Abstract
Recent reports claimed that glucosylsphingosine (GS) is highly accumulated and specifically evoking itch-scratch responses in the skins of atopic dermatitis (AD) patients. However, it was unclear how GS can trigger itch-scratch responses, since there were no known molecular singling pathways revealed yet. In the present study, it was verified for the first time that GS can activate mouse serotonin receptor 2a (mHtr2a) and 2b (mHtr2b), but not 2c (mHtr2c) that are expressed in HEK293T cells. Specifically, effects of GS on all mouse serotonin receptor 2 subfamily were evaluated by calcium imaging techniques. The GS-induced intracellular calcium increase was dose-dependent, and antagonists such as ketanserin (Htr2a antagonist) and RS-127445 (Htr2b antagonist) significantly blocked the GS-induced responses. Moreover, the proposed GS-induced responses appear to be mediated by phospholipase C (PLC), since pretreatment of a PLC inhibitor U-73122 abolished the GS-induced responses. Additionally, the GS-induced calcium influx is probably mediated by endogenous TRPC ion channels in HEK293T cells, since pretreatment of SKF-96365, an inhibitor for TRPC, significantly suppressed GS-induced response. In conclusion, the present study revealed for the first time that GS can stimulate mHtr2a and mHtr2b to induce calcium influx, by utilizing PLC-dependent pathway afterwards. Considering that GS is regarded as a pruritogen in AD, the present study implicates a novel GS-induced itch signaling pathway.
Keywords: Glucosylsphingosine, Calcium imaging, Serotonin receptor 2 subfamily, G-protein coupled receptor, Phospholipase C
INTRODUCTION
Glucosylsphingosine (GS) is generally recognized as an accumulated byproduct resulted by deficiency of glucocer-ebrosidases in Gaucher disease (Orvisky et al., 2000, 2002). However, recent studies revealed that GS is also increased in the stratum corneum of atopic dermatitis (AD) patients (Ishibashi et al., 2003; Okamoto et al., 2003). Interestingly, GS is known to cause itch-scratching responses (Kim et al., 2010), suggesting that GS is an itch-inducing agent or so-called ‘pruritogen.’
Itch is an unpleasant sensation that causes a desire to scratch that is evoked by pruritogen via itch-mediating sensory neurons (Ikoma et al., 2006). Generally, it was once thought that histamine is the only pruritogen, but recent studies have revealed that there are many different “histamine-independent” pruritogens as well, such as chloroquine, cowhage, β-alanine, proteases, and serotonin (Ajayi et al., 1989; Thomsen et al., 2001; Jinks and Carstens, 2002; Liu et al., 2009; Aghahowa et al., 2010; Papoiu et al., 2011; Nakagawa and Hiura, 2013; Moser and Giesler, 2014). Especially, serotonin is a pruritogen of interest in this study, since itch-scratch responses caused by GS in mice were significantly reduced by the pretreatment of cyproheptadine, a non-specific blocker of serotonin receptor 2 subfamily, but not by antihistamine ketotifen (Kim et al., 2010). Therefore, it seems plausible that GS may induce itch sensation with the aid of serotonin receptor 2 subfamily, although detailed molecular mechanisms are yet elusive.
Therefore, in the present study, the aim of the study was focused on identifying possible molecular mechanisms by which GS may induce itch sensations. Specifically, it was investigated whether GS is able to activate three subfamily of serotonin receptor 2 subfamily (Htr2a, Htr2b and Htr2c). Since serotonin receptor 2 subfamily is a member of G-protein coupled receptor (GPCR) with Gαq/11, its activation will lead to elevation of intracellular calcium levels, which can be measured by calcium imaging techniques. Furthermore, in-depth examinations of GS-mediated responses were performed such as involvement of phospholipase C (PLC). Although it has already been suggested that serotonin receptor 2 subfamily could be involved in GS-induced itch-scratch behaviors (Yamaguchi et al., 1999; Thomsen et al., 2001; Jinks and Carstens, 2002; Nojima and Carstens, 2003), there is no actual study that pursued to identify the interrelationship between GS and serotonin receptor 2 subfamily.
MATERIALS AND METHODS
Reagents
Glucosylsphingosine (GS) was purchased from Avanti Polar Lipids Inc (Alabaster, AL, USA). α-Me-5HT, RS-102221 hydrochloride, U-73122 and SKF-96365 were bought from Sigma-Aldrich (Incheon, Korea). RS-127445 hydrochloride was purchased from Tocris bioscience (Bristol, UK).
