Abstract
Excessive activation of microglia causes the continuous production of neurotoxic mediators, which further causes neuron degeneration. Therefore, inhibition of microglial activation is a possible target for the treatment of neurodegenerative disorders. Balanophonin, a natural neolignoid from Firmiana simplex, has been reported to have anti-inflammatory and anti-cancer effects. In this study, we aimed to evaluate the anti-neuroinflammatory effects and mechanism of balanophonin in lipopolysaccharide (LPS)-stimulated BV2 microglia cells. BV2 microglia cells were stimulated with LPS in the presence or absence of balanophonin. The results indicated that balanophonin reduced not only the LPS-mediated TLR4 activation but also the production of inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), Interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α), in BV2 cells. Balanophonin also inhibited LPS-induced inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX2) protein expression and mitogen activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK. Interestingly, it also inhibited neuronal cell death resulting from LPS-activated microglia by regulating cleaved caspase-3 and poly ADP ribose polymerase (PARP) cleavage in N2a cells. In conclusion, our data indicated that balanophonin may delay the progression of neuronal cell death by inhibiting microglial activation.
Keywords: Firmiana simplex, Neuroinflammation, Balanophonin, Microglia, Neuroprotection, Apoptosis
INTRODUCTION
Neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and ischemia, are related to chronic neuroinflammation (McGeer and McGeer, 1995, 1999, 2004; Campbell, 2004). Following the invasion of pathogens or injuries, the immune system responds by inducing inflammation. In acute conditions, this response defends tissue against pathogens and promotes healing; however, chronic inflammation may cause damage to host tissue (Kim and Joh, 2006). Microglia cells are key immune cells in the brain and are rapidly activated in emergency situations, such as brain injury or immunological conditions. The morphology of microglia after activation changes to a ramified state with extensively branched processes (Kreutzberg, 1996; Lynch, 2009). Chronically activated microglial cells secrete neurotoxic factors, such as nitric oxide (NO), various pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and prostaglandin E2 (PGE2), and chemokines, which are responsible for neuronal damage in the majority of cases (Minghetti and Levi, 1998; Hanada and Yoshimura, 2002; Block and Homg, 2005; Kaminska., 2005; Kim and Joh, 2006; da Fonseca et al., 2014).
Synthetic and natural products effectively regulate the neuroinflammatory cascade, either by inhibiting chronic microglial activation or by inhibiting activated microglial-induced neuronal death. For example, dextromethorphan inhibited microglial activation, thereby protecting dopaminergic neurons against inflammatory pathogenesis (Liu et al., 2003). In addition, non-steroidal anti-inflammatory drugs (NSAIDs) exhibited neuroprotective effects in transgenic animal models (Klegeris and McGeer, 2005). However, adverse effects, high cost, extensive time for new drug discovery, and other drawbacks of synthetic drugs make them second choices for both treatment and prevention of neuroinflammation and neurodegeneration. Numerous scientific studies indicated that anti-inflammatory herbal medicine reduced microglial activation, protected neuronal cells, and retarded the progression of neurodegenerative disease. For example, wogonin, a flavonoid from medicinal herbs, showed neuroprotective activity by inhibiting inflammatory activation of microglia (Lee et al., 2003). Curcumin (Lee et al., 2007; Yang et al., 2008), Apigenin (Ha et al., 2008), 6-gingerol (Tripathi et al., 2007), and 6-shogaol (Ha et al., 2012) also inhibited microglial activation and protected neurons. These results indicated that neurodegenerative diseases may be treatable by regulating the mechanism of inflammation related to microglial activation. Therefore, we investigated the anti-inflammatory effect of Balanophonin in vitro.
Lipopolysaccharide (LPS), a constituent of the outer membrane of gram-negative bacteria, is a compound that activates microglia and stimulates the neuroinflammatory pathway. In the case of bacterial invasion, LPS binds to CD14 and TLR4 to promote the inflammatory signaling pathway. LPS-activated microglial cells initiate the intracellular signaling pathway involving mitogen activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK. MAPKs, respectively, cause activation of transcription factors that regulate the expression of inducible NO synthase (iNOS), cyclooxygenase-2 (COX2), pro-inflammatory cytokines (e.g., TNF-α, IL-1β), and chemokines (Kaminska, 2005). Increased iNOS causes continuous production of a high level of NO and COX2 is an enzyme that produces PGE2. Therefore, increased expressions of iNOS and COX2 result in high production of NO and PGE2 in the medium. Accumulation of these pro-inflammatory cytokines amplifies the inflammatory response and causes damage to neurons (Minghetti and Levi, 1998; Murphy, 2000; Minghetti, 2004; St-Onge et al., 2007).
Damage to neurons by pro-inflammatory mediators may potentially cause neurodegenerative diseases. Therefore, many previous studies indicated that downregulation of microglial cell activation reduced neuron death and the possibility of the neurodegenerative diseases described above. In addition, microglial activation and function may play a more important role in mediating these diseases than in protecting neurons (Liu and Hong, 2003; Block and Hong, 2005). Therefore, it is important to explore a potential agent that downregulates microglial activation and provides an alternative explanation for the neuroprotective effects of potential candidates in various neurodegenerative disease models.
Several studies have suggested that inhibition of microglial activation was an effective strategy for treating inflammation-related neuronal diseases. Many previous studies reported the therapeutic potential of anti-inflammatory agents for the prevention and treatment of neurodegenerative disease accompanied by microglia activation. Dextromethorphan was effective in protecting dopaminergic neurons against inflammatory pathogenesis by inhibiting microglial activation (Liu et al., 2003). NSAIDs had a neuroprotective effect in transgenic animal models (Klegeris and McGeer, 2005). In addition, active novel compounds from natural products might be potential anti-neuroinflammatory agents. Numerous scientific studies indicated that anti-inflammatory herbal medicine reduced microglial activation, protected neuronal cells, and retarded the progression of neurodegenerative disease (Lee et al., 2003, 2007; Ha et al., 2012).
