Abstract
Background and aim
Inflammatory changes in the stomach caused by Helicobacter pylori indirectly and directly affect liver function. Moreover, the bacteria may worsen the course of the liver cirrhosis. The study aimed at evaluating the incidence of H. pylori infection among patients with liver cirrhosis, depending on the etiology and injury stage, scored according to Child–Pugh classification. Stage of esophageal varices and endoscopic inflammatory lesions in the stomach were evaluated, depending on the presence of H. pylori infection.
Patients and methods
The study included 147 patients with liver cirrhosis: 42 were infected with hepatitis C virus, 31 were infected with hepatitis B virus, 56 had alcoholic liver cirrhosis, and 18 had primary biliary cirrhosis. Diagnosis of H. pylori infection was performed based on the presence of immunoglobulin G antibodies in serum.
Results
H. pylori infection was found in 46.9% of patients. The incidence of H. pylori infection among patients with postinflammatory liver cirrhosis was significantly higher (P=0.001), as compared with patients with alcoholic liver cirrhosis. Ammonia concentration was significantly higher in patients infected with H. pylori, compared with noninfected individuals (129 vs. 112 μmol/l; P=0.002). Incidence of H. pylori infection in patients without esophageal varices was significantly lower compared with patients with esophageal varices (14 vs. 60%; P<0.001).
Conclusion
H. pylori infection is significantly more frequent among patients with postinflammatory liver cirrhosis (infected with hepatitis C virus or hepatitis B virus) than in patients with alcoholic liver cirrhosis or primary biliary cirrhosis. H. pylori infection correlates with elevated concentration of blood ammonia and the incidence of esophageal varices.
Keywords: ammonia, esophageal varices, Helicobacter pylori, liver cirrhosis
Introduction
Helicobacter pylori is a microaerophile, a Gram-negative bacillus, resistant to the activity of gastric juice. The bacteria may take the vegetative form (spiral shape) or sporulation form. H. pylori lives mainly on the surface of epithelial cells of mucous membranes of the prepyloric part of the stomach. The cilia present on the bacteria allow it to move into intercellular spaces and adhere to the surface of cells. Infection with these bacteria is one of the most common in the world. In highly developed countries, 50% of the population is infected, whereas in the developing countries the proportion reaches as much as 90% 1.
H. pylori infection causes local (limited to the gastric mucous membrane) and general increase of proinflammatory cytokines interleukin (IL)-1, IL-2, IL-4, IL-6, IL-8, IL-10, IL-17, interferon-β, and tumor necrosis factor-α 2. This phenomenon not only leads to stimulation of local inflammatory reaction but also exacerbates different inflammatory reactions in the organism. H. pylori underlies chronic atrophic gastritis, metaplasia, and dysplasia, leading to the development of gastric cancer. According to the WHO, the bacteria is a class I carcinogenic factor. It may influence extragastric organ disturbances, exacerbating cardiovascular diseases, metabolic diseases, disturbing normal liver function, especially in patients with liver cirrhosis 3.
In the group of patients infected with H. pylori, morphologic studies on liver of patients infected with hepatitis B virus (HBV), hepatitis C virus (HCV), and patients with chronic noninfectious liver diseases have demonstrated the presence of H. pylori DNA in the perihepatic tissues. Such an infection is caused by disturbances of immunologic functions in this group of patients 4. H. pylori infection influences the disturbances of lipid metabolism, manifesting with hypertriglyceridemia and hypercholesterolemia, with concurrent fall in high-density lipoprotein. This is especially important in the metabolism of hepatocytes, their steatosis, and liver fibrosis 5.
In the course of liver cirrhosis, pathological lesions often appear in the mucous membranes of the stomach. Portal gastropathy is a chronic inflammatory state that occurs most frequently. H. pylori infection in the group of patients with liver cirrhosis may influence exacerbation of inflammatory lesions in the stomach, which could directly and indirectly lead to impairment of liver function. This is especially dangerous in patients with advanced liver injury. Studies on this group of patients point to high importance of cytopathological effect of H. pylori on hepatocytes 4.
