Abstract
Paclitaxel (PTX) is one of the most frequently used anticancer agent for treating refractory ovarian cancer, metastatic breast cancer and non-small cell lung cancer. However, its oral administration is impeded by very low bioavailability (<5%) due to the Pglycopprotein (P-gp) efflux pump effect. This study investigated in vitro and in vivo P-gp inhibitory effects of adamantyl derivatives AC-603 and AC-786 in rats. Two adamantyl derivatives tested in this study increased the cytotoxicity of daunomycin (DNM) in P-gp overexpressed cell line by inhibiting P-gp efflux function. Pharmacokinetics of PTX with orally co-administered P-gp inhibitors were assessed in rats to improve PTX absorption. The pharmacokinetic parameters of PTX were determined in rats after intravenous (2 mg/kg) or oral (25 mg/kg) administration in the presence or absence of verapamil (a positive control), AC-603 or AC-786 (0.5 mg/kg or 5 mg/kg). Compared to control group (PTX alone), experimental groups (PTX with AC-603 or AC-786) significantly increased the area under the plasma concentration-time curve of PTX following oral administration by 1.7–2.2 fold. The volume of distribution and total clearance of PTX were decreased, while other parameters were not significantly changed. In conclusion, co-administration of AC-603 or AC-786 enhanced the relative bioavailability of orally administered PTX as compared to control.
Keywords: Paclitaxel, Adamantyl derivatives, Verapamil, P-glycoprotein, Oral bioavailability
INTRODUCTION
Paclitaxel (PTX) is an anticancer agent, which has a significant activity against a wide variety of tumors, including refractory ovarian cancer, metastatic breast cancer, and non-small cell lung cancer (Wang et al., 2000). Paclitaxel hyper-stabilizes microtubule structure by binding to tubulin, the “building block” of microtubules, during cell division (Kumar, 1981).
The currently marketed PTX (Taxol [Bristol-Myers Squibb, New York, USA] 6 mg/mL PTX in a 1:1 Cremophor EL [Sigma-Aldrich, Sigma-Aldrich, St. Louis, MO, USA]/ethanol mixture) is dissolved in a 0.9% NaCl solution for clinical use and administered IV infusion (Colin et al., 2014). Unfortunately, severe adverse reactions of vasodilatation, breathing problem, lethargy and hypotension have been reported after PTX infusion, and it is well known that the vehicle used in formulation, Cremophor EL, contributes largely to the reactions (Van Zuylen et al., 2001). Thus, a significant amount of effort has dedicated to minimize the adverse effects caused by IV Cremophor EL. One of the strategies to avoid the reaction is to administer PTX orally by formulating PTX to an oral dosage form (Theis et al., 1995). However, oral administration of PTX has disadvantages of its low bioavailability (<5%) due to its poor aqueous solubility, limited permeability and metabolism by cytochrome P450 enzymes in the gut and liver (Sezgin-Bayindir et al., 2013; Cai et al., 2014). For the reasons, PTX is administered primarily by IV infusion (Sparreboom et al., 1997).
Other limitation would be the presence P-glycoprotein (Pgp) efflux pump preventing from the absorption of PTX in the intestine (Sparreboom et al., 1997) which led to multidrug resistance (MDR). It makes many potent anticancer drugs ineffective and is largely responsible for the failure of cancer chemotherapy (Ambudkar et al., 1999). MDR is primarily resulted from the overexpression of ATP-binding cassette (ABC) drug efflux transporters, such as ABCB1 (P-gp), ABCC1-7 (MDR related protein 1–7), and ABCG2 (breast cancer resistance protein) (Min et al., 2009). These proteins tend to pump out a number of chemically unrelated therapeutic agents (Min et al., 2009). P-gp also transports chemotherapeutic agents to the outside of cells, making the chemotherapy almost ineffective in many cases (Amin, 2013). P-gp expressed at high concentrations not only in tumor cells but also in normal tissues including intestines, liver, kidney, spinal cord, blood brain barrier, testis, adrenal gland and placenta. P-gp transporters are present on apical or luminal surface of epithelial or endothelial cells (Wu et al., 2011). PTX is a substrate for the P-gp which is a well-known limiting factor in the intestinal uptake of drugs (Hendrikx et al., 2013).