Gene cloning
Total RNA from mouse dorsal root ganglia (DRG) was isolated by using easy-spin total RNA extraction kit (Intron Bio-technology Inc., Gyeonggi, Korea), first-strand cDNA was prepared with Prime script first strand cDNA synthesis kit (Takara). For cloning of mHtr2a (NM_008312.4) and mHtr2c (NM_172812.2), custom-synthesized oligomers were designed to cover full-length coding sequence regions of each gene, and the two genes were successfully incorporated into pcDNA 3.1 by using “Single step ligation independent cloning (SLIC)” method (Jeong et al., 2012). In case of mouse Htr2b, the gene was purchased from Korea Human Gene Bank (Medical Genomics Research Center, KRIBB, Daejeon, Korea), and subcloned into pCMV-SPORT6 expression vector. All genes were sequenced and showed 100% identity to NCBI Genbank database.
Cell culture and gene transfection
HEK293T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) that contains 10% of fetal bovine serum (FBS), 2 mM of L-glutamine, and penicillin/Zell Shield. Grown cells were transfected by FuGENE HD transfection Reagent (Promega) according to manufacturer’s instruction. 24–48 hrs after gene transfection, calcium imaging experiments were performed.
Calcium imaging: measurement of intracellular Ca2+ changes
Intracellular Ca2+ changes were measured using a fluorescence microscope (Nikon Eclipse Ti-U, Nikon Instruments, Tokyo, Japan). Transfected HEK293T cells were loaded with 5 μM Fluo-3/AM (Invitrogen, Eugene, OR, USA) and incubated for 40 min at 37°C. Then, it was washed with 1× NBS solution (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, 5.5 mM HEPES, adjusted to pH 7.4). After the incubation, the medium was washed out, and compounds were applied to elicit intracellular calcium increase. Fluorescence was detected with wavelengths of excitation at 488 nm, and emission at 515 nm. Fluorescent microscopic images were recorded to the computer for 2 min at time intervals of 1.5 sec. Image analysis was performed using ImageJ (NIH, Public Domain, USA) with custom-made scripts for automatic cell count and ratio metric image production. Intracellular Ca2+ changes were expressed as F/F0, where F indicates the intensity of fluorescence, whereas F0 indicates the initial fluorescence intensity.
Statistical analysis
All data were presented as mean ± SEM. Student’s t-test was applied for comparison between two groups. For comparison among more than three groups, one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison posttest was used.
RESULTS
GS induces intracellular calcium level increase via mouse serotonin receptor 2a
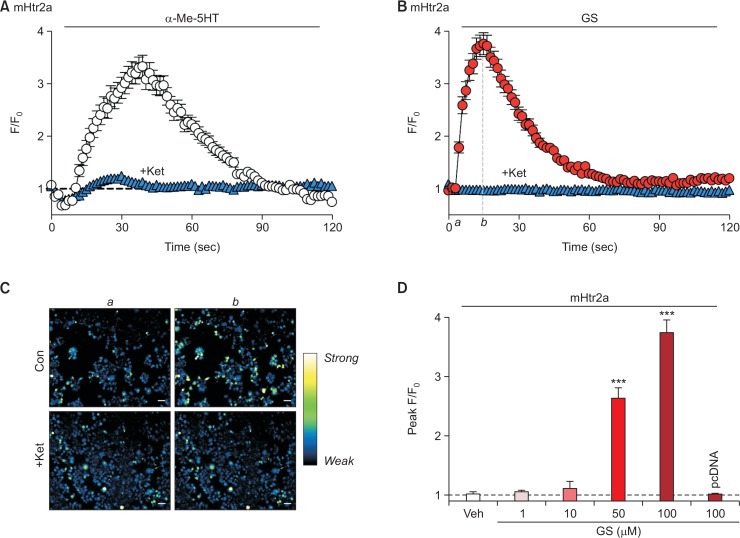
Prior to investigating the effect of GS on mouse serotonin receptor 2a (mHtr2a), it was first examined whether the cloned mHtr2a was functionally active in transiently-expressed HEK-293T cells. For this reason, HEK293T cells expressing mHtr2a were treated with α-Me-5HT, an agonist for serotonin receptor 2 subfamily, and its response was measured by calcium imaging. It turned out, as shown in Fig. 1A, that treatment of 10 μM α-Me-5HT significantly increased the intracellular fluorescence intensity, which is proportional to the intracellular calcium concentration. As expected, the increment by α-Me-5HT was not observed in cells that were only transfected with the vector pcDNA3.1 alone (data not shown). Moreover, the response to α-Me-5HT was virtually abolished by pretreatment of 500 nM ketanserin, a specific antagonist for serotonin receptor 2a (“+Ket” in Fig. 1A). These data suggest that the cloned mHtr2a was indeed functionally active in HEK293T cells, and thus considered ready to be used in subsequent experiments.
