Abstract
Purpose
T-cell–replete HLA-haploidentical donor hematopoietic transplantation using post-transplant cyclophosphamide was originally described using bone marrow (BM). With increasing use of mobilized peripheral blood (PB), we compared transplant outcomes after PB and BM transplants.
Patients and Methods
A total of 681 patients with hematologic malignancy who underwent transplantation in the United States between 2009 and 2014 received BM (n = 481) or PB (n = 190) grafts. Cox regression models were built to examine differences in transplant outcomes by graft type, adjusting for patient, disease, and transplant characteristics.
Results
Hematopoietic recovery was similar after transplantation of BM and PB (28-day neutrophil recovery, 88% v 93%, P = .07; 100-day platelet recovery, 88% v 85%, P = .33). Risks of grade 2 to 4 acute (hazard ratio [HR], 0.45; P < .001) and chronic (HR, 0.35; P < .001) graft-versus-host disease were lower with transplantation of BM compared with PB. There were no significant differences in overall survival by graft type (HR, 0.99; P = .98), with rates of 54% and 57% at 2 years after transplantation of BM and PB, respectively. There were no differences in nonrelapse mortality risks (HR, 0.92; P = .74) but relapse risks were higher after transplantation of BM (HR, 1.49; P = .009). Additional exploration confirmed that the higher relapse risks after transplantation of BM were limited to patients with leukemia (HR, 1.73; P = .002) and not lymphoma (HR, 0.87; P = .64).
Conclusion
PB and BM grafts are suitable for haploidentical transplantation with the post-transplant cyclophosphamide approach but with differing patterns of treatment failure. Although, to our knowledge, this is the most comprehensive comparison, these findings must be validated in a randomized prospective comparison with adequate follow-up.
INTRODUCTION
Allogeneic hematopoietic cell transplantation using a T-cell–replete HLA-haploidentical donor transplantation, with post-transplant cyclophosphamide to control alloreactivity, has recently been demonstrated as a safe and effective alternative in patients who lack timely access to a matched related or unrelated donor.1-5 In retrospective comparisons adjusted for confounding covariables, recipients of transplants using HLA-haploidentical donors with a post-transplant cyclophosphamide strategy were shown to have equivalent survival and nonrelapse mortality to recipients of matched related and matched unrelated donor transplants and with evidence of lower incidence and severity of chronic graft-versus-host disease (GVHD).3,4,6,7
As originally developed by the Baltimore group, HLA-haploidentical transplantation with post-transplant cyclophosphamide was performed using a bone marrow (BM) graft. Mobilized peripheral blood (PB) grafts are considered more convenient and are widely used for HLA-matched related and unrelated donor transplantations. In these patients, prospective comparisons have demonstrated similar outcomes to BM transplants except more rapid hematopoietic recovery and a higher incidence of chronic GVHD with PB when using myeloablative conditioning and conventional GVHD prophylaxis.8,9 For HLA-haploidentical transplantation with post-transplant cyclophosphamide, single centers have reported relatively good outcomes using mobilized PB.10-14 In a recent small, matched-pair comparison, nonablative haploidentical transplants with post-transplant cyclophosphamide with PB had similar times to engraftment, rates of acute and chronic GVHD, and overall survival but lower relapse compared with BM.15 In the absence of prospective trials of outcomes after haploidentical transplants using post-transplant cyclophosphamide and PB grafts, we used data reported to the Center for International Blood and Marrow Transplant Research to study outcomes after transplantation of PB compared with BM for hematologic malignancy.
PATIENTS AND METHODS
Patients
The Center for International Blood and Marrow Transplant Research is a group of over 350 transplant centers that contribute data prospectively on consecutive transplants performed at each individual center. Forty-two centers contributed patients, and transplants were performed between 2009 and 2014 in the United States. Eligible patients were ≥ 18 years of age with acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, non-Hodgkin lymphoma, or Hodgkin lymphoma. All received BM or PB from haploidentical related donors and a uniform GVHD prophylaxis regimen (tacrolimus or cyclosporine with mycophenolate and post-transplant cyclophosphamide). The reduced-intensity conditioning regimen was uniform for both graft types (total-body irradiation [TBI] 2 Gy, cyclophosphamide 29 mg/kg, fludarabine 150 mg/m2). Myeloablative regimens included TBI (≥ 10 Gy with fludarabine or cyclophosphamide) or busulfan and cyclophosphamide with or without fludarabine. Excluded were transplant regimens that were melphalan-based (n = 109) and busulfan with fludarabine (n = 13) because these were exclusively used for BM transplants. Also excluded were regimens that included antithymocyte globulin or alemtuzumab (n = 92) or CD34 selected PB grafts (n = 139). Patients provided written informed consent for research. The institutional review board of the National Marrow Donor Program approved this study.