Based on our previous research using the natural product library, we selected Firmiana simplex as a potential candidate for an anti-inflammatory agent. Firmiana simplex (F. simplex), commonly called the Chinese parasol tree, is a deciduous tree in Korea in the Sterculiaeae family. This plant is very common in Asia, especially in Korea and China (Woo et al., 2016). It has large leaves and stems and is a popular ornamental tree in North America (Zhang et al., 2013). Extracts from this tree have been used as traditional medicines to alleviate stomach disorders and diarrhea in Korea (Son et al., 2005). Although this plant is common, its constituents have rarely been studied. According to recent studies, F. simplex contains hepatoprotective constituents in the stem bark (Kim et al., 2015), neolignans with antipsychotic effects (Son et al., 2005), and cytotoxic triterpenes effective against cancer cells (Woo et al., 2015). In addition, activated Firmiana simplex Leaf (FSL) has effects on the removal of Cd(II) from aqueous solution (Li et al, 2009; Tang et al., 2010).
Interestingly, in a previous study, we found bioactive lignan derivatives in the CHCl3-soluble fraction of the 80% MeOH extract of the stems of F. simplex. Among 19 compounds, our compound, named Balanophonin, strongly inhibited NO production without cell death in LPS-activated BV2 cells (Woo et al., 2016).
Therefore, the present study was designed to validate the anti-inflammatory and neuroprotective effects of Balanophonin in vitro. To evaluate the anti-inflammatory effects of Balanophonin, we used BV2 and neuro2a (N2a) neuroblastoma cells as representative cells for microglia and neurons, respectively. The LPS-activated microglial BV2 cell line was treated with Balanophonin. In addition, we treated the N2a neuroblastoma cells with an LPS-activated microglia-conditioned medium to evaluate the neuroprotective effect resulting from suppression of microglial activation.
MATERIALS AND METHODS
Reagents
Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), and penicillin streptomycin (PS) were obtained from Invitrogen (Carlsbad, CA, USA). LPS was purchased from Sigma (St. Louis, MO, USA). NG-Monomethyl-L-arginine mono-acetate salt (L-NMMA) was obtained from Abcam (Cambridge, MA, USA). ELISA kits for TNF-α and IL-1β was purchased from R&D Systems (Minneapolis, MN, USA). Antibodies for Western blot were as purchased from various companies. MAPK proteins (p-38, p-p38, ERK, p-ERK, JNK, p-JNK), iNOS and cleaved caspase-3 (c-caspase-3) were purchased from Cell Signaling (Beverly, MA, USA). Additionally, COX-2, Bax and Bcl-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Tubulin was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Plant derived compound
Balanophonin, one of the bioactive compounds from the stem of F. simplex, was provided by the Natural Products Laboratory, School of Pharmacy, Sungkyunkwan University, Suwon, Korea (Woo et al., 2016). Balanophonin was mixed with DMSO and prepared the 20 μM stock solution.
Cell culture
BV2, the immortalized mouse microglial cell line, originally developed by Dr. V. Bocchini at the University of Perugia (Perugia, Italy), was provided by Dr. E. Choi at Korea University (Seoul, Korea). These cells have functional and phenotypic characteristics of reactive microglia. Mouse N2a, a murine neuroblastoma cell line, was obtained from the American Type Culture Collection (Manassas, VA, USA). BV2 and N2a cells were maintained in DMEM supplemented with 10% FBS and 1% PS at 37°C in a humidified incubator with 5% CO2.
Cell activation
BV2 cells were seeded at a density of 4×105 cells/well and N2a cells seeded at 5×105 cells/well in DMEM. The cells were then incubated for 24 h at 37°C. After 24 h cell seeding, cells were treated with Balanophonin for 30 min before LPS administration. 100 ng/mL of Lipopolysaccharides (LPS) was treated to the same plate treated with compounds except to the untreated control group. Plate was designed as untreated control, LPS only treated, LPS and compound treated group. DMEM with 2% FBS and 1% PS was used in treating the Balanophonin. LPS (Lipopolysaccharide from Escherichia Coli 055; B5) obtained from Sigma Aldrich (Cat No; L2637) was dissolved in PBS to make the desired stock solution and stored in refrigerator for prolong use.
Measurement of cell viability in BV2 cells
Cell viability was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. BV2 cells were seeded at a density of 4×105 cells/well in a 96-well plate. After incubation for 24 h, cells were treated with 1, 5, and 10 μM of Balanophonin for 30 min before LPS (100 ng/mL) administration, and then incubated for 24 h at 37°C. In addition, BV2 cells were treated with 10, 20 μM of L-NMMA as a positive control.
Measurement of NO production
Nitrite (NO2), a soluble oxidation product of NO, was measured in culture medium using the Gries reaction. BV2 cells were treated as above (section 2.5) and the culture medium was mixed with Gries reagent (0.1%N-napthyl-ethylene-diamine dihydrochloride and 1% sulfanilamide in 5% phosphoric acid). NaNO2, the standard compound, was used to calculate the concentration of NO. Absorbance was detected at 570 nm.
Western blot analysis
BV2 cells were seeded in 6-well plates at a density of 4×105 cells/well, incubated for 24 h at 37°C, and treated with Balanophonin (1, 5, and 10 μM) and LPS for various times (30 min for MAPKs, 6 h for iNOS and COX2, and 24h for apoptosis related proteins). The cells were harvested and proteins were extracted with Radio immunoprecipitation assay (RIPA) lysis solution. Protein samples were loaded and separated at 8–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk for 1h and incubated overnight with primary antibodies for ERK, p-ERK, JNK, p-JNK, p38, p-p38, iNOS, COX2, Bax, Bcl-2, c-caspase-3, caspase 3, poly ADP ribose polymerase (PARP), and tubulin at 4°C. Secondary antibodies were then applied for 1 h. Detection was performed with enhanced chemiluminescence (ECL) Western Blotting Detection Reagents. Analysis of band density was measured by Image Lab™ Software, Version 5.0 (Bio-Rad, Hercules, CA, USA).