Aim
The study aimed at determining the incidence of H. pylori infection among patients with liver cirrhosis. The presence of this infection was analyzed depending on the cause of liver cirrhosis and failure stage. Incidence of H. pylori infection was evaluated depending on the stage of esophageal varices and endoscopic changes of gastric mucous membranes. The effect of H. pylori infection on ammonia concentration was determined.
Patients and methods
The study was conducted to assess patients with liver cirrhosis in the North-East of Poland. The study included patients with postinflammatory liver cirrhosis, connected with HCV or HBV infection, primary biliary cirrhosis (PBC), and alcoholic liver cirrhosis. The severity of liver cirrhosis was estimated based on Child–Pugh score. An ultrasound examination was performed in all patients, determining liver parenchyma and signs of portal hypertension, using USG Power Doppler technique. Liver cirrhosis was estimated on the basis of clinical evaluation, liver biopsy, or elastography as a noninvasive method for liver fibrosis evaluation.
Endoscopy of the upper part of the gastrointestinal tract was performed in all patients. Stage of esophageal varices was established based on three-stage OMED classification, as varices bulging only slightly above the surface of mucous membrane; medium ones, bulging up to one-third of the lumen; and large ones, bulging over one-third of the esophageal lumen 6.
Gastric mucous membrane inflammation was estimated on the basis of endoscopic image, with the use of Sydney classification, updated in Houston 7.
H. pylori infection diagnosis was performed on the basis of the presence of specific immunoglobulin G antibodies in the serum, with the use of the qualitative and quantitative test Enzygnost anti-H. pylori II/IgG (Siemens, Siemens company, Germany; in population of adults: sensitivity is 96.8%, and overall specificity is 98.8%).
In some patients, without significant abnormal blood coagulation measures and low platelet count, measurement of bacterial urease activity was performed in the biopsy specimen of gastric mucous membrane excised during endoscopic procedure.
Ammonia blood concentration was estimated with immunochemical tests, using COBAS C501 machine, Roche company, USA.
Statistical analysis was performed using χ2 and Mann–Whitney U-tests. Statistical significance was established as P value less than 0.05.
After approval of the study by the Bioethical Committee at the Medical University of Bialystok, the patients provided informed consent to participate in the research.
Results
Among 147 patients with liver cirrhosis included in the study, there were 44 women and 103 men aged 58 years on average (from 23 to 86 years). Liver cirrhosis was caused by HCV infection (42 patients), HBV infection (31 patients), alcohol abuse (56 patients), and PBC (18 patients) (Table 1).
Table 1.
Characteristics of patients with liver cirrhosis
Irrespective of the cause of cirrhosis, 27% patients were graded as Child–Pugh class A, 47% as Child–Pugh class B, and 26% as Child–Pugh class C. Class C included mostly patients with alcoholic liver cirrhosis (41%) (Table 1).
H. pylori infection was diagnosed in 69 (46.9%) patients, usually among those chronically infected with HBV or HCV.
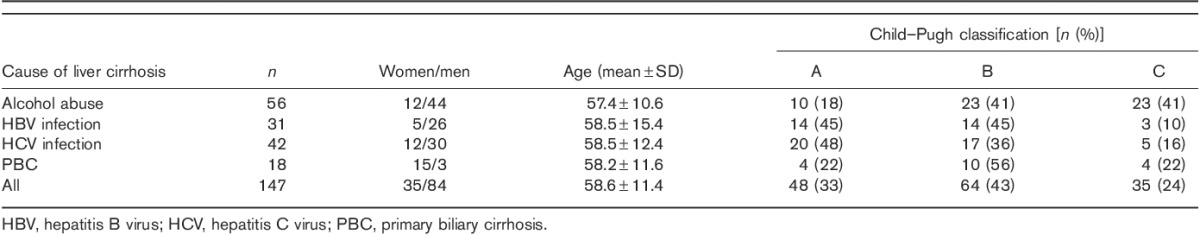
The incidence of H. pylori infection among patients with postinflammatory liver cirrhosis was significantly higher (P=0.001), as compared with patients with alcoholic liver cirrhosis (Fig. 1).
Fig. 1.
