Abstract
Purpose
The Children’s Oncology Group AAML0631 trial for newly diagnosed pediatric acute promyelocytic leukemia (APL) was a phase III historically controlled trial to determine the survival of patients receiving arsenic trioxide (ATO) consolidation and reduced doses of anthracyclines.
Patients and Methods
Patients age 2 to 21 years with de novo APL confirmed by PML-RARα polymerase chain reaction were stratified as standard risk (SR) or high risk (HR) on the basis of diagnostic WBC count. All patients received all-trans retinoic acid (ATRA) during induction, each consolidation course, and maintenance. All patients received two cycles of ATO therapy during consolidation 1, an additional two (SR) or three (HR) consolidation courses that included high-dose cytarabine and anthracycline, and maintenance therapy comprising ATRA, oral methotrexate, and mercaptopurine.
Results
One hundred one patients (66 SR and 35 HR) were evaluable for outcome. The 3-year overall survival was 94%, and event-free survival (EFS) was 91%. For SR and HR patients with APL, the overall survival was 98% versus 86% (P = .003), and EFS was 95% versus 83% (P = .03), respectively. The EFS for SR patients in AAML0631 was noninferior to that of patients in the AIDA 0493 historical control, which used a significantly higher anthracycline dose and did not include ATO consolidation. Relapse risk for patients in AAML0631 from end consolidation 1 (after ATO treatment) was only 4% at 3 years and did not differ significantly between SR and HR patients.
Conclusion
ATO consolidation cycles were well tolerated in pediatric patients with APL and allowed significant reduction in cumulative anthracycline doses while maintaining excellent survival and a low relapse risk for both SR and HR patients with APL.
INTRODUCTION
Historically, treatment of pediatric acute promyelocytic leukemia (APL) has included high doses of anthracyclines, with risk of cardiac toxicity.1,2 This risk increases with higher cumulative doses (≥ 300 mg/m2) and younger age at exposure.3 Anthracycline-based chemotherapy in combination with all-trans retinoic acid (ATRA) has improved survival for pediatric APL.4,5 In the Gruppo Italiano Malattie EMatologiche dell’Adulto–Associazione Italiana di Ematologia Oncologia Pediatrica AIDA 0493 trial, treatment with ATRA, anthracyclines, and high-dose cytarabine resulted in overall survival (OS) of approximately 90%.5
More recently, APL clinical trials have evaluated treatment with arsenic trioxide (ATO). The North American Intergroup study Cancer and Leukemia Group B (CALGB) 9710 demonstrated that adults with APL receiving two cycles of ATO consolidation had significantly improved OS and decreased relapse risk (RR).6 ATO has been used with great success as a single agent and in combination with ATRA in the treatment of children with APL.7-9 The pivotal APL0406 trial demonstrated that ATRA and ATO was not inferior to ATRA and chemotherapy in adult SR APL.10
Our clinical trial (AAML0631) is a phase III historically controlled Children’s Oncology Group trial for pediatric APL incorporating ATO consolidation with an approximate 40% reduction in anthracycline dosing compared with the historical control trial AIDA 0493.5 Here, we report the safety and tolerability of ATO consolidation in pediatric patients with APL treated in AAML0631 and demonstrate that ATO consolidation allowed a significant reduction in anthracycline dosing while maintaining excellent outcomes.
PATIENTS AND METHODS
Patients
AAML0631 was a nonrandomized phase III cooperative group trial in which event-free survival (EFS) from AIDA 0493 was used as the benchmark for the standard-risk (SR) group (WBC count at presentation < 10,000 cells/μL), whereas outcome for the smaller high-risk (HR) group (WBC count ≥ 10,000 cells/μL) is descriptively reported because of limitations of power. AIDA 0493 enrolled 983 patients with APL of all ages between January 1993 and June 2000, but the pediatric cohort reported by Testi et al5 included 107 patients (69 SR, 38 HR) age < 18 years. Inclusion criteria for our trial AAML0631 were age ≥ 2 to < 22 years, clinical diagnosis of de novo APL made by examining marrow or peripheral blood, and no prior leukemia treatment except for steroids, hydroxyurea, and leukapheresis. There were no performance score or organ function exclusions in AAML0631, whereas the comparator study, AIDA 0493, did exclude patients with WHO performance status (PS) ≥ 4 or creatinine, alkaline phosphatase, and ALT or AST greater than three times upper normal limit. Patients with preexisting prolonged QT syndrome were excluded from AAML0631 because of the risk of QT interval prolongation during ATO therapy. The National Cancer Institute’s central institutional review board and institutional review boards at each enrolling center approved the study; patients and their families provided informed consent or assent. The trial was conducted in accordance with the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov as NCT00866918.
