Abstract
Increasing concern is being given to the association between risk of cancer and exposure to low-dose bisphenol A (BPA), especially in young-aged population. In this study, we investigated the effects of repeated oral treatment of low to high dose BPA in juvenile Sprague-Dawley rats. Exposing juvenile rats to BPA (0, 0.5, 5, 50, and 250 mg/kg oral gavage) from post-natal day 9 for 90 days resulted in higher food intakes and increased body weights in biphasic dose-effect relationship. Male mammary glands were atrophied at high dose, which coincided with sexual pre-maturation of females. Notably, proliferative changes with altered cell foci and focal inflammation were observed around bile ducts in the liver of all BPA-dosed groups in males, which achieved statistical significance from 0.5 mg/kg (ANOVA, Dunnett’s test, p<0.05). Toxicokinetic analysis revealed that systemic exposure to BPA was greater at early age (e.g., 210-fold in Cmax, and 26-fold in AUC at 50 mg/kg in male on day 1 over day 90) and in females (e.g., 4-fold in Cmax and 1.6-fold in AUC at 50 mg/kg vs. male on day 1), which might have stemmed from either age- or gender-dependent differences in metabolic capacity. These results may serve as evidence for the association between risk of cancer and exposure to low-dose BPA, especially in young children, as well as for varying toxicity of xenobiotics in different age and gender groups.
Keywords: Bisphenol A, Toxicokinetics, Bile duct proliferation, Juvenile animals
INTRODUCTION
Bisphenol A (BPA, 4,4′-(propane-2,2-diyl)diphenol), a major component of epoxy resins and polycarbonate plastics, is broadly used in the manufacture of plastic wares, coating materials for food and beverage containers, and thermal receipt papers (Le et al., 2008; Swedenborg et al., 2009; Goodman et al., 2017). Average daily exposure of adult to BPA was estimated to fall in the range of 0.1 to 5 μg/kg body weight per day (Vandenberg et al., 2013), which would persist throughout the whole life. Indeed, BPA was detected in the urine in most adults in US and Korea (>90%) with the average concentration of 1.28–2.64 μg/L (Calafat et al., 2008; Vandenberg et al., 2010; Miyaguchi et al., 2015; Park et al., 2016).
Generally, the toxicity of BPA is considered rather weak which was based on conventional toxicity assessments in rodents. US Environment Protection Agent determined NOAEL of BPA at 50 mg/kg/day while European Union set it at 5 mg/kg/day. However, concerns have been raised on the endocrine disrupting effects and the potential carcinogenesis from exposure to low-dose BPA, which generally do not follow monotonic dose response relationship; therefore, effects at lower doses could not be neglected. Indeed, BPA is known to induce cellular alterations associated with proliferation and carcinogenicity through interacting with multiple hormone receptors (Zoeller et al., 2005; vom Saal et al., 2007; Vogel, 2009; Kwintkiewicz et al., 2010; Sui et al., 2012), and genetic/epigenetic toxicity (Tsutsui et al., 1998; Iso et al., 2006; Izzotti et al., 2009). Due to this, the safety of BPA on young children is questioned since these populations are extremely sensitive to endocrine disruption and at a risk of higher exposure due to widespread use of plastic nursing wares, toys and the inappropriate behaviors like pica and suckling (Edginton and Ritter, 2009).
Many researches have been undertaken to investigate the toxicity of BPA on juvenile populations and demonstrated potential harmful effects of BPA exposure on neuromuscular system (Jones et al., 2016), central nerve system and behavior (Rebuli et al., 2014, 2015), adipogenicity and energy metabolism (Rönn et al., 2013), heart (Ljunggren et al., 2016), immune responses (Ménard et al., 2014), endocrine system (Ahmed, 2016), the development of reproductive organs (Delclos et al., 2014) and liver (Xia et al., 2014). In most of these studies, however, bona fide signs of carcinogenicity could not be shown for low doses of BPA. Doses of BPA adopted in these studies ranged from 2.5 to 5,000 μg/kg bw/day, which are most probably designed to cover doses lower than 5 mg/kg, the most stringent NOAEL in EU (Park et al., 2016), and at the same time, the highest human exposure level reported; 5 μg/kg and current TDI, 50 μg/kg. At these low dose exposure scenarios, only minimal signs of weight gains and metabolic abnormality (Marmugi et al., 2012), increased hepatocyte apoptosis (Xia et al., 2014), behavioral changes, and neurological alterations were significant enough for observation. To elucidate the risk of low-dose exposure to BPA in young children, we considered it crucial to adopt extended dosing periods starting from early ages with toxicokinetic profiles of BPA over maturation to adult, to address functional maturation of liver.
Herein, BPA was repeatedly administered to juvenile SD rats from post-natal day 6 through their adulthood through toxicokinetic examinations. To this end, we adopted a sensitive analytical tool, LC/MSMS (limit of detection about 0.5 ppb), and immunohistochemical analysis with Ki-67 antibody to closely examine the signs of proliferation of liver to provide a novel insight for understanding the potential adverse health effects of BPA on young children.