Fig. 1.
GS can induce intracellular calcium increase through mHtr2a in HEK293T cells. (A) HEK293T cells transient expressing mouse Htr2a (mHtr2a) was treated with α-Me-5HT (10 μM), a non-specific agonist for serotonin receptor 2 family. As a control, α-Me-5HT successfully induced an increase of intracellular calcium levels (empty circle), which is substantially suppressed by pretreatment of ketanserin (“+Ket,” 500 nM for 5 min), a specific antagonist for Htr2a (filled blue triangle). (B) GS (100 μM) increased intracellular calcium levels in mHtr2a-expressing HEK293T cells (filled red circle), which was significantly inhibited by pretreatment of 500 nM ketanserin (filled blue triangle). (C) Representative pseudocolor microscopic images of cells at initial (a) and peak (b) time points denoted in (B). Scale bar indicates 50 μm. (D) A comparison of peak F/F0 from mHtr2a after treatment of different ranges of GS concentration. Notice that the response shows dose-dependent manner, and cells that do not express mHtr2a exerts no intracellular calcium increase at all (“pcDNA”). ***p<0.001, when compared to vehicle-treated control group (“Veh”).
Surprisingly, these mHtr2a-expressing cells exhibited a significant rise of intracellular calcium levels upon GS treatment (Fig. 1B, 1C). To clarify whether the increase was due to mH-tr2a activation, pretreatment of 500 nM ketanserin was also performed before 100 μM of GS application. As a result, it was found that ketanserin pretreatment almost completely abolished the GS-induced calcium increase (Fig. 1B, 1C), indicating that the observed calcium increase by GS was indeed mediated by mHtr2a. Moreover, GS-induced responses showed dose-dependent manner (Fig. 1D), which is in agreement with the finding that GS-induced calcium increase is mediated by mHtr2a. As expected, cells transfected with pcDNA3.1 alone did not show any intracellular calcium changes to 100 μM GS (“pcDNA” in Fig. 1D). Taken together, it was clearly revealed that GS is able to evoke intracellular calcium increase via mHtr2a.
GS also induces intracellular calcium level increase via mouse serotonin receptor 2b
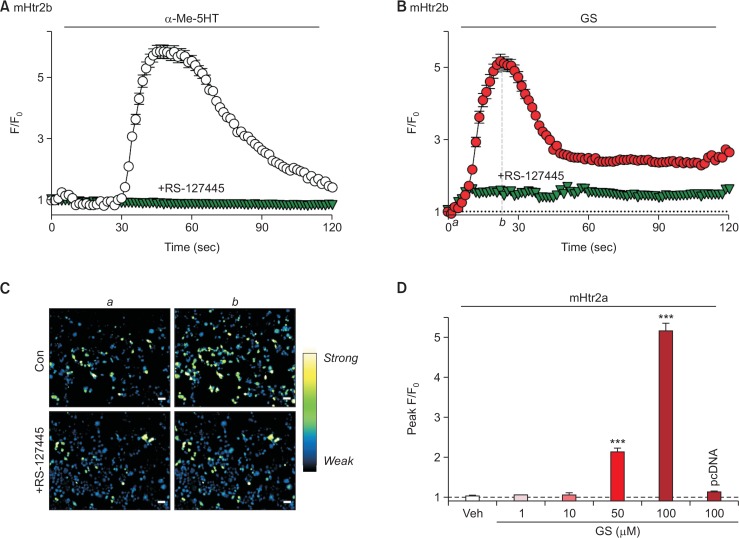
Similar approach has been applied to mouse serotonin receptor 2b (mHtr2b) as well. As shown in Fig. 2A, it was also found that the mHtr2b was functionally active when transiently expressed in HEK293T cells, verified by treatment of 10 μM α-Me-5HT. Again, the responses were significantly inhibited by pretreatment of mHtr2b-specific antagonist, RS-127445 (500 nM for 5 min, Fig. 2A). Interestingly, GS (100 μM) also triggered a strong increment of intracellular calcium in cells transfected with mHtr2b, and pretreatment of RS-127445 significantly reduced the GS-induced responses (Fig. 2B, 2C). Similar to mHtr2a, the intracellular calcium increase by GS through mHtr2b showed dose-dependent manner, and there was almost complete lack of response when cells did not express mHtr2b (“pcDNA” in Fig. 2D). Overall, it was found that mHtr2b can also be activated by GS, as well as mHtr2a.
