Abstract
The prognostic significance of Ki-67 in patients with gastric cancer (GC) remains controversial. The aim of our meta-analysis is to evaluate its association with clinicopathological characteristics and prognostic value in patients with GC. PubMed, EMBASE, and Web of Science were systematically searched up to May 2017. Twenty-two studies including 3,825 patients with GC were analyzed. The meta-analysis indicated that the incidence difference of Ki-67 expression in GC patients was significant when comparing the older group to younger group (odds ratio [OR] =1.44, 95% confidence interval [CI] 1.19, 1.75), lymph node positive group to negative group (OR =1.49, 95% CI 1.20, 1.84), the large size tumor group to the small size tumor group (OR =1.27, 95% CI 1.24, 1.68) and the TNM stage III+IV group to TNM stage I+II group (OR =2.28, 95% CI 1.66, 3.12). However, no statistical differences existed in gender. The detection of Ki-67 significantly correlated with the overall survival of patients (hazard ratio =1.51, 95% CI 1.31, 1.72). Our study suggested that Ki-67 overexpression was associated with poor prognosis in GC patients. Ki-67 positive rates may be associated with age, lymph node metastasis, tumor size, and TNM staging system in GC patients.
Keywords: Ki-67, gastric cancer, meta-analysis, prognostic
Introduction
Gastric cancer (GC) is one of the most common malignancies and ranks the second cause of cancer deaths worldwide.1 Over the last 10 years, diagnostic capabilities and therapeutic methods have been developed, but the prognosis for GC patients still remains poor, especially in those in the advanced stage.2 Tumor biomarkers reflect the pathological characteristics of tumor cells; therefore, they often have diagnostic significance or prognostic value. The expression level of many prognostic biomarkers is associated with patient survival and can be particularly valuable in terms of guiding the clinical treatment of patients; some prognostic biomarkers also have potential as novel therapeutic targets. Therefore, novel prognostic biomarkers and therapeutic targets need be identified for the management of GC.
Ki-67, as a well-characterized nuclear protein, is encoded by the MKI-67 gene that is associated with the transcription of ribosomal RNA and cell proliferation and is involved in the development of tumor.3,4 Some studies reported that Ki-67 is only expressed in the G1, S, and G2 stages of the cell cycle during mitosis, but not the G0 stage; hence, Ki-67 usually is regarded as a valuable biomarker of cell growth and proliferation.5,6 Many retrospective articles have reported that the high level of Ki-67 is associated with poor prognosis in patients with GC.7–19 However, the results of other articles are inconclusive and no consensus has been reached, and some studies reported that Ki-67 expression is prognostically irrelevant in GC patients.20–23 Therefore, it is necessary to evaluate the prognostic and clinicopathological value of Ki-67 expression in GC. Accordingly, we performed a systematic review and meta-analysis to evaluate the prognostic value of Ki-67 quantitatively and explore the associations of Ki-67 with the clinicopathological features of GC.
Methods
Search strategy
We performed a thorough search for available literature in electronic databases of PubMed, EMBASE and Web of Science until May 2017. The search terms “Ki-67 AND (GC OR gastric neoplasm OR gastric carcinoma OR gastric malignancy)” were applied, and we initially identified 783 entries for further examination. The references cited by the primary studies were also reviewed in this search strategy. The most recent or larger sample size studies were selected when duplicated data were published.
Selection criteria
The 22 eligible studies had to meet the following criteria: 1) studies in which expression of Ki-67 in the primary GC tissue was evaluated by immunohistochemistry (IHC); 2) studies that investigated the association between Ki-67 expression and prognosis of patients (overall survival [OS]), or studies that described the correlation between Ki-67 and clinicopathological features in GC; and 3) only published studies written in English. The exclusion criteria were as follows: 1) studies that were reviews, letters or conference papers; 2) studies that were not performed in humans; and 3) articles that failed to report sufficient data for determining desired meta-analysis outcomes. All evaluations were separately undertaken by two authors to guarantee the precision of the selection process.