MATERIALS AND METHODS
Materials
Bisphenol A (BPA, CAS 80-05-7) was purchased from Sigma-Aldrich (St. Louis, CA, USA). Dose formulations were prepared on the day of dosing with 0.3% carboxyl methylcellulose for oral gavage administration as appropriate. LC-MS grade water (Tedia, NJ, USA), acetonitrile (Burdick & Jackson, MI, USA), and ammonium acetate (Sigma-Aldrich) were used in the mobile phase of LC system.
Animals and husbandry
F0 generation male and female Sprague-Dawley (Crl:CD) rats were obtained from Orient Bio Inc (Gyeonggi, Korea). For obtaining pregnant animals, two females were placed in the cage with one male overnight. F1 generation male and female pups were selected on postnatal day (PND) 4. All procedures were in compliance with Animal Welfare Act and Guide for the Care and Use of Laboratory Animals (by ILAR publication) and assessed by the Institutional Animal Care and Use Committee (IACUC) of KIT (The approval number is 1605-0157). The animal room was maintained in a controlled environment (temperature: 23 ± 3°C, humidity: 30–70%, light cycle: 12-hour light/12-hour dark cycle, light intensity: 150–300 Lux, air changes: 10 to 20 times/hour). A standard rodent pellet diet (Lab Diet® #5053 PMI Nutrition International, USA; irradiated by gamma-ray) and filtered, ultraviolet light-irradiated municipal tap water were provided to the animals ad libitum.
Animal experiments
PND 4 rats were considered to be equivalent to preterm human babies (Bowman et al., 2011) and the PND 7 are believed to be similar to humans at birth (Beck et al., 2006). On PND 4, each pups are cross-fostered between litters such that each litter is comprised of pups originating from at least 6 different litters, with no litter-mates of the same sex, and containing no pups from the original litter. Pups were assigned to toxicology and toxicokinetic groups. Male and female pups were administered with BPA by oral gavage once daily at dose level of 0, 0.5, 5, 50 and 250 mg/kg/day from PND 6 to 96 for 13-week. Parameters including mortality, clinical sign, body weight, food consumption, sexual maturation, organ weight, clinical pathology, macro- and microscopic findings were examined. For treatment groups, approximately 0.5 mL of blood were serially taken form caudal vein and put into blood collecting tubes containing EDTA-2K before (0), and at 0.25, 0.5, 1, 2, 4, 8 and 24 hours after administration of BPA on day 1 and day 91, respectively. Serum was collected and then stored in −80°C prior to analysis. Liver tissues were fixed in formalin, embedded in paraffin, and undergone section to stain with H&E.
Ki-67 immunohistochemistry and quantitative analysis
For examination of bile duct cell proliferation capacity, we processed immunohistochemical staining for Ki-67 as described previously (Kim et al., 2016). To perform immunohistochemistry, samples were fixed in 4% paraformaldehyde and then embedded in paraffin. The sections were deparaffinized and rehydrated sequentially. Antigen retrieval was carried out using pH 6.0 Target Retrieval solution (DAKO, Carpinteria, CA, USA) in a pressure cooker for 15 min. After cooling for more than 1 h on ice, the sections were incubated in 3% H2O2 for 30 min to block endogeneous peroxidase activity. The sections were washed twice with PBS and incubated with Protein block serum-free (DAKO) for 2 h at 25°C to reduce non-specific signal. Sections were incubated overnight at 4°C with primary antibody specific for Ki-67 (abcam, Cambridge, UK) at a 1:200 dilution. After washing three times with PBS, the sections were incubated for 15 min with HRP-conjugated rabbit secondary antibody (DAKO) at 25°C. For immunohistochemistry, DAB (DAKO) was used for antibody development and Mayer’s Hematoxylin (DAKO) was used for counter staining.
For quantitative analysis, all bile duct area in each group samples was captured using Olympus BX43 microscope 40× lens (Olympups Optical Co., Tokyo, Japan). Using the Image J (National Institutes of Health, USA), we counted total cell of each group bile duct and calculated the percentages of Ki-67 positive cells, respectively.
Toxicokinetic analysis
The BPA calibration standards were prepared firstly by making 300 ppm stock solution of the substance in methanol. Then, the list of standards was prepared by spiking blank rat serum and acetonitrile to obtain standards ranging from 0.5 ppb to 100 ppm. The standards then centrifuged at 14,000 × g for 5 min at 4°C, and later the supernatant was used.
