Abstract
Neuroimmunologists seek to understand the interactions between the central nervous system (CNS) and the immune system, both under homeostatic conditions and in diseases. Unanswered questions include those relating to the diversity and specificity of the meningeal T cell repertoire; the routes taken by immune cells that patrol the meninges under healthy conditions and invade the parenchyma during pathology; the opposing effects (beneficial or detrimental) of these cells on CNS function; the role of immune cells after CNS injury; and the evolutionary link between the two systems, resulting in their tight interaction and interdependence. This Review summarizes the current standing of and challenging questions related to interactions between adaptive immunity and the CNS and considers the possible directions in which these aspects of neuroimmunology will be heading over the next decade.
Although the initial experiments carried out by Medawar and colleagues (1), as well as others (2), have demonstrated that the immune response to the central nervous system (CNS) antigens does exist (although it is distinct from the response in other tissues), the CNS was traditionally viewed as an immune-privileged site. This depiction set a clear separation between the nervous and immune systems until quite recently (3, 4). Therefore, the CNS was generally assumed to be largely devoid of immune entities, the microglia (a macrophage-like cell) being an acknowledged exception (5). Any sign of immune presence within the CNS parenchyma was perceived as a hallmark of pathology.
Numerous seminal works have investigated the interaction between innate immunity and the CNS under conditions such as stress, bulimia or anorexia, fever, and others (6–8). The development of an animal model [experimental allergic (or autoimmune) encephalomyelitis (EAE)] of a major neuroinflammatory disease [multiple sclerosis (MS)] led to closer scrutiny of the interactions between adaptive immunity and the CNS (9). For more than 80 years, studies with the EAE model resulted in many breakthrough findings in the fields of immunology and neuroscience (10–20).
For a long time, the immune system was commonly viewed solely as the body’s defense mechanism against pathogens. In the early 1990s, however, Matzinger proposed the “danger” theory, in which the immune system responds not only to signals from pathogens but also to danger signals released from damaged tissues, even in the case of sterile injuries (21). Around the same time, Cohen advanced the idea of the “immunological homunculus” (22), assigning the immune system physiological roles in tissue maintenance and homeostasis. These studies broadened our understanding of what the immune system does and, in combination with other works on the role of adaptive immunity in the injured CNS (23, 24), led to a wider understanding that the two systems may be more closely connected than was previously thought.
In this article, I will provide a brief overview of multifaceted interactions between adaptive immunity and the CNS as we see them today and will highlight critical questions currently confronting neuroimmunologists.
Neuroimmune interactions in CNS disorders
In MS, immune cells in the brain and spinal cord attack the myelin sheath that encases nerves. EAE, the animal model of MS, has provided many insights into how the immune system interacts with the CNS in this disease [reviewed in (25–28)]. Briefly, the devastating effects of MS and EAE were initially ascribed primarily to autoimmune CD4+ T cells [in EAE, the CD4+ T cells were reactive to proteins found in the myelin, particularly myelin basic protein, proteolipid protein, and myelin oligodendrocyte glycoprotein (MOG) (15, 17)]. Over the years, our understanding of the complexity of this disease has grown as a result of intensive research on its underlying mechanisms. The current consensus is that many other immune cells besides CD4+ T cells—including CD8+ T cells, B cells, neutrophils, natural killer cells, and monocytes and macrophages—are involved in MS pathology (29–33). This research also revealed that the damage incurred in EAE, and likely also in MS, is mediated by the immune system, and the overall outcome of the inflammatory process in this disease is unequivocally detrimental.
Many, if not all, neurodegenerative diseases also exhibit some sort of immune association. In Alzheimer’s disease (AD), for example, phagocytes are thought to play an important role in disease progression, although the identity of the major phagocytes in AD brains remains unclear. More specifically, whether microglia or blood monocyte–derived macrophages that engraft the parenchyma contribute to disease pathology is unknown (34–38).
