased anxiety and increased expectations of pain relief, along with accompanying release of endogenous opioids and/or cannabinoids, underlie many instances of placebo analgesia.5, 8 Thus, similar mechanisms may have been at play in the present study. Our finding that participants highest in anxiety exhibited the strongest relationship between patient feelings about clinicians and decreases in pain perception provides support for this hypothesis. Several possibilities exist for the specific role anxiety may have played in our study. One possibility is that patients who were the most anxious benefitted the most from a positive clinician-patient relationship. Alternatively, anxiety might have enhanced patients’ attention toward positive aspects of the clinician-patient relationship, such as similarities or trust. In terms of clinical implications, this finding suggests that those individuals highest in anxiety may gain the greatest benefit from interventions that increase feelings of similarity and trust toward their clinician.
The present study also contributes to our understanding of the impact that the clinician-patient relationship can have on the immediate pain experience. Previous studies of the effects of clinician-patient group concordance and feelings of similarity and trust on health outcomes have focused on indirect health outcomes such as patient satisfaction and treatment adherence.16, 22, 30, 37, 39, 60 There is limited evidence that health outcomes can be impacted by other aspects of the clinician-patient relationship, such as clinician-patient communication.33 To our knowledge, however, this is the first study to demonstrate that feelings related to clinician-patient group concordance can impact pain report, a validated subjective health outcome encompassing both underlying nociceptive processes as well as social, psychological, and contextual factors.
These results may have implications for disparities in pain treatment. Clinician-patient racial and ethnic concordance is the most thoroughly studied example of clinician-patient group concordance impacting health outcomes such as patient satisfaction.16, 39 Furthermore, the increased level of ethnically discordant clinician-patient interactions experienced by minority Americans,1 combined with the lower levels of trust,16, 22 positive affect,30 and communication37, 60 that characterizes these interactions, has led to the hypothesis that clinician-patient ethnic discordance may contribute to the higher pain reported by minority patients.13, 23, 24 Further research is needed to evaluate to what degree our findings using modified minimal groups generalize to real-world sociocultural groups such as racial and ethnic groups and to interventions that increase feelings of interpersonal similarity and trust.
Counter to our original hypothesis, we did not find a direct effect of clinician-patient group concordance on pain report. However, our finding that group concordance significantly predicts clinician-patient feelings, which in turn significantly predict patient pain, suggests patients’ feelings about clinicians are a likely mediator of the relationship between clinician-patient group concordance and patient pain. Such a mediation model lacking a direct effect would still be consistent with the latest statistical recommendations for mediation analysis.46 In the present study, due to the fact that each patient only interacted with two different clinicians, it was not possible to conduct a multilevel mediation analysis to test for the indirect effect of group concordance on pain. Future efforts should also seek to explicitly specify a causal mediation model that can test potential indirect effects of group concordance on pain report.
These results should be interpreted in light of several limitations. First, while we do not believe that the modified minimal group manipulation resulted in a consistently high degree of similarity between members of the same group, it is possible that for certain dyads there could have been a degree of true similarity influencing patient pain report. However, there was random variation in the beliefs of participants arriving for any given study session, and we prevented participants from talking with one another outside of the simulated clinician-patient interactions. Therefore, we believe that the group manipulation used for the study is unlikely to have resulted in a consistently high degree of true similarity between dyads. Second, it is possible that, due to the within-subjects design, where participants were aware that their interaction partner was either similar or dissimilar to them in terms of personal beliefs and values, demand effects may have subsequently influenced patient participants’ ratings of clinician similarity/trust and pain in response to the thermal stimulation. However, because participants were never explicitly informed about our interest in the effect of feelings about one’s clinician on pain rating, we believe it is unlikely that demand is responsible for the entirety of the observed effects. Third, in order to increase the believability and ecological validity of the simulated clinical interactions, we only measured clinician-patient ratings of similarity and trust after the experimental pain procedure. As a result, the aversive experience of the pain may have influenced patient participants’ subsequent ratings of clinician participants. However, because group assignment occurred prior to pain and was also related to patients’ feelings about their clinicians, we believe that the patients’ feelings about their clinicians were not solely driven by their experiences of pain. Finally, although our use of artificial sociocultural groups potentially increases the generalizability of our findings to different sociocultural groups, it is unclear how the effects of our experimentally created sociocultural groups relate to the effects of real-world groups such as race, gender, or sexual orientation. Similarly, although our use of simulated clinical interactions increased experimental control, it is unclear if findings would be similar in a clinical context.