Fifty microliter aliquot of samples were treated with acetonitrile (1:4, v/v), and centrifuged at 14,000 × g for 5 minutes at 4°C in order to separate protein (Johnson et al., 2010). BPA was separated using Agilent 1290 Ultra Performance Liquid Chromatography with Thermo Hypersil GOLD aQ 100 mm×2.1 mm column (1.9 μm particle size), and two mobile phases: 1 mM ammonium acetate in water (A) and acetonitrile (B) in isocratic elution. Injection volume was 3 μL at a flow rate of 0.3 mL/min and a column temperature of 50°C. BPA was detected and quantified using Agilent 6490 triple quad (QQQ) LC/MS multiple reaction monitoring (MRM) mode in the negative ionization, showing that the patterns of ion dissociation matched with standards (Buscher et al., 2015; Wilczewska et al., 2016). The parent and daughter m/z for BPA detection and quantification in this study were 227.10 and 211.95, respectively at retention time of 1.4 min. LOD and LOQ were obtained with the value of 0.55 ppb and 1.68 ppb, respectively.
For obtaining pharmacokinetic parameters (Cmax, Tmax and area under curve), non-compartment model was assumed and calculated with calculated with WINNONLIN ver. 5.2 (Pharsight, Sunnyvale, CA, USA) as described previously (Noh et al., 2016).
Statistics
Statistical analyses for comparisons of the various dose groups with the vehicle control group were conducted using Pristima System (Version 6.4, Xybion Medical Systems Corporation, USA). Difference from control (0 mg/kg) group was examined using Student t-test. Multiple comparison tests for different dose groups were conducted. Variance of homogeneity was examined using the Bartlett’s Test. Homogeneous data was analyzed using the Analysis of Variance (ANOVA) and the significance of inter-group differences were analyzed using Dunnett’s Test. Heterogeneous data was analyzed using Kruskal-Wallis Test and the significance of inter-group differences between the control and treated groups were assessed using Dunn’s Rank Sum Test.
RESULTS
Effects of oral treatment of BPA on body weight change and food consumption
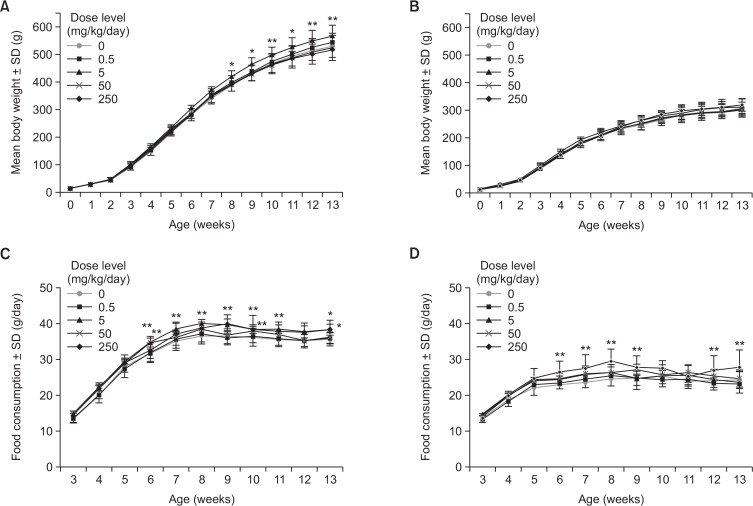
Increased body weight and food consumption were noted in 5 mg/kg male group from 8 weeks of age which persisted until the end of treatment (13 weeks, Fig. 1A, 1B). Interestingly, other dose groups (0.5, 50 and 250 mg/kg) in male and all dose groups in female did not showed any effects on body weights. However, increased food consumption was noted sporadically across 50 and 250 mg/kg BPA in males and consistently in 250 mg/kg BPA in female (Fig. 1C, 1D).
Fig. 1.
Body weight and food consumption of juvenile rats exposed to BPA for 90 days. Body weight of male (A) and female (B) rats orally exposed to BPA at 0, 0.5, 5, 50 and 250 mg/kg/day. Food consumptions of male (C) and female (D) rats. Data are mean ± SD of 10 animals. *p<0.05, **p<0.01, Student t-test for the difference from 0 mg/kg/day.
Effects of oral treatment of BPA on hematology and blood chemistry
Hematology revealed that white blood cell (WBC) and absolute lymphocyte (LYM) were increased at 50 and 250 mg/kg in both sexes. Additionally, relative LYM was elevated at 250 mg/kg in males (Supplementary Table 1). Absolute monocyte (MON) was increased at dose higher than 5 mg/kg in females. Absolute and relative large unstained cells (LUC) were increased at 5 mg/kg in males, and absolute LUC were increased at 50 and 250 mg/kg in females. Activated partial thromboplastin time (APTT) was decreased at dose higher than 50 mg/kg in males and 5 mg/kg in females. Blood chemistry showed that creatinine (CRE) was decreased at doses higher than 0.5 mg/kg in male (Supplementary Table 2). Albumin/globulin (A/G) ratio was decreased at doses higher than 50 mg/kg in male and 5 mg/kg in female, respectively. Total bilirubin (TBIL) was increased at 0.5, 50 and 250 mg/kg in males, and at doses higher than 5 mg/kg in female. At 250 mg/kg group, increased γ-glutamyl transpeptidase (GGT) and Alkaline phosphatase (ALP) were observed in males and females, respectively.
Other statistically significant changes noted in dosing groups compared with the control were not considered BPA-related, as these values were within an acceptable range for biological variation.