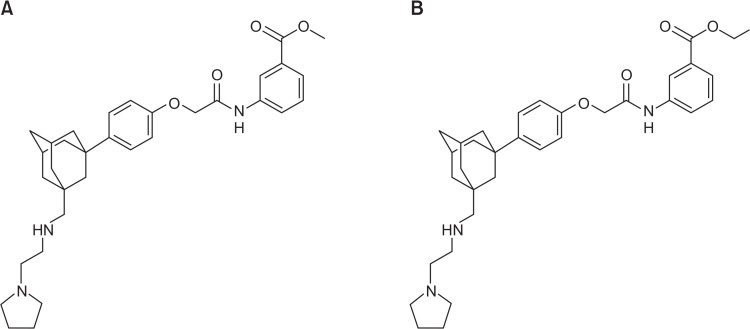
Above all, it is considered for P-gp to be clinically more significant than the other transporters, therefore, is viewed as a therapeutic target for MDR cancer cells to respond to anticancer drugs (Min et al., 2009). Several adamantyl derivatives were synthesized for P-gp inhibitors to overcome MDR using structure-activity relationship (SAR) and cell lines and also assessed in P-gp dependent MDR cancer cell lines. Many adamantyl derivatives showed potential P-gp inhibitory effect without considerable intrinsic cytotoxicity (Min et al., 2009). Previously, despite the rigorous efforts, a number of candidates have failed because of their intrinsic toxicities, like verapamil and cyclosporine, and pharmacokinetic interactions in vivo (Min et al., 2009). Although there are some leading agents are under clinical trials, they have not been applied clinically yet. Recently, 20 different P-gp inhibitors with adamantyl derivatives synthesized, AC-603 (Fig. 1A) and AC-786 (Fig. 1B) were found to be the most promising which exhibited reversal activity of adamantly derivatives in P-gp overexpressed MES-SA/DX5 cells (Min et al., 2009). The result suggests that these highly potent and selective compounds deserve further investigation. Thus, in this study, the P-gp inhibitory function of AC-603 and AC-786 was evaluated in vitro and their absorption enhancing effect was assessed in vivo.
Fig. 1.
Chemical structures of AC-603 (A) and AC-786 (B). (A) Adamantyl analogues AC-603. (B) Adamantyl analogues AC-786.
MATERIALS AND METHODS
Materials
Paclitaxel and 4-Hydroxybenzoic acid n-hexyl ester (internal standard) were purchased from Samyang Genex (Daejeon, Korea) and Tokyo Kassei Kogyo (Tokyo, Japan), respectively. Antibiotic-antimycotic agent, 0.25% trypsin-1 mM EDTA and RPMI 1640 medium were supplied by GIBCO (Rockville, MD, USA). Fetal bovine serum and Tris, N-(2-hydroxyethyl) peperazine-N′-2-ethanesulfonic acid (HEPES) were obtained from HyClone (Logan, UT, USA) and USB (Cleveland, OH, USA), respectively. Daunomycin was supplied by EMD Chemicals (San Diego, CA, USA). Diethyl ether and Phosphoric acid were purchased from Samchun pure chemical (Pyeongtaek, Korea) and Showa Chemical (Tokyo, Japan), respectively. Heparin sodium injection was obtained from Hanlim Pharm (Yongin, Korea). All other agents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Human breast cancer cell line (MCF-7) and P-gp overexpressed human breast cancer cell line (MCF-7/ADR) were kindly gifted by Dr. Marilyn E. Morris (State University of New York at Buffalo, USA).
In vitro daunomycin cytotoxicity assay
To determine the effect of adamantyl derivatives on P-gp function, cytotoxicity assay was carried out in MCF-7/ADR cells (P-gp overexpressed human breast cancer cell line). The tumor cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 10 mM HEPES, 24 mM NaHCO3, and 1% antibiotic-antimycotic agent, and maintained at 37°C in a humidified 5% CO2 atmosphere. MCF-7/ADR cells were seeded in 96-well plates at 5,500 cells per well and incubated for 24 h. Daunomycin (DNM) was diluted with cell culture media and treated on the plates to achieve the final concentrations of 0, 0.05, 0.1, 0.5, 1, 5, 10, 25, 50 and 100 μM with or without 5 μM of adamantly derivatives, AC-603 and AC-786 or Verapamil (VER). VER is one of the first generation of P-gp inhibitors. DNM containing 0.4% DMSO and DNM with 5 μM VER were used as negative and positive control, respectively. Each DNM concentration was assayed in three wells and in three independent experiments. After 2 h incubation, cells were washed and replaced with 200 μL of fresh culture media and then incubated for additional 72 h. After 72 h incubation, 50% TCA was treated on the wells to affix cells on the bottom of plates and then removed. Sulforhodamine B (SRB, 0.4%) was used to dye cell membrane and the cells were washed with 1% acetic acid. Dyed cells were treated with 10 mM Tris-base and dissolved on the orbital shaker for about 80 minutes at 163–167 rpm. The cell cytotoxicity was assessed using ELISA reader supplied by Molecular Devices. After placing plates on the ELISA reader, the light intensity of remained cells was measured at a wavelength of 515 nm. IC50 values were calculated with Table curve Windows program.