Helicobacter pylori infection in different groups of patients. HBV, hepatitis B virus; HCV, hepatitis C virus; PBC, primary biliary cirrhosis.
H. pylori infection among all the patients with liver cirrhosis with respect to Child–Pugh classification was comparable, irrespective of the stage of liver failure. However, in relation to patients with alcoholic liver failure and those infected with HBV or HCV, increase of H. pylori incidence and worsening of liver function was observed (the increase of incidence was not statistically significant).
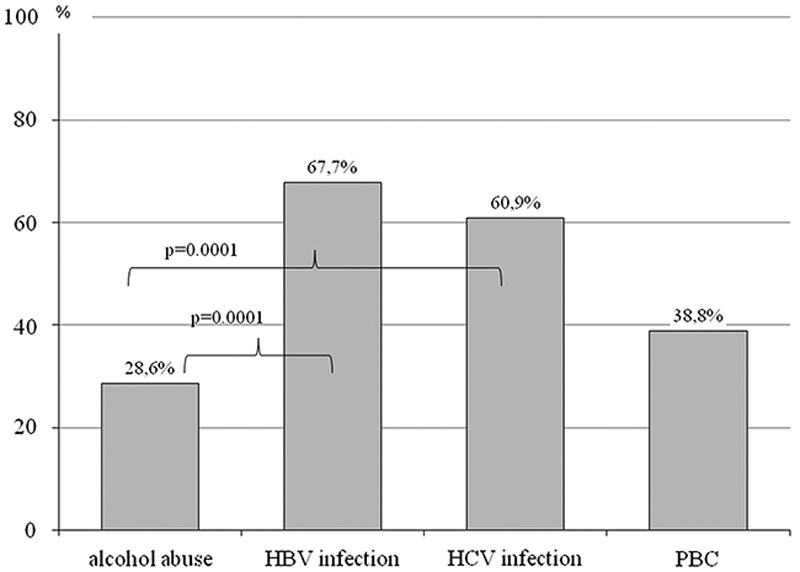
Among the patients without esophageal varices, H. pylori infection was found in 14% cases, which was a significantly lower number than in the case of patients with esophageal varices (60%; P<0.005) (Tables 2 and 3).
Table 2.
The presence of esophageal varices in relation to the severity of liver failure, and the cause of cirrhosis
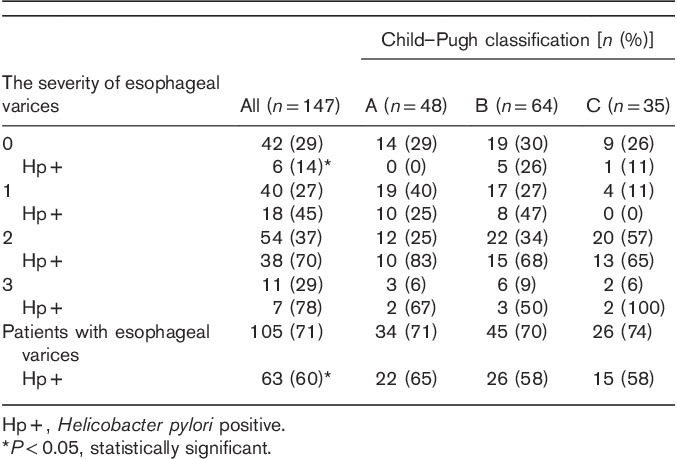
Table 3.
Endoscopic gastric changes in relation to causative agents of liver cirrhosis
In the performed analysis of the incidence of H. pylori infection versus advancement stage of esophageal varices, a statistically significant correlation was found between the stage of varices and incidence of H. pylori infection (Fig. 2).
Fig. 2.