Effects of oral treatment of BPA on the sexual maturation and organ weights
To examine the effects of BPA on sexual maturation of male and female juvenile rats, prepuptial separation and vaginal opening time were evaluated respectively (Supplementary Table 3). Female exposed to high dose BPA (250 mg/kg) showed premature vaginal opening (30.4 ± 0.52 day vs 32.2 ± 1.75, p<0.01), alluding the pro-estrogenic effects of BPA. The body weight at the time of vaginal opening was significantly lighter in the BPA (250 mg/kg) exposed group, alluding the premature sexual development.
Measurement of absolute (Supplementary Table 4) and relative organ weights (Supplementary Table 5) revealed that the lung of male rats dosed with 50 and 250 mg/kg BPA showed increased weights.
Histopathological examination of the liver of rats exposed to BPA
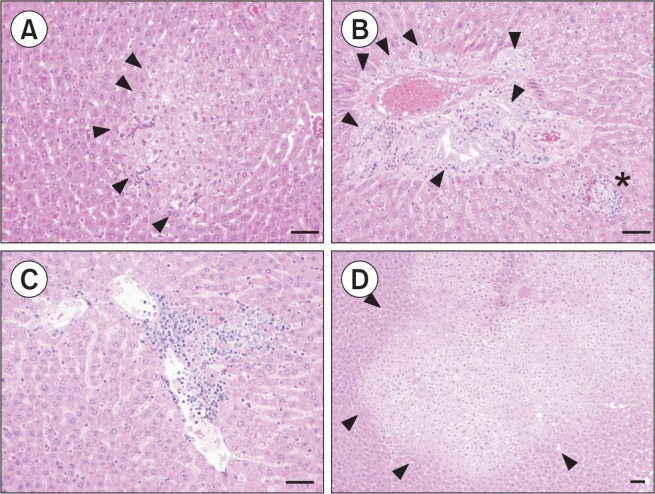
To analyze the characteristics of BPA effects in the liver, morphological changes were examined for male animals by a pathologist (Table 1). Fig. 2 summarizes representative pathologic findings present in the 0.5 mg/kg BPA treated group showing altered cell foci, bile duct hyperplasia and persistent focal inflammatory cell infiltration. As shown in Fig. 2, the eosinophilic altered cell foci were observed in BPA 0.5 mg/kg and 5 mg/kg male group (Fig. 2A, 2D, arrow head). 0.5 mg/kg group also showed bile duct hyperplasia (Fig. 2B, arrow head) with focal inflammatory cell infiltration (Fig. 2B, asterisk). The bile duct hyperplasia in the BPA treated group showed distinct pathological features that the proliferative epithelial cell islets of bile duct significantly extended into the portal triad mesenchyma without lymphocyte infiltration (Fig. 2B, arrow head). Multi focal lymphocytic inflammatory cells infiltration was also observed in liver parenchyma (Fig. 2C). Fig. 2D shows that eosinophilic altered cell foci graded two plus in 5 mg/kg group (arrow head).
Table 1.
Histopathological findings and grading of the liver of males
| Dose level (mg/kg/day) | 0 | 0.5 | 5 | 50 | 250 |
|---|---|---|---|---|---|
| No. of animals observed | 5 | 5 | 5 | 5 | 5 |
| Altered cell foci | + (0/5) | + (1/5) | + (0/5) | + (1/5) | + (2/5) |
| ++ (0/5) | ++ (1/5) | ++ (2/5) | ++ (2/5) | ++ (1/5) | |
| +++ (0/5) | +++ (0/5) | +++ (0/5) | +++ (1/5) | +++ (2/5) | |
| Bile duct hyperplasia | + (0/5) | + (3/5) | + (1/5) | + (1/5) | + (0/5) |
| ++ (0/5) | ++ (1/5) | ++ (2/5) | ++ (2/5) | ++ (2/5) | |
| +++ (0/5) | +++ (1/5) | +++ (2/5) | +++ (2/5) | +++ (3/5) | |
| Focal inflammation | + (0/5) | + (2/5) | + (2/5) | + (4/5) | + (2/5) |
| ++ (0/5) | ++ (0/5) | ++ (3/5) | ++ (0/5) | ++ (2/5) | |
| +++ (0/5) | +++ (0/5) | +++ (0/5) | +++ (1/5) | +++ (1/5) |
( ): Number of animals, Affected animals/Total examined animals.
Fig. 2.
Histopatholgical findings in the liver of BPA-treated juvenile rats. BPA 0.5 mg/kg: Photomicrograph of the eosinophilic altered cell foci (arrow head) with grade + (A), BPA 0.5 mg/kg: Photomicrograph of minimal bile duct hyperplasia (arrow head) with lymphocytic cell infiltration (asterisk) with grade ++ (B). BPA 0.5 mg/kg: Focal inflammatory cell infiltration with grade + (C). BPA 5 mg/kg: Photomicrograph of the eosinophilic altered cell foci (arrow head) with grade ++ (D). (Bar=50 μm).