In vivo pharmacokinetic studies
Healthy male Sprague-Dawley rats (6–7 weeks old), weighing 240–295 g, were purchased from Orient Bio Inc (Seongnam, Korea). All animal procedures for animal studies were approved by Institutional Animal Care and Use Committee (IACUC) at Ewha Womans University, Seoul, Korea. They were kept in an environmentally controlled breeding room for at least five days before starting the experiments.
The rats were given surgical procedure for blood sampling one day before the experiments. The common carotid artery of the rat was surgically cannulated using polyethylene tubing (PE-60) for blood sampling. After surgery, rats were housed individually to allow them to recover. Before the experiments, rats were fasted overnight with free access to water. The rats were assigned randomly to eight groups. PTX was dissolved in Cremophor® EL and anhydrous ethanol (1/1, v/v), followed by dilution of the drug solution with isotonic saline (1/2, v/v) before use. AC-603, AC-786 and VER were also dissolved using Taxol® formulation. Two groups of rats were intravenously (IV) or orally (PO) administered with PTX, 2 mg/kg or 25 mg/kg, respectively, as control. To the other six groups, 25 mg/kg of PTX was orally co-administered with VER (0.5, 5 mg/kg), AC-603 (0.5, 5 mg/kg), or AC-786 (0.5, 5 mg/kg). For the IV injection group, blood samples (0.2 mL) were taken from the common carotid artery at 0, 0.033, 0.083, 0.25, 0.5, 1, 2, 3, 4, 6, 10 and 24 h after the IV injection. For the oral administration groups, 0.2 mL of blood samples were taken at 0, 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10 and 24 h after the oral administration. The same volume of heparinized 0.9% NaCl solution (100 IU/mL, 0.2 mL) was administered to keep the blood volume and to prevent blood clotting. Plasma samples (100 μL) were obtained after centrifugation for 15 min at 13,000 rpm immediately after collection and stored at −20°C until used for HPLC analysis.
HPLC analysis and sample preparation
The analysis was carried out on an Agilent HP1100 series system. The chromatographic separation was performed on a Capcell-pak C18 MG120 column (3.0×250 mm, 5 μm, Shiseido, Tokyo, Japan). The mobile phase was composed of acetonitrile and 0.1% phosphoric acid (1/1, v/v) at a flow rate of 0.5 mL/min. The UV-detector wavelength was set at 227 nm. A volume of 5 μL of n-hexyl 4-hydroxybenzoate (internal standard, IS) was added to 50 μL of each plasma sample and then 45 μL of acetonitrile were added to precipitate the plasma protein. The mixture was vortexed for 2 min, followed by centrifugation at 13,000 rpm for 15 min to remove all the precipitated material. The auto-sampler was programmed to inject 40 μL of the sample solution into the HPLC system. An analytical method validation for PTX was performed according to the FDA guidance for Industry (U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research and Center for Veterinary Medicine, 2001).
Pharmacokinetic evaluation of PTX
The pharmacokinetic parameters of PTX after a single intravenous or oral administration to rats were investigated by non-compartmental analysis using WinNonlin® Professional version 5.2 software (Pharsight Corporation, Mountain View, CA, USA). The pharmacokinetic parameters estimated from the plasma concentration-time profiles were as follows: the area under the plasma concentration-time curve from 0 h to the last sampling time (AUClast), the area under the plasma concentration-time curve from 0 h to infinity (AUCinf), elimination half-life (t1/2), elimination rate constant (Ke), apparent volume of distribution (Vd), total clearance (Clt), apparent volume of distribution following oral administration (Vd/F) and oral clearance (Clt/F). The maximum plasma concentration (Cmax) and the time required to reach Cmax (Tmax) were measured directly from the concentration-time data.