End Points
The primary end point was overall survival. Death from any cause was considered an event. Neutrophil recovery was defined as achieving an absolute neutrophil count of ≥ 0.5 × 109/L for 3 consecutive days, and platelet recovery was defined as platelets ≥ 20 × 109/L, unsupported by transfusion for 7 days. Primary and secondary graft failures were considered as a single outcome. Primary graft failure was defined as failure to achieve an absolute neutrophil count of ≥ 0.5 × 109/L for 3 consecutive days or donor chimerism < 5% (PB CD3+ or BM). Secondary graft failure was defined as initial donor engraftment followed by graft loss, evidenced by a persistent decline in the absolute neutrophil count (< 0.5 × 109/L) or loss of donor chimerism < 5% or a second transplantation in patients with documented clinical remission.16 Grades 2 to 4 and 3 to 4 acute GVHD and chronic GVHD were based on reports from each transplant center using standard criteria.17,18 Relapse/progression was defined as disease recurrence (morphologic, cytogenetic, or molecular) or progression, and nonrelapse mortality was defined as death in remission. Progression-free survival (PFS) was defined as surviving in remission (relapse/progression or death were considered events). GVHD-free, relapse-free survival (GRFS) events included grade 3 to 4 acute GVHD, chronic GVHD requiring systemic therapy, relapse or progression, or death from any cause within the first year after transplantation.19 Surviving patients were censored at last follow-up.
Statistical Methods
Differences between groups were compared using the χ2 statistic for categorical variables. The probabilities of overall survival, PFS, and GRFS were calculated using the Kaplan-Meier estimator.20 The probabilities of neutrophil and platelet recovery, acute and chronic GVHD, nonrelapse mortality, and relapse/progression were calculated using the cumulative incidence estimator to accommodate competing risks.21 Cox regression models were built to study the effect of graft type (BM v PB) and other factors associated with overall mortality, grade 2 to 4 and grade 3 to 4 acute GVHD, chronic GVHD, relapse/progression, nonrelapse mortality and treatment failure (inverse of PFS), and GRFS.22 Variables tested included: graft type, age, sex, performance score, hematopoietic cell transplant comorbidity index (HCT-CI) score, cytomegalovirus (CMV) serostatus, disease, disease status, disease risk index (DRI; a composite of disease, disease status at transplant, cytogenetic risk for leukemias and disease, and disease status at transplant for lymphomas),23 transplant conditioning regimen intensity, and transplant period. All variables tested met the assumptions for proportionality, and there were no first-order interactions between graft type and other variables held in the final multivariable model. All variables that attained a P value ≤ .05 were held in the final multivariable model, with the exception of the variable for graft type that was held in all steps of model building and the final model regardless of the level of significance. Transplant center effect on survival was tested using the frailty approach.24 All P values are two sided. All analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
RESULTS
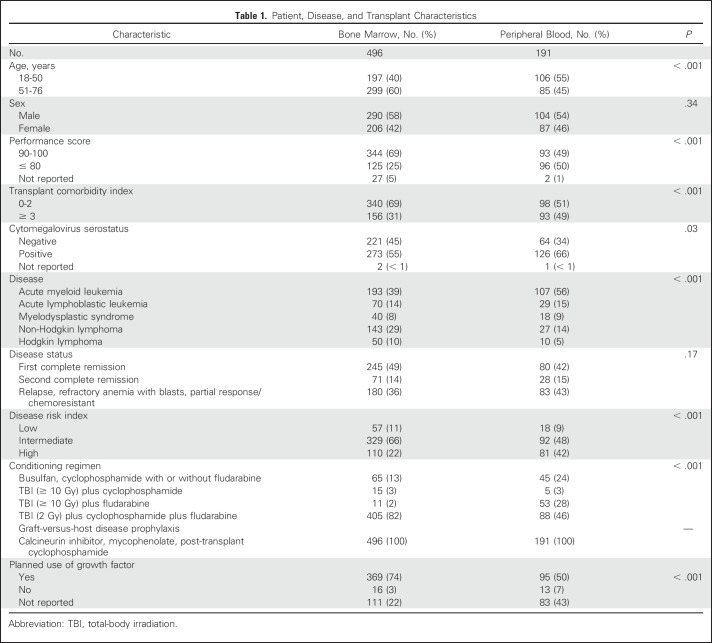
Patient, Disease, and Transplant Characteristics
The characteristics of the study population by graft type are listed in Table 1. The median age of BM recipients was 58 years (range, 18 to 76 years) and that of PB recipients was 47 years (range, 19 to 73 years). Compared with recipients of BM, recipients of PB were younger and less likely to have performance scores of 90 or 100, to have HCT-CI scores of 0 to 2, to be CMV seronegative, and to have received a reduced-intensity conditioning regimen. Recipients of BM were less likely to have undergone transplantation for acute myeloid leukemia (AML) and less likely to have a high DRI. The majority of BM recipients received a reduced-intensity conditioning regimen that was uniform for both graft types. Although myeloablative regimens were restricted to TBI ≥ 10 Gy with fludarabine or cyclophosphamide and busulfan and cyclophosphamide with or without fludarabine, BM recipients were less likely to receive a TBI-containing regimen compared with PB recipients. Because the majority of PB transplants were performed after 2011, the median follow-up of PB recipients was 20 months (range, 6 to 72 months) compared with 35 months (range, 3 to 74 months) for BM recipients.