Future studies should seek to enhance the realism of the simulated clinical interactions to increase the generalizability of the findings, such as through the use of real-world sociocultural groups (race, ethnicity, gender) and through the use of actual clinicians and/or patients as participants. Other efforts to enhance the realism of the clinical simulation in future studies, such as a more extended simulated clinical interaction approximating a physician’s office visit, would serve to retain experimental control while also increasing the ecological validity and clinical applicability of the scientific findings.
In conclusion, our findings suggest important new links between clinician-patient group concordance, patients’ feelings of similarity and trust toward clinicians, and the pain experienced during medical care. These findings increase our understanding of top-down pain modulation and the health effects of the clinician-patient relationship. Furthermore, these findings suggest that teaching clinicians new ways to find common ground with their patients might be an effective way to reduce pain and pain-related disparities without necessitating changes to the demographics of the clinician workforce.
Perspective.
We present novel evidence that the clinician-patient relationship can impact the pain experienced during medical care. We found that “patients” in simulated clinical interactions who reported feeling more similarity and trust toward their “clinicians” reported less pain, suggesting that increasing feelings of clinician-patient similarity and trust may reduce pain disparities.
Acknowledgments
We thank Luke Chang for his help with study design and consultation on data analysis, Megan Powell for her help with data collection, and Natalia Medina for her help with data analysis.
Footnotes
Disclosures: This work was supported by the National Institutes of Health grants 2R01MH076136 and R01DA035484 (TDW, PI). The authors have no conflicts of interest to declare.
References
- 1.AAMC. Diversity in the Physician Workforce: Facts & Figures 2014. Association of American Medical Colleges; 2014. [Google Scholar]
- 2.Arnau J, Bono R, Blanca MJ, Bendayan R. Using the linear mixed model to analyze nonnormal data distributions in longitudinal designs. Behavior research methods. 2012;44(4):1224–1238. doi: 10.3758/s13428-012-0196-y. [DOI] [PubMed] [Google Scholar]
- 3.Aslaksen PM, Myrbakk IN, Høifødt RS, Flaten MA. The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain. 2007;129(3):260–268. doi: 10.1016/j.pain.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Association, WVS. World Values Survey, Wave 5, 2005–2008, Official Aggregate v. 20140429. Madrid, Spain: Asep/JDS; 2014. [Google Scholar]
- 5.Atlas LY, Wager TD. A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions. Handb Exp Pharmacol. 2014;225:37–69. doi: 10.1007/978-3-662-44519-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartoshuk LM, Fast K, Snyder DJ. Differences in Our Sensory Worlds: Invalid Comparisons With Labeled Scales. Current Directions in Psychological Science. 2005;14(3):122–125. [Google Scholar]
- 7.Bates D, MM, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. 2015. [Google Scholar]
- 8.Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev. 2013;93(3):1207–46. doi: 10.1152/physrev.00043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta J-K. Neurobiological mechanisms of the placebo effect. The journal of neuroscience. 2005;25(45):10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollen KA, Jackman RW. Regression diagnostics an expository treatment of outliers and influential cases. Sociological Methods & Research. 1985;13(4):510–542. [Google Scholar]