Peripheral myeloid cells are not the only cells involved in neurodegenerative diseases, as T lymphocytes were recently proposed to play a role in animal models of several neurodegenerative conditions, including AD and amyotrophic lateral sclerosis (ALS). For example, AD-susceptible mice progress to disease more rapidly when they lack an adaptive immune system (39). This suggests that T cells may be protecting the diseased brain, much as they do after CNS injury (24, 40, 41), as discussed in detail below. Aging is associated with T cell dysfunction; consequently, AD progression in mouse models is slowed down and its outcome improved by enhanced functioning of effector T cells (42). Likewise, mouse models of ALS on a T cell–deficient background show more rapid disease progression (43). Although the mechanism of immune-mediated neuroprotection is not fully understood, accumulating evidence from different neurological diseases points to a beneficial role for both peripheral (macrophage, T cell) and resident (microglial) immune cells.
Neuroimmune interactions after CNS injury
Acute injury to the CNS, such as a contusive spinal cord injury or optic nerve crush injury, results in global immune changes throughout the body (44), most prominently within the deep cervical lymph nodes, the meningeal spaces (including the cerebrospinal fluid), and the site of injury itself (45–49). The adaptive response at the site of injury is preceded by the innate immune response, in which the injured CNS promptly releases alarmins, such as interleukin (IL)–33, adenosine triphosphate, and HMGB1, which activate glia and recruit granulocytes and monocytes to the site of injury (46, 50). IL-33 is particularly important for monocyte recruitment, and injury outcomes are worse in mice lacking alarmins or monocytes. Compared with other organs, the CNS expresses very high amounts of IL-33 (51). However, the reason for this is unclear. It might simply be that IL-33 also has other, as yet unknown, effects in the CNS or that IL-33 represents a mechanism that has evolved to recruit immune cells upon CNS injury, perhaps to fight pathogens invading the exposed nervous tissue and to help heal the wound. This mechanism might have been later adopted to also heal sterile CNS injuries.
In the case of infection, innate immunity leads to a pathogen-specific adaptive immune response. This, in turn, further regulates the innate response, skewing it (once the infection has cleared) from an antipathogenic to a tissue-building phenotype. The adaptive response then regulates itself by suppressing effector T cells and retaining a small population of memory clones that can be easily and rapidly reactivated upon subsequent exposure to the same infection. A similar scenario is likely to occur in response to CNS injury (49), although the antigenic specificity of T cells that respond to the injury is unknown. The T cell response may be specific to CNS-restricted antigens (24). However, depending on the experimental conditions, T cells may also be activated in a T cell receptor (TCR)–independent manner (45). If the response is activated as a result of drainage of CNS antigens into draining lymph nodes, T cells will likely mount a response to these self-antigens.
The above scenario raises a question: What prevents these potentially self-reactive T cells from attacking the brain? Because the failure of such putative mechanisms of tolerance within the CNS might result in the development of autoimmune diseases such as MS, it is of crucial importance to identify and understand these mechanisms. In most, if not all, experimental animal models of CNS injury, the net outcome of the spontaneous T cell response to injury is neuroprotective. However, an uncontrolled autoimmune T cell response may result in destructive outcomes. The regulation of the immune response after injury, therefore, is crucially important (45, 47, 52).
Neuroimmune interactions in homeostasis
Whether the endogenous responses to CNS injury and neurodegeneration are exclusively autoimmune in nature requires further study. If responding T cells recognize self-antigens and are yet beneficial, what would be the evolutionary advantage of having a task force with the potential for attacking healthy tissue? After injury to the CNS, would the beneficial capacity of such T cells possess sufficient evolutionary force to favor such a trait? One plausible explanation that might accommodate this possibility is that the immune response to injury is an extreme manifestation of a CNS-specific immunity that is always present and whose role is to protect the healthy CNS in its daily functioning.