The absolute bioavailability (AB, %) of PTX was calculated by the following equation:
The relative bioavailability (RB, %) of PTX was calculated by the following equation:
, where AUCoral control is the AUC obtained from the oral administration of PTX alone, and AUCco-admin is the AUC obtained from the oral co-administration of PTX with VER, AC-603, or AC-786.
Statistical analysis
All the means are presented with their standard deviation (mean ± SD). All data acquired from this study used Graph-Pad Instat 3.0 statistics program (GraphPad Software, Inc., La Jolla, CA, USA) for Windows to calculate the descriptive statistics. To determine any significant difference between the control and PTX treated with compounds for each variable, one-way ANOVA was executed and SNK (Student-Newman-Keuls) was performed for post verification. The p values <0.05 were considered statistically significant.
RESULTS
In vitro daunomycin cytotoxicity assay
In vitro growth inhibition of MCF-7/ADR cells was studied in the presence of adamantyl derivatives, AC-603 and AC-786 (Table 1) (Naik et al, 2011). For in vitro study, DNM was selected as a P-gp substrate anticancer drug because PTX has very low solubility. PTX was precipitated at high concentrations, while DNM was soluble at all the concentrations used for cytotoxicity assay. The IC50 values of DNM in the presence of these compounds, AC-603 and AC-786, significantly reduced, indicating that these compounds enhanced the cytotoxicity of DNM in MCF-7/ADR cells by inhibiting P-gp efflux function (Table 1). However, DMN is known for the substrate of both P-gp and Breast Cancer Resistance Protein (BCRP), so that later in vivo test, we continued to study using PTX which is a substrate of P-gp.
Table 1.
Effect of 5 μM adamantyl derivatives on the IC50 values of DNM in MCF-7/ADR cells
| Compounds | IC50 Values (μM) |
|---|---|
| Negative Control | 10.37 ± 0.53 |
| Verapamil (positive control) | 2.75 ± 0.54* |
| AC-603 | 1.16 ± 0.05* |
| AC-786 | 1.15 ± 0.03* |
p<0.001 compared with control.
Values are expressed as mean ± SD from triplicate experiment.
Analytical method validation
The peaks of PTX and IS were well separated from endogenous interfering peaks in rat plasma, confirming the specificity of the method. The retention times of PTX and IS were approximately 13.7 and 23.4 min, respectively. The analytical method exhibited excellent linearity over the concentration range of 0.02–10.0 μg/mL with a high correlation coefficient (r=0.9999).