Fig. 2.
GS can also induce intracellular calcium increase through mHtr2b in HEK293T cells. (A) HEK293T cells transient express mHtr2b was treated with α-Me-5HT (10 μM). As a control, α-Me-5HT successfully induced an increase of intracellular calcium levels (empty circle), which is substantially suppressed by pretreatment of RS-127445 (500 nM for 5 min), a specific antagonist for Htr2b (inverted triangle). (B) GS (100 μM) increased intracellular calcium levels in mHtr2b-expressing HEK293T cells (filled circle), which was significantly inhibited by pretreatment of 500 nM RS-127445 (inverted triangle). (C) Representative pseudocolor microscopic images of cells at initial (a) and peak (b) time points denoted in (B). Scale bar indicates 50 μm. (D) A comparison of peak F/F0 from mHtr2b after treatment of different ranges of GS concentration. Notice that the response shows dose-dependent manner, and cells that do not express mHtr2b exert no intracellular calcium increase at all (“pcDNA”). ***p<0.001, when compared to vehicle-treated control group (“Veh”).
GS does not activate mouse serotonin receptor 2c
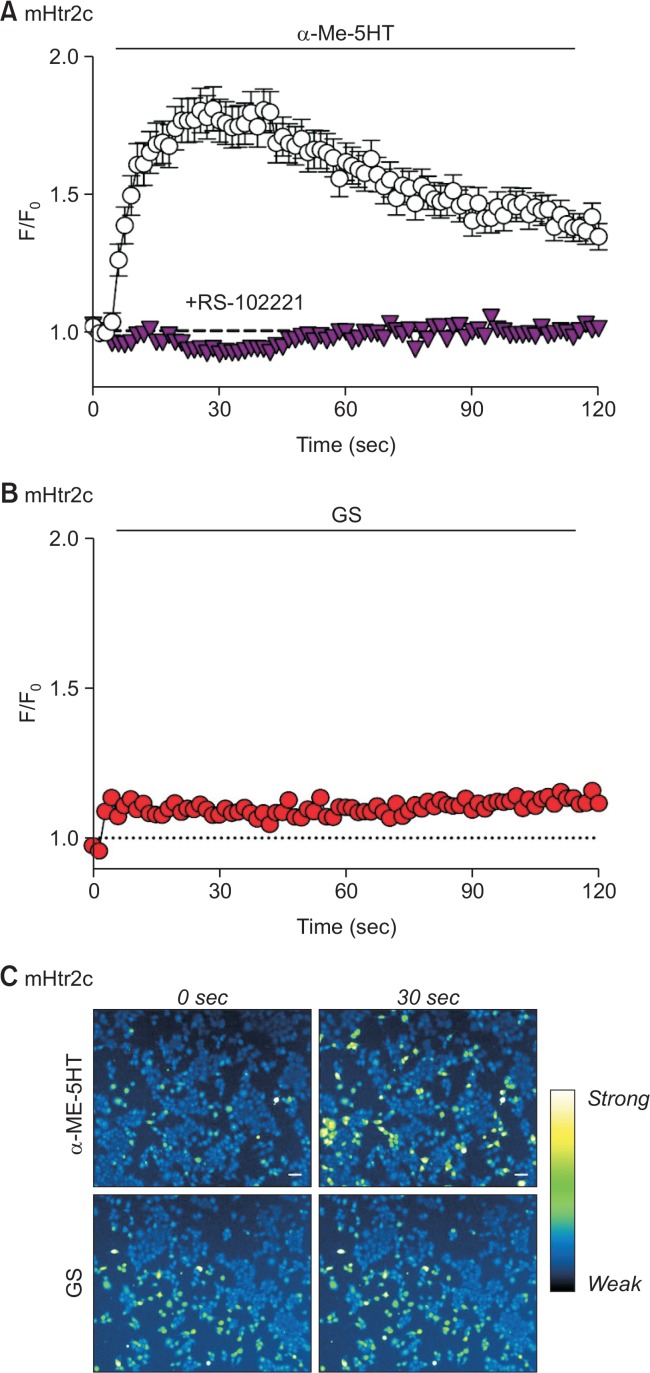
In order to verify possible GS-induced calcium increase on the last member of serotonin receptor 2 subfamily, experiments with mouse serotonin receptor 2c (mHtr2c) were performed as well. Likewise, mHtr2c expressed in HEK293T cells showed significant intracellular calcium increment upon treatment of 10 μM α-Me-5HT, and the responses were specifically inhibited by RS-102221, an inhibitor for mHtr2c (Fig. 3A). However, when 50 μM of GS was applied on mHtr2c, it was found that no remarkable responses have appeared (Fig. 3B, 3C). Moreover, 100 μM of GS also failed to evoke any intracellular calcium increment at all (data not shown). Thus, it was concluded that GS did not activate mHtr2c sufficiently, implying that mHtr2c may not be strongly involved in GS-induced responses.