The above rationale led me and colleagues to examine the role of adaptive immunity in cognitive function (53). Unexpectedly, mice deficient in T lymphocytes exhibited cognitive impairment, and passive transfer of mature T cells improved their cognitive function. Further research on the effect of immunity on CNS function has yielded greater knowledge of the immune cell populations required for learning and memory (mainly CD4+ T cells) and provided some initial insights into their antigenic specificity and the location from which their beneficial effects are mediated (53–57). The jury is still out with regard to the antigenic specificity of the T cells that is required for proper cognitive function. Our studies indicate that OTII mice (TCR transgenic mice, bearing ~90% of T cells with specificity to ovalbumin) exhibit cognitive impairment, whereas injecting them with MOG-reactive T cells (CNS protein–specific T cells) improves cognitive function, suggesting an autoimmune nature of procognitive T cells (58, 59). T cells likely mediate their beneficial effects from the meningeal spaces—that is, the regions between the three membranes that make up the brain and the spinal cord–enveloping meninges (Fig. 1). Data supporting this notion are numerous, though not yet conclusive. For example, meningeal T cells demonstrate changes in phenotype and activation in mice undergoing cognitive tasks or exposed to stress (55). Moreover, treatment of mice with an antibody targeting the VLA-4 integrin, which attenuates the migration of immune cells (primarily T cells and monocytes) across the blood-brain barrier (BBB) and the blood-meningeal barrier (BMB) (as well as across gut barriers), results in cognitive impairment (55). Furthermore, elimination of the deep cervical lymph nodes that drain the CNS results in disturbed meningeal T cells (as well as meningeal myeloid cells) and is associated with impaired learning behavior (58, 59). However, these findings are mainly correlative. Further investigation is needed to pinpoint the role of T cells within meningeal barriers on CNS function.
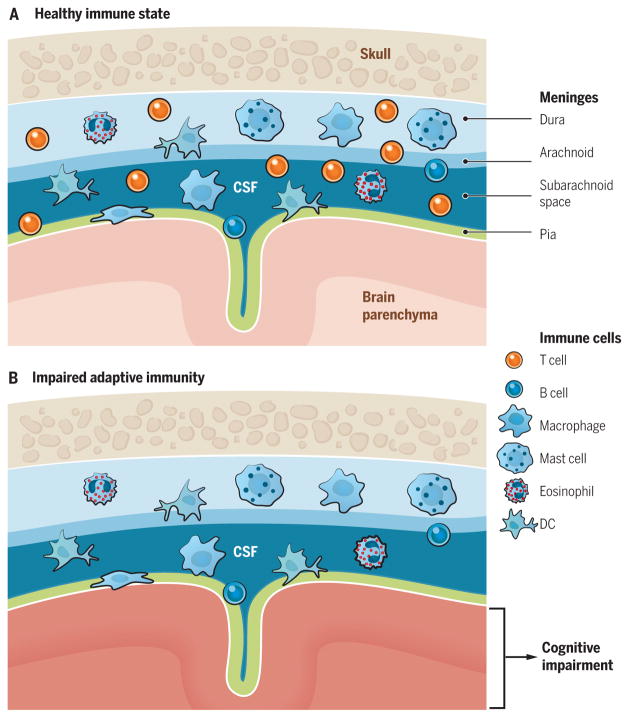
Fig. 1. Meningeal immunity in “surveillance” of brain function.
(A) Representation of meninges (pia mater, lining the brain parenchyma; dura mater, attached to skull; arachnoid, attached to dura mater; subarachnoid space, space between arachnid and pia mater, where the CSF flows) and their coverage by the immune cells. Recent evidence suggests that meningeal immune cells, primarily T cells, affect brain function. (B) Elimination of meningeal T cells by using genetically modified mice, pharmacologically trapping T cells in the deep cervical lymph nodes, or preventing their migration to meningeal spaces results in impaired cognitive function. The precise mechanism of how meningeal T cells regulate cognitive function is still not fully understood. DC, dendritic cell.
Although the role of meningeal immunity in CNS function is not yet fully understood, its composition and maintenance represent an interesting aspect of tissue immunology. The meningeal T cell population is dynamic: Treatment with FTY720 (a sphingosine 1-phosphate receptor-1 agonist that traps lymphocytes in lymph nodes) or VLA-4–targeting antibodies reduces the number of T cells and monocytes in the meninges (55, 58, 59). Moreover, removal of the deep cervical lymph nodes results in an increase in T cell number in meningeal spaces, pointing to their circulation between these anatomical locations. In parabiotic wild-type (WT) mice, lymphocyte exchange occurs in the meninges (suggesting that the immunity within meningeal barriers is dynamic), but the rate of exchange is lower than the 50:50 ratio seen in the blood. This ratio changes, however, when TCR transgenic mice are attached parabiotically to WT mice: The amount of WT cells entering the meninges of TCR transgenic mice is at least doubled relative to WT:WT parabionts (59). These results further indicate that meningeal immunity is dynamic and T cells are presumably enriched for a specifically selected repertoire. Despite the importance of meningeal immunity in neuroinflammatory diseases such as MS, as well in neuroviral infections, an understanding of immune cell trafficking into this compartment is only emerging (60–62). Also, migration of these cells out of the meninges was only vaguely understood until recently.