Patients were stratified by diagnostic WBC count into SR (WBC count < 10,000 cells/µL) or HR (WBC ≥ 10,000 cells/µL) groups. Cytogenetic data were reviewed centrally. For patients to remain on protocol therapy and be evaluable for outcome analysis, t(15;17) needed to be confirmed by PML-RARα (promyelocytic leukemia and retinoic acid receptor alpha fusion gene) polymerase chain reaction (PCR). The centralized laboratory testing the samples used real-time quantitative reverse-transcription PCR (RQ-PCR). PML-RARα PCR was performed on patient samples at end of consolidation therapy and every 3 months thereafter until 1 year off therapy.
Treatment Protocol
ATRA was given at a dose of 25 mg/m2/d divided twice daily on days 1 to 30 of induction and days 1 to 14 of each consolidation course and each maintenance cycle (Table 1; Data Supplement). Induction comprised ATRA and idarubicin, and consolidation comprised two cycles of ATRA and ATO. Patients received two (SR) or three (HR) additional cycles of chemotherapy consolidation. Patients received approximately 2 years (nine cycles of 12 weeks each) of maintenance therapy comprising ATRA, 6-mercaptopurine, and oral methotrexate. Patients received three (SR) or four (HR) prophylactic treatments of intrathecal cytarabine.
Table 1.
Treatment Regimens for the COG AAML0631 and GIMEMA-AIEOP AIDA 0493 Trials

Statistical Analysis
Data from AAML0631 are current as of September 30, 2015. The Kaplan-Meier method was used to estimate OS (time from study entry to death) and EFS (time from study entry until failure to achieve complete remission [CR] at end of consolidation 1, relapse, or death).12 Disease-free survival (DFS) was evaluated from the end of consolidation 1 (after completion of the ATO cycles) until relapse or death. Cumulative incidence methods were used to calculate RR, defined as time from the end of consolidation 1 for patients in CR to relapse or death, where deaths without relapse were considered competing events.13 Appearance of promyeloblasts or abnormal promyelocytes (≥ 5%) or two consecutive positive PML-RARα RQ-PCR tests in the bone marrow was considered disease relapse. Death before achieving CR, death within 14 days of diagnosis from any cause, or refractory disease (hematologic CR not achieved by the end of consolidation 1) were defined as failures. The significance of predictor variables was tested by the log-rank statistic for OS and EFS and the Gray’s statistic for RR. All estimates are reported with two times the Greenwood SEs. Patients lost to follow-up were censored at their date of last known contact.
The study was designed to have 90% power with one-sided testing at the 20% level of statistical significance to detect an 11% decrease in EFS (increase in the hazard of failure of 1.76) for SR patients treated in this trial compared with a benchmark on the basis of results from AIDA 0493. A one-sample log-rank test was used to assess whether the EFS experience for SR patients in this study was inferior to that resulting from an exponential mixture cure model that adequately modeled EFS for SR patients in AIDA 0493 (A.M. Testi, personal communication, February 2006).5,14 To monitor insufficient EFS for SR patients, the Lan-DeMets criterion with an α-spending function αt2 (truncated at 3 SDs) and 20% type I error was used. Interim monitoring boundaries were not crossed.