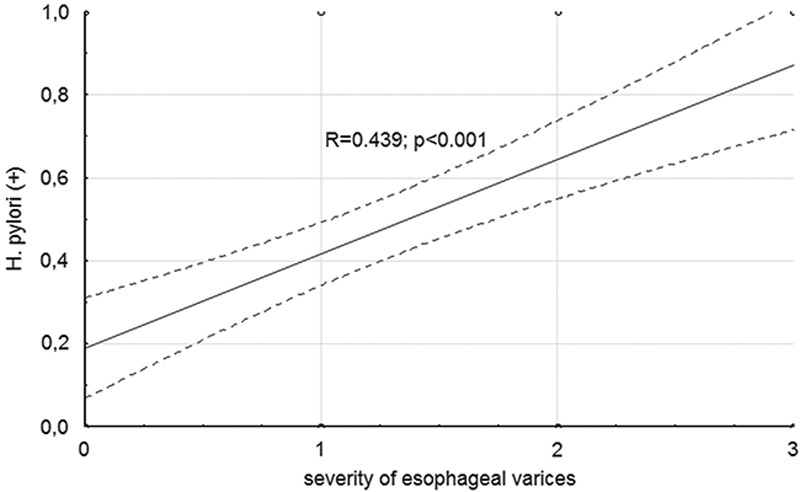
The occurrence of Helicobacter pylori infection in respect to the advancement of esophageal varices.
Reflux, erythematous, or atrophic gastropathy was most often diagnosed in the studied patients. No statistically significant differences of incidence of gastric mucous lesions were found between different ethiological factors of cirrhosis (Fig. 3).
Fig. 3.
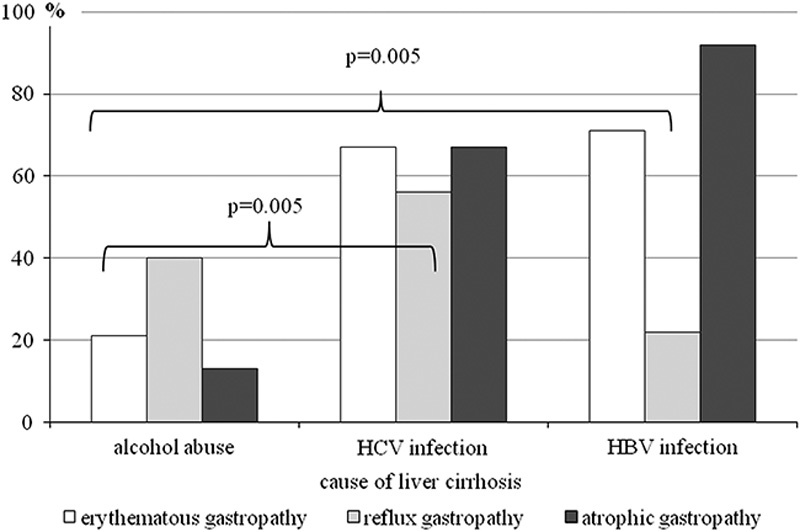
Infection with Helicobacter pylori in respect to changes of the gastric mucosa and liver cirrhosis agent. HBV, hepatitis B virus; HCV, hepatitis C virus.
H. pylori infection was significantly more frequent among patients with postinflammatory liver cirrhosis, compared with alcoholic liver cirrhosis, with respect to diagnosed reflux (this did not apply to HBV infection), erythematous, or atrophic gastropathy.
Significantly higher ammonia concentration was found in patients with H. pylori infection (Table 4). There were no statistically significant differences in ammonia concentration related to the stage of liver injury or the cause of liver cirrhosis. The only exception was significant difference between the patients with alcoholic liver cirrhosis and PBC (Fig. 4).
Table 4.
The ammonia concentration according to the degree of liver damage and infection with Helicobacter pylori
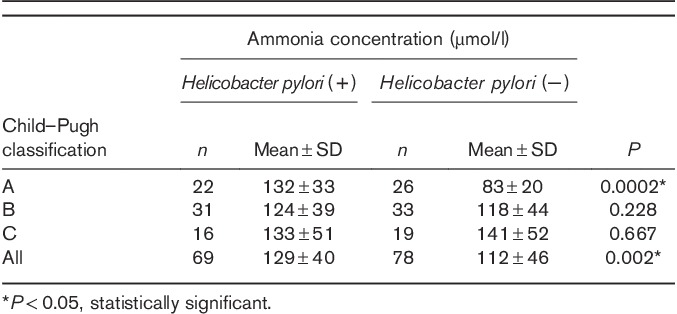
Fig. 4.