Systemic exposure to BPA after oral treatment of BPA on day 1 and day 91
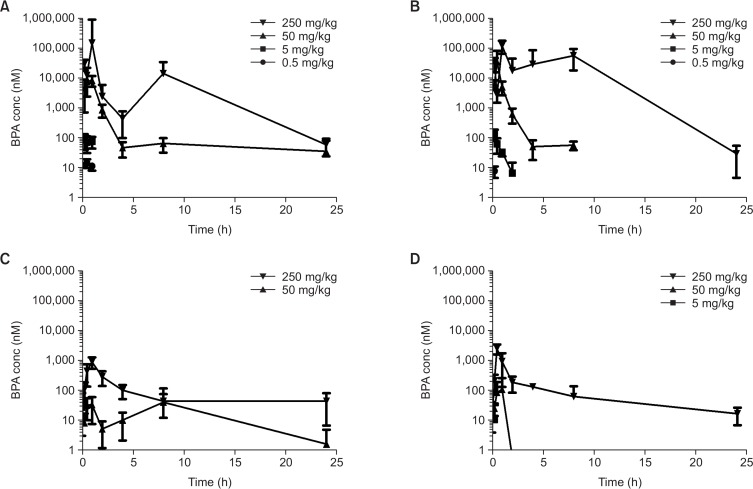
To examine the toxicokinetic behavior of BPA in juvenile rats, population pharmacokinetics was applied since full-time coursed sampling is impossible in juvenile rats due to limited blood volume. For each time point over 0 to 24 h after administration, 4 plasma samples were collected from 4 animals. During day 1 administration, each experimental group has similar BPA concentration-time curve pattern, with Tmax obtained mostly within 1 h after administration (Fig. 3A, 3B). Second peaks were observed in high dose groups (250 mg/kg male and female), indicating the entero-hepatic recirculation of BPA. Area under curve (AUC) and Cmax exhibited non-linear (supra-linear) dose-response relationship (Table 2), suggesting that clearance pathways might be easily saturated at high doses. In gender-wise comparison, female rats showed higher BPA concentrations and exposure.
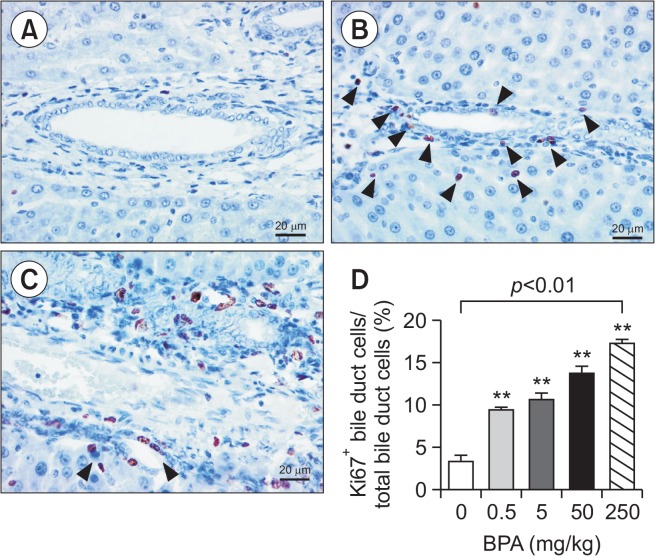
Fig. 3.
Bile duct proliferation in the liver of BPA-treated juvenile rats. Photomicrograph of the immunohistochemistry for Ki-67 to evaluate bile duct proliferation, BPA 0 mg/kg (A), 0.5 mg/kg with arrow heads indicating Ki-67+ cells (B) and 250 mg/kg (C). Ratio of Ki-67+ bile duct cells over total bile duct cells (mean ± SEM of counting in 7–45 sections). **p<0.01 One-way ANOVA followed by Dunnett’s post hoc analysis.
Table 2.
Toxicokinetic parameters
| Dose level (mg/kg/day) | 0.5 | 5 | 50 | 250 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Day 1 | Day 91 | Day 1 | Day 91 | Day 1 | Day 91 | Day 1 | Day 91 | |
| Male | ||||||||
| Tmax (Tmax1/Tmax2, h) | 0.5 | - | 0.25 | - | 1 | 1/8 | 1/8 | 1 |
| Cmax (Cmax1/Cmax2, nM) | 14.7 | - | 86.6 | - | 8387.8 | 33.3/40.0 | 146,219.7/14,286.8 | 883.2 |
| Area under curve (AUC0-t, nmole*h/L) | 17.1 | - | 105.8 | - | 12,834.2 | 493.8 | 269,943.3 | 2,369.4 |
| Female | ||||||||
| Tmax (h) | 0.25 | - | 0.25 | 0.25 | 0.5 | 1 | 1/8 | 0.5 |
| Cmax (nmole/L, nM) | 7.5 | - | 130.0 | 10.9 | 34,635.6 | 108.0 | 121,141.4/56,091.0 | 2445.9 |
| Area under curve (AUC0-t, nmole*h/L) | 1.9 | - | 89.3 | 4.4 | 19,961.1 | 123.0 | 773,664.0 | 3,088.5 |
Parameters were calculated with average concentrations of each time point.