RESULTS
Patient Characteristics
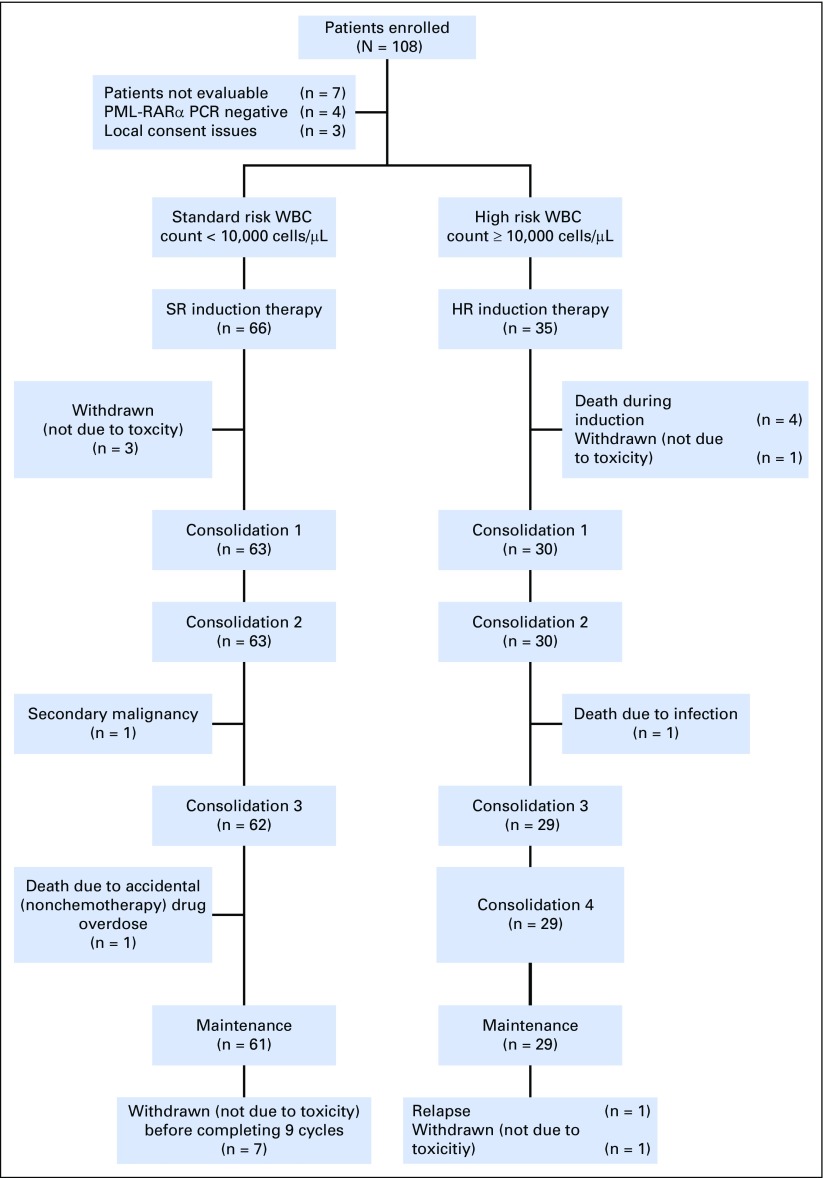
This trial accrued patients between March 2009 and November 2012. Of the 108 patients enrolled, 101 (66 SR and 35 HR) were evaluable for treatment outcome. Nonevaluable patients included four whose samples were PML-RARα PCR negative and three who were excluded because of improper consent procedures at local sites (Fig 1).
Fig 1.
CONSORT diagram for the study. HR, high risk; PCR, polymerase chain reaction; PML-RARα, promyelocytic leukemia and retinoic acid receptor alpha fusion gene; SR, standard risk.
Patient characteristics for HR and SR APL groups are presented in Table 2. Cytogenetic analysis revealed only the classic t(15;17) in 63% (n = 62) of patients, but an additional one, two, three, and more than three cytogenetic abnormalities were present in 31% (n = 30), 3% (n = 3), 2% (n = 2), and 1% (n = 2) of patients, respectively. Patients with SR APL in AAML0631 and the historical comparator AIDA 0493 had similar characteristics except for differences in racial/ethnic diversity and distribution of PS scores (Table 2).
Table 2.
Characteristics for Patients Enrolled in AIDA 0493 and COG AAML0631

Lumbar puncture was not required at initial diagnosis, but in 28 patients with diagnostic lumbar puncture, seven had blasts present in an atraumatic CSF specimen. There was no central review of CSF pathology. Six of these seven patients remain in remission, and one patient died of infectious complications during consolidation therapy.