How do immune cells enter and exit the CNS?
Access of immune cells to the CNS parenchyma is likely secondary to their infiltration into the meninges during inflammation (60). It is therefore important to understand the routes through which immune cells find their way into and out of the meninges, as well as what keeps them in the meninges as opposed to freeing them to return to the periphery or infiltrate the parenchyma.
Two plausible routes might explain how immune cells access the meninges: through the meningeal blood vessels or, alternatively, via the choroid plexus. The choroid plexus is located within each ventricle of the brain and is composed of epithelial cells surrounding the capillaries and the stromal cells. The endothelial cells in the choroid plexus, unlike elsewhere in the CNS, are fenestrated (63). Because the role of choroid plexus epithelial cells is to produce cerebrospinal fluid (CSF) by filtering the blood, the choroid plexus is highly vascularized, allowing for the presence of many immune cells. However, this does not necessarily mean that cells are able to penetrate the epithelial layer and thus gain entry into the meningeal spaces/CSF. To access the meninges, immune cells from blood vessels supplying the choroid plexus would need to traverse the endothelial barrier (an explainable step) and then the choroid plexus epithelial cell barrier with tight gap junctions (an unusual step for lymphocytes) to enter the CSF. For a cell to penetrate the meningeal vessels and enter the CSF, it has to cross the BMB. The BMB differs from the BBB and lacks some of the latter’s components, such as astrocyte endfeet (64, 65), making it easier for cells to penetrate (Fig. 2, A and B).
Fig. 2. Meningeal and parenchymal access of immune cells.
(A) During the steady state, Tcells (and presumably other immune cells) circulate through the meningeal spaces. Their primary entry site is via the meningeal blood vessels, where the immune cells need to cross the blood-meningeal barrier (BMB) to enter the meningeal space. Blood-borne immune cells do not cross the blood-brain barrier (BBB) in a healthy situation. (B) Choroid plexus endothelial cells are fenestrated, which allows immune cells to easily cross them. For the immune cells to make their way into the CSF, however, they need to also cross a tight barrier of choroid plexus epithelial cell layer connected by tight junctions. (C and C′) Under pathological conditions such as inflammation, immune cells extravasate through the meningeal vessels and then cross the pial layer to infiltrate the brain parenchyma (C) or, more plausibly, the meningeal inflammatory environment results in the production of chemokines that, upon diffusion into the parenchyma (across pia), recruit peripheral immune cells across the BBB (C′).
A recent study suggests that meningeal blood vessels recruit T cells into the meningeal spaces (60). Though activation of T cells in the meninges and their detachment are probably necessary for the cells to access the parenchyma, the route from the meningeal spaces/CSF to the parenchyma is not well understood. Meningeal cells have the capability to transmigrate across pia mater to reach parenchyma (48), but the mechanisms guiding such a process are still unclear. Under neuroinflammatory conditions, the gradient of chemokines produced and induced by meningeal immune cells might result in transmigration of immune cells across the BBB (66). Both routes might contribute to immune infiltration in patients with MS [particularly given the differential homing of encephalitogenic CD8+ T cells (67)] and need to be studied further (Fig. 2C).
CNS drainage: New concepts for old
Much is still unclear about the entry of cells into the meningeal spaces and the CNS parenchyma. Until recently, the CNS routes by which immune cells and macromolecules exit the meninges and the CNS were even less clear. All bodily tissues are served by two kinds of vessels: (i) blood vessels that convey blood, oxygen, and nutrients to the tissue and (ii) lymphatic vessels that remove the tissue’s waste products. These vascular routes are shared by immune cells, which use blood vessels as a means of accessing the tissue and lymphatic vessels as the main exit routes. It is therefore likely that immune cells surveying a tissue leave it by the same exit path as that taken by the tissue’s waste products. If this is the case in the meninges, then we need to understand the drainage system of the brain (or, for that matter, of the entire CNS) to comprehend how immune cells exit the CSF/meninges. Because of the lack of lymphatic vessels in the CNS parenchyma, its drainage pathways have remained unclear. A new (or revised) hypothesis proposed that the perivascular space [formed between a blood vessel’s endothelial cells and the astrocytic endfeet processes and termed as “glymphatic” (68)] serves as a channel that allows CSF to enter the parenchyma along the arteries (Fig. 3). Pulsation of the vessels allows perivascular fluid, along with its macromolecules (of a particular size range), to diffuse from the periarterial spaces into the parenchyma, from which it is subsequently reabsorbed at the perivenular space, owing to the expression of aquaporin 4 (a protein that conducts water through the cell membrane) by perivenular astrocytes. On the way from the periarterial to the perivenular space, the fluid “washes” the parenchyma, carrying tissue-generated waste products with it. Those waste products are then carried through the perivenular space back into the CSF (69) (Fig. 3). But what drains the CSF?