Fig. 3.
GS failed to induce intracellular calcium increase through mHtr2c in HEK293T cells. (A) HEK293T cells transient express mHtr2c was treated with α-Me-5HT (10 μM). As a control, α-Me-5HT successfully induced an increase of intracellular calcium levels (empty circle), which is substantially inhibited by pretreatment of RS-102221 (500 nM for 5 min), a specific antagonist for Htr2c (inverted triangle). (B) GS (50 μM) failed to induce intracellular calcium increase in HEK293T cells expressing mHtr2c. (C) Representative pseudocolor microscopic images after treatment of α-Me-5HT or GS on mHtr2c-expressing cells at initial (0 sec) and peak (30 sec) time points. Scale bar indicates 50 μm.
GS acts on mHtr2a and mHtr2b to evoke calcium influx via PLC-dependent pathway
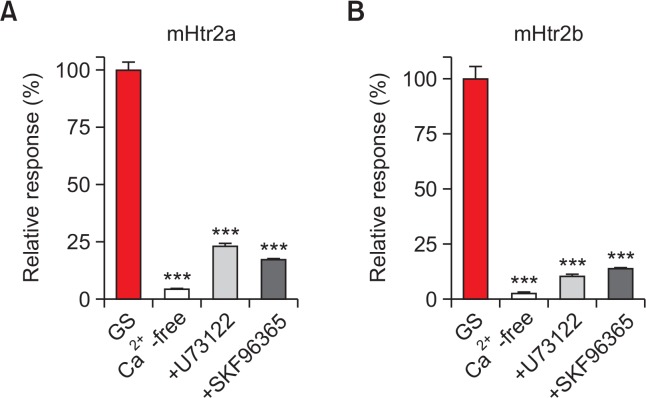
After finding out that GS induced intracellular calcium increment through activation of mHtr2a and mHtr2b, further investigation was performed to identify the underpinning mechanisms of calcium increment upon GS application. To verify the source of calcium increase, calcium-free media condition was prepared to eliminate extracellular calcium presence. As shown in Fig. 4, calcium-free condition failed to evoke any noticeable intracellular calcium increment by 100 μM of GS on both mHtr2a and mHtr2b, respectively. Thus, these data suggest that the GS-induced intracellular calcium increment was due to an influx from extracellular media, implying involvement of some calcium-permeable ion channels in HEK293T cells.
Fig. 4.
mHtr2a and mHtr2b may cause calcium influx through phospholipase C (PLC)-dependent TRPC activation after GS treatment. (A) An intracellular calcium increase through mHtr2a by GS in HEK293T cells (GS) was significantly suppressed by calcium-free media, U-73122 (an inhibitor of PLC), and SKF-96365 (a non-specific TRPC blocker). (B) Similar results for mHtr2b in HEK293T cells. ***p<0.001.
It is well known that serotonin receptor 2 subfamily is a member of GPCR, and its G alpha subunit falls into Gq/11 category (Bockaert et al., 2006). Thus, activation of serotonin receptor 2 subfamily will lead to phospholipase C (PLC) stimulation. To confirm this, pretreatment of U-73122, an inhibitor for PLC, was applied before GS treatment on either mHtr2a- or mHtr2b-expressing HEK293T cells. As expected, the pretreatment of U-73122 significantly abolished the GS-induced calcium influx on both mHtr2a and mHtr2b, respectively (Fig. 4).