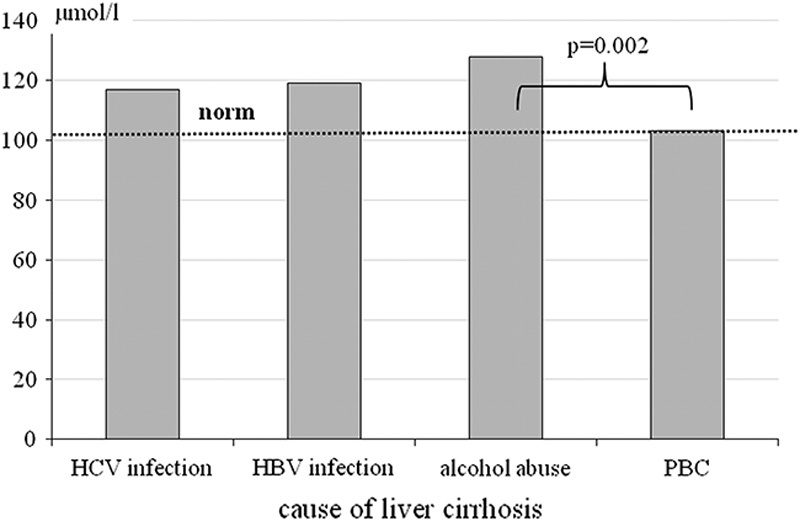
The ammonia concentration according to the cause of cirrhosis. HBV, hepatitis B virus; HCV, hepatitis C virus; PBC, primary biliary cirrhosis.
Discussion
One of the first studies performed on mice and rats infected with H. pylori have shown the effect of this infection on the degree of fibrosis and development of liver cirrhosis 8. Stalke et al. 9 who studied patients with chronic liver injury, have demonstrated the presence of bacteria from the genus Helicobacter spp. in liver tissue of 26% of patients. Both experimental and clinical studies show detrimental effect of H. pylori infection on the progression of liver injury, especially on the stage of fibrosis. The impact of infection on metabolic changes connected with carbohydrates, synthesis of high-energy molecules (mainly ATP), and increased concentration of proinflammatory cytokines may be one of the reasons for this effect. H. pylori infection may contribute to different organ injuries. Irrespectively from the effect onto the gastric mucosa, H. pylori infection causes multiorgan injuries, including chronic injury of the pancreas, which indirectly influences the function of the liver 10. Research by Sumida et al. 11 clearly points to high significance of H. pylori infection in patients with nonalcoholic fatty liver for the development of nonalcoholic steatohepatitis. Eradication of this bacteria significantly simplifies the therapy of fatty liver.
Meta-analysis of 21 studies evaluating H. pylori infection among patients with liver cirrhosis did not find higher incidence of infection in this group versus people without liver cirrhosis 12. It is, however, worth mentioning that the analysis included mainly patients with alcoholic liver cirrhosis. Also in our own research, the presence of H. pylori infection was found in 46.9% of patients, which is proportional to the incidence of this infection in the general population (up to 50%). In our observations, incidence of H. pylori infection among people infected with HCV or HBV was significantly higher (60.9–67.7%). These observations comply with the research by Hanafy et al. 13, who have demonstrated H. pylori infection in 70% (281/400) of patients chronically infected with HCV. A meta-analysis of 20 studies, performed by Wang et al. 14, clearly shows higher incidence of H. pylori infection among HCV-positive patients versus people not infected with the virus. El-Masry et al. 15 argue that the incidence of H. pylori infection among patients with liver cirrhosis and those infected with HCV increases with more pronounced liver failure. Moreover, Wang et al. 14 demonstrate that the highest proportion of patients with H. pylori infection in the group of those who are HCV-positive can be detected in the case of hepatocellular carcinoma (HCC) development. It seems that very frequent coinfection with H. pylori and HCV among people with HCC may contribute to increased incidence of this tumor, because HCV and H. pylori are deemed to be carcinogenic. Unfavorable effect of H. pylori infection among patients with HCV may be diverse. In the past, eradication of this bacteria led to increased platelet number, which allowed administration of antiviral treatment (with interferon). Moreover, the efficacy of antiviral therapies was better among people previously subjected to eradication of H. pylori 13,16. At the moment, during the time of broad use of direct-acting antivirals, this probably does not seem so important; however, such studies have not been performed.