Table 1.
Patient, Disease, and Transplant Characteristics
Hematopoietic Recovery
The median times to neutrophil and platelet recovery were slower after transplantation of BM compared with PB (17 v 16 days for neutrophils, P < .001; and 26 v 25 days for platelets, P = .03). Despite this, there were no significant differences in day-28 rates of neutrophil recovery after transplantation of BM and PB grafts (88%; 95% CI, 85 to 91; and 93%; 95% CI, 89 to 96, respectively; P = .07) and in day-100 rates of platelet recovery (88%; 95% CI, 85 to 91; and 85%; 95% CI, 80 to 90; P = .33). The 1-year cumulative incidence of primary or secondary graft failure rates after transplantation of BM and PB were 9% (95% CI, 7 to 12) and 12% (95% CI, 8 to 17; P = .23), respectively.
GVHD
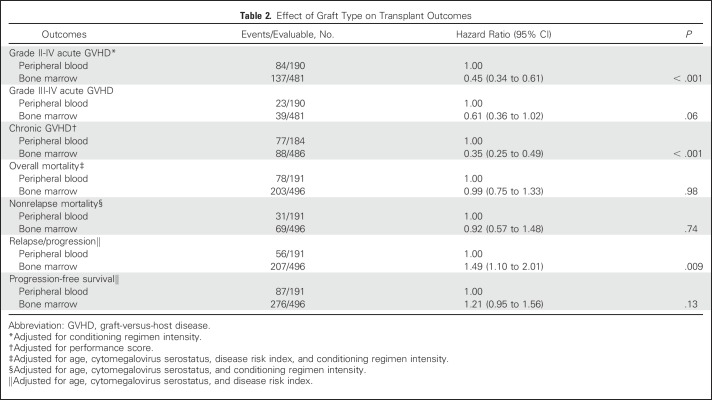
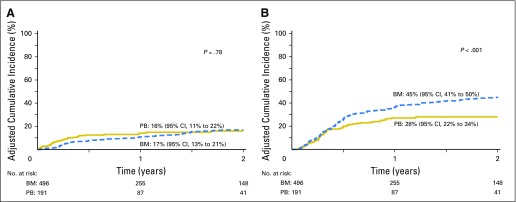
Compared with transplantation of PB, grade 2 to 4 acute GVHD was lower after transplantation of BM, but there were no differences in risks of grade 3 to 4 acute GVHD (Table 2; Fig 1A). Independent of graft type, grade 2 to 4 acute GVHD was higher with reduced-intensity regimens (HR, 1.51; 95% CI, 1.09 to 2.10; P = .01). The 6-month incidence of grade 2 to 4 acute GVHD was 25% (95% CI, 21% to 29%) and 42% (95% CI, 35% to 50%) after transplantation of BM and PB, respectively. Chronic GVHD risks were also lower after transplantation of BM (Table 2). Risks were higher for patients with performance scores < 90 (HR, 1.50; 95% CI, 1.08 to 2.08; P = .01). The 2-year incidence of chronic GVHD was 20% (95% CI, 16% to 24%) and 41% (95% CI, 33% to 48%), after transplantation of BM and PB, respectively (Fig 1B). Despite differences in chronic GVHD risk by graft type, there were no differences in its severity by graft type (P = .64). Among BM recipients (n = 90) with chronic GVHD, severity was graded as mild for 62%, moderate for 28%, and severe for 10%. Corresponding severity rates for PB recipients (n = 78) was 58%, 30%, and 12%, respectively. Chronic GVHD rates were lower with BM grafts in the setting of both myeloablative (HR, 0.35; 95% CI, 0.23 to 0.52; P < .001) and reduced-intensity (HR, 0.28; 95% CI, 0.14 to 0.55; P < .001) regimens.
Table 2.
Effect of Graft Type on Transplant Outcomes
Fig 1.
(A) The 6-month incidence of grades 2 to 4 (left panel) acute graft-versus-host disease (GVHD) adjusted for conditioning regimen intensity were 25% (95% CI, 21% to 29%) and 42% (95% CI, 35% to 50%) after bone marrow (BM) and peripheral blood (PB) transplants, respectively. The 6-month incidence of grades 3 to 4 (right panel) acute GVHD adjusted for conditioning regimen intensity were 7% (95% CI, 5% to 10%) and 10% (95% CI, 6% to 15%) after BM and PB transplants, respectively. (B) The 2-year incidence of chronic GVHD adjusted for performance score were 20% (95% CI, 16% to 24%) and 41% (95% CI, 33% to 48%) after BM and PB transplants, respectively.