Data extraction
All candidate articles were carefully assessed by two independent reviewers (Gang Liu and Disheng Xiong) for possible inclusion; any disagreements were resolved by discussion between the two reviewers or consultation with a third reviewer (Zhengjie Huang). The following information was captured from all included studies: first author, country, publication year, number of patients, patient age and gender, positive expression rate of Ki-67, detection method, cutoff value, hazard ratios (HRs) for OS and their 95% confidence intervals (CIs) and clinicopathological features. HRs and 95% CIs were directly extracted from articles or estimated from Kaplan–Meier survival curves by the open digitizing program (Engauge Digitizer) and Tierney’s methods.20
Statistical analysis
The meta-analysis was performed using Review Manager version 5.3 (Cochrane Collaboration, Oxford, UK). Heterogeneity across eligible studies was evaluated by Cochran’s Q test and I2 test (a P-value <0.10 for the Q-test or I2 >50% represented statistically significant heterogeneity). The fixed-effects model or random-effects model was used depending on the aforementioned heterogeneity analysis. HRs and 95% CIs were applied to estimate the impact of Ki-67 on OS, while ORs and 95% CIs were used to assess the association between Ki-67 expression and clinicopathological characteristics in GC. Potential publication bias was assessed using Begg’s funnel plot and Egger’s test. All of the generated P-values <0.05 were defined as statistically significant.
Results
Search results
A flowchart of the study selection process is shown in Figure 1. The search strategy generated 783 articles, of which 164 were considered potentially valuable after reading titles and abstracts. Then, the full text was retrieved for detailed evaluation and 37 were subsequently excluded for various reasons, including not prognostic or clinicopathological study. Eventually, 22 of the studies as of May 2017 (with a total of 3,825 patients) satisfied the criteria for this meta-analysis.7–19
Figure 1.
Flow diagram of the meta-analysis process.
Characteristics of studies
The main characteristics of the 22 studies are summarized in Table 1. The 22 eligible studies were published between 2006 and 2017. Of them, 13 studies originated from China, three were from Korea, one was from Greece, one was from Poland, one was from Japan, one was from Tunisia, one was from Germany, and one was from Egypt. Among the studies, 18 were performed to analyze OS. Hazard ratio (HR) and 95% CI data were extracted directly from the 14 studies and estimated by Kaplan–Meier survival curves in four studies. Various clinicopathological data were reported in 22 studies (age in eight studies, gender in eight studies, lymph nodes metastasis in nine studies, TNM stage in seven studies, tumor size in six studies and histological differentiation in eight studies).
Table 1.
Characteristics of the included studies
| Reference | Country | Case number | Gender (m/f) | Age (y) | Detection method (cutoff) | Increased Ki-67, n (%) | Survival analysis | HR (95% CI) | HR (obtained) |
|---|---|---|---|---|---|---|---|---|---|