- 11.Brown JL, Sheffield D, Leary MR, Robinson ME. Social support and experimental pain. Psychosomatic Medicine. 2003;65(2):276–283. doi: 10.1097/01.psy.0000030388.62434.46. [DOI] [PubMed] [Google Scholar]
- 12.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–11. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Management. 2012;2(3) doi: 10.2217/pmt.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chassany O, Boureau F, Liard F, Bertin P, Serrie A, Ferran P, Keddad K, Jolivet-Landreau I, Marchand S. Effects of training on general practitioners’ management of pain in osteoarthritis: A randomized multicenter study. Journal of Rheumatology. 2006;33(9):1827–1834. [PubMed] [Google Scholar]
- 15.Cikara M, Van Bavel JJ. The Neuroscience of Intergroup Relations: An Integrative Review. Perspect Psychol Sci. 2014;9(3):245–74. doi: 10.1177/1745691614527464. [DOI] [PubMed] [Google Scholar]
- 16.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907–15. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 17.Craig KD. The Social Communication Model of Pain. Canadian Psychology-Psychologie Canadienne. 2009;50(1):22–32. [Google Scholar]
- 18.Cremeans-Smith JK. Fear of pain and the frequency with which healthy individuals engage in physical activity. International Journal of Sport and Exercise Psychology. 2016:1–13. [Google Scholar]
- 19.David RA, Rhee M. The impact of language as a barrier to effective health care in an underserved urban Hispanic community. Mount Sinai Journal of Medicine. 1998;65(5–6):393–397. [PubMed] [Google Scholar]
- 20.Descartes R. Treatise of man [1664] Vol. 34. Cambridge, MA: Harvard University Press; 1972. [Google Scholar]
- 21.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. The Lancet. 2001;357(9258):757–762. doi: 10.1016/s0140-6736(00)04169-6. [DOI] [PubMed] [Google Scholar]
- 22.Doescher MP, Saver BG, Franks P, Fiscella K. Racial and ethnic disparities in perceptions of physician style and trust. Arch Fam Med. 2000;9(10):1156–63. doi: 10.1001/archfami.9.10.1156. [DOI] [PubMed] [Google Scholar]
- 23.Edwards RR. The association of perceived discrimination with low back pain. J Behav Med. 2008;31(5):379–89. doi: 10.1007/s10865-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 24.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61(3):346–54. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59(2):195–206. doi: 10.1016/j.neuron.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Greffrath W, Baumgärtner U, Treede R-D. Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. Pain. 2007;132(3):301–311. doi: 10.1016/j.pain.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Gurka MJ, Edwards LJ, Muller KE, Kupper LL. Extending the Box–Cox transformation to the linear mixed model. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2006;169(2):273–288. [Google Scholar]
- 28.Hall MA, Zheng B, Dugan E, Camacho F, Kidd KE, Mishra A, Balkrishnan R. Measuring patients trust in their primary care providers. Medical care research and review. 2002;59(3):293. doi: 10.1177/1077558702059003004. [DOI] [PubMed] [Google Scholar]
- 29.Jepma M, Jones M, Wager TD. The dynamics of pain: evidence for simultaneous site-specific habituation and site-nonspecific sensitization in thermal pain. J Pain. 2014;15(7):734–46. doi: 10.1016/j.jpain.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. American Journal of Public Health. 2004;94(12):2084. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT, Park M, Rivers AL, McManus C, Kokkotou E, Drossman DA, Goldman P, Lembo AJ. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336(7651):999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaptchuk TJ, Miller FG. Placebo Effects in Medicine. N Engl J Med. 2015;373(1):8–9. doi: 10.1056/NEJMp1504023. [DOI] [PubMed] [Google Scholar]