Wang et al. 17 analyzed 15 studies evaluating the effect of H. pylori infection on the progress of chronic HBV infection. In the analyzed groups, the incidence of infection was from 30 to over 80%. Results of studies pointed to a correlation between the progression of inflammatory changes in the liver, development of liver cirrhosis and occurrence of HCC, and infection with the bacteria 17. In the study by Zhang et al. 18 performed in 225 patients chronically infected with HBV and compensated liver cirrhosis with thrombocytopenia, eradication of H. pylori irrespectively of the antiviral therapy positively impacted the progression of the disease and increased platelet count.
H. pylori synthesizes urease, catalyzing urea decomposition to ammonia and carbon dioxide. However, among patients with subclinical hepatic encephalopathy, H. pylori infection does not result in higher concentration of ammonia in the blood 19. Observation of patients with liver cirrhosis, especially caused by inflammatory reaction, points to a higher incidence of symptomatic hepatic encephalopathy among people infected with H. pylori, compared with people without this infection 20. On the basis of our own observations, we did not find higher ammonia concentration among patients infected with H. pylori, as compared with uninfected individuals. Chen et al. 21 indicate the potential for H. pylori infection to increase serum ammonia levels, which may be responsible for the mental disorders associated with hepatic encephalopathy. This may be a direct effect on hepatocytes or on intestinal flora and increased intestinal permeability for ammonia produced in bacteria 21.
The research of Sathar et al. 22 demonstrated much more frequent severe inflammatory reaction of gastric mucosa among patients with liver cirrhosis infected with H. pylori. However, these observations seem to be obvious. Infection with this bacteria often accompanies inflammatory lesions in the stomach, leading to gastric ulcer development. In our own patient population we also found high incidence of H. pylori infection among patients with severe inflammatory lesions of the gastric mucosa 22. Interestingly, according to our observations, esophageal varices occur more often in the group of patients infected with H. pylori. Moreover, the correlation between the stage of varices and incidence of H. pylori infection may point to significant, direct effect of this bacteria on liver function 3. Licinio et al. 23 argue for high probability of the influence of H. pylori infection on increase of portal hypertension, which is one of the most important causes of the development of esophageal varices.
Conclusion
H. pylori infection is significantly more frequent among patients with postinflammatory liver cirrhosis related to HCV or HBV infection than in patients with alcoholic liver cirrhosis or PBC. The type of inflammatory lesions in the stomach is not dependent on the causative factor of liver cirrhosis. The incidence of esophageal varices correlates with the incidence of H. pylori infection.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mitchell H, Katelaris P. Epidemiology, clinical impacts and current clinical management of Helicobacter pylori infection. Med J Aust 2016; 10:376–380. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Xu Y, Wang L, Zhang F, Zhang J, Fu X, et al. Association between TNFA gene polymorphisms and Helicobacter pylori infection: a meta-analysis. PLoS One 2016; 1:e0147410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waluga M, Kukla M, Żorniak M, Bacik A, Kotulski R. From the stomach to other organs: Helicobacter pylori and the liver. World J Hepatol 2015; 18:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva LD, Rocha AM, Rocha GA, de Moura SB, Rocha MM, Dani R, et al. The presence of Helicobacter pylori in the liver depends on the Th1, Th17 and Treg cytokine profile of the patient. Mem Inst Oswaldo Cruz 2011; 106:748–754. [DOI] [PubMed] [Google Scholar]
- 5.Buzás GM. Metabolic consequences of Helicobacter pylori infection and eradication. World J Gastroenterol 2014; 18:5226–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zych W, Habior A. Hemorrhage from esophageal varices. Post Nauk Med 2014; 1:9–16. [Google Scholar]