The intra- and inter-day precision and accuracy acceptance criteria for each QC sample (0.1, 0.5 and 5 μg/mL) was ≤15% and all the values successfully met the criteria. The LLOQ (the signal-to-noise ratio >5) was determined to be 0.02 μg/mL. All the values for precision and accuracy of LLOQ were within the acceptable range (± 20%). The mean relative extraction recoveries of PTX were in the range of 85–115% at the concentrations of 0.1, 1 and 5 μg/mL. The mean relative recovery of IS was 96.1 ± 0.10% at 5 μg/mL which was used for the analysis. As expected, the efficiency of PTX and IS extraction from rat plasma was coherent, regardless of concentration.
Pharmacokinetic evaluation of PTX
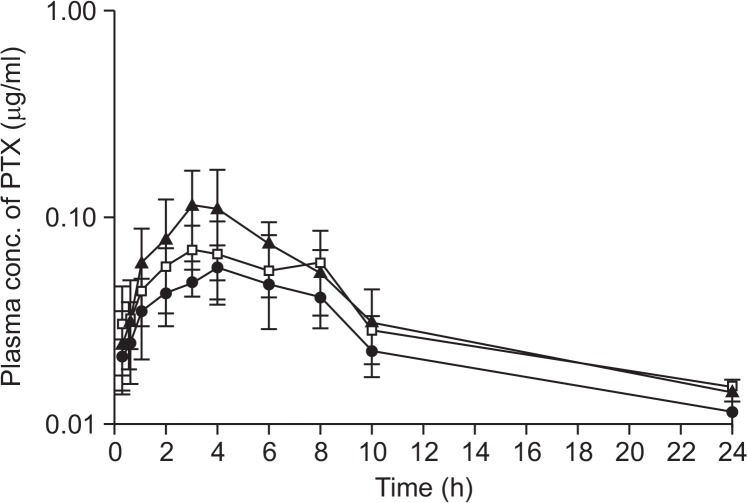
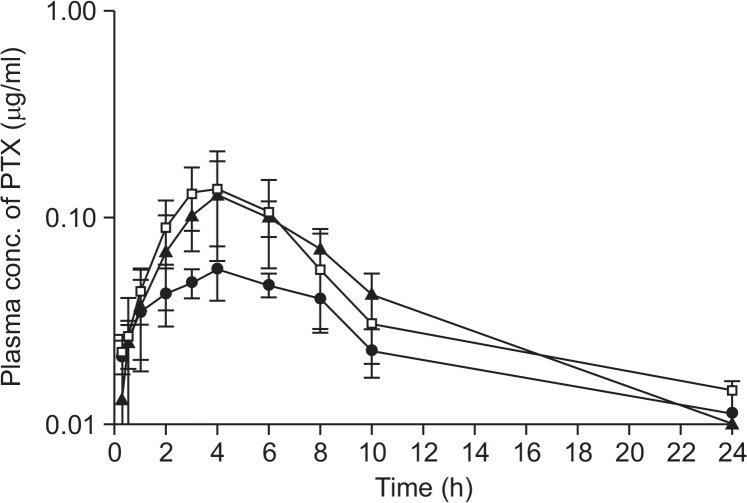
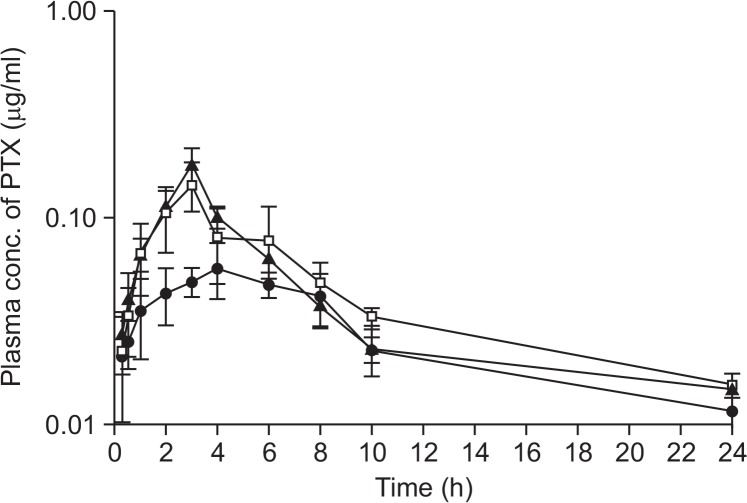
The mean plasma concentration-time curves for PTX obtained after oral co-administration of PTX (25 mg/kg) with two different doses of VER (0.5 mg/kg and 5 mg/kg, Fig. 2), AC-603 (0.5 mg/kg and 5 mg/kg, Fig. 3) and AC-786 (0.5 mg/kg and 5 mg/kg, Fig. 4) are shown in each Figure. In addition, all of them were compared with the mean plasma concentration-time curve obtained after oral administration of PTX (25 mg/kg) alone (control). All the relevant pharmacokinetic parameters for PTX were summarized in Table 2. The data were used to calculate the absolute bioavailability (AB, %) of PTX after oral administration in the absence or presence of compounds (Table 2).
Fig. 2.
Mean plasma concentration-time profiles after oral administration of PTX alone or with VER to rats. Bars represent the SD (n=4–5). PTX (25 mg/kg) control (•), PTX (25 mg/kg) co-administration with VER 0.5 mg/kg (□) or 5 mg/kg (▲).
Fig. 3.
Mean plasma concentration-time profiles after oral administration of PTX alone or with AC-603 to rats. Bars represent the SD (n=4-5). PTX (25 mg/kg) control (•), PTX (25 mg/kg) co-administration with AC-603 0.5 mg/kg (□) or 5 mg/kg (▲).
Fig. 4.
Mean plasma concentration-time profiles after oral administration of PTX alone or with AC-786 to rats. Bars represent the SD (n=4-5). PTX (25 mg/kg) control (•), PTX (25 mg/kg) co-administration with AC-786 0.5 mg/kg (□) or 5 mg/kg (▲).
Table 2.