Measurement of PGE2, TNF-α and IL-1β
BV2 cells were seeded in 6-well plates at a density of 4×105 cells/well and incubated for 24 h. Thereafter, cells were pretreated with balanophonin at concentrations of 1 to 10 μM for 30 min before stimulation with LPS (100 ng/mL) for 24 h. Culture medium was used to measure the inhibitory effect of Balanophonin on the production of PGE2, TNF-α and IL-1β. PGE2 was measured using a competitive enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI, USA). TNF-α and IL-1β were measured using an ELISA development kit (R&D Systems).
Measurement of cell viability, neurite outgrowth and neurite length measurement assay in N2a cells
The N2a cell line was used to measure neuronal cell viability in conditioned media. To make the conditioned media, BV2 cells were pretreated with balanophonin at concentrations of 1 to 10 μM and activated with LPS. After 24 h of treatment, the conditioned medium was transferred to N2a cells. Cell viability was measured by MTT assay. Similarly treated N2a cells were placed in to the IncuCyte ZOOM imaging system (Essen Instruments, Ann Arbor, MI, USA) and the pictures were captured after 24 h of CM treatment and neurite length was also measured at the 2 h of regular interval.
Statistical analysis
The data was analyzed using GraphPad PRISM 5 (GraphPad Software Inc., La Jolla, CA, USA). All results were expressed as mean ± SD. Statistical differences between experimental groups were measured using one-way ANOVA with Tukey’s multiple comparison tests.
RESULTS
Effect of balanophonin on viability of and NO production in BV2 cells
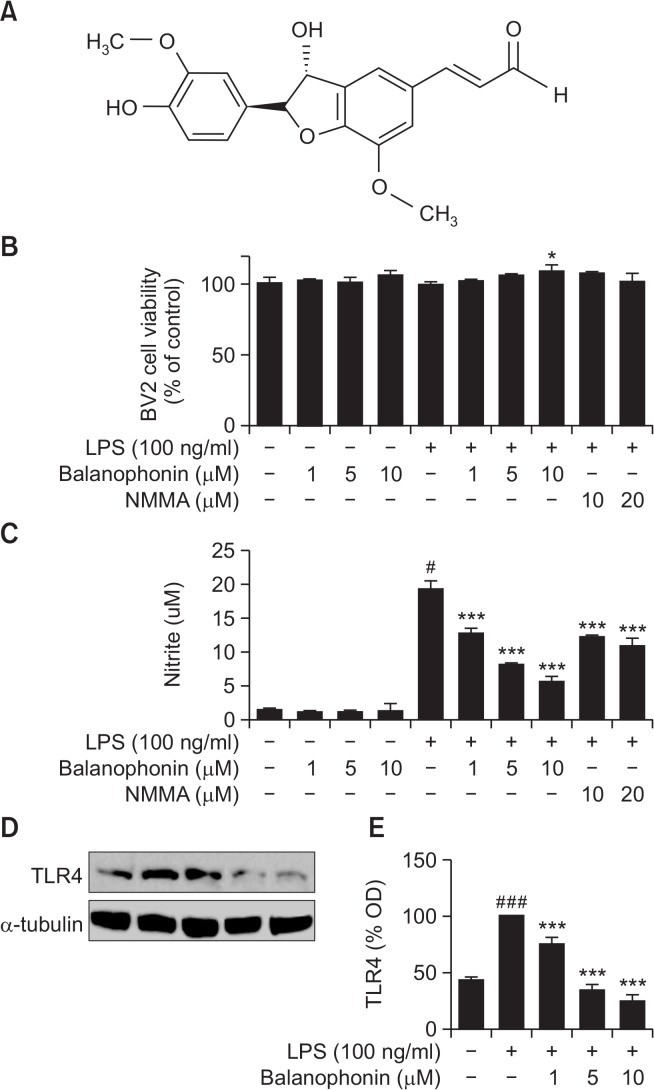
The structure of balanophonin is shown in Fig. 1A. Balanophonin is a bioactive compound extracted from the one of the CHCl3-soluble fractions of the stem of F. simplex (Woo et al., 2016). To evaluate the cytotoxicity of this compound, we measured the cell viability of LPS-activated microglial cells and performed an MTT assay using the mouse microglial cell line, BV2, in vitro. There was no change in cell viability in any group compared to that observed for control cells (Fig. 1B). To evaluate the effect of balanophonin on NO production, BV2 cells were treated with various concentrations of balanophonin (1, 5, and 10 μM) in the presence or absence of LPS (100 ng/mL). Balanophonin reduced NO production in LPS-activated BV2 cells at all concentrations (1, 5, and 10 μM) compared to that of LPS-only treated group. The relative production of NO at concentrations of 1, 5, and 10 μM of balanophonin was 66 ± 4.31%, 42 ± 0.56%, and 29 ± 4.16% of that of the LPSonly treated group respectively. L-NMMA, the positive control and an iNOS inhibitor, produced NO at 63 ± 1.01% (10 μM of NMMA) and 56 ± 6.23% (20 μM of NMMA) of that of the LPS-only treated group (Fig. 1C). As LPS is Toll like receptor 4 (TLR4) agonist, we performed the experiment to check whether it alter some changes in receptor activation or not. Interestingly, balanophonin start to show its effect even from the receptor activation via downregulating it. It is clearly shown in (Fig. 1D, 1E).
Fig. 1.
Effects of balanophonin on the viability of and NO production in microglial cell line BV2. Structure of balanophonin. BV2 cells were treated with 1, 5, and 10 μM of balanophonin for 30 min before being induced by LPS and then incubated for 24 h. The concentration of LPS was 100 ng/mL. (A) Structure of balanophonin. (B) Effect of balanophonin on cell viability. Cell viability was assessed using MTT assay. (C) Effect of balanophonin on nitrite production. Production of nitrite in the culture media was measured by Griess reaction. (D) Effect of balanophonin on the TLR4 inactivation. (E) Densitometric analysis of TLR4 as percentage of LPS treated group. α-Tubulin was used as a loading control. Each value is presented as mean ± SD of at least three independent experiments. *p<0.05, ***p<0.001 indicate statistically significant differences, respectively, compared with LPS alone-treated group and #p<0.05, ###p<0.001 indicate statistically significant while comparing with untreated control group.