Similar patterns but with greatly reduced levels of BPA were observed on day 91 (Fig. 3C, 3D). On day 91, BPA was not detectable in 0.5 and 5 mg/kg in male and 0.5 mg/kg in female, reflecting that clearance might be considerably increased during maturation into adulthood.
DISCUSSION
This study demonstrated that BPA, when administered to juvenile rats for 90 days even at doses as low as 0.5 mg/kg/day, induces altered cell foci, bile duct hyperplasia, and persistent focal inflammatory cell infiltration in the liver, providing a strong evidence that risk of cancer is associated with low-dose BPA exposure. Carcinogenic and proliferative effects of BPA have been a topic of intense debate. Recently, Weinhouse et al. (2014) reported that perinatal exposure to 50 mg/kg BPA could induce neoplastic and pre-neoplastic lesions in mice liver at the age of 10 months. In particular, they observed a hyperplastic nodule around bile duct, which is in accordance with our findings. However, mice are not a standard species for repeated dose toxicity or carcinogenicity bioassay. Also, the dose level is exceedingly high considering that the average human exposure remains below 10 μg/kg/day. In this context, we attempted to add an important line of evidence by showing the proliferative effects of BPA in standard experimental animal species (rats) at lower dose levels (up to 0.5 mg/kg/day, which can be calculated to human equivalent dose of 80 μg/kg/day), in order to provide strong evidence for pro-hepatocarcinogenic effects of BPA, which needs further investigation.
Mechanism behind BPA-induced bile duct proliferation remains unclear in the results of the current study. However, both non-genotoxic and genotoxic pathways are likely to be involved in the proliferative effects of BPA. Generally, proliferative effects of BPA is considered to emerge from its endocrine disrupting activities, since it could interact with multiple hormone receptors including estrogen (vom Saal et al., 2007), androgen (Vogel, 2009), pregnane X receptors (Sui et al., 2012), thyroid (Zoeller et al., 2005), and peroxisome proliferator-activated receptor-γ (Kwintkiewicz et al., 2010). Direct injury of DNA by BPA cannot be ruled out. Many studies have shown that BPA induces the formation of DNA adducts (Izzotti et al., 2009), genetic mutations, cellular transformation (Tsutsui et al., 1998), and positive Comet assay results (Iso et al., 2006), which may explain the bile duct proliferation observed in the current study, although further studies are required to clarify it.
The systemic exposure to BPA was greater at the early age and in female than in male, suggesting the higher susceptibility of juvenile populations to BPA. It has been reported that the oral systemic bioavailability of BPA is low ranging around 20% which is attributable to extensive metabolism to sulfate- or glucuronide-conjugated forms. The results from male rats dosed orally in the current study largely confirm the findings of previous investigators (Churchwell et al., 2014; Draganov et al., 2015) but Churchwell et al. (2014) showed only pooled data from both sexes. Our data provided remarkably different profiles between male and females, which may considerably expands the knowledge on the pharmacokinetic behaviors of BPA. Cmax and AUC of BPA augmented exponentially as doses increase, indicating that the clearance was saturated and other factors increasing the exposure of BPA at high doses. Previously, Doerge et al. (2010) showed that BPA displays linear dose-exposure relationship between doses of 50 to 200 μg/kg in rodents. Doses we employed were much higher than this range and different pattern was anticipated. BPA undergoes extensive phase II metabolism to glucuronide or sulfateconjugated forms (Doerge et al., 2010) and in rodents, biliary excretion was predominant, which was corroborated by the appearance of second Cmax peaks alluding entero-hepatic recirculation. This shall increase the systemic exposure of BPA to the extent exceeding dose-proportion (e.g. Cmax at 250 mg/kg in male on day 1 was 146,219.7 nM, almost 10,000 fold of Cmax at 0.5 mg/kg, 14.7 nM even though dose was 500 fold). Cmax and AUC of BPA were considerably lower at the time of day 91 compared to day 1, reflecting that juvenile animals may be much more susceptible to BPA exposure which might be attributable to metabolic inefficiency.
We demonstrated that repeated oral treatment of BPA to the juvenile rats from the post-natal day 9 for 90 days caused increases in body weights and food consumption, and premature female sexual development. The increases of body weight achieved a statistical significance at 5 mg/kg in male rats which were apparent from 6 weeks of dosing, about the time when the puberty of rats starts (Leibowitz et al., 2009), and persisted through the adulthood until the end of the study. Interestingly, these effects were of a non-linear dose-response relationship in which higher doses, 50 or 250 mg/kg, only showed marginal differences, demonstrating the non-monotonic dose-effect relationship of BPA-induced health effects and at the same time, importance of low-dose effects. Collectively, we demonstrated that repeated oral exposure to BPA in juvenile rats led to bile duct proliferation, which may support the higher risk of BPA exposure in younger generations. Greater exposure in this age group may attribute to bile duct proliferation by low-dose BPA, at least in part, which calls for further studies in the future.