Toxicity Data
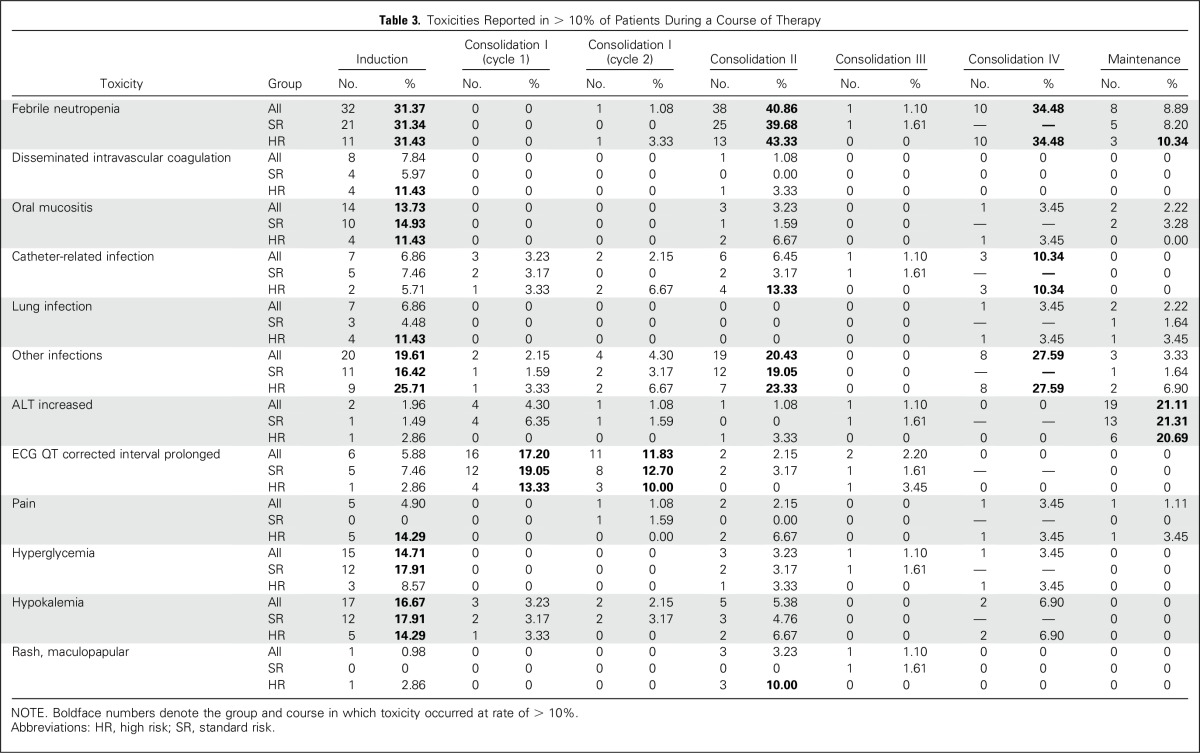
Adverse events included grade ≥ 3 nonhematologic toxicities and grades 1 to 5 QTc interval prolongation, ventricular arrhythmia, or left ventricular systolic dysfunction. The percentage of patients with adverse events varied by treatment course and was highest during induction and high-dose cytarabine–containing courses as follows: induction (82%, n = 84), consolidation 1 cycle 1 (34%, n = 32), consolidation 1 cycle 2 (30%, n = 28), consolidation 2 (73%, n = 68), consolidation 3 (13%, n = 12), consolidation 4 (69%, n = 20), and maintenance cycles combined (53%, n = 48; Table 3). The most common adverse events were fever/neutropenia and infection. Differentiation syndrome (an APL-associated cardiopulmonary distress syndrome) was diagnosed in 20% (n = 20) of patients during induction, and its prevalence was 31% in HR APL and 13% in SR APL. Fifteen of these patients required temporary holding of ATRA in induction, but all were able to restart ATRA at reduced dose and advance to full dosing. The prevalence of ATRA-associated pseudotumor cerebri was ≤ 6% in each course. Targeted cardiac and liver toxicities were analyzed for the two cycles of ATO during consolidation 1. Grade 1 or 2 QTc interval prolongation occurred in 16% (n = 15) and 12% (n = 11) of patients during the ATO cycles. One patient experienced grade 3 QTc interval prolongation during ATO consolidation, and there were no grade 4 or 5 events for this toxicity. There was one event each of grade 1 ventricular arrhythmia and grade 1 left ventricular systolic dysfunction during ATO consolidation. Starting in maintenance, yearly echocardiogram and ECG are to be obtained to 10 years from diagnosis, and the only cardiac events reported off therapy are one grade 1 QTc interval prolongation and one grade 2 ventricular arrhythmia. There have been no cardiac deaths. Liver toxicity was minimal during ATO cycles, with < 5% of patients having grade ≥ 3 increased levels of aspartate aminotransferase or alanine aminotransferase and one patient having elevated levels of bilirubin.
Table 3.
Toxicities Reported in > 10% of Patients During a Course of Therapy
Response Data
Bone marrow evaluation at the end of induction relied on local assessment (without central review) of hematologic CR (hCR), which was defined as < 5% promyeloblasts/abnormal promyelocytes by morphology. The hCR rate was 81%; however, patients could remain on protocol therapy regardless of the hCR response at the end of induction. PML-RARα RQ-PCR analysis at end of consolidation revealed a molecular remission rate of 100% (PCR data missing for seven patients).