Inhibitory effect of balanophonin on production of pro-inflammatory factors in BV2 cells
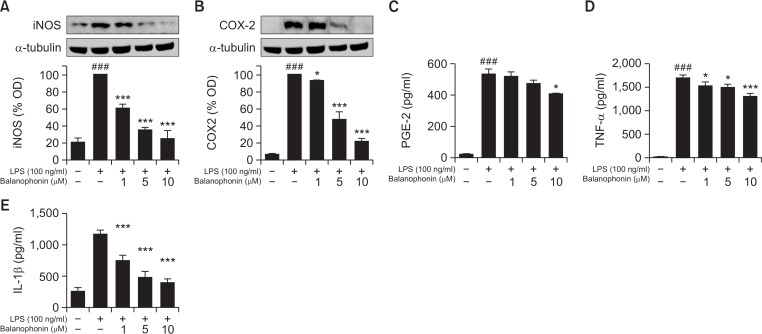
LPS-activated microglial cells produced various pro-inflammatory factors, including iNOS, COX2, TNF-α, and IL-1β. These factors affect the inflammatory signaling pathway. Since iNOS is closely related to NO production and COX2 is an enzyme that produces PGE2, Western blot analysis was performed to measure the expression levels of iNOS and COX2. BV2 cells were pretreated with balanophonin for 30 min and stimulated with LPS for 6 h. To measure cytokine levels, BV2 cells were treated for 24 h in the presence or absence of LPS (100 ng/mL) and evaluated using respective competitive ELISA kit. As shown in Fig. 2, compared to the control group, the LPS-only treated group had high levels of iNOS, COX2, PGE2, TNF-α, and IL-1β. However, iNOS decreased in a concentration-dependent manner in the balanophonin-treated groups (1–10 μM). There was a statistically significant change in iNOS expression in LPS-activated BV2 cells treated even with 1, 5 and 10 μM of Balanophonin with 60 ± 4.62, 35 ± 3.30 and 24 ± 9.89% respectively (Fig. 2A). Similar pattern of result was observed in COX2 expression too. COX-2 expression was decreased in a concentration-dependent manner and the change (47 ± 9.59 and 21 ± 3.94% of that expressed in the LPS-only treated group) highly significant at the 5 and 10 μM Balanophonin treatment (Fig. 2B). PGE2 levels were not significant at 1 and 5 μM but it’s significantly reduced with 10 μM Balanophonin treatments supporting the fact of reduced COX-2 (Fig. 2C). In addition, we performed an experiment to find the role of balanophonin to inhibit the pro-inflammatory cytokine production. Balanophonin significantly reduced the secretion of TNF-α and IL-1β in the BV2 cells where inhibition of TNF-α levels seems lesser than that of IL-1β in concentration dependent manner (Fig. 2D). However, TNF-α levels decreased by two thirds in the group treated with 20 μM of Balanophonin and one-third in the group treated with 50 μM of Balanophonin (data not shown). Balanophonin at concentrations of 1, 5, and 10 μM promisingly reduced IL-1β secretion in a concentration-dependent manner (65 ± 7.76, 45 ± 8.28, and 38 ± 4.96% of that of the LPS-only treated group) (Fig. 2E).
Fig. 2.
Effects of balanophonin on iNOS and COX2 protein expression and TNF-α and IL-6 production in LPS-induced microglial cells. (A) Effect of balanophonin on the production of iNOS. (B) Effect of balanophonin on the production of COX2. α-Tubulin was used as a loading control. (C) Effect of balanophonin on PGE-2 production in BV2. (D) Effect of balanophonin on TNF-α production in BV2. (E) Effect of balanophonin on IL-1β production in BV2. Each value is presented as mean ± SD of at least three independent experiments. *p<0.05, and ***p<0.001 indicate statistically significant differences, respectively, compared with LPS alone-treated group and ###p<0.001 indicate statistically significant while comparing with untreated control group.
Effect of balanophonin on activation of MAPKs
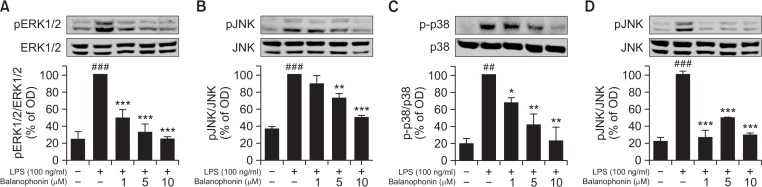
Many reports suggest that MAPKs are important factors in the inflammatory response (Kaminska, 2005). Phosphorylation of ERK1/2, JNK, and p38 plays a key role in their signaling cascade and activates transcription factors. As shown in Fig. 3, Balanophonin effectively inhibited MAPK activation. In activated BV2 cells, balanophonin decreased the phosphorylation of MAPKs such as pERK, pJNK, and p-p38 at all concentrations (Fig. 3) in concentration dependent manner. In particular, p-p38, pERK significantly decreased after treatment with balanophonin even at lower concentration (1–10 μM). However, balanophonin seems equally potent to inhibit the phosphorylation of all factors at the higher concentration (10 μM) (Fig. 3B). As increased pJNK through activated microglia is directly related to neuronal apoptosis, via induced pro-inflammatory cytokines and other inflammatory factors. We compared the potency of balanophonin with well-known JNK inhibitor SP600125 using Western blot analysis. Balanophonin showed almost equally potency to inhibit the JNK phosphorylation as that of SP600125 (Fig. 3D). We further evaluated the effect of balanophonin in activated microglia-induced neuronal apoptosis.
Fig. 3.
Effect of balanophonin on MAPK expression in LPS-activated BV-2 cells. (A) Effect of balanophonin on the production of pERK1/2. (B) Effect of balanophonin on the production of pJNK. (C) Effect of balanophonin on the production of p-p38. (D) Effect of balanophonin and SP600125 on the Phosphorylation of JNK. α-Tubulin was used as a loading control. Each value is presented as mean ± SD of at least three independent experiments. *p<0.05, **p<0.01 and ***p<0.001 indicate statistically significant differences respectively, when compared with LPS alone-treated group and ##p<0.01, ###p<0.001 indicate statistically significant while comparing with untreated control group.