Fig. 4.
Toxicokinetic profiles of BPA on day 1 and 91 after repeat dose. Plasma concentrations of BPA of male (A) and female rats (B) on day 1, and male (C) and female rats (D) on day 91. Data are mean ± SD of 10 animals.
Acknowledgments
This research was supported by grants from Ministry of Food and Drug Safety in 2015 (15162MFDS631) and the Korea Mouse Phenotyping Center (2016M3A9D5A01952416).
Footnotes
CONFLICT OF INTEREST
All authors have no interests to declare for this study.
REFERENCES
- Ahmed RG. Maternal bisphenol A alters fetal endocrine system: Thyroid adipokine dysfunction. Food Chem Toxicol. 2016;95:168–174. doi: 10.1016/j.fct.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Beck MJ, Padgett EL, Bowman CJ, Wilson DT, Kaufman LE, Varsho BJ, Stump DG, Nemec MD, Holson JF. Nonclinical juvenile toxicity testing. In Developmental and Reproductive Toxicology: A Practical Approach. 2006. pp. 263–328.
- Bowman CJ, Chmielewski G, Lewis E, Ripp S, Sawaryn CM, Cross DM. Juvenile toxicity assessment of anidulafungin in rats: an example of navigating case-by-case study design through scientific and regulatory challenges. Birth Defects Res B Dev Reprod Toxicol. 2011;92:333–344. doi: 10.1002/bdrb.20301. [DOI] [PubMed] [Google Scholar]
- Buscher B, van de Lagemaat D, Gries W, Beyer D, Markham DA, Budinsky RA, Dimond SS, Nath RV, Snyder SA, Hentges SG. Quantitative analysis of unconjugated and total bisphenol A in human urine using solid-phase extraction and UPLC-MS/MS: method implementation, method qualification and troubleshooting. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1005:30–38. doi: 10.1016/j.jchromb.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell MI, Camacho L, Vanlandingham MM, Twaddle NC, Sepehr E, Delclos KB, Fisher JW, Doerge DR. Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats. Toxicol Sci. 2014;139:4–20. doi: 10.1093/toxsci/kfu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, Davis KJ, Patton RE, da Costa GG, Woodling KA, Bryant MS, Chidambaram M, Trbojevich R, Juliar BE, Felton RP, Thorn BT. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139:174–197. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol. 2010;247:158–165. doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Draganov DI, Markham DA, Beyer D, Waechter JM, Dimond SS, Budinsky RA, Shiotsuka RN, Snyder SA, Ehman KD, Hentges SG. Extensive metabolism and route-dependent pharmacokinetics of bisphenol A (BPA) in neonatal mice following oral or subcutaneous administration. Toxicology. 2015;333:168–178. doi: 10.1016/j.tox.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Edginton AN, Ritter L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ Health Perspect. 2009;117:645–652. doi: 10.1289/ehp.0800073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JE, Peterson MK, Hixon ML, Shubin SP. Derivation of an oral Maximum Allowable Dose Level for Bisphenol A. Regul Toxicol Pharmacol. 2017;86:312–318. doi: 10.1016/j.yrtph.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Iso T, Watanabe T, Iwamoto T, Shimamoto A, Furuichi Y. DNA damage caused by bisphenol A and estradiol through estrogenic activity. Biol Pharm Bull. 2006;29:206–210. doi: 10.1248/bpb.29.206. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Kanitz S, D’Agostini F, Camoirano A, De Flora S. Formation of adducts by bisphenol A, an endocrine disruptor, in DNA in vitro and in liver and mammary tissue of mice. Mutat Res. 2009;679:28–32. doi: 10.1016/j.mrgentox.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Yu T, Strobel FH, Jones DP. A practical approach to detect unique metabolic patterns for personalized medicine. Analyst. 2010;135:2864–2870. doi: 10.1039/c0an00333f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BA, Wagner LS, Watson NV. The effects of Bisphenol A exposure at different developmental time points in an androgen-sensitive neuromuscular system in male rats. Endocrinology. 2016;157:2972–2977. doi: 10.1210/en.2015-1574. [DOI] [PubMed] [Google Scholar]
- Kim CS, Kim J, Lee YM, Sohn E, Kim JS. Esculetin, a coumarin derivative, inhibits aldose reductase activity in vitro and cataractogenesis in galactose-fed rats. Biomol. Ther. (Seoul) 2016;24:178–183. doi: 10.4062/biomolther.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator-activated receptor-γ mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect. 2010;118:400–406. doi: 10.1289/ehp.0901161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Akabayashi A, Alexander J, Karatayev O, Chang GQ. Puberty onset in female rats: relationship with fat intake, ovarian steroids and the peptides, galanin and enkephalin, in the paraventricular and medial preoptic nuclei. J Neuroendocrinol. 2009;21:538–549. doi: 10.1111/j.1365-2826.2009.