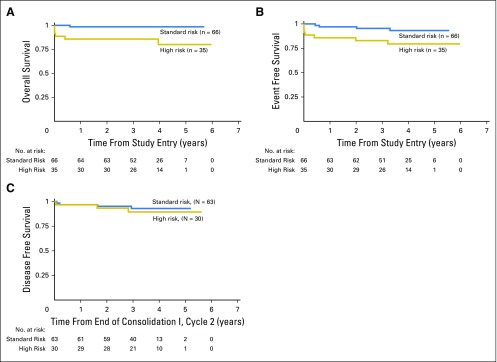
The median follow-up for patients alive at last contact was 3.73 (range, 0.003 to 5.97) years. The 3-year OS was 94% ± 5%, and the 3-year EFS was 91% ± 6%. The 3-year OS for SR and HR patients with APL was 98% ± 3% and 86% ± 12%, and 3-year EFS was 95% ± 5% and 83% ± 13%, respectively (Fig 2). For patients who survived to receive ATO in consolidation 1, the 3-year DFS was 93% ± 6% for SR and 89% ± 12% for HR APL (Fig 2). The RR for all patients was only 4% ± 4% at 3 years. The SR patients with APL in our trial had a 2-year EFS of 97% (including molecular relapse as an event) compared with that of 91% for patients in AIDA 0493 (which censored molecular relapses). This outcome met the protocol’s predetermined statistical criteria to conclude that the current therapy is not inferior to that in AIDA 0493 (P = .93).
Fig 2.
Survival by risk group including (A) overall survival, (B) event-free survival, and (C) disease-free survival.
Events in this trial included three relapses, two second malignant neoplasms, and seven deaths (six HR and one SR), including one death post relapse. Four deaths (all HR) occurred during induction: two related to coagulopathy and two due to multiorgan dysfunction, in which differentiation syndrome likely contributed. There were two postinduction on-therapy deaths: one SR patient with APL died of accidental (nonchemotherapy) drug overdose during consolidation 3, and one HR patient with APL died of Serratia marcescens sepsis during consolidation 2. Of the two patients with second malignancies, one had skin cancer (carcinoma in situ) at 5 months after APL diagnosis and one had acute monoblastic/monocytic leukemia at 40 months after APL diagnosis.
The three relapses occurred in one SR and two HR patients with APL. The SR patient with APL had a combined molecular bone marrow and morphologic CNS relapse during maintenance, nearly 24 months after diagnosis. This patient achieved second remission with chemotherapy and is alive at 4 years off therapy without stem-cell transplantation. One HR APL patient had a combined hematologic bone marrow and CNS relapse during maintenance at 23 months after diagnosis. This patient received an autologous stem-cell transplantation with cyclophosphamide, total-body irradiation, and cranial radiation boost and is alive at 6 months after relapse. The other HR patient with APL had a molecular bone marrow relapse after completion of therapy at 38 months after diagnosis. This patient received allogeneic (double umbilical cord blood) transplant with fludarabine, cyclophosphamide, and total-body irradiation but died 9 months after relapse while in remission as a result of graft-versus-host disease.
DISCUSSION
Treatment of pediatric APL with anthracyclines and ATRA has resulted in excellent outcomes, but in this young population intensive chemotherapy regimens may cause significant toxicity, including late cardiotoxicity. Our trial demonstrates that chemotherapy with ATO consolidation can result in a low relapse rate in both SR and HR APL. The use of ATO allowed an approximate 40% decrease in cumulative anthracycline dosing without compromising survival in SR APL compared with a benchmark from the AIDA 0493 trial. Furthermore, the 3-year EFS 83% and OS 86% for the HR group compares favorably to reported outcomes in other pediatric APL trials, including the 3-year EFS of 74% in AIDA 0493 and 5-year OS of 83% in Spanish Cooperative Group for Hematological Malignancies Treatment, Spanish Society of Hematology (PETHEMA) LPA96 and LPA99 trials (A.M. Testi, personal communication, April 2017).5,15
An important goal of this trial was to study toxicities in pediatric patients treated with ATO and reduced anthracycline doses. Most deaths of patients receiving therapy occurred during induction because of disease complications of differentiation syndrome and coagulopathy. One patient died as a result of infection during consolidation 2, which includes mitoxantrone (a highly myelosuppressive anthracycline). This finding highlights the importance of recent efforts to transition to ATRA and ATO consolidation without traditional cytotoxic chemotherapy. ATO is associated with cardiac toxicity, particularly QTc prolongation.16,17 In this trial, ATO therapy did result in prolonged QTc interval, but these were almost exclusively grades 1 and 2. There were no heart failure events or cardiac deaths. Reducing the anthracycline dose from 600 mg/m2 daunorubicin equivalents in AIDA 0493 to 335 mg/m2 (for SR APL) and 385 mg/m2 (for HR APL) in the current trial represents a significant improvement in reducing the risk of acute and chronic treatment toxicity.