Pharmacokinetic parameters of PTX after IV injection (2 mg/kg), and after oral administration of PTX (25 mg/kg) alone, or with other compounds (VER, AC-603 or AC-786) in rats (n=4–5)
| PK parameters | PTX IV | PTX PO alone | PTX with VER | PTX with AC-603 | PTX with AC-786 | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| 0.5 mg/kg | 5 mg/kg | 0.5 mg/kg | 5 mg/kg | 0.5 mg/kg | 5 mg/kg | |||
| C0(ng/mL) | 3328.1 ± 1160.1 | - | - | - | - | - | - | - |
| Cmax (ng/mL) | - | 60.6 ± 10.3 | 84.7 ± 14.4 | 125.3 ± 63.0 | 167.1 ± 58.6* | 133.9 ± 55.2 | 144.1 ± 39.2 | 180.1 ± 37.2** |
| Tmax (h) | - | 4.2 ± 1.1 | 5.0 ± 2.6 | 3.3 ± 0.5 | 4.3 ± 1.3 | 4.3 ± 1.3 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| AUClast(ng·hr/mL) | 1655.7 ± 498.8 | 408.9 ± 47.2 | 604.9 ± 111.7 | 715.3 ± 259.9* | 824.2 ± 198.2* | 903.8 ± 161.7** | 716.9 ± 194.0* | 738.7 ± 73.8* |
| AUCinf(ng·hr/mL) | 1794.3 ± 522.0 | 564.4 ± 115.8 | 785.9 ± 125.7 | 875.6 ± 304.5 | 933.5 ± 215.7* | 1021.8 ± 158.1* | 910.1 ± 158.0 | 831.7 ± 72.2 |
| t1/2(h) | 4.55 ± 1.02 | 4.26 ± 1.62 | 5.45 ± 2.86 | 3.28 ± 0.88 | 2.41 ± 0.70 | 3.09 ± 0.83 | 4.16 ± 1.71 | 2.84 ± 0.28 |
| Ke(1/h) | 0.161 ± 0.047 | 0.182 ± 0.066 | 0.153 ± 0.071 | 0.227 ± 0.078 | 0.312 ± 0.112 | 0.234 ± 0.050 | 0.191 ± 0.085 | 0.246 ± 0.025 |
| Vd(L) | 2.06 ± 0.36 | - | - | - | - | - | - | - |
| Vd/F (L) | - | 73.6 ± 14.9 | 64.6 ± 27.2 | 39.4 ± 19.0* | 24.8 ± 10.0** | 28.7 ± 7.1** | 45.3 ± 21.1* | 33.0 ± 6.3* |
| Clt(L/h) | 0.328 ± 0.097 | - | - | - | - | - | - | - |
| Clt/F (L/h) | - | 12.7 ± 2.8 | 8.5 ± 1.4** | 8.3 ± 3.3** | 7.1 ± 1.4** | 6.5 ± 1.2** | 7.4 ± 1.1** | 8.0 ± 0.8** |
| Absolute BA (%) | - | 2.0 | 2.9 | 3.5 | 4.0 | 4.4 | 3.5 | 3.6 |
| Relative BA (%) | - | 100.0 | 147.5 | 174.7 | 201.0 | 220.7 | 174.7 | 180.3 |
p<0.05,
p<0.01 compared with PTX PO (25 mg/kg) alone, Data are presented as mean ± SD (n=4–5).
After oral co-administration of PTX with each compound including a positive control, VER, the mean area under the plasma concentration-time curve (AUClast) were significantly increased as compared with control group (p<0.05 or p<0.01), except for the case of orally co-administrated with 0.5 mg/kg of VER (Table 2). All the compounds tested resulted in meaningful enhancement in the absolute and relative bioavailability of PTX. With 5 mg/kg of AC-603 and AC-786, the relative bioavailability (RB, %) was significantly increased to 220.7% and 180.3%, respectively (Fig. 3, 4, Table 2). The Vd/F and Clt/F of PTX were significantly decreased by co-administration with the compounds without changes in Tmax and t1/2. The mean Cmax values were also increased in all the co-administered groups (Table 2).
DISCUSSION
The main purpose of this study was to improve oral bioavailability of PTX by intestinal P-gp inhibition using adamantyl derivatives (Fig. 1). In previous study, AC-603 and AC-786 were found to be two most potent P-gp inhibitors among twenty adamantyl derivatives. The dose-dependency of P-gp inhibitory function was not studied in vitro. As shown in the Table 1, AC-603 and AC-786 at a concentration of 5 μM significantly reduced the IC50 values of DNM, which are lower than the value with the positive control of verapamil, in P-gp overexpressed cell line, MCF-7/ADR (Table 1). The results suggest that AC-603 and AC-786 inhibit P-gp function in the cell level. Based on this in vitro result, we evaluated the effect of AC-603 and AC-786 on oral bioavailability of PTX in rats.