Effect of balanophonin on expression of survival-related factors, neurite outgrowth and neurite length in N2a cells against activated microglia-induced toxicity to neuron
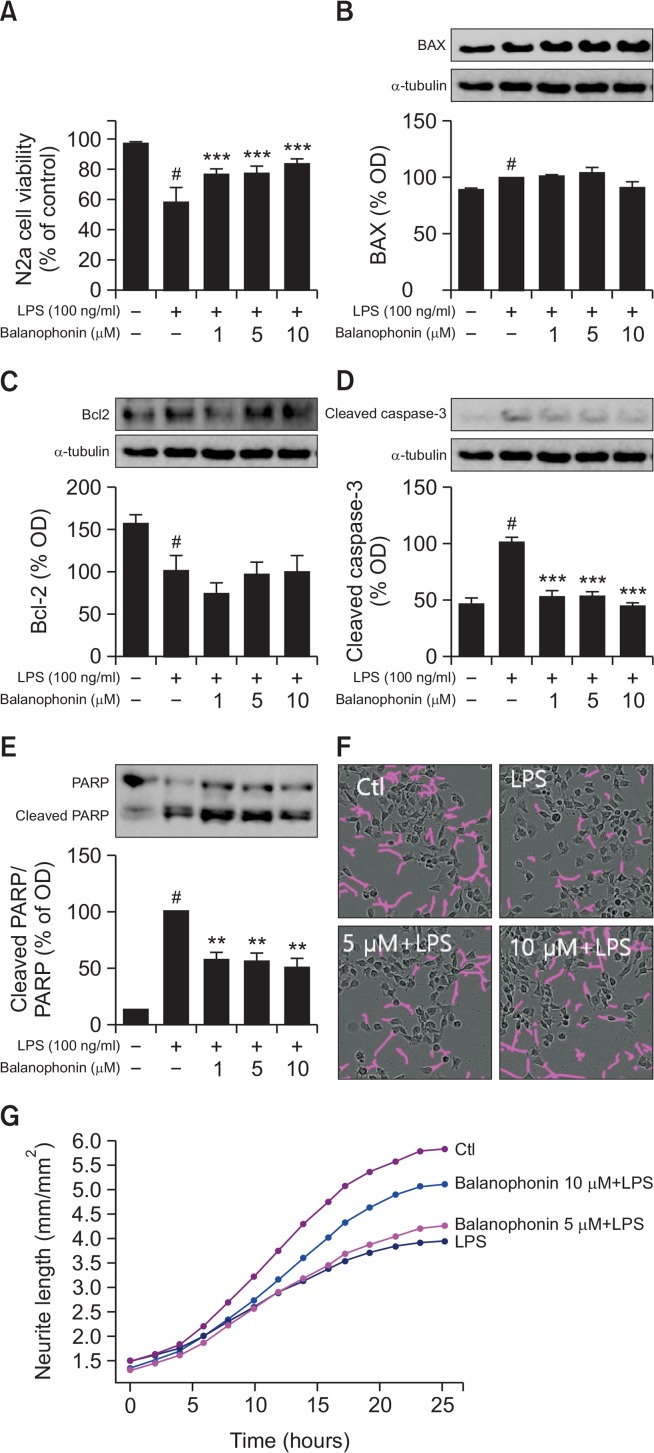
Apoptosis is a key pathway in cell survival. We used neuroblastoma N2a cells as a representative cell for neurons. Basically in the normal brain microglia, neuron and astrocytes lives together for the basic function of brain however in the laboratory experiments we performed experiments in separate cells. so in order to know the role of activated microglia to the normal neurons, first BV2 cells were activated with LPS for 24 h followed by the balanophonin treatment and the treated cell supernatant was transferred to the normal neuron cells as N2a cells so that we could demonstrate that activated microglia release inflammatory mediators and pro-inflammatory cytokines in the medium that is responsible for the neuronal death and neurodegeneration. To evaluate the effect of balanophonin on N2a cell survival by inhibiting the activation of microglia, BV2 cells were treated with balanophonin with or without LPS (100 ng/mL). After 24 h of BV2 treatment, N2a cells were incubated with the previously treated BV2 cultured medium for another 24 h. Immediately after transferring CM to N2a cells, we measured neurite length in every 2 h interval. Balanophonin significantly increased the neurite outgrowth and neurite length against LPS induced toxicity to neuron. To measure cell viability, N2a cells were treated in the same way and evaluated with an MTT assay. Similarly, N2a cells were harvested and evaluated by Western blot. Four apoptotic factors were evaluated with Western blot: Bax, Bcl-2, c-caspase-3, and PARP cleavage. Compared with the control group, the LPS-only treated group exhibited decreased cell viability and increased apoptotic cascades. The Balanophonin-treated groups exhibited increased cell viability in a concentration-dependent manner; the change was significant at 10 μM (Fig. 4A). Balanophonin had no effect on Bax production in N2a cells (Fig. 4B). Bcl-2, an anti-apoptotic protein, slightly increased in a concentrationdependent manner, but the change was not significant (Fig. 4C). 10 μM balanophonin pretreatment effectively inhibited c-caspase-3 (to 44 ± 2.71% of that of the LPS-only treated group) in N2a cells that were cultured in conditioned medium (Fig. 4D). PARP is inactivated by activated caspase-3 cleavage, after which, neurons disappear by the process of apoptosis (Sairanen et al., 2009). Balanophonin inhibited caspase-3 activation and PARP cleavage (Fig. 4E).
Fig. 4.
Neuroprotective effect of balanophonin in N2a cells against cytotoxicity of LPS-activated BV2 cells. (A) Effect of balanophonin on N2a cell viability. (B) Expression level of Bax. (C) Expression level of Bcl-2. (D) Expression level of c-caspase-3. (E) Expression level of PARP and cleaved-PARP. α-Tubulin was used as a loading control. (F) N2a cell morphology with or without Balanophonin treatment. Purple lines represents the neurite outgrowth. (G) Neurite length measurement graph. Each value is presented as mean ± SD of at least three independent experiments. **p<0.01, and ***p<0.001 indicate statistically significant differences, respectively, compared with LPS alone-treated group and #p<0.05 indicate statistically significant while comparing with untreated control group.