Two patients had second malignancies in this trial. The occurrence of squamous cell carcinoma in situ is of interest, because exposure to arsenic as an environmental toxin is linked to this disease.18,19 A study by the MD Anderson Cancer Center found the incidence of second neoplasms in patients receiving ATO/ATRA therapy was not greater than for those receiving ATRA and chemotherapy. Of 106 patients treated with ATO/ATRA, two patients had second neoplasms, including a case of melanoma.20 Acute monoblastic/monocytic leukemia occurred as a second malignancy in the current trial, and the Spanish PETHEMA group found a 2.2% rate of therapy-related myeloid neoplasms in > 1,000 patients with APL (mostly adults) treated with ATRA and anthracyclines but without ATO.21 Given the type and timing of therapy-related AML in the current study, we predict that anthracycline or etoposide exposure was the causative agent; however, the contribution of other chemotherapeutic agents or ATO remains unclear.22
Relapses (including molecular relapse) occurred in three patients in this trial. The 3-year relapse rate was 4% ± 4%, which compares favorably to that in AIDA 0493. In AIDA 0493, the definition of relapse during most of the accrual period included hematologic relapse only, and the protocol was later amended to include molecular relapse. Before this amendment, 85 children achieved molecular remission at the end of consolidation, 21 (25%) of whom developed hematologic relapse. The median time to relapse was 26 months (range, 6 to 72 months), which suggests that the favorable 3-year relapse rate in the current study represents a true decrease in relapse attributable to ATO. Similarly, the CALGB 9710 trial demonstrated a significant reduction in relapse among adult patients with APL randomly assigned to ATO consolidation.6
Some previous reports on adult APL suggest that additional cytogenetic abnormalities (ACAs) other than t(15;17) portend a poor outcome.23-25 In contrast, several recent and large studies including ATRA therapy have shown no effect of ACAs on outcome.26-28 We found no significant difference in outcome on the basis of presence of ACAs.
In the current study, all patients received oral chemotherapy maintenance. In contrast, AIDA 0493 initially randomly assigned patients to four maintenance arms: mercaptopurine and methotrexate (6MP/MTX), ATRA alone, 6MP/MTX alternating with ATRA, or observation. Long-term follow-up revealed no difference in DFS among the maintenance arms.29 Similarly, the North American Intergroup C9710 study found no difference in outcomes between a limited cohort of patients receiving observation versus ATRA maintenance or in a larger cohort of patients receiving ATRA versus ATRA plus 6MP/MTX.30,31 It will be important to determine whether maintenance therapy can be safely removed from pediatric APL therapy that includes ATO.
This study is limited by its nonrandomized design, and the outcome of patients in the HR arm was not initially designed to have statistical power for comparison. However, because of the rarity of pediatric APL, a nonrandomized design was warranted to evaluate the safety and efficacy of ATO. The survival benchmark for this study was based on results from the pediatric cohort in AIDA 0493, which represents the largest (N = 107) and best outcomes previously published for a pediatric phase III trial in APL, although the inclusion and exclusion criteria had some variations from our current study, as noted in the methods.5 The PS of SR patients with APL in our trial suggested less impairment (majority with PS 0) than for AIDA 0493. It is unclear whether this represents a true difference in PS or whether there is a difference in national tendencies for reporting of these scores, because all patients in AIDA 0493 had PS of 1 or 2, whereas, in AAML0631, the PS ranged from 0 to 4. The current trial was an exclusively pediatric study that included a similarly large number (N = 101) of patients. Another study limitation was the lack of central review of end induction remission status, and the reported CR rate was lower than that for historical comparisons. Without a central review, the reported CR rate might be inaccurate, but end induction remission status did not result in a change in therapy in this trial. SR patients with APL with persistent molecular disease at the end of consolidation were eligible for augmented therapy, but no patients required this intensification. The excellent molecular remission rate and the low relapse rate confirm the effectiveness of this regimen for both SR and HR APL.