| Joo et al (2006)25 | Korea | 118 | 84/35 | 52/67 (<59 y/≥59 y) | IHC (strong staining) | 62 (52.5) | NR | NR | NR |
| Chen et al (2008)7 | China | 86 | 57/29 | Mean 57.6 | IHC (>10%) | 64 (74.4) | OS | 1.62 (1.45–1.81) | Curve |
| Tsamandas et al (2009)8 | Greece | 110 | 71/39 | Mean 66.0 | IHC (>5%) | 15 (13.6) | OS | 2.15 (1.07–4.32) | Direct |
| Czyzewska et al (2009)19 | Poland | 100 | 67/33 | 34/65 (<60 y/≥60 y) | IHC (>50%) | 37 (37.0) | OS | 1.12 (0.86–1.46) | Curve |
| Lee et al (2010)20 | Korea | 245 | 171/74 | 202/43 (≤65 y/>65 y) | IHC (>10%) | 164 (66.9) | OS | 0.56 (0.38–0.83) | Direct |
| Zhao et al (2010)26 | China | 316 | NR | Mean 58.1 | IHC (score ≥5) | 196 (62.0) | NR | NR | NR |
| Yu et al (2012)9 | China | 315 | 165/150 | 177/138 (<60 y/≥60 y) | IHC (>50%) | 76 (24.1) | OS | 1.75 (1.13–2.34) | Direct |
| Xiao et al (2013)21 | Japan | 431 | 301/130 | Mean 66.4 | IHC (score ≥1) | 362 (84.0) | OS | 0.98 (0.82–1.17) | Direct |
| Kang et al (2013)22 | Korea | 184 | 123/61 | Mean 61.0 | IHC (>10%) | 100 (54.3) | OS | 0.18 (0.03–1.06) | Direct |
| He et al (2013)22 | China | 166 | 113/53 | 43/38 (<60 y/≥60 y) | IHC (>25%) | 94 (56.0) | OS | 1.85 (1.30–2.63) | Direct |
| Ayed et al (2014)11 | Tunisia | 54 | 34/20 | 21/33 (≤50 y/>50 y) | IHC (>5%) | 11 (20.4) | OS | 4.17 (1.08–16.1) | Direct |
| Ezziddin et al (2014)12 | Germany | 74 | 42/32 | Mean 62.5 | IHC (>10%) | 14 (18.9) | OS | 3.10 (1.40–6.90) | Direct |
| Yang et al (2014)14 | China | 159 | 118/41 | Mean 58.0 | IHC (>30%) | 115 (72.3) | OS | 1.84 (1.21–2.80) | Curve |
| Zhou et al (2015)27 | China | 40 | 28/12 | 18/22 (≤60 y/>60 y) | IHC (>5%) | 28 (70.0) | NR | NR | NR |
| Li et al (2015)15 | China | 69 | 51/18 | Mean 55.0 | IHC (>50%) | 42 (60.9) | OS | 1.62 (1.28–2.05) | Curve |
| Yang et al (2015)13 | China | 161 | 108/53 | NR | IHC (>50%) | 98 (60.9) | OS | 2.50 (1.34–4.65) | Direct |
| Deng et al (2016)23 | China | 62 | 48/14 | Mean 63.4 | NR | 28 (45.2) | OS | 1.56 (0.72–3.38) | Direct |
| Huang et al (2016)17 | China | 693 | 515/178 | Mean 60.3 | IHC (>50%) | 335 (48.3) | OS | 1.46 (1.22–1.75) | Direct |
| Wang et al (2016)17 | China | 173 | 117/56 | NR | IHC (>50%) | 114 (65.9) | OS | 2.97 (1.94–4.55) | Direct |
| Zhu et al (2016)18 | China | 150 | 100/50 | NR | IHC (>50%) | 89 (59.3) | OS | 2.93 (1.52–5.65) | Direct |
| Badary et al (2017)28 | Egypt | 42 | 26/16 | Mean 53.0 | IHC (>10%) | 22 (52.4) | NR | NR | NR |
| Zhang et al (2017)24 | China | 77 | 44/33 | 41/36 (≤48 y/>48 y) | IHC (>5%) | 48 (62.3) | OS | 1.43 (0.67–3.04) | Direct |
Note: Age data shown as mean or number of patients per the age ranges specified in parentheses.
Abbreviations: CI, confidence interval; f, female; HR, hazard ratio; IHC, immunohistochemistry; m, male; NR, not reported; OS, overall survival; y, years.
Meta-analysis
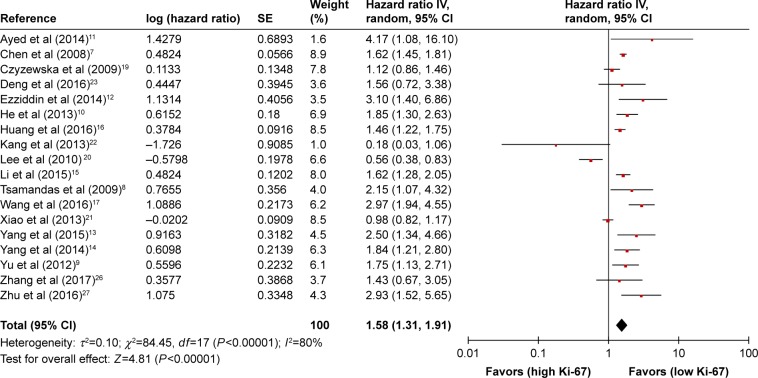
Eighteen studies were included in the meta-analysis of OS. A random-effects model utilized to calculate the pooled HR and 95% CI on account of the heterogeneity test reported a P<0.00001 and an I2 value of 80.0%. The results suggested that Ki-67 overexpression was associated with poor OS of GC (HR =1.58, 95% CI =1.31–1.91, P<0.00001) (Figure 2).