- 33.Kelley JM, Kraft-Todd G, Schapira L, Kossowsky J, Riess H. The Influence of the Patient-Clinician Relationship on Healthcare Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE. 2014;9(4):e94207. doi: 10.1371/journal.pone.0094207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. British Journal of Clinical Psychology. 1983;22(4):245–249. doi: 10.1111/j.2044-8260.1983.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 35.Koban L, Wager TD. Beyond conformity: Social influences on pain reports and physiology. Emotion. 2016;16(1):24–32. doi: 10.1037/emo0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenig HG, Büssing A. The Duke University Religion Index (DUREL): a five-item measure for use in epidemological studies. Religions. 2010;1(1):78–85. [Google Scholar]
- 37.Koerber A, Gajendra S, Fulford RL, BeGole E, Evans CA. An exploratory study of orthodontic resident communication by patient race and ethnicity. Journal of Dental Education. 2004;68(5):553–562. [PubMed] [Google Scholar]
- 38.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests in Linear Mixed Effects Models. 2015. [Google Scholar]
- 39.Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43(3):296–306. [PubMed] [Google Scholar]
- 40.Lee LJ, Batal HA, Maselli JH, Kutner JS. Effect of Spanish interpretation method on patient satisfaction in an urban walk-in clinic. Journal of General Internal Medicine. 2002;17(8):640–645. doi: 10.1046/j.1525-1497.2002.10742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine FM, De Simone LL. The effects of experimenter gender on pain report in male and female subjects. Pain. 1991;44(1):69–72. doi: 10.1016/0304-3959(91)90149-R. [DOI] [PubMed] [Google Scholar]
- 42.Loeser JD, Melzack R. Pain: an overview. The Lancet. 1999;353(9164):1607–1609. doi: 10.1016/S0140-6736(99)01311-2. [DOI] [PubMed] [Google Scholar]
- 43.Losin EA, Cross KA, Iacoboni M, Dapretto M. Neural processing of race during imitation: self-similarity versus social status. Hum Brain Mapp. 2014;35(4):1723–39. doi: 10.1002/hbm.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyby PS, Aslaksen PM, Flaten MA. Is fear of pain related to placebo analgesia? J Psychosom Res. 2010;68(4):369–77. doi: 10.1016/j.jpsychores.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Lyby PS, Aslaksen PM, Flaten MA. Variability in placebo analgesia and the role of fear of pain--an ERP study. Pain. 2011;152(10):2405–12. doi: 10.1016/j.pain.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 46.MacKinnon DP. Introduction to statistical mediation analysis. Routledge; 2008. [Google Scholar]
- 47.McNeil DW, Rainwater AJ., 3rd Development of the Fear of Pain Questionnaire--III. J Behav Med. 1998;21(4):389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 48.Merskey H, Albe-Fessard D, Bonica J, Carmon A, Dubner R, Kerr F, Lindblom U, Mumford J, Nathan P, Noordenbos W, Pagni C, Renaer M, Sternbach R, Sunderland S. Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6(3):249. [PubMed] [Google Scholar]
- 49.Metzger RL. A reliability and validity study of the State-Trait Anxiety Inventory. Journal of Clinical Psychology. 1976 [Google Scholar]
- 50.Mistiaen P, Osch M, Vliet L, Howick J, Bishop F, Di Blasi Z, Bensing J, Dulmen S. The effect of patient–practitioner communication on pain: a systematic review. European Journal of Pain. 2015 doi: 10.1002/ejp.797. [DOI] [PubMed] [Google Scholar]
- 51.Nieuwenhuis R, te Grotenhuis M, Pelzer B. Influence.ME: Tools for Detecting Influential Data in Mixed Effects Models. R Journal. 2012;4(2):38–47. [Google Scholar]
- 52.Osman A, Breitenstein JL, Barrios FX, Gutierrez PM, Kopper BA. The Fear of Pain Questionnaire-III: further reliability and validity with nonclinical samples. Journal of behavioral medicine. 2002;25(2):155–173. doi: 10.1023/a:1014884704974. [DOI] [PubMed] [Google Scholar]