The favorable results of this study incorporating ATO consolidation with reduced anthracycline dose provide a new benchmark for outcome in pediatric APL. The Italian-German APL0406 and the Australasian APML4 studies demonstrate that intensified ATO consolidation can allow further reduction or elimination of traditional chemotherapy.10,32 The Children’s Oncology Group is currently investigating similar approaches in pediatric patients with APL in an actively accruing trial. The AAML0631 trial also demonstrates that early deaths attributable to coagulopathy and differentiation syndrome remain an obstacle in the cure of APL. Further research of supportive care and treatment strategies for these complications should be an objective of APL trials.
ACKNOWLEDGMENT
We thank the Children’s Oncology Group (COG) institutions and the investigators who cared for children enrolled in this trial. We thank the patients and their families for their willingness to participate in this trial. We thank COG protocol coordinator Tanya Wallas-Shannon and COG research coordinator Caroline Wang for all their efforts in the development and conduct of this trial. We thank Vani Shanker, ELS, for scientific editing.
Footnotes
Supported by Chair’s Grant No. U10CA098543, National Clinical Trials Network Operations Center Grant No. U10CA180886, Statistics and Data Center Grant No. U10CA098413, and National Clinical Trials Network Statistics and Data Center Grant No. U10CA180899 of the Children’s Oncology Group from the National Cancer Institute, and by the St Baldrick’s Foundation and the Andrew McDonough B+ Foundation.
Presented in part at the Annual Meetings of the American Society of Hematology, December 6-9, 2014 (San Francisco, CA), and December 5-8, 2015 (Orlando, FL).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical trial information: NCT00866918.
AUTHOR CONTRIBUTIONS
Conception and design: Todd A. Alonzo, Cecilia H. Fu, Soheil Meshinchi, Alan S. Gamis, James H. Feusner, John J. Gregory Jr
Collection and assembly of data: Matthew A. Kutny, Todd A. Alonzo, Susana C. Raimondi, Betsy A. Hirsch, Soheil Meshinchi, John J. Gregory Jr
Data analysis and interpretation: Matthew A. Kutny, Todd A. Alonzo, Robert B. Gerbing, Yi-Cheng Wang, Susana C. Raimondi, Betsy A. Hirsch, James H. Feusner, John J. Gregory Jr
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Arsenic Trioxide Consolidation Allows Anthracycline Dose Reduction for Pediatric Patients With Acute Promyelocytic Leukemia: Report From the Children's Oncology Group Phase III Historically Controlled Trial AAML0631
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Matthew A. Kutny
No relationship to disclose
Todd A. Alonzo
No relationship to disclose
Robert B. Gerbing
Stock or Other Ownership: Pfizer
Yi-Cheng Wang
No relationship to disclose
Susana C. Raimondi
No relationship to disclose
Betsy A. Hirsch
No relationship to disclose
Cecilia H. Fu
No relationship to disclose
Soheil Meshinchi
No relationship to disclose
Alan S. Gamis
Consulting or Advisory Role: Novartis
James H. Feusner
No relationship to disclose
John J. Gregory Jr
Consulting or Advisory Role: Celgene
REFERENCES
- 1.Aldouri MA, Lopes ME, Yacoub M, et al. : Cardiac transplantation for doxorubicin-induced cardiomyopathy in acute myeloid leukaemia. Br J Haematol 74:541, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Thomas X, Le QH, Fiere D: Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Ann Hematol 81:504-507, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Shankar SM, Marina N, Hudson MM, et al. : Monitoring for cardiovascular disease in survivors of childhood cancer: Report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics 121:e387-e396, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Gregory J, Kim H, Alonzo T, et al. : Treatment of children with acute promyelocytic leukemia: Results of the first North American Intergroup trial INT0129. Pediatr Blood Cancer 53:1005-1010, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testi AM, Biondi A, Lo Coco F, et al. : GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood 106:447-453, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Powell BL, Moser B, Stock W, et al. : Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 116:3751-3757, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George B, Mathews V, Poonkuzhali B, et al. : Treatment of children with newly diagnosed acute promyelocytic leukemia with arsenic trioxide: A single center experience. Leukemia 18:1587-1590, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Zhang Y, Li J, et al. : Single-agent arsenic trioxide in the treatment of children with newly diagnosed acute promyelocytic leukemia. Blood 115:1697-1702, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhu X, Zou Y, et al. : Effect of arsenic trioxide on the treatment of children with newly diagnosed acute promyelocytic leukemia in China. Int J Hematol 93:199-205, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Lo-Coco F, Avvisati G, Vignetti M, et al. : Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 369:111-121, 2013 [DOI] [PubMed] [Google Scholar]