Figure 2.
HRs and 95% CIs in studies assessing the relationship between Ki-67 expression and OS.
Abbreviations: CI, confidence interval; HR, hazard ratio; IV, inverse variance; OS, overall survival; SE, standard error.
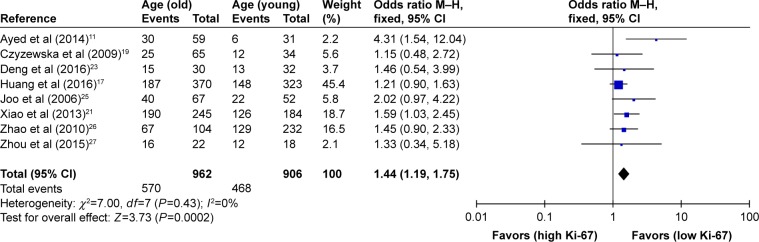
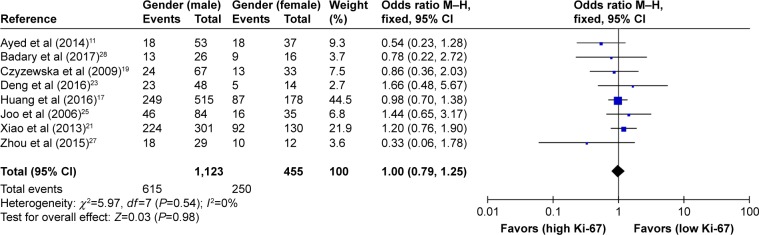
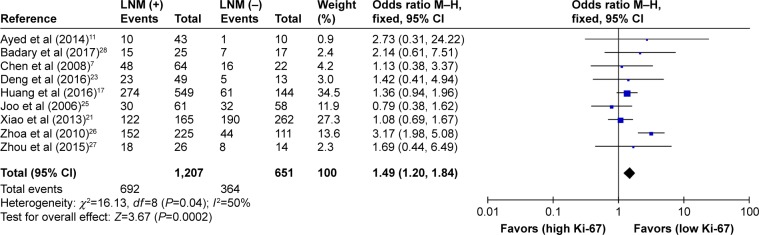
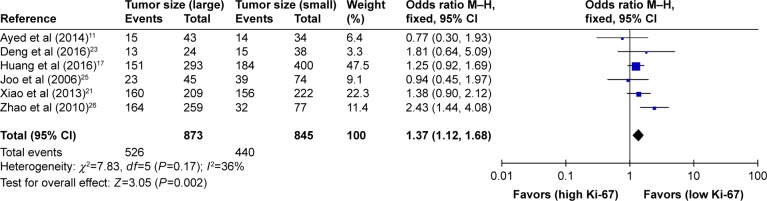
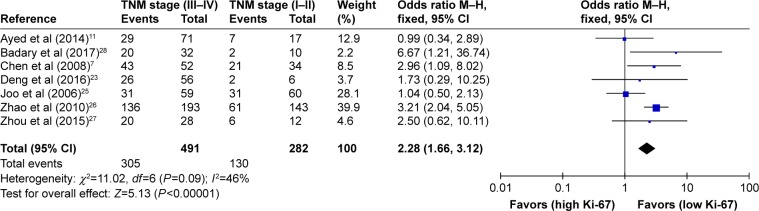
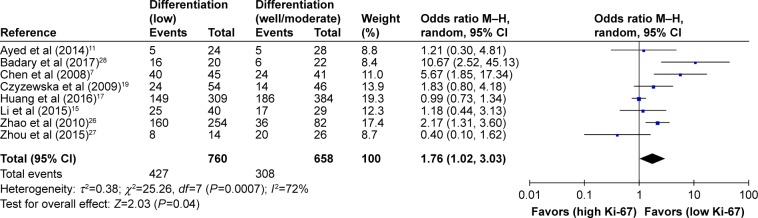
As shown in Table 2, the correlation between Ki-67 overexpression and age, gender, lymph node metastasis, tumor size, tumor TNM stage and histological differentiation was also explored in our meta-analysis (Figures 3–8). According to the results of evidence synthesis, we found that Ki-67 overexpression significantly correlated with age (OR =1.44, 95% CI [1.19, 1.75]) (Figure 3), lymph node metastasis (OR =1.49, 95% CI [1.20, 1.84]) (Figure 5), tumor size (OR =1.27, 95% CI [1.24, 1.68]) (Figure 6), tumor TNM stage (OR =2.28, 95% CI [1.66, 3.12]) (Figure 7) and histological differentiation (OR =1.76, 95% CI [1.02, 3.03]) (Figure 8). However, no statistical differences existed in gender (OR =1.00, 95% CI [0.67, 1.25]) (Figure 4).