Overall Survival
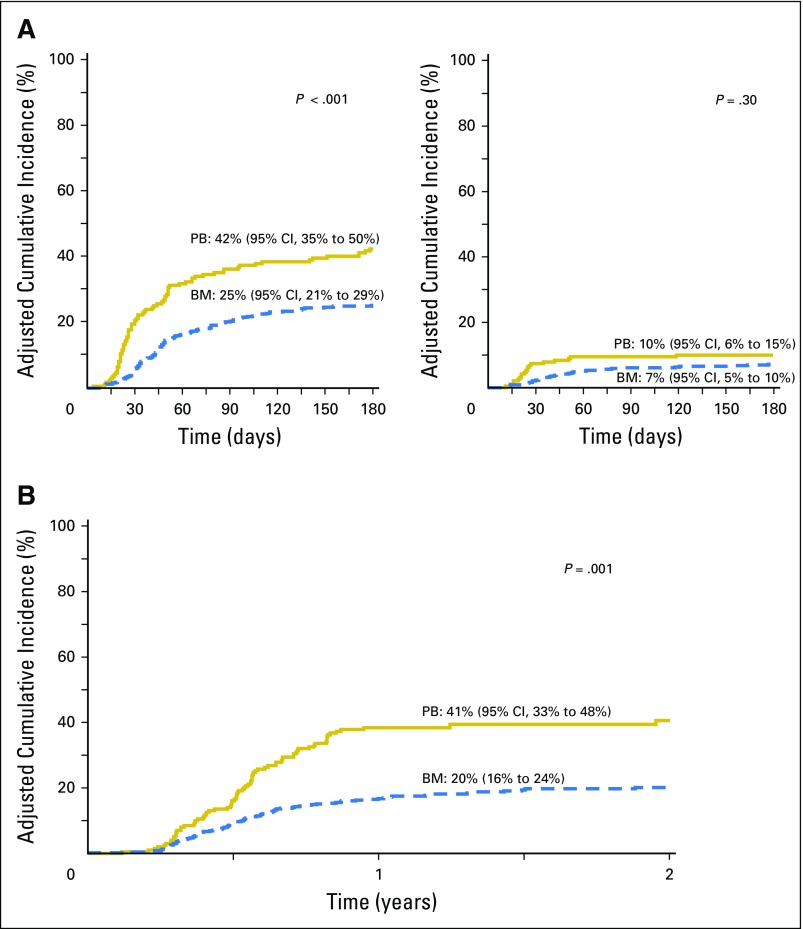
The risks of overall mortality did not differ by graft type (Table 2; Fig 2). Other factors associated with higher mortality included age, older than 55 years of age (HR, 1.72; 95% CI, 1.34 to 2.21; P < .001), CMV seropositivity (HR, 1.47; 95% CI, 1.15 to 1.89; P = .002), high DRI (HR, 2.12; 95% CI, 1.31 to 3.45; P < .001), and myeloablative conditioning regimen (HR, 1.39; 95% CI, 1.04 to 1.85; P = .03). The effect of graft type on survival was further tested, adjusting for age, performance score, HCT-CI score, CMV seropositivity, disease type, disease status, DRI, and transplant conditioning regimen; consistent with the main model, overall mortality risks did not differ by graft type (Appendix Table A1, online only). The effect of the transplant center on overall survival was explored, and no relationship was found (P = .82). There were no differences in causes of death by graft type (P = .13); recurrent disease was the most common cause of death, accounting for 69% of deaths in BM recipients and 67% in PB recipients. There were no differences in proportion of deaths attributed to GVHD, infection, interstitial pneumonitis, or organ failure by graft type.
Fig 2.
The 2-year probabilities of overall survival adjusted for age, cytomegalovirus serostatus, disease risk index, and transplant conditioning regimen intensity were 54% (95% CI, 49% to 59%) and 57% (95% CI, 49% to 65%) after bone marrow (BM) and peripheral blood (PB) transplants.
Nonrelapse Mortality
The risk of nonrelapse mortality also did not differ by graft type (Table 2; Fig 3A). Independent of graft type, nonrelapse mortality risks were higher for patients who were older than 55 years of age (HR, 2.56; 95% CI, 1.67 to 3.92; P < .001), were CMV seropositive (HR, 1.96; 95% CI, 1.26 to 3.04; P = .003), and received myeloablative conditioning regimens (HR, 1.86; 95% CI, 1.16 to 3.00; P = .01).
Fig 3.
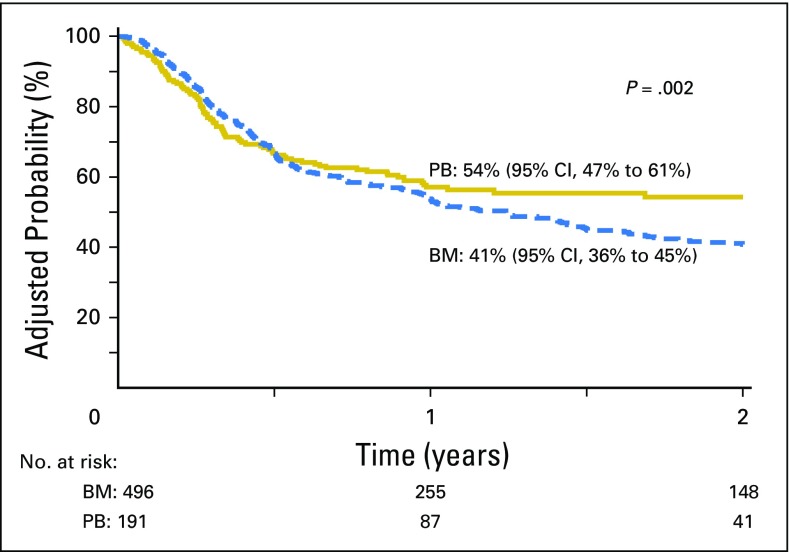
(A) The 2-year incidence of nonrelapse mortality adjusted for age, cytomegalovirus serostatus, and transplant conditioning regimen intensity was 17% (95% CI, 13% to 21%) and 16% (95% CI, 11% to 22%) after bone marrow (BM) and peripheral blood (PB) transplants, respectively. (B) The 2-year incidence of relapse/progression adjusted for disease risk index was 45% (95% CI, 41% to 50%) and 28% (95% CI, 22% to 34%) after BM and PB transplants.