DISCUSSION
In our previous study, we found promising bioactive lignan compounds from the stems of F. simplex. Among 19 compounds, balanophonin showed an outstanding inhibitory effect on NO production without cytotoxicity. The IC50 value of Balanophonin was 7.07 μM and the viability was 115% for LPS-activated BV2 cells in comparison to that of the positive control, L-NMMA (IC50 value 16.27 μM Cell viability 98.2%), indicating that balanophonin was less cytotoxic and had a greater inhibitory effect on NO production (Woo et al., 2016). Because of the potent effect of balanophonin, in this study, we investigated its detailed mechanism in relation to neuroinflammation and neuronal death. Cell viability was not different between balanophonin-only treated groups (1, 5, and 10 μM) and control groups, indicating that balanophonin was not cytotoxic. In the present study, after treatment with 10 μM of Balanophonin, cell viability and inhibition of NO production were far better than that after treatment with L-NMMA as a positive control. Balanophonin concentration dependently increased the cell viability against LPS treatment in BV-2 cells. Cell viability at 10 μM was significant to that of LPS treated group. Additionally, IC50 value of balanophonin was half of that of L-NMMA. This result signified that balanophonin more potently inhibited NO production than the positive control, L-NMMA, did and was not cytotoxic (Fig. 1). As LPS is TLR4 receptor agonist, it will activate the TLR4 and that will further induce the MAPK signaling for further neuroinflammatory cascades. Hence we evaluated the expression of TLR4 after LPS and balanophonin treatment. Interestingly, LPS activates almost 5 times higher than untreated control and that was reversed by the balanophonin treatment. Higher concentration of Balanophonin (5, 10 μM) brings TLR4 almost back to the limit as that of untreated control. When inflammatory responses occur in the brain, a series of molecular processes starts with microglial activation. iNOS and COX2 are highly expressed only in inflammatory sequences. NO production and release of pro-inflammatory cytokines, such as TNF-α, IL-1β, and PGE2, also increase and are complicating factors in neuroinflammation (Minghetti and Levi, 1998; Hanada and Yoshimura, 2002). iNOS-induced NO plays a key role in various signaling pathways and leads to neuronal cell death from excessive production (Murphy, 2000). Because Balanophonin significantly inhibited the NO production, induced by the LPS-activated microglia, we further investigated the expression of iNOS in the same environment. Balanophonin downregulated iNOS expression in a concentration dependent manner (Fig. 2A), supporting the fact that balanophonin inhibited NO release was through the decreased iNOS expression. COX2, a rate-limiting enzyme for the synthesis of PGE2, is overexpressed while an inflammatory response is in process and is a key parameter in the neurotoxic inflammatory pathway (Minghetti, 2004; St-Onge et al., 2007). Recently, a study reported that selective COX2 inhibitors, such as celecoxib, delayed neurodegenerative diseases by inhibiting inflammatory responses (Sánchez-Pernaute et al., 2004). Therefore, we also evaluated COX2 expression and found that it was downregulated after treatment with Balanophonin in BV2 cells. Significant inhibition of PGE2 with 10 μM of balanophonin treatment further support the potency of balanophonin to reduce the expression of COX-2 and followed by the decreased PGE2 production in the environment (Fig. 2B, 2C). Furthermore, TNF-α and IL-1β are also important pro-inflammatory cytokines in the inflammatory pathway, we evaluated the concentration of these cytokines in Balanophonin and LPS-treated BV2 cell-cultured medium. Balanophonin treatment concentration dependently downregulated the production of TNF-α in the media however this effect is significant only at 10 μM balanophonin treatment but not in 1 and 5 μM. Additionally, higher concentrations (20 μM, data not shown) of balanophonin reduced TNF-α production almost 40% than that of only LPS-treated group. Balanophonin showed the potency to decrease the secretion of IL-1β even with low concentrations of Balanophonin (Fig. 2E). This indicated that balanophonin-mediated anti-inflammatory effects might be through the significant decrease of pro-inflammatory cytokines, such as TNF-α, IL-1β, and inflammatory mediators, such as NO, iNOS, COX2 and PGE2.
Signaling pathways play important roles in the mechanism of anti-inflammatory compounds. They mediate the signaling through which the transcription of inflammatory proteins takes place, which in turn further induces the inflammatory cascades. In this study, we also investigated the MAPK signaling pathway in LPS-activated microglia in the presence or absence of balanophonin. ERK1/2, JNK, and p38 MAPK play key roles in the signal transduction cascade and help activate transcription factors such as Nuclear factor-κB (NF-κB) (Kaminska, 2005). As a result, iNOS, COX2, and pro-inflammatory cytokines, such as TNF-α and IL-1β, are overexpressed and NO and PGE2 synthesis increases (Hanada and Yoshimura, 2002; Kim et al., 2004; Xie et al., 2004; Waetzig et al., 2005). In this study, balanophonin inhibited the phosphorylation of MAPK proteins in activated BV2 cells. Compared with the LPS-only treated group, treatment with balanophonin reduced the expression of pERK1/2, pJNK, and p-p38. Downregulation of ERK, p38 and JNK seems to be promising and significant even at the concentration of 1–10 μM (Fig. 3). This result indicated that the anti-inflammatory effects of balanophonin occurred primarily by inhibition of pERK and p-p38 in the MAPK pathway. However, phosphorylation of JNK plays an important role in activated microglia induced neurodegeneration and neuronal apoptosis. Hence we target the JNK inhibitory activity of balanophonin for further mechanism and we compare the pJNK inhibitory activity of balanophonin together with well-known JNK inhibitor SP600125 with same concentration. Surprisingly, balanophonin showed similar potency to inhibit the JNK phosphorylation as that of standard JNK inhibitor SP600125. Balanophonin at 5 μM shows a similar potency to inhibit the JNK phosphorylation than 10 μM of JNK inhibitor compound while 10 μM of Balanophonin showed similar potency to that of SP600125. Our data support the fact that Balanophonin might be a potential candidate to inhibit the phosphorylation of JNK, ERK and P38. Because increased pJNK through activated microglia is directly related to neuronal apoptosis (Robitaille et al., 2008; Tu et al., 2011) and our compound effectively reduced pJNK, we further evaluated the effect of balanophonin in activated microglia-induced neuronal apoptosis.