Table 2.
Association of Ki-67 expression and clinicopathological parameters
| Clinicopathological parameters | Number of studies | Number of patients | OR (95% CI) | P-value | Heterogeneity
|
|
|---|---|---|---|---|---|---|
| I2 (%) | P-value | |||||
| Age (older vs younger) | 8 | 1,868 | 1.44 (1.19–1.75) | 0.0002 | 0 | 0.43 |
| Gender (male vs female) | 8 | 1,578 | 1.00 (0.76–1.25) | 0.98 | 0 | 0.54 |
| Lymph node metastasis (N+ vs N−) | 9 | 1,858 | 1.49 (1.20–1.84) | 0.0002 | 50 | 0.04 |
| Tumor size (large vs small) | 6 | 1,718 | 1.37 (1.12–1.68) | 0.002 | 36 | 0.17 |
| TNM stage (III+IV vs I+II) | 7 | 773 | 2.28 (1.66–3.12) | <0.00001 | 46 | 0.09 |
| Differentiation (low vs well/moderate) | 8 | 735 | 1.76 (1.02–3.03) | 0.04 | 72 | 0.0007 |
Abbreviations: CI, confidence interval; N, node; OR, odds ratio.
Figure 3.
Forest plot of studies evaluating the association between Ki-67 and clinical parameters (older vs younger age) in gastric cancer with fixed-effects model.
Abbreviations: CI, confidence interval; M–H, Mantel–Haenszel.
Figure 4.
Forest plot of studies evaluating the association between Ki-67 and clinical parameters (male vs female gender) in gastric cancer with fixed-effects model.
Abbreviations: CI, confidence interval; M–H, Mantel–Haenszel.
Figure 5.
Forest plot of studies evaluating the association between Ki-67 and clinical parameters (N+ vs N− lymph node metastasis) in gastric cancer with fixed-effects model.
Abbreviations: CI, confidence interval; LNM, lymph node metastasis; M–H, Mantel–Haenszel.
Figure 6.
Forest plot of studies evaluating the association between Ki-67 and clinical parameters (large vs small tumor size) in gastric cancer with fixed-effects model.
Abbreviations: CI, confidence interval; M–H, Mantel–Haenszel.
Figure 7.
Forest plot of studies evaluating the association between Ki-67 and clinical parameters (III+IV vs I+II TNM stage) in gastric cancer with fixed-effects model.
Abbreviations: CI, confidence interval; M–H, Mantel–Haenszel.
Figure 8.
Forest plot of studies evaluating the association between Ki-67 and clinical parameters (low vs well/moderate differentiation) in gastric cancer with random-effects model.
Abbreviations: CI, confidence interval; M–H, Mantel–Haenszel.
Due to the heterogeneity, we performed the subgroup analyses, as is shown in Table 3, by stratifying the combined data according to ethnicity (Asian vs Caucasian), cutoff of staining (<50% vs ≥50%) and number of patients (<100 vs ≥100).
Table 3.