Relapse/Progression
Relapse/progression was higher after BM compared with PB transplants (Table 2; Fig 3B), adjusted for an intermediate (HR, 2.28; 95% CI, 1.32 to 3.94; P = .003) and a high (HR, 3.81; 95% CI, 2.17 to 6.69; P < .001) DRI. The effect of graft type on relapse/progression may be influenced by disease type (P = .05); therefore, in subset analysis, the effect of disease type was tested separately for leukemia/myelodysplastic syndrome (MDS) and lymphoma. For patients with leukemia/MDS (predominantly AML), relapse risks were higher with transplantation of BM compared with PB (HR, 1.73; 95% CI, 1.23 to 2.44; P = .002) after adjusting for DRI. Although there were no differences in the proportion of patients with de novo AML, BM recipients were older (difference of 8 years in median age), but had better performance and HCT-CI scores, were more likely to be in remission at transplantation, and were more likely to have received reduced-intensity conditioning (Appendix Table A2, online only). We did not observe differences in relapse risks by graft type for patients with lymphoma (HR, 0.87; 95% CI, 0.48 to 1.58; P = .64). Transplant conditioning regimen intensity was not associated with relapse/progression (P = .09).
Because higher risk of relapse/progression with transplantation of BM could potentially be attributed to lower GVHD rates, acute and chronic GVHD were tested as time-dependent covariables to study graft-versus-tumor effects. Relapse risks were higher after transplantation of BM, adjusting for acute GVHD (HR, 2.22; P = .02), chronic GVHD (HR, 1.92; P = .02), and acute and chronic GVHD (HR, 2.13; P = .01), suggesting that the lower relapse rate observed using PB could not adequately be explained by the higher rates of GVHD associated with this graft source.
PFS
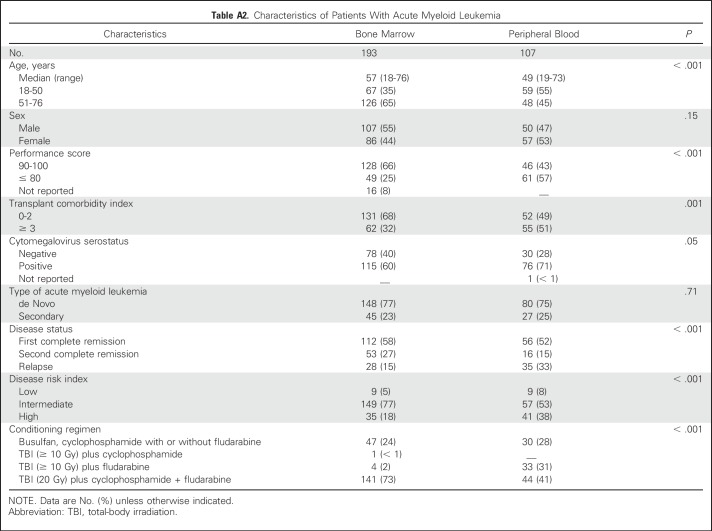
Although the overall PFS did not differ by graft type (Table 2), the 2-year probabilities of PFS adjusted for age, CMV serostatus, and DRI support differences by graft type (Fig 4). PFS was worse for patients who were older than 55 years of age (HR, 1.31; 95% CI, 1.06 to 1.61; P = .01), had CMV seropositivity (HR, 1.26; 95% CI, 1.02 to 1.57; P = .03), had an intermediate DRI (HR, 1.59; 95% CI, 1.06 to 2.40; P = .03), and had a high DRI (HR, 2.46; 95% CI, 1.61 to 3.77; P < .001). Because the effect of graft type may be influenced by disease type (P = .05), PFS was studied separately for leukemia/MDS and lymphoma. For patients with leukemia/MDS (predominantly AML), PFS was lower with transplantation of BM (HR, 1.35; 95% CI, 1.01 to 1.80; P = .04). We did not observe differences in PFS by graft type for lymphoma (HR, 0.78; 95% CI, 0.49 to 1.27; P = .32). Transplant conditioning regimen intensity was not associated with PFS (P = .18).
Fig 4.