The pharmacokinetic studies were carried out using two administration routes (IV and PO) to compare the effects of compounds (VER, AC-603 and AC-786) on PTX bioavailability (Fig. 2–4). Throughout the entire experiments, the administered volume of the compounds was the same in order to minimize the solvent effect. PTX was largely distributed to body organs (2.06 L). It has been reported to be disposed much higher in the liver and bile than in the other tissues (Choi et al., 2004). PTX was cleared from the body quickly (0.328 L/h) and the absolute bioavailability of PTX was only 2.0%. The Vd/F and Clt/F of PTX were significantly decreased (p<0.05 or p<0.01) in the presence of both compounds without considerable alterations in Tmax and t1/2, suggesting slower oral clearance and lower organ distribution. After oral co-administration of PTX with each compound, the mean area under the plasma concentrationtime curve (AUClast) were significantly enhanced except the case of orally co-administrated with 0.5 mg/kg of VER, as compared with control group (p<0.05 or p<0.01) (Table 2). Even though the dose of each compound increased 10-fold from 0.5 mg/kg to 5 mg/kg, there was only a slight change of insignificant increase in the relative bioavailability (from 201.0 to 220.7% at AC-603 and from 174.7 to 180.3% at AC-786). This result can be explained by two different possible ways. The first explanation is that a small amount of compound can be sufficient to have P-gp inhibition. This might be a positive impact for patients who take multi-drug regimen. The second one is that the poor solubility of AC-603 and AC-786 may limit its P-gp inhibitory effect in the intestine at high dose. Saturation of P-gp would be one of the possible mechanisms responsible for such nonlinearity (Pan et al., 2016). Thus, for P-gp inhibitor in vivo, its luminal exposure to P-gp may reach a level that is much higher than the affinity. At the same time, the introduction of PTX, P-gp substrate, would indicate that P-gp saturated by excess P-gp inhibitor are present which may not response proportionally. If it is the case, we can suggest to employ the microemulsion formulation to enhance the solubility of poorly water soluble compounds to increase the rate of absorption by reducing the droplet size (<100 nm) (Patel et al., 2010). There was similar efforts of increase in absolute oral bioavailability (1.8-fold) of PTX achieved by the combination of pretreatment with verapamil, a P-gp inhibitor, and coadministration with the lipid vehicle mixture (Cai et al., 2016). In addition, microemulsion of AC-603 and AC-786 might have advantages over the PTX formulation. It was reported that the amount of Cremophor® EL in the PTX microemulsion was markedly reduced as compared with the Taxol® formulation (0.8 g in microemulsion vs. 2.6 g in Taxol® formulation) (van Zuylen et al., 2001; Wang et al., 2011). Therefore, microemulsion containing less Cremophor® EL might reduce side effects associated with Cremophor® EL. Microemulsion containing PTX with P-gp inhibitor and the reduced amount of Cremophor ® EL can be beneficial because the entrapped PTX in the micelle formed by Cremophor® EL reduced the uptake of PTX in the intestine (Malingre et al., 2001).
In conclusion, adamantyl derivatives, AC-603 and AC-786, significantly improved oral absorption of PTX, resulting in increased bioavailability via the inhibition of intestinal P-gp. These results suggest that the co-administration of AC-603 and AC-786 could provide a therapeutic benefit on the oral delivery of P-gp substrate drugs.
Acknowledgments
This work was supported by the Research Grant funded by Ewha Womans University.