- 11. Children’s Oncology Group: Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers: Version 4.0. http://www.survivorshipguidelines.org/ [PubMed]
- 12.Kaplan E, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 13.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, NJ, John Wiley & Sons, 2002 [Google Scholar]
- 14.Woolson RF: Rank tests and a one-sample logrank test for comparing observed survival data to a standard population. Biometrics 37:687-696, 1981 [Google Scholar]
- 15.Ortega JJ, Madero L, Martín G, et al. : Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: A multicenter study by the PETHEMA Group. J Clin Oncol 23:7632-7640, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Chiang CE, Luk HN, Wang TM, et al. : Prolongation of cardiac repolarization by arsenic trioxide. Blood 100:2249-2252, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Roboz GJ, Ritchie EK, Carlin RF, et al. : Prevalence, management, and clinical consequences of QT interval prolongation during treatment with arsenic trioxide. J Clin Oncol 32:3723-3728, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Yu HS, Liao WT, Chai CY: Arsenic carcinogenesis in the skin. J Biomed Sci 13:657-666, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Wong SS, Tan KC, Goh CL: Cutaneous manifestations of chronic arsenicism: Review of seventeen cases. J Am Acad Dermatol 38:179-185, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Eghtedar A, Rodriguez I, Kantarjian H, et al. : Incidence of secondary neoplasms in patients with acute promyelocytic leukemia treated with all-trans retinoic acid plus chemotherapy or with all-trans retinoic acid plus arsenic trioxide. Leuk Lymphoma 56:1342-1345, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montesinos P, González JD, González J, et al. : Therapy-related myeloid neoplasms in patients with acute promyelocytic leukemia treated with all-trans-retinoic acid and anthracycline-based chemotherapy. J Clin Oncol 28:3872-3879, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Godley LA, Larson RA: Therapy-related myeloid leukemia. Semin Oncol 35:418-429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiorns LR, Swansbury GJ, Mehta J, et al. : Additional chromosome abnormalities confer worse prognosis in acute promyelocytic leukaemia. Br J Haematol 96:314-321, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Slack JL, Arthur DC, Lawrence D, et al. : Secondary cytogenetic changes in acute promyelocytic leukemia--prognostic importance in patients treated with chemotherapy alone and association with the intron 3 breakpoint of the PML gene: A Cancer and Leukemia Group B study. J Clin Oncol 15:1786-1795, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Schlenk RF, Germing U, Hartmann F, et al. : High-dose cytarabine and mitoxantrone in consolidation therapy for acute promyelocytic leukemia. Leukemia 19:978-983, 2005 [DOI] [PubMed] [Google Scholar]
- 26.De Botton S, Chevret S, Sanz M, et al. : Additional chromosomal abnormalities in patients with acute promyelocytic leukaemia (APL) do not confer poor prognosis: Results of APL 93 trial. Br J Haematol 111:801-806, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Hernández JM, Martín G, Gutiérrez NC, et al. : Additional cytogenetic changes do not influence the outcome of patients with newly diagnosed acute promyelocytic leukemia treated with an ATRA plus anthracyclin based protocol. A report of the Spanish group PETHEMA. Haematologica 86:807-813, 2001 [PubMed] [Google Scholar]
- 28.Cervera J, Montesinos P, Hernández-Rivas JM, et al. : Additional chromosome abnormalities in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Haematologica 95:424-431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avvisati G, Lo-Coco F, Paoloni FP, et al. : AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: Very long-term results and role of maintenance. Blood 117:4716-4725, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Powell BL, Moser BK, Stock W, et al: Adding mercaptopurine and methotrexate to alternate week ATRA maintenance therapy does not improve the outcome for adults with acute promyelocytic leukemia (APL) in first remission: Results from North American Leukemia Intergroup Trial C9710. Blood 118:258, 2011 (abstr) [Google Scholar]
- 31.Coutre SE, Othus M, Powell B, et al. : Arsenic trioxide during consolidation for patients with previously untreated low/intermediate risk acute promyelocytic leukaemia may eliminate the need for maintenance therapy. Br J Haematol 165:497-503, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iland HJ, Bradstock K, Supple SG, et al: All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 120:1570-1580; quiz 1752, 2012. [DOI] [PubMed]