Subgroup analysis of pooled HR for gastric cancer patients with Ki-67 overexpression
| Subgroup | Studies | Pooled HR | 95% CI | P-value | Model | Heterogeneity I2 (%) | P-value |
|---|---|---|---|---|---|---|---|
| Ethnicity | |||||||
| Asian | 14 | 1.54 | 1.25–1.89 | <0.0001 | Random | 82 | <0.00001 |
| Caucasian | 4 | 2.04 | 1.08–3.86 | 0.03 | Random | 71 | 0.01 |
| Cutoff of staining | |||||||
| <50% | 10 | 1.52 | 1.09–2.12 | 0.01 | Random | 78 | <0.00001 |
| ≥50% | 7 | 1.78 | 1.40–2.27 | <0.0001 | Random | 72 | 0.002 |
| Number of patients | |||||||
| <100 | 6 | 1.64 | 1.49–1.81 | <0.00001 | Fixed | 0 | 0.48 |
| ≥100 | 12 | 1.51 | 1.15–1.97 | 0.003 | Random | 84 | <0.00001 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Publication bias
As shown in Figure 9, Begg’s and Egger’s tests, as well as funnel plots, were conducted to estimate publication bias in the present meta-analysis. The funnel plots indicated that the included studies had no evident asymmetry. These findings suggested that significant publication bias did not exist in our meta-analysis.
Figure 9.
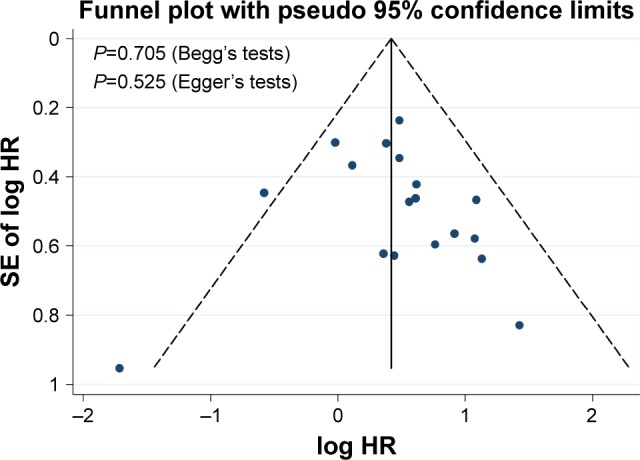
Funnel plot and Begg’s and Egger’s tests for evaluation of potential publication bias.
Abbreviations: HR, hazard ratio; SE, standard error.
Discussion
In recent years, despite improvement in diagnosis and therapy, GC is still characterized by late clinical presentation, rapid progression and poor survival. Considering the high morbidity and mortality of GC, researchers have been dedicated in identifying available new prognostic markers to achieve better clinical decision making regarding therapy and outcomes, in past decades. As far as we know, Ki-67 has been declared as a predictor of cell proliferation, malignant potential and recurrence of cancer. Ki-67 expression in GC is controversial. For example, a number of retrospective articles have reported that high level of Ki-67 is associated with poor prognosis in patients with GC. However, some studies suggested that Ki-67 overexpression is prognostically irrelevant in GC patients. Therefore, we performed a systematic review and meta-analysis to evaluate the prognostic value of Ki-67 and explore the associations of Ki-67 with the clinicopathological features of GC.
In our study, a combined analysis of 22 eligible clinical studies revealed a critical role of Ki-67 overexpression in GC progression and prognosis. The meta-analysis results suggested that GC patients with low Ki-67 expression showed better OS than those with high Ki-67 expression. In addition, we performed meta-analyses to determine the relationship between Ki-67 overexpression and the clinical features of GC, and correlations between Ki-67 upregulation and age, gender, lymph node metastasis, tumor size, TNM stage and histological differentiation were observed. Finally, we revealed that high level of Ki-67 was significantly associated with high risk of age, lymph node metastasis, tumor size, tumor TNM stage and histological differentiation. However, no statistical differences existed in gender. Although with highly significant heterogeneity, this new result also had statistical significance, illustrating that high Ki-67 expression was significantly associated with poor OS of patients with GC.