REFERENCES
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- Amin ML. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights. 2013;7:27–34. doi: 10.4137/DTI.S12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Deng X, Li Z, An D, Shen T, Zhong M. Effects of lipid vehicle and P-glycoprotein inhibition on the mesenteric lymphatic transport of paclitaxel in unconscious, lymph duct-cannulated rats. Drug Deliv. 2016;23:147–153. doi: 10.3109/10717544.2014.907841. [DOI] [PubMed] [Google Scholar]
- Choi JS, Jo BW, Kim YC. Enhanced paclitaxel bioavailability after oral administration of paclitaxel or prodrug to rats pretreated with quercetin. . Eur J Pharm Biopharm. 2004;57:313–318. doi: 10.1016/j.ejpb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Colin P, De Smet L, Vervaet C, Remon JP, Ceelen W, Van Bocxlaer J, Boussery K, Vermeulen A. A model based analysis of IPEC dosing of paclitaxel in rats. . Pharm Res. 2014;31:2876–2886. doi: 10.1007/s11095-014-1384-5. [DOI] [PubMed] [Google Scholar]
- Hendrikx JJ, Lagas JS, Rosing H, Schellens JH, Beijnen JH, Schinkel AH. P-glycoprotein and cytochrome P450 3A act together in restricting the oral bioavailability of paclitaxel. Int. J. Cancer. 2013;132:2439–2447. doi: 10.1002/ijc.27912. [DOI] [PubMed] [Google Scholar]
- Kumar N. Taxol-induced polymerization of purified tubulin. Mechanism of action. . J Biol Chem. 1981;256:10435–10441. [PubMed] [Google Scholar]
- Malingre MM, Schellens JH, Van Tellingen O, Ouwehand M, Bardelmeijer HA, Rosing H, Koopman FJ, Schot ME, Ten Bokkel Huinink WW, Beijnen JH. The co-solvent Cremophor EL limits absorption of orally administered paclitaxel in cancer patients. Br. J. Cancer. 2001;85:1472–1477. doi: 10.1054/bjoc.2001.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KH, Xia Y, Kim EK, Jin Y, Kaur N, Kim ES, Kim DK, Jung HY, Choi Y, Park MK, Min YK, Lee K, Lee K. A novel class of highly potent multidrug resistance reversal agents: disubstituted adamantyl derivatives. Bioorg Med Chem Lett. 2009;19:5376–5379. doi: 10.1016/j.bmcl.2009.07.127. [DOI] [PubMed] [Google Scholar]
- Naik R, Jeon C, Min H, Choi HK, Min KH, Lee K. Synthesis and bioactivity of novel adamantyl derivatives as potent MDR reversal agents. Bull Korean Chem Soc. 2011;32:4444–4446. doi: 10.5012/bkcs.2011.32.12.4444. [DOI] [Google Scholar]
- Pan Y, Hsu V, Grimstein M, Zhang L, Arya V, Sinha V, Grillo JA, Zhao P. The application of physiologically based pharmacokinetic modeling to predict the role of drug transporters: scientific and regulatory perspectives. J. Clin. Pharmacol. 2016;56(Suppl 7):S122–S131. doi: 10.1002/jcph.740. [DOI] [PubMed] [Google Scholar]
- Patel V, Kukadiya H, Mashru R, Surti N, Mandal S. Development of microemulsion for solubility enhancement of clopidogrel. Iran J Pharm Res. 2010;9:327–334. [PMC free article] [PubMed] [Google Scholar]
- Sezgin-Bayindir Z, Onay-Besikci A, Vural N, Yuksel N. Niosomes encapsulating paclitaxel for oral bioavailability enhancement: preparation, characterization, pharmacokinetics and biodistribution. J Microencapsul. 2013;30:796–804. doi: 10.3109/02652048.2013.788088. [DOI] [PubMed] [Google Scholar]
- Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, Borst P, Nooijen WJ, Beijnen JH, van Tellingen O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci USA. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis JG, Liau-Chu M, Chan HS, Doyle J, Greenberg ML, Koren G. Anaphylactoid reactions in children receiving high-dose intravenous cyclosporine for reversal of tumor resistance: the causative role of improper dissolution of Cremophor EL. J Clin Oncol. 1995;13:2508–2516. doi: 10.1200/JCO.1995.13.10.2508. [DOI] [PubMed] [Google Scholar]
- Department US of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research and Center for Veterinary Medicine Guidance for industry: Bioanalytical methods validation. 2001 Available from: http://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf/.
- van Zuylen L, Verweij J, Sparreboom A. Role of formulation vehicles in taxane pharmacology. Invest. New Drugs. 2001;19:125–141. doi: 10.1023/A:1010618632738. [DOI] [PubMed] [Google Scholar]
- Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000;88:2619–2628. doi: 10.1002/1097-0142(20000601)88:11<2619::AID-CNCR26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu KC, Zhao BX, Zhao X, Wang X, Chen S, Nie SF, Pan WS, Zhang X, Zhang Q. A novel paclitaxel microemulsion containing a reduced amount of Cremophor EL: pharmacokinetics, biodistribution, and in vivo antitumor efficacy and safety. J Biomed Biotechnol. 2011;2011:854872. doi: 10.1155/2011/854872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CP, Ohnuma S, Ambudkar SV. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:609–620. doi: 10.2174/138920111795163887. [DOI] [PMC free article] [PubMed] [Google Scholar]