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren SA, Iggland M, Rönn M, Lind L, Lind P, Karlsson H. Altered heart proteome in fructose-fed Fisher 344 rats exposed to bisphenol A. Toxicology. 2016;347–349:6–16. doi: 10.1016/j.tox.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Ménard S, Guzylack-Piriou L, Lencina C, Leveque M, Naturel M, Sekkal S, Harkat C, Gaultier E, Olier M, Garcia-Villar R. Perinatal exposure to a low dose of bisphenol A impaired systemic cellular immune response and predisposes young rats to intestinal parasitic infection. PLoS ONE. 2014;9:e112752. doi: 10.1371/journal.pone.0112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, Priymenko N, Bertrand-Michel J, Pineau T, Guillou H, Martin PG, Mselli-Lakhal L. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology. 2012;55:395–407. doi: 10.1002/hep.24685. [DOI] [PubMed] [Google Scholar]
- Miyaguchi T, Suemizu H, Shimizu M, Shida S, Nishiyama S, Takano R, Murayama N, Yamazaki H. Human urine and plasma concentrations of bisphenol A extrapolated from pharmacokinetics established in in vivo experiments with chimeric mice with humanized liver and semi-physiological pharmacokinetic modeling. Regul Toxicol Pharmacol. 2015;72:71–76. doi: 10.1016/j.yrtph.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Noh K, Oh do G, Nepal MR, Jeong KS, Choi Y, Kang MJ, Kang W, Jeong HG, Jeong TC. Pharmacokinetic interaction of chrysin with caffeine in rats. Biomol. Ther. (Seoul) 2016;24:446–452. doi: 10.4062/biomolther.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Hwang MS, Ko A, Jeong DH, Lee JM, Moon G, Lee KS, Kho YH, Shin MK, Lee HS, Kang HS, Suh JH, Hwang IG. Risk assessment based on urinary bisphenol A levels in the general Korean population. Environ Res. 2016;150:606–615. doi: 10.1016/j.envres.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Rönn M, Kullberg J, Karlsson H, Berglund J, Malmberg F, Örberg J, Lind L, Ahlström H, Lind PM. Bisphenol A exposure increases liver fat in juvenile fructose-fed Fischer 344 rats. Toxicology. 2013;303:125–132. doi: 10.1016/j.tox.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Rebuli ME, Camacho L, Adonay ME, Reif DM, Aylor DL, Patisaul HB. Impact of low-dose oral exposure to bisphenol A (BPA) on juvenile and adult rat exploratory and anxiety behavior: A CLARITY-BPA consortium study. Toxicol Sci. 2015;148:341–354. doi: 10.1093/toxsci/kfv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Cao J, Sluzas E, Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Patisaul HB. Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol Sci. 2014;140:190–203. doi: 10.1093/toxsci/kfu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Ai N, Park SH, Rios-Pilier J, Perkins JT, Welsh WJ, Zhou C. Bisphenol A and its analogues activate human pregnane X receptor. Environ Health Perspect. 2012;120:399–405. doi: 10.1289/ehp.1104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedenborg E, Ruegg J, Makela S, Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol. 2009;43:1–10. doi: 10.1677/JME-08-0132. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Tamura Y, Yagi E, Hasegawa K, Takahashi M, Maizumi N, Yamaguchi F, Barrett JC. Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syrian hamster embryo cells. Int. J. Cancer. 1998;75:290–294. doi: 10.1002/(sici)1097-0215(19980119)75:2<290::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, Hunt PA, Newbold RR, Rubin BS, Saili KS. Low dose effects of bisphenol A: An integrated review of in vitro, laboratory animal, and epidemiology studies. Endocr Disrupt. 2013;1:e26490. doi: 10.4161/endo.26490. [DOI] [Google Scholar]
- Vogel SA. The politics of plastics: the making and unmaking of bisphenol a “safety”. Am. J. Public Health. 2009;99(Suppl 3):S559–S566. doi: 10.2105/AJPH.2008.159228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse C, Anderson OS, Bergin IL, Vandenbergh DJ, Gyekis JP, Dingman MA, Yang J, Dolinoy DC. Dose-dependent incidence of hepatic tumors in adult mice following perinatal exposure to bisphenol A. Environ Health Perspect. 2014;122:485–491. doi: 10.1289/ehp.1307449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczewska K, Namiesnik J, Wasik A. Troubleshooting of the determination of bisphenol A at ultra-trace levels by liquid chromatography and tandem mass spectrometry. Anal Bioanal Chem. 2016;408:1009–1013. doi: 10.1007/s00216-015-9215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Jiang Y, Li Y, Wan Y, Liu J, Ma Y, Mao Z, Chang H, Li G, Xu B, Chen X, Xu S. Early-life exposure to bisphenol a induces liver injury in rats involvement of mitochondria-mediated apoptosis. PLoS ONE. 2014;9:e90443. doi: 10.1371/journal.pone.0090443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.