Furthermore, pretreatment of SKF-96365, a blocker for non-specific TRPC (non-selective cation ion channel), has been pretreated before GS application. It turned out that GS-induced calcium influx via mHtr2a or mHtr2b was greatly diminished by SKF-96365 pretreatment, suggesting that the calcium influx might have been mediated through TRPC ion channels in HEK293T cells (Fig. 4).
In conclusion, the present study revealed for the first time that GS can stimulate mHtr2a and mHtr2b to induce calcium influx by utilizing PLC-dependent pathway, leading to TRPC activation in HEK293T cell line. Considering that GS is involved in certain itch sensation such as that of AD patients, the current finding may pave a way to elucidate the underlying molecular mechanisms of GS-induced itch pathways.
DISCUSSION
Glucosylsphingosine (GS) is an endogenous sphingolipid that has been well recognized for its involvement with Gaucher disease (Orvisky et al., 2000, 2002; Dekker et al., 2011; Rolfs et al., 2013). However, recent studies have also suggested that GS could play a role as a novel pruritogen (Kim et al., 2010). Indeed, the level of GS is elevated in the skin of AD patients (Ishibashi et al., 2003; Okamoto et al., 2003), and is able to exert itch-scratch responses when injected in mice (Kim et al., 2010). However, the detailed molecular mechanism of how GS can trigger these itch responses were remained elusive.
Itch is an unpleasant sensation that cause a desire to scratch, and it is transmitted through various itch pathways (Nakagawa and Hiura, 2013). Generally, sensation including itch occurs via stimulation of peripheral sensory neurons. Specifically, the signal triggered at the afferent fibers of dorsal root ganglia (DRG) is conveyed to spinal cord. Thereafter, the signal ascends to the thalamus, and arrives at the certain cerebral region for interpretation of the appropriate senses (Davidson and Giesler, 2010). The present study focused to mimic the environment of the very initial stages of itch signal transmission, where pruritogen acts on certain receptors and/or ion channels to trigger production of electrical signal. In this regard, the current study essentially suggests a possible mechanism how GS can initiate itch signal production with mHtr2a and/or mHtr2b.
Serotonin plays various role in our body, and mostly recognized as a neurotransmitter that is related to depression, anxiety, chronic pain, aggression and so on. More importantly, serotonin is also involved in itch. For instance, enhancing serotonergic tone by administered serotonin potentiated itch sensation, whereas mice lacking serotonin or serotonergic neurons in the brainstem exhibited significantly decreased scratching behavior (Zhao et al., 2014). Moreover, serotonin also induced scratching not only in healthy subjects but also in AD patients (Schworer et al., 1995; Schworer and Ramadori, 1995; Hosogi et al., 2006).
One of the interesting finding in the current study is that mHtr2a and mHtr2b, but not mHtr2c, can be activated by GS. Surprisingly, it is in agreement with previous finding that mHtr2a and mHtr2b are present in mouse DRG, but not mHtr2c at all (Nicholson et al., 2003; Lin et al., 2011). Thus, it strengthens an idea that mHtr2a and/or mHtr2b is required to transmit GS-induced itch, since DRG already holds the prerequisite receptors for GS. However, the lack of responses from mHtr2c upon GS treatment is still elusive. At the moment, it is difficult to speculate the reason why mHtr2c was not responsive to GS. One plausible reason is that the similarity of protein sequences among mHtr2 receptors. However, when protein sequence homology was verified in silico among three serotonin receptor 2 subfamily, it was found that mHtr2a is more closely similar to mHtr2c, rather than mHtr2b (data not shown). Thus, it implicates that the lack of GS-induced response from mHtr2c was not due to protein sequence difference. Another possible explanation would be that mHtr2c intrinsically has low sensitivity to α-Me-5HT, as shown in Fig. 3A, so that 100 μM of GS was not sufficient to evoke any noticeable response through less-sensitive mHtr2c. Further studies are definitely required, but it seems that mHtr2c is less likely to hold noticeable sensitivity to GS, unlike mHtr2a and mHtr2b.