- 7.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 10:1161–1181. [DOI] [PubMed] [Google Scholar]
- 8.Goo MJ, Ki MR, Lee HR, Yang HJ, Yuan DW, Hong IH, et al. Helicobacter pylori promotes hepatic fibrosis in the animal model. Lab Invest 2009; 89:1291–1303. [DOI] [PubMed] [Google Scholar]
- 9.Stalke P, Al-Soud WA, Bielawski KP, Bakowska A, Trocha H, Stepinski J, et al. Detection of Helicobacter species in liver and stomach tissues of patients with chronic liver diseases using polymerase chain reaction-denaturing gradient gel electrophoresis and immunohistochemistry. Scand J Gastroenterol 2005; 40:1032–1041. [DOI] [PubMed] [Google Scholar]
- 10.Rabelo-Gonçalves EM, Roesler BM, Zeitune JM. Extragastric manifestations of Helicobacter pylori infection: possible role of bacterium in liver and pancreas diseases. World J Hepatol 2015; 30:2968–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumida Y, Kanemasa K, Imai S, Mori K, Tanaka S, Shimokobe H, et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J Gastroenterol 2015; 9:996–1004. [DOI] [PubMed] [Google Scholar]
- 12.Feng H, Zhou X, Zhang G. Association between cirrhosis and Helicobacter pylori infection: a meta-analysis. Eur J Gastroenterol Hepatol 2014; 26:1309–1319. [DOI] [PubMed] [Google Scholar]
- 13.Hanafy AS, El Hawary AT, Hamed EF, Hassaneen AM. Impact of Helicobacter pylori eradication on refractory thrombocytopenia in patients with chronic HCV awaiting antiviral therapy. Eur J Clin Microbiol Infect Dis 2016; 7:1171–1176. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Li WT, Zheng YX, Zhao SS, Li N, Huang Y, et al. The association between Helicobacter pylori infection and chronic hepatitis C: a meta-analysis and trial sequential analysis. Gastroenterol Res Pract 2016; 2016:8780695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Masry S, El-Shahat M, Badra G, Aboel-Nour MF, Lotfy M. Helicobacter pylori and hepatitis C virus coinfection in Egyptian patients. J Glob Infect Dis 2010; 1:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takashima T, Enomoto H, Iwata Y, Nishikawa H, Yoh K, Hasegawa K, et al. Effects of Helicobacter pylori eradication on the platelet count in hepatitis C virus-infected patients. J Clin Med Res 2016; 12:854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Chen RC, Zheng YX, Zhao SS, Li N, Zhou RR, et al. Helicobacter pylori infection may increase the risk of progression of chronic hepatitis B disease among the Chinese population: a meta-analysis. Int J Infect Dis 2016; 50:30–37. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XH, He Y, Feng R, Xu LP, Jiang Q, Jiang H, et al. Helicobacter pylori infection influences the severity of thrombocytopenia and its treatment response in chronic hepatitis B patients with compensatory cirrhosis: a multicenter, observational study. Platelets 2016; 3:223–229. [DOI] [PubMed] [Google Scholar]
- 19.Rekha C, Phanidhar S, Sagar AV, Revathi A, Asra WA. Role of Helicobacter pylori and hyperammonemia in subclinical hepatic encephalopathy in cirrhosis of liver. Indian J Clin Biochem 2007; 2:136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kountouras J, Deretzi G, Zavos C, Katsinelos P. Helicobacter pylori infection and liver cirrhosis: possible association with hepatic encephalopathy and/or post-hepatic encephalopathy cognitive impairment in patients with portal hypertension. Ann Gastroenterol 2014; 3:285. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen SJ, Wang LJ, Zhu Q, Cai JT, Chen T, Si JM. Effect of H. pylori infection and its eradication on hyperammonemia and hepatic encephalopathy in cirrhotic patients. World J Gastroenterol 2008; 12:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathar SA, Kunnathuparambil SG, Sreesh S, Narayanan P, Vinayakumar KR. Helicobacter pylori infection in patients with liver cirrhosis: prevalence and association with portal hypertensive gastropathy. Ann Gastroenterol 2014; 1:48–52. [PMC free article] [PubMed] [Google Scholar]
- 23.Licinio R, Losurdo G, Carparelli S, Iannone A, Giorgio F, Barone M, et al. Helicobacter pylori, liver cirrhosis, and portal hypertension: an updated appraisal. Immunopharmacol Immunotoxicol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