Activated microglial cells produce and release neurotoxic and pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6, NO, PGE2, and numerous chemokines). Those cytokines and chemokines produced by activated microglia can induce neuronal damage (Block and Hong, 2005; Kaminska, 2005; Kim and Joh, 2006). In this study, balanophonin-treated and LPS-activated microglial conditioned medium was transferred to N2a cells in order to evaluate neuronal apoptosis in the LPS-only treated group. Neuronal death was decreased in the Balanophonin-treated group. Interestingly, we found that Balanophonin inhibited neuronal cell death and improved cell survival by decreasing microglial activation (Fig. 4A). In case of inflammation-related neuron degeneration induced with microglial activation, neurons went through the apoptotic process. In particular, the activation of JNK precedes neuronal cell death by inflammation and apoptosis (Cao et al., 2004). In the apoptotic process, anti-apoptotic proteins, such as Bcl-2, are inhibited and activated Bax complex makes channels through which cytochrome c passes from the inside to the outside of mitochondria. A group of cytochrome c and caspase 9 proteins makes an apoptosome, which cleaves pro-caspase 3 (Burguillos et al., 2011). Thereafter, caspase-3 is activated, which increases PARP cleavage. PARP is enzymatic machinery involved in single-strand DNA breakage repair. After PARP is inactivated by caspase cleavage, neurons disappear by the process of apoptosis (Sairanen et al., 2009). In order to determine the effect of balanophonin on neuron cell protection, we evaluated the expression of apoptotic proteins. Our data indicated that balanophonin inhibited caspase-3 activation and PARP cleavage (Fig. 4D, 4E). Balanophonin decreased neuronal degeneration by inhibiting the neuronal apoptotic pathway via reducing microglial activation and pro-inflammatory-cytokine release. characterized by increased neurite outgrowth and neurite length against LPS activated microglia mediated toxicity to N2a cells (Fig. 4F, 4G). Additionally, we have shown the neurite outgrowth picture of N2a cells after CM treatment. Formation or breakdown of neurite length is the normal process. So some of the broken neurite outgrowth are not coupled with cells. Furthermore, we have also measured the neurite length of the cell against LPS treated group. Moreover, our data showed that balanophonin might significantly protect neurons against neurotoxicity induced by LPS-activated microglia (Fig. 5).
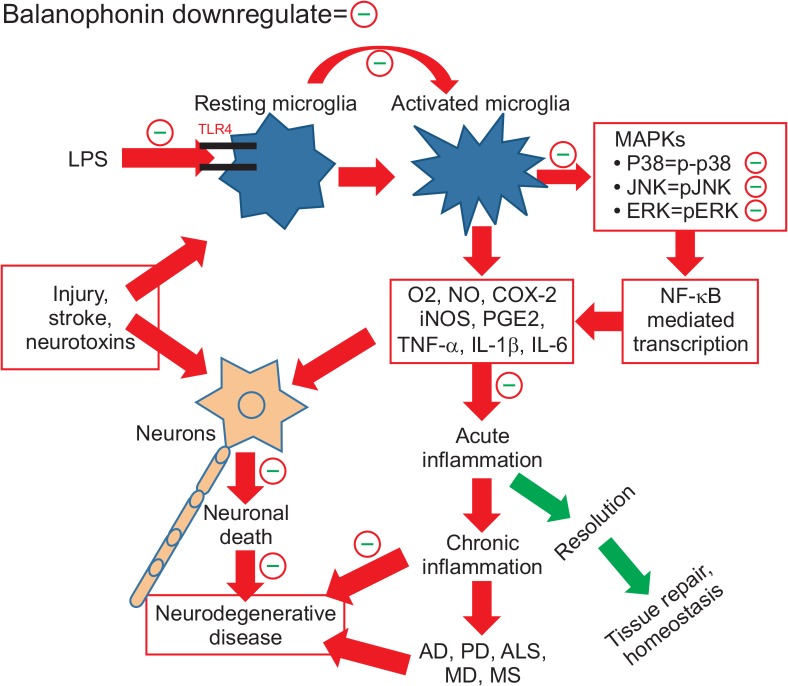
Fig. 5.
Graphical summary of the balanophonin mediated antineuroinflammatory and neuroprotective effect in LPS activated microglia and activated microglia induced neuronal death.
In summary, our investigation revealed that balanophonin attenuated neuronal death by inhibiting microglial activation. In detail, balanophonin reduced iNOS and COX2 expression levels and down regulated the MAPK (EKR1/2, JNK, and p38 MAPK) pathway, pJNK in particular. Additionally, balanophonin inhibited NO production and pro-inflammatory cytokines, such as TNF-α and IL-1β. Inhibition of microglial activation by treatment with balanophonin increased neuronal cell survival by reducing caspase-3 activation and PARP inactivation compared with the LPS-only treated group. It was further confirmed by the increased neurite outgrowth and neurite length of N2a cells with pretreatment of balanophonin against LPS. F. simplex is a traditional medicinal plant found in Asia, particularly in Korea and China, and has potential biological activity against human ailments. Balanophonin, extracted from F. simplex, is a natural product worthy of further study to learn more about its biological effects. In this study, we evaluated balanophonin as a promising compound for the treatment of neuro-inflammation related diseases by inhibiting microglial activation and neurodegeneration via inhibiting activated microglia-induced apoptosis. Therefore, balanophonin could be a good candidate for treating neurodegenerative diseases and the further study of its structure derivative will be needed to develop it as a new drug.
Acknowledgments
This work was supported by the grant of Gachon University, Fund from Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (grant no. HI14C1135) and by iPET (Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries), Ministry of Agriculture, Food and Rural Affairs (NO. 114006-04).