Fig. 3. Schematic representation of the glymphatic system.
Periarterial space (formed between a blood vessel’s endothelial cells and the astrocytic endfeet processes) allows CSF to follow the arteries into the parenchyma. CSF, along with macromolecules within it, diffuses from the periarterial spaces as an interstitial fluid into the parenchyma, “washes” the parenchyma, and is reabsorbed into perivenular space, to be then carried back and mixed with the CSF.
Humans produce about six times their CSF volume per day, most of which drains into venous sinuses through the arachnoid granulations. This path is unlikely to allow immune cells to traffic out because immune cells normally do not leave tissues through reverse transmigration into the blood vessels. An alternative pathway might be exiting via the cribriform plate, a porous plate on the skull that allows olfactory nerves to exit the brain and innervate the nasal cavity (70). Immune cells and CNS antigens and other macromolecules could leave the CNS along the olfactory nerves and enter the nasal mucosa, from which they are reabsorbed by the nasal mucosa lymphatics and drained into the deep cervical lymph nodes (71, 72). The absence of classical lymphatics in the CNS was the distinctive trait that led to the proposal of this path (Fig. 4).
Fig. 4. CNS drainage: New concepts for old.
(A) Before the discovery of meningeal lymphatic vessels, the old concept of CNS drainage was based on the fact that water from CSF is drained through arachnoid granulations, whereas macromolecules and immune cells from the CNS and the CSF are drained through the cribriform plate into nasal lymphatics and, from there, to CNS-draining deep cervical lymph nodes. (B) Discovery of the meningeal lymphatic vessels led to the hypothesis that they may drain meningeal immune cells and macromolecules from the parenchyma and the CSF, whereas the contribution of the cribriform plate as a drainage route for immune cells under homeostatic conditions needs to be reassessed. This route may be more active during neuroinflammatory conditions. Additional studies are needed to better characterize the contribution of each route of drainage for immune cells and macromolecules from the CNS and the CSF under homeostatic and pathological conditions.
This hypothesis of drainage into the deep cervical lymph nodes was recently challenged when the reported existence of meningeal lymphatics was proposed as the direct migratory route for immune cells and macromolecules from the CSF into the deep cervical lymph nodes (73, 74). A scrupulous search of the literature revealed that meningeal lymphatic vessels and CNS-draining lymphatic connections were suggested in the past (75–77) but were apparently overlooked by contemporary researchers (70, 71). Markers that permit an unambiguous definition of lymphatic vessels have only recently been developed (78, 79). Many organs—such as the eye (80) and, most recently, the CNS (73, 74)—once believed to be devoid of lymphatic vessels appear to be drained by lymphatic vessels. Although meningeal lymphatic vessels drain macromolecules from within the brain parenchyma, their location is extraparenchymal. A study published nearly 40 years ago indicated that the brains of patients with MS harbor lymphatic vessels (76). Verifying these findings with modern tools would be an important achievement, because although the healthy parenchyma is not vascularized by lymphatic vessels but rather drained via lymphatic vessels in the meninges, it is plausible that in inflammatory conditions such as MS, the meningeal lymphatic vessels may extend, physiologically or pathologically, into the CNS parenchyma. Definitive proof that this happens is not easy to obtain because no exclusive markers of lymphatic endothelial cells currently exist, and many of the molecules expressed by lymphatic endothelial cells are also expressed by other cells in the CNS (79). Development of novel whole-organism imaging techniques will make it possible to conduct a closer examination of routes for the removal of waste material from the brain parenchyma and may even lead to the discovery of lymphatic vessels within the parenchyma itself. Such putative parenchymal vessels may not be fully functional and, in the healthy adult animal, may not even be organized into vessels. However, if they grow and form functional vessels during the course of inflammation, it might be possible to achieve more efficient drainage of immune cells. Similar mechanisms may apply to the generation of tertiary lymphatic structures associated with CNS inflammation in MS patients and in some animal models (81, 82).