Since GS is a sphingolipid, it can basically penetrate the plasma membrane, omitting the need for membrane receptors to exert further physiological signals in cells. However, the present study found that GS requires serotonin receptor 2 subfamily as a mediator to efficiently relay its signals. As serotonin receptor 2 subfamily are members of GPCR related to the class Gαq/11 (Bockaert et al., 2006), it is well known that activation of the receptor will lead to stimulation of the effector, PLC. Indeed, it was found that a PLC inhibitor U-73122 greatly inhibited the intracellular calcium increase after GS treatment in both mHtr2a and mHtr2b, supporting previous reports. PLC then catalyzes cleavage of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) into inositol (1,4,5) trisphosphate (IP3) and diacylglycerol (DAG). IP3 is known to increase intracellular calcium released from internal calcium store (store depletion) that may activate ion channels such as TRPC. TRPC ion channels are regarded as channels responsible for store-operated calcium entry (SOCE) (Salido et al., 2009). DAG, on the other hand, can also activate subset of TRPC such as TRPC3/6/7, without depleting calcium stores.
The observed intracellular calcium increment by GS through mHtr2a and mHtr2b could be either from extracellular environment or intracellular calcium stores. In order to clarify this, calcium-free external media experiments were performed, and it turned out that calcium-free condition almost completely abolished the GS-induced calcium increase, suggesting that the GS-induced calcium increase was an influx from external environment. Moreover, the actual calcium-permeable ion channel in the current experimental condition appears to be TRPC, since SKF-96365 − a non-specific TRPC blocker − greatly decreased the GS-induced calcium influx. Overall, it can be summarized that GS first stimulate mHtr2a/b, which then activate PLC to produce IP3 and DAG. Both IP3 and DAG can lead to opening of TRPC, which are endogenously expressed in HEK293T cells.
However, since SOCE is a complex series of event with various molecular players involved, it requires more in-depth study to fully identify details of the proposed mechanisms. As the present study is confined to HEK293T cells, and HEK293T cells have some endogenous TRPC channels (Zagranichnaya et al., 2005), the present finding should be interpreted in a way that the current experiment system is suitable for detecting GS-induced calcium influx, because of endogenous TRPC present in HEK293T cells.
Although the present study demonstrated a possible relationship between GS and serotonin receptor 2 subfamily, more experimental evidence is mandatory to fully support the claim. For instance, the current study is only performed in HEK293T cells in vitro. Moreover, verification in itch-mediating peripheral sensory neuron has not been finished, not to mention that itch-scratch in vivo behavior tests are missing. Although our team is in process of resolving these shortfalls, it should be mentioned that the current finding that GS acts on mHtr2a and mHtr2b is worth reporting in every aspect. Together with future experiments, we hope that our findings facilitate the molecular basis of GS-induced itch sensation, shedding a light to find out a way to alleviate GS-induced itch in AD patients. Considering that both GS and serotonin affect itch in AD patients, the present study raises a possibility that Htr2a and/or Htr2b could be regarded as candidate therapeutical target genes to alleviate itch in AD patients.
Acknowledgments
This work was supported by Gachon Institute of Pharmaceutical Sciences Research Fund 2015 (GCU-2015-5131) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016009938).
REFERENCES
- Aghahowa SE, Obianwu HO, Isah AO, Arhewoh IM. Chloroquine-induced pruritus. Indian J Pharm Sci. 2010;72:283–289. doi: 10.4103/0250-474X.70471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi AA, Oluokun A, Sofowora O, Akinleye A, Ajayi AT. Epidemiology of antimalarial-induced pruritus in Africans. Eur J Clin Pharmacol. 1989;37:539–540. doi: 10.1007/BF00558141. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker N, van Dussen L, Hollak CE, Overkleeft H, Scheij S, Ghauharali K, van Breemen MJ, Ferraz MJ, Groener JE, Maas M, Wijburg FA, Speijer D, Tylki-Szymanska A, Mistry PK, Boot RG, Aerts JM. Elevated plasma glucosylsphingosine in Gaucher disease: relation to phenotype, storage cell markers, and therapeutic response. Blood. 2011;118:e118–e127. doi: 10.1182/blood-2011-05-352971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Arikawa J, Okamoto R, Kawashima M, Takagi Y, Ohguchi K, Imokawa G. Abnormal expression of the novel epidermal enzyme, glucosylceramide deacylase, and the accumulation of its enzymatic reaction product, glucosylsphingosine, in the skin of patients with atopic dermatitis. Lab Invest. 2003;83:397–408. doi: 10.1097/01.LAB.0000059931.66821.92. [DOI] [PubMed] [Google Scholar]