REFERENCES
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- Campbell A. Inflammation, neurodegenerative diseases, and environmental exposures. Ann N Y Acad Sci. 2004;1035:117–132. doi: 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- Cao J, Semenova MM, Solovyan VT, Han J, Coffey ET, Courtney MJ. Distinct requirements for p38α and c-Jun N-terminal kinase stress-activated protein kinases in different forms of apoptotic neuronal death. J Biol Chem. 2004;279:35903–35913. doi: 10.1074/jbc.M402353200. [DOI] [PubMed] [Google Scholar]
- da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int. 2008;52:878–886. doi: 10.1016/j.neuint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh MS, Kim SY. 6-Shogaol, a ginger product, modulates neuroinflammation: a new approach to neuroprotection. Neuropharmacology. 2012;63:211–223. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–421. doi: 10.1016/S1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kim JW, Yang H, Cho N, Kim B, Kim YC, Sung SH. Hepatoprotective constituents of Firmiana simplex stem bark against ethanol insult to primary rat hepatocytes. Pharmacogn Mag. 2015;11:55–60. doi: 10.4103/0973-1296.149704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1β production. Neurobiol. Aging. 2004;25:431–439. doi: 10.1016/S0197-4580(03)00126-X. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res. 2005;2:355–365. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, Cho GJ, Choi WS, Suk K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003;17:1943–1944. doi: 10.1096/fj.03-0057fje. [DOI] [PubMed] [Google Scholar]
- Lee HS, Jung KK, Cho JY, Rhee MH, Hong S, Kwon M, Kim SH, Kang SY. Neuroprotective effect of curcumin is mainly mediated by blockade of microglial cell activation. Pharmazie. 2007;62:937–942. [PubMed] [Google Scholar]
- Li Z, Tang X, Chen Y, Wei L, Wang Y. Activation of firmiana simplex leaf and the enhanced Pb(II) adsorption performance: equilibrium and kinetic studies. J Hazard Mater. 2009;169:386–394. doi: 10.1016/j.jhazmat.2009.03.108. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, Li G, Zhang W, An L, Liu B, Hong JS. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J Pharmacol Exp Ther. 2003;305:212–218. doi: 10.1124/jpet.102.043166. [DOI] [PubMed] [Google Scholar]
- Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40:139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation of the brain in Alzheimer’s disease: implications for therapy. J Leukoc Biol. 1999;65:409–415. doi: 10.1002/jlb.65.4.409. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:S3–S7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol. 1998;54:99–125. doi: 10.1016/S0301-0082(97)00052-X. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- Murphy S. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 2000;29:1–13. doi: 10.1002/(SICI)1098-1136(20000101)29:1<1::AID-GLIA1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Rankine EL, Hughes PM, Botham MS, Perry VH, Felton LM. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. Eur J Neurosci. 2006;24:77–86. doi: 10.1111/j.1460-9568.2006.04891.x. [DOI] [PubMed] [Google Scholar]
- Robitaille K, Daviau A, Lachance G, Couture JP, Blouin R. Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ. 2008;15:1522–1531. doi: 10.1038/cdd.2008.77. [DOI] [PubMed] [Google Scholar]
- Sairanen T, Szepesi R, Karjalainen-Lindsberg ML, Saksi J, Paetau A, Lindsberg PJ. Neuronal caspase-3 and PARP-1 correlate differentially with apoptosis and necrosis in ischemic human stroke. Acta Neuropathol. 2009;118:541–552. doi: 10.1007/s00401-009-0559-3. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson’s disease. J. Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YK, Lee MH, Han YN. A new antipsychotic effective neolignan from firmiana simplex. Arch Pharm Res. 2005;28:34–38. doi: 10.1007/BF02975132. [DOI] [PubMed] [Google Scholar]
- St-Onge M, Flamand N, Biarc J, Picard S, Bouchard L, Dussault AA, Laflamme C, James MJ, Caughey GE, Cleland LG, Borgeat P, Pouliot M. Characterization of prostaglandin E2 generation through the cyclooxygenase (COX)-2 pathway in human neutrophils. Biochim. Biophys. Acta. 2007;1771:1235–1245. doi: 10.1016/j.bbalip.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Tang X, Hu M, Li Z, Chen Y, Lou P. Removal of Cd(II) from aqueous solution with activated Firmiana Simplex Leaf: behaviors and affecting factors. J Hazard Mater. 2010;179:95–103. doi: 10.1016/j.jhazmat.2010.02.062. [DOI] [PubMed] [Google Scholar]
- Tripathi S, Maier KG, Bruch D, Kittur DS. Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J Surg Res. 2007;138:209–213. doi: 10.1016/j.jss.2006.07.051. [DOI] [PubMed] [Google Scholar]
- Tu YF, Tsai YS, Wang LW, Wu HC, Huang CC, Ho CJ. Overweight worsens apoptosis, neuroinflammation and blood-brain barrier damage after hypoxic ischemia in neonatal brain through JNK hyperactivation. J. Neuroinflammation. 2011;8:40. doi: 10.1186/1742-2094-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch UR. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia. 2005;50:235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- Woo KW, Choi SU, Kim KH, Lee KR. Ursane saponins from the stems of Firmiana simplex and their cytotoxic activity. J Braz Chem Soc. 2015;26:1450–1456. [Google Scholar]
- Woo KW, Suh WS, Subedi L, Kim SY, Kim A, Lee KR. Bioactive lignan derivatives from the stems of Firmiana simplex. Bioorg Med Chem Lett. 2016;26:730–733. doi: 10.1016/j.bmcl.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45:170–179. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang D, Yang Z, Hu X, Qian S, Liu J, Wilson B, Block M, Hong JS. Curcumin protects dopaminergic neuron against LPS induced neurotoxicity in primary rat neuron/glia culture. Neurochem Res. 2008;33:2044–2053. doi: 10.1007/s11064-008-9675-z. [DOI] [PubMed] [Google Scholar]
- Zhang L-X, Hu Q-H, Wang C-B. Emergency evaluation of environmental sustainability of poultry farming that produces products with organic claims on the outskirts of mega-cities in China. Ecol Eng. 2013;54:128–135. doi: 10.1016/j.ecoleng.2013.01.030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.