As we all know, Ki-67 is an antigen that corresponds to a nuclear non-histone protein expressed by cells in the proliferative phases G1, G2, M, and S. Scholzen and Gerdes have reported that quiescent cells in the G0 phase do not express the Ki-67 antigen, making Ki-67 a good marker to detect the cell proliferative fraction.24 Many studies have shown that Ki-67 expression is a valuable independent prognostic predictor for the survival of patients with GC,25–27 which is consistent with the present study. The detection of expression of Ki-67 in GC provides prognostic information for patients with the disease.6,28,29 In the present meta-analysis, the overexpression of Ki-67 is significantly increased in patients with clinical grade, tumor TNM stage, metastases occurrence and poor prognosis. This may help us to use these markers as a meaningful prognostic and predictive biomarker in each patient with GC.
In summary, the present meta-analysis shows that Ki-67 overexpression can predict an unfavorable prognosis of GC patients. Our results also indicate an association of Ki-67 upregulation with clinicopathological features such as age, lymph node metastasis, tumor size, tumor TNM stage and histological differentiation. More multicenter and prospective studies are needed to clarify the clinical relevance and precise molecular explanation for the abnormal expression of Ki-67.
Acknowledgments
This study was supported by grants from the Science and Technology Project of Natural Science Foundation of Fujian Province (number 2016J01639), the Medical Innovations Topic in Fujian Province (numbers 2016-CXB-8 and 2012-CXB-29) and the Project of Xiamen Scientific and Technological Plan (number 3502Z20134011).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42(2):219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS. Gastric cancer – new therapeutic options. N Engl J Med. 2006;355(1):76–77. doi: 10.1056/NEJMe068121. [DOI] [PubMed] [Google Scholar]
- 3.Bullwinkel J, Baron-Luhr B, Ludemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206(3):624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 4.Schonk DM, Kuijpers HJ, van Drunen E, et al. Assignment of the gene(s) involved in the expression of the proliferation-related Ki-67 antigen to human chromosome 10. Hum Genet. 1989;83(3):297–299. doi: 10.1007/BF00285178. [DOI] [PubMed] [Google Scholar]
- 5.Rahmanzadeh R, Huttmann G, Gerdes J, Scholzen T. Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis. Cell Prolif. 2007;40(3):422–430. doi: 10.1111/j.1365-2184.2007.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Li X, Wang GL, Wang Y, Zhu YY, Zhu J. Clinicopathological significance of overexpression of TSPAN1, Ki67 and CD34 in gastric carcinoma. Tumori. 2008;94(4):531–538. doi: 10.1177/030089160809400415. [DOI] [PubMed] [Google Scholar]
- 8.Tsamandas AC, Kardamakis D, Tsiamalos P, et al. The potential role of Bcl-2 expression, apoptosis and cell proliferation (Ki-67 expression) in cases of gastric carcinoma and correlation with classic prognostic factors and patient outcome. Anticancer Res. 2009;29(2):703–709. [PubMed] [Google Scholar]
- 9.Yu HF, Zhao G, Ge ZJ, et al. High RIN1 expression is associated with poor prognosis in patients with gastric adenocarcinoma. Tumour Biol. 2012;33(5):1557–1563. doi: 10.1007/s13277-012-0409-0. [DOI] [PubMed] [Google Scholar]
- 10.He WL, Li YH, Yang DJ, et al. Combined evaluation of centromere protein H and Ki-67 as prognostic biomarker for patients with gastric carcinoma. Eur J Surg Oncol. 2013;39(2):141–149. doi: 10.1016/j.ejso.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Ayed DB, Khabir A, Abid M, et al. Clinicopathological and prognostic significance of p53, Ki-67, and Bcl-2 expression in Tunisian gastric adenocarcinomas. Acta Histochem. 2014;116(8):1244–1250. doi: 10.1016/j.acthis.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Ezziddin S, Attassi M, Yong-Hing CJ, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55(2):183–190. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Zhu J, Huang H, et al. PFTK1 promotes gastric cancer progression by regulating proliferation, migration and invasion. PLoS One. 2015;10(10):e0140451. doi: 10.1371/journal.pone.0140451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Wang X, Zhao Q, et al. Combined evaluation of the expression of NUCKS and Ki-67 proteins as independent prognostic factors for patients with gastric adenocarcinoma. Tumour Biol. 2014;35(8):7505–7512. doi: 10.1007/s13277-014-1880-6. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Deng W, Ma J, et al. Prognostic evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol. 2015;32(1):433. doi: 10.1007/s12032-014-0433-6. [DOI] [PubMed] [Google Scholar]
- 16.Huang G, Chen S, Wang D, et al. High Ki67 expression has prognostic value in surgically-resected T3 gastric adenocarcinoma. Clin Lab. 2016;62(1–2):141–153. doi: 10.7754/clin.lab.2015.150610. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Li M, Zhang X, et al. Upregulation of CDK7 in gastric cancer cell promotes tumor cell proliferation and predicts poor prognosis. Exp Mol Pathol. 2016;100(3):514–521. doi: 10.1016/j.yexmp.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Wang Y, Huang H, et al. Upregulation of KPNβ1 in gastric cancer cell promotes tumor cell proliferation and predicts poor prognosis. Tumour Biol. 2016;37(1):661–672. doi: 10.1007/s13277-015-3839-7. [DOI] [PubMed] [Google Scholar]
- 19.Czyzewska J, Guzinska-Ustymowicz K, Pryczynicz A, Kemona A, Bandurski R. Immunohistochemical evaluation of Ki-67, PCNA and MCM2 proteins proliferation index (PI) in advanced gastric cancer. Folia Histochem Cytobiol. 2009;47(2):289–296. doi: 10.2478/v10042-009-0042-y. [DOI] [PubMed] [Google Scholar]
- 20.Lee HE, Kim MA, Lee BL, Kim WH. Low Ki-67 proliferation index is an indicator of poor prognosis in gastric cancer. J Surg Oncol. 2010;102(3):201–206. doi: 10.1002/jso.21583. [DOI] [PubMed] [Google Scholar]
- 21.Xiao LJ, Zhao S, Zhao EH, et al. Clinicopathological and prognostic significance of Ki-67, caspase-3 and p53 expression in gastric carcinomas. Oncol Lett. 2013;6(5):1277–1284. doi: 10.3892/ol.2013.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang Y, Jung WY, Lee H, et al. Prognostic significance of heat shock protein 70 expression in early gastric carcinoma. Korean J Pathol. 2013;47(3):219–226. doi: 10.4132/KoreanJPathol.2013.47.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Y, Chen X, Ye Y, et al. Histological characterisation and prognostic evaluation of 62 gastric neuroendocrine carcinomas. Contemp Oncol (Pozn) 2016;20(4):311–319. doi: 10.5114/wo.2016.61852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang QX, Gao R, Xiang J, et al. Cell cycle protein Bora serves as a novel poor prognostic factor in multiple adenocarcinomas. Oncotarget. 2017;8(27):43838–43852. doi: 10.18632/oncotarget.16631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joo YE, Chung IJ, Park YK, et al. Expression of cyclooxygenase-2, p53 and Ki-67 in gastric cancer. J Korean Med Sci. 2006;21(5):871–876. doi: 10.3346/jkms.2006.21.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10:218. doi: 10.1186/1471-2407-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Li Y, Zheng J, Liu K, Zhang H. Detecting of gastric cancer by Bcl-2 and Ki67. Int J Clin Exp Pathol. 2015;8(6):7287–7290. [PMC free article] [PubMed] [Google Scholar]
- 28.Badary DM, Abdel-Wanis ME, Hafez MZ, Aboulhagag NA. Immunohistochemical analysis of PTEN, HER2/neu, and ki67 expression in patients with gastric cancer and their association with survival. Pathophysiology. 2017;24(2):99–106. doi: 10.1016/j.pathophys.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Kikuyama S, Kubota T, Shimizu K, Miyakita M. Ki-67 antigen expression in relation to clinicopathological variables and prognosis in gastric cancer. Oncol Rep. 1998;5(4):867–870. doi: 10.3892/or.5.4.867. [DOI] [PubMed] [Google Scholar]