The 2-year probabilities of progression-free survival adjusted for age, cytomegalovirus serostatus and disease risk index were 41% (95% CI, 36% to 45%) and 54% (95% CI, 47% to 61%) after bone marrow (BM) and peripheral blood (PB) transplants.
GVHD-Free Relapse-Free Survival
Four hundred seventy-three of 496 BM and 182 of 191 PB recipients were evaluable for GRFS. GRFS was higher after BM compared with PB transplants (HR, 0.75; 95% CI, 0.61 to 0.92; P = .006) after adjusting for performance score and DRI, the other factors associated with GRFS. The 1-year adjusted probability of GRFS was 41% (95% CI, 37 to 45) and 27% (95% CI, 21 to 34) after transplantation of BM and PB, respectively (P < .001). Relapse was the predominant event in BM recipients (58%), and acute grade 3 to 4 and chronic GVHD requiring systemic treatment was the predominant event in PB recipients (46%).
DISCUSSION
To our knowledge, this was the largest analysis that compared outcomes for T-cell–replete haploidentical-related donor transplant with post-transplant cyclophosphamide using either BM or PB graft, adjusted for patient and disease characteristics that were associated with these outcomes to correct for imbalances between the treatment groups. Heterogeneity of transplant characteristics of the study population was limited by including a single reduced-intensity conditioning regimen (Baltimore regimen), one nonirradiation myeloablative regimen (busulfan and cyclophosphamide with or without fludarabine), and TBI-containing (≥ 10 Gy) regimen with fludarabine or cyclophosphamide, and all patients received uniform GVHD prophylaxis (calcineurin inhibitor, mycophenolate, and post-transplant cyclophosphamide). Because PB transplants were more recent, with the majority being performed after 2011, outcomes were censored at 2 years to accommodate differences in the length of follow-up of patients who received BM and PB. After a carefully controlled analysis, we did not observe a difference in hematopoietic recovery, nonrelapse mortality, or survival after transplantation of BM compared with PB grafts. However, acute and chronic GVHD risks were higher after transplantation of PB, and relapse risks were higher after transplantation of BM for patients with leukemia/MDS but not lymphoma. Consequently, 2-year PFS was higher with PB compared with BM transplants. Yet, GRFS was significantly better after transplantation of BM. Most BM recipients, including those with leukemia/MDS, received a reduced-intensity conditioning regimen, which may in part have contributed to higher relapse risk.25 Because nonrelapse mortality risks were modest after both BM and PB transplants, the higher relapse risk was not offset by lower nonrelapse mortality in the BM group. Lower relapse rates after PB transplants could not be accounted for by higher GVHD with this graft.
The results demonstrate that there was no difference in hematopoietic recovery between the graft types. Similar results have been demonstrated in smaller comparisons15,26,27 and contrast with the finding that in the matched related and unrelated donor setting, neutrophil recovery occurs 4 to 6 days earlier and platelet recovery occurs 6 to 8 days earlier with PB.8,28,29 We hypothesize that the much smaller difference in hematopoietic recovery times between PB and BM in haploidentical-related donor transplants may be the result of the use of 100 mg/kg of cyclophosphamide post-transplant and in part to the use of growth factor, which was more common in the setting of BM transplants. In this regard, it is notable that a recent trial of post-transplant cyclophosphamide for HLA-matched related or unrelated donor PB transplantation30,31 also reported similar median times to neutrophil recovery. We did not find a difference in graft failure rates after transplantation of BM and PB. This differs from the Blood and Marrow Transplant Clinical Trials Network trial where, in the setting of myeloablative conditioning, transplantation of BM from unrelated donors was associated with higher graft failure.9 Differences between that trial and the current analysis may relate to the fact that ours was limited to mismatched related donors, a large number of nonablative transplants, and use of post-transplant cyclophosphamide. Our results must also be interpreted with caution because post-transplant lineage-specific chimerism data were not available for all patients.
Acute grade 2 to 4 but not grade 3 to 4 acute GVHD was higher with PB and consistent with that reported by the European Group for Blood and Marrow Transplantation32 but differed from reports with substantially fewer patients that failed to show higher grade 2 to 4 acute GVHD with PB.12,15 The higher overall chronic GVHD after PB transplants in the current analysis also differed from the smaller reports12,15 and the larger series from Europe.32 The reason behind the differences between our study and others is unclear other than ours was a larger population and all patients received a uniform GVHD prophylaxis regimen that consisted of calcineurin inhibitor, mycophenolate, and cyclophosphamide post-transplant. Because choice of graft type differed by transplanting center, differences in attribution of clinical findings to GVHD versus other etiologies and in assessments between centers cannot be eliminated as possible contributing factors to the differences between ours and the other reports. We did not assess the functional health of long-term survivors, which may be affected by a higher incidence of chronic GVHD.33
Although we performed a careful comparison adjusting for factors associated with transplant outcomes, there are likely to be several unknown and unmeasured factors that were not tested. For example, data on molecular factors associated with AML prognosis were available on only a small subset of patients and therefore could not be adequately adjusted for. We do not have data on immune reconstitution, a limitation of our analysis. Our results indicate that both BM and PB grafts are valid options for haploidentical-related donor transplantation for adults with hematologic malignancy and ideally must be studied further in the setting of a randomized trial. The lower relapse risk with transplantation of PB was not associated with better survival in the short-term, underscoring the importance of follow-up beyond the 2-year period.