- 53.Piercy MA, Sramek JJ, Kurtz NM, Cutler NR. Placebo response in anxiety disorders. Ann Pharmacother. 1996;30(9):1013–9. doi: 10.1177/106002809603000917. [DOI] [PubMed] [Google Scholar]
- 54.Pitkin Derose K, Hays RD, McCaffrey DF, Baker DW. Does physician gender affect satisfaction of men and women visiting the emergency department? Journal of general internal medicine. 2001;16(4):218–226. doi: 10.1046/j.1525-1497.2001.016004218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.RCoreTeam. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- 56.Roberts MH, Klatzkin RR, Mechlin B. Social Support Attenuates Physiological Stress Responses and Experimental Pain Sensitivity to Cold Pressor Pain. Ann Behav Med. 2015;49(4):557–69. doi: 10.1007/s12160-015-9686-3. [DOI] [PubMed] [Google Scholar]
- 57.Roelofs J, Peters ML, Deutz J, Spijker C, Vlaeyen JW. The Fear of Pain Questionnaire (FPQ): further psychometric examination in a non-clinical sample. Pain. 2005;116(3):339–346. doi: 10.1016/j.pain.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Shapiro A, Shapiro E. Patient-provider relationship than the placebo effect. In: Matrazzo J, et al., editors. Behavioral Health: A Handbook of Health Enhancement and Disease Prevention. Wiley-Interscience; New York: 1984. pp. 371–383. [Google Scholar]
- 59.Shapiro AK, Mike V, Barten H, Shapiro E. Study of the placebo effect with a self-administered placebo test. Compr Psychiatry. 1973;14(6):535–48. doi: 10.1016/0010-440x(73)90039-4. [DOI] [PubMed] [Google Scholar]
- 60.Siminoff LA, Graham GC, Gordon GH. Cancer communication patterns and the influence of patient characteristics: Disparities in information-giving and affective behaviors. Patient Education and Counseling. 2006:355–360. doi: 10.1016/j.pec.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Sitzia J. How valid and reliable are patient satisfaction data? An analysis of 195 studies. International Journal for Quality in Health Care. 1999;11(4):319–328. doi: 10.1093/intqhc/11.4.319. [DOI] [PubMed] [Google Scholar]
- 62.Spielberger CD. Manual for the State-Trait Anxiety Inventory STAI (form Y)(“ self-evaluation questionnaire”) 1983 [Google Scholar]
- 63.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423–33. [PMC free article] [PubMed] [Google Scholar]
- 64.Street RL, O’Malley KJ, Cooper LA, Haidet P. Understanding concordance in patient-physician relationships: personal and ethnic dimensions of shared identity. Ann Fam Med. 2008;6(3):198–205. doi: 10.1370/afm.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clinical Journal of Pain. 2001;17(1):52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Tajfel H. Experiments in intergroup discrimination. Scientific American. 1970;223(5):96–102. [PubMed] [Google Scholar]
- 67.Tajfel H. The social psychology of intergroup relations. Annual review of psychology. 1982;33:1–39. [Google Scholar]
- 68.Tajfel H, Turner JC. An integrative theory of intergroup conflict. The social psychology of intergroup relations. 1979;33:47. [Google Scholar]
- 69.Thom DH, Wong ST, Guzman D, Wu A, Penko J, Miaskowski C, Kushel M. Physician Trust in the Patient: Development and Validation of a New Measure. The Annals of Family Medicine. 2011;9(2):148–154. doi: 10.1370/afm.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Bavel JJ, Cunningham WA. A Social Identity Approach to Person Memory: Group Membership, Collective Identification, and Social Role Shape Attention and Memory. Pers Soc Psychol Bull. 2012 doi: 10.1177/0146167212455829. [DOI] [PubMed] [Google Scholar]
- 71.Williams B. Patient satisfaction: a valid concept? Social science & medicine. 1994;38(4):509–516. doi: 10.1016/0277-9536(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 72.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution. 2010;1(1):3–14. [Google Scholar]