- Jeong JY, Yim HS, Ryu JY, Lee HS, Lee JH, Seen DS, Kang SG. One-step sequence- and ligation-independent cloning as a rapid and versatile cloning method for functional genomics studies. Appl Environ Microbiol. 2012;78:5440–5443. doi: 10.1128/AEM.00844-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim KM, Noh M, Yoo HJ, Lee CH. Glucosylsphingosine induces itch-scratch responses in mice. Biomol. Ther. (Seoul) 2010;18:316–320. doi: 10.4062/biomolther.2010.18.3.316. [DOI] [Google Scholar]
- Lin SY, Chang WJ, Lin CS, Huang CY, Wang HF, Sun WH. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. J Neurosci. 2011;31:1410–1418. doi: 10.1523/JNEUROSCI.4682-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr Itch elicited by intradermal injection of serotonin, intracisternal injection of morphine, and their synergistic interactions in rats. Neuroscience. 2014;274:119–127. doi: 10.1016/j.neuroscience.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Hiura A. Four Possible Itching Pathways Related to the TRPV1 Channel, Histamine, PAR-2 and Serotonin. Malays. J Med Sci. 2013;20:5–12. [PMC free article] [PubMed] [Google Scholar]
- Nicholson R, Small J, Dixon AK, Spanswick D, Lee K. Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci Lett. 2003;337:119–122. doi: 10.1016/S0304-3940(02)01256-9. [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. 5-Hydroxytryptamine (5-HT)2 receptor involvement in acute 5-HT-evoked scratching but not in allergic pruritus induced by dinitrofluorobenzene in rats. J Pharmacol Exp Ther. 2003;306:245–252. doi: 10.1124/jpet.103.049239. [DOI] [PubMed] [Google Scholar]
- Okamoto R, Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Imokawa G. Sphingosylphosphorylcholine is upregulated in the stratum corneum of patients with atopic dermatitis. J Lipid Res. 2003;44:93–102. doi: 10.1194/jlr.M200225-JLR200. [DOI] [PubMed] [Google Scholar]
- Orvisky E, Park JK, LaMarca ME, Ginns EI, Martin BM, Tayebi N, Sidransky E. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. Mol Genet Metab. 2002;76:262–270. doi: 10.1016/S1096-7192(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Orvisky E, Sidransky E, McKinney CE, LaMarca ME, Samimi R, Krasnewich D, Martin BM, Ginns EI. Glucosylsphingosine accumulation in mice and patients with type 2 Gaucher disease begins early in gestation. Pediatr Res. 2000;48:233–237. doi: 10.1203/00006450-200008000-00018. [DOI] [PubMed] [Google Scholar]
- Papoiu AD, Tey HL, Coghill RC, Wang H, Yosipovitch G. Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch. PLoS ONE. 2011;6:e17786. doi: 10.1371/journal.pone.0017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs A, Giese AK, Grittner U, Mascher D, Elstein D, Zimran A, Bottcher T, Lukas J, Hubner R, Golnitz U, Rohle A, Dudesek A, Meyer W, Wittstock M, Mascher H. Glucosyl-sphingosine is a highly sensitive and specific biomarker for primary diagnostic and follow-up monitoring in Gaucher disease in a non-Jewish, Caucasian cohort of Gaucher disease patients. PLoS ONE. 2013;8:e79732. doi: 10.1371/journal.pone.0079732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salido GM, Sage SO, Rosado JA. TRPC channels and store-operated Ca2+ entry. Biochim. Biophys. Acta. 2009;1793:223–230. doi: 10.1016/j.bbamcr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Schworer H, Hartmann H, Ramadori G. Relief of cholestatic pruritus by a novel class of drugs: 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists: effectiveness of ondansetron. Pain. 1995;61:33–37. doi: 10.1016/0304-3959(94)00145-5. [DOI] [PubMed] [Google Scholar]
- Schworer H, Ramadori G. Cholestatic pruritus--pathophysiology and therapy with special reference to treatment with 5-hydroxytryptamine subtype 3 receptor antagonists. Z Gastroenterol. 1995;33:265–274. [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol. 2001;81:250–254. doi: 10.1080/00015550152572868. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35:77–83. doi: 10.1016/S0168-0102(99)00070-X. [DOI] [PubMed] [Google Scholar]
- Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Liu XY, Jeffry J, Karunarathne WK, Li JL, Munanairi A, Zhou XY, Li H, Sun YG, Wan L, Wu ZY, Kim S, Huo FQ, Mo P, Barry DM, Zhang CK, Kim JY, Gautam N, Renner KJ, Li YQ, Chen ZF. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron. 2014;84:821–834. doi: 10.1016/j.neuron.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]