ACKNOWLEDGMENT
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, Department of the Navy, Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Appendix
Table A1.
Effect of Graft Type on Transplant Outcomes Adjusted for Age, Performance Score, Hematopoietic Cell Transplant Comorbidity Score, Cytomegalovirus Seropositivity, Disease, Disease Status, Disease Risk Index, and Conditioning Regimen
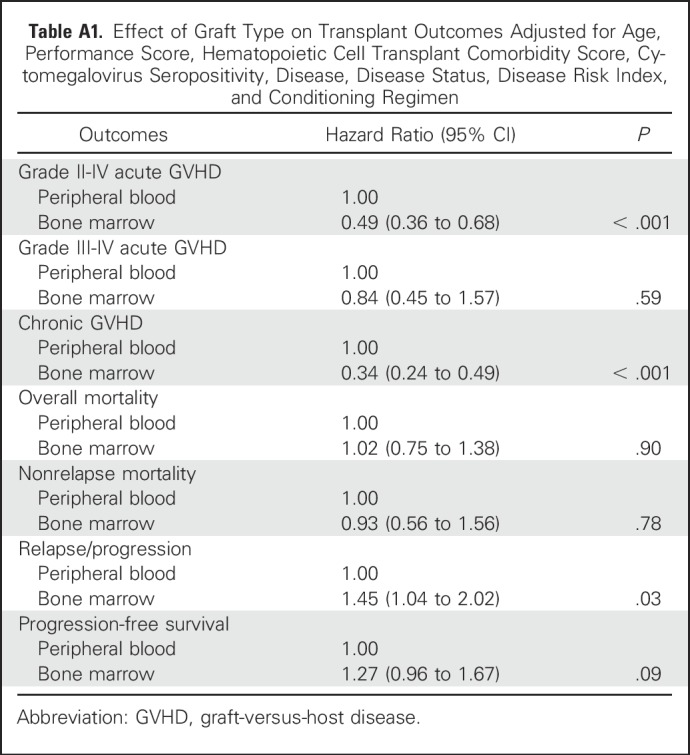
Table A2.
Characteristics of Patients With Acute Myeloid Leukemia
Footnotes
The Center for International Blood and Marrow Transplant Research is supported primarily by Public Health Service Grant/Cooperative Agreement No. 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; Grant No. 5U10HL069294 from NHLBI and NCI; a contract (No. HHSH250201200016C) with the Health Resources and Services Administration/US Department of Health and Human Services; and Grants No. N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research.
Presented in part at the 58th Annual Meeting of the American Society of Hematology, San Diego, CA, December 3-6, 2016.
See accompanying Editorial on page 2984
AUTHOR CONTRIBUTIONS
Conception and design: Asad Bashey, Mei-Jie Zhang, Andrew St. Martin, Trevor Argall, Omotayo Fasan, Sameh Gaballa, Ephraim Joseph Fuchs, Mary Eapen
Collection and assembly of data: Shannon R. McCurdy, Andrew St. Martin, Stefan O. Ciurea
Data analysis and interpretation: Asad Bashey, Mei-Jie Zhang, Shannon R. McCurdy, Andrew St. Martin, Claudio Anasetti, Stefan O. Ciure, Omotayo Fasan, Sameh Gaballa, Mehdi Hamadani, Pashna Munshi, Monzr M. Al Malki, Ryotaro Nakamura, Paul V. O'Donnell, Miguel-Angel Perales, Kavita Raj, Rizwan Romee, Scott Rowley, Vanderson Rocha, Rachel B. Salit, Melhem Solh, Robert J. Soiffer, Ephraim Joseph Fuchs
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell–Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Asad Bashey
No relationship to disclose
Mei-Jie Zhang
No relationship to disclose
Shannon R. McCurdy
No relationship to disclose
Andrew St. Martin
No relationship to disclose
Trevor Argall
No relationship to disclose
Claudio Anasetti
No relationship to disclose
Stefan O. Ciurea
Stock or Other Ownership: Cyto-Sen Therapeutics
Consulting or Advisory Role: Company: Spectrum Pharmaceuticals, MolMed
Omotayo Fasan
No relationship to disclose
Sameh Gaballa
No relationship to disclose
Mehdi Hamadani
Honoraria: Celgene
Consulting or Advisory Role: MedImmune, Celerant, Janssen Oncology
Speakers' Bureau: Sanofi/Genzyme
Research Funding: Takeda, Spectrum Pharmaceuticals
Pashna Munshi
No relationship to disclose
Monzr M. Al Malki
No relationship to disclose
Ryotaro Nakamura
Honoraria: MolMed, Amgen
Research Funding: Helocyte (Inst)
Paul V. O'Donnell
No relationship to disclose
Miguel-Angel Perales
Consulting or Advisory Role: Seattle Genetics, Incyte, Merck
Kavita Raj
Consulting or Advisory Role: Celgene, Jazz Pharmaceuticals
Speakers' Bureau: MSD
Travel, Accommodations, Expenses: Celgene
Rizwan Romee
No relationship to disclose
Scott Rowley
Stock or Other Ownership: GlaxoSmithKline, Cladrius
Consulting or Advisory Role: Chimerix
Vanderson Rocha
No relationship to disclose
Rachel B. Salit
No relationship to disclose
Melhem Solh
Consulting or Advisory Role: Celgene
Speakers' Bureau: Celgene, Amgen, Seattle Genetics
Travel, Accommodations, Expenses: Celgene, Amgen, Seattle Genetics
Robert J. Soiffer
Consulting or Advisory Role: Sandoz, AstraZeneca/MedImmune, Juno, Kiadis Pharma
Ephraim Joseph Fuchs
Stock or Other Ownership: Cellunova
Consulting or Advisory Role: Novartis
Research Funding: Cellunova
Patents, Royalties, Other Intellectual Property: Cellunova
Mary Eapen
No relationship to disclose
REFERENCES
- 1.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 2.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 4.Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20:1573–1579. doi: 10.1016/j.bbmt.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 5.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125:3024–3031. doi: 10.1182/blood-2015-01-623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashey A, Zhang X, Jackson K, et al. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: A multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22:125–133. doi: 10.1016/j.bbmt.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Blaise D, Kuentz M, Fortanier C, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: A report from the Société Française de Greffe de Moelle. J Clin Oncol. 2000;18:537–546. doi: 10.1200/JCO.2000.18.3.537. [DOI] [PubMed] [Google Scholar]
- 9.Anasetti C, Logan BR, Lee SJ, et al. Increased incidence of chronic graft-versus-host disease (GVHD) and no survival advantage with filgrastim-mobilized peripheral blood stem cells (PBSC) compared to bone marrow (BM) transplants from unrelated donors: Results of blood and marrow transplant clinical trials network (BMT CTN) Protocol 0201, a phase III, prospective, randomized trial Blood 118:12011 [Google Scholar]
- 10.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: Results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18:1859–1866. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Bhamidipati PK, DiPersio JF, Stokerl-Goldstein K, et al. Haploidentical transplantation using G-CSF-mobilized T-cell replete PBSCs and post-transplantation CY after non-myeloablative conditioning is safe and is associated with favorable outcomes. Bone Marrow Transplant. 2014;49:1124–1126. doi: 10.1038/bmt.2014.108. [DOI] [PubMed] [Google Scholar]
- 12.Castagna L, Crocchiolo R, Furst S, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20:724–729. doi: 10.1016/j.bbmt.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Raj K, Pagliuca A, Bradstock K, et al. Peripheral blood hematopoietic stem cells for transplantation of hematological diseases from related, haploidentical donors after reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20:890–895. doi: 10.1016/j.bbmt.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solomon SR, Sizemore CA, Sanacore M, et al: Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant 21:1299-1307, 2015. [DOI] [PubMed]
- 15.O’Donnell PV, Eapen M, Horowitz MM, et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: A matched-pair analysis. Bone Marrow Transplant. 2016;51:1599–1601. doi: 10.1038/bmt.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48:537–543. doi: 10.1038/bmt.2012.239. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 18.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 19.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–1338. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 23.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: A Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–1500. doi: 10.1002/(sici)1097-0258(19990630)18:12<1489::aid-sim140>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Luger SM, Ringdén O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47:203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castagna L, Crocchiolo R, Bramanti S, et al: Lower relapse and better PFS among chemosensitive patients undergoing allogeneic transplantation by haploidentical compared with HLA-identical donor: Results on a cohort of 94 patients with Hodgkin’s lymphoma. Blood 122:2144, 2013. [Google Scholar]
- 27.Bradstock K, Bilmon I, Kwan J, et al. Influence of stem cell source on outcomes of allogeneic reduced-intensity conditioning therapy transplants using haploidentical related donors. Biol Blood Marrow Transplant. 2015;21:1641–1645. doi: 10.1016/j.bbmt.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 29.Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–1531. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 30.Mielcarek M, Furlong T, O’Donnell PV, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–1508. doi: 10.1182/blood-2015-10-672071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moiseev IS, Pirogova OV, Alyanski AL, et al. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transplant. 2016;22:1037–1042. doi: 10.1016/j.bbmt.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Ruggeri A, Labopin M, Bacigalupo A, et al. Use of bone marrow or peripheral blood stem cell grafts in non t cell depleted haploidentical transplants using post-transplant cyclophosphamide, an ALWP-EBMT analysis. ASH Annual Abstracts, Blood. 128:1165. [Google Scholar]
- 33.Lee SJ, Logan B, Westervelt P, et al. Comparison of patient-reported outcomes in 5-year survivors who received bone marrow vs peripheral blood unrelated donor transplantation: Long-term follow-up of a randomized clinical trial. JAMA Oncol. 2016;2:1583–1589. doi: 10.1001/jamaoncol.2016.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]