Why are the nervous and immune systems so important for each other’s functioning?
This Review has focused on the adaptive immune system’s effects on the brain. I did not attempt to review the recent works on the new roles of microglia in healthy and diseased CNS function (83–88), as the subject warrants a full analysis of its own. Space restrictions here also prohibit inclusion of another relevant topic in neuroimmunology: interaction between the immune system and the peripheral nervous system (89–94).
Many molecules classically defined as “immune” are also crucial for CNS development and function (95–98). This fact further contributes to the growing appreciation that the two systems are molecularly and cellularly equipped for close communication. The interaction of the two systems, for so long considered to function separately, begs a fundamental question: Why are they so closely related, with each so capable of affecting the other?
The answer is to be found by looking into the evolutionary development of their coexistence (99). Assuming that pathogens represent a major driving force of evolution and that the counterforce is antipathogen immunity, it is plausible that the interface between pathogens and immunity influenced the evolution of our almost infinitely complex nervous system. Moreover, some behavioral traits may have evolved as a result of an “arms race” between the nervous and immune systems and the pathogens. As an example, because many of our ancestors died from infections, sickness behavior might have evolved to prevent the spread of the infective agent. This might have led to the evolvement of cytokine ability to affect the brain in a manner expressed as social withdrawal (100). On the other hand, the infective agent would favor social behavior on the part of the host so that the pathogen could spread. For example, mice infected with the parasite Toxoplasma gondii lose their fear response to cats, their predator and the parasite’s natural host (101). The study indicated that this may occur by direct parasite infection of mouse neurons; however, it did not rule out that a loss of fear might have been mediated by cytokines produced by the immune system. If so, behavioral development might similarly have been shaped through interactions with pathogens. Recent work in my lab suggests that social behavior and an antipathogen response (for example, through an interferon-γ signaling pathway) might have coevolved (102). For the evolution of social behavior both within and between groups, an antipathogenic immune response would be needed to protect individuals from pathogen spread. Along similar lines, inability to fight an infection might have resulted in traits of self-isolation or other antisocial behavior.
Neuroimmunology: Quo vadis?
The major questions in neuroimmunology are too numerous to be listed in a few pages. However, since the emphasis of this Review is on the adaptive arm of the immune system and its effects on CNS function in physiology and pathology, it seems justifiable to discuss the unanswered questions of neuroimmunology from that perspective.
Recently discovered meningeal lymphatic vessels (73, 74) seem to represent an important exit route for immune cells (and macromolecules) from the CNS and/or CSF. The way into the CNS and/or the CSF is still a matter of debate. Although the most-recent studies have convincingly demonstrated that meningeal blood vessels are the main route for immune cells into the CSF and meningeal spaces (60), how T cells access the parenchyma is not yet clear. One possibility is that meningeal T cells infiltrate the parenchyma when a regulatory mechanism fails. Another is that meningeal myeloid cells produce chemokines that allow peripheral T cells to migrate across the blood vessels into the parenchyma (Fig. 2).
A question closely related to the matter of entry and exit routes is the antigenic specificity of meningeal T cells affecting the brain. One plausible scenario might be that T cells in the meninges are largely specific for CNS self-antigens (autoimmune T cells). Interstitial fluid from the CNS drains, at least partially, into the CSF, where it is sampled by meningeal myeloid cells, which then present the acquired antigens to T cells. Such tonic activation allows autoimmune T cells to maintain a particular homeostatic cytokine profile that controls the phenotype of meningeal myeloid cells and thus allows the brain to function properly. When control mechanisms fail, these T cells may acquire an unfavorable phenotype and then invade the parenchyma.
Another possibility is that the meninges do not necessarily select for CNS self-reactive T cells but instead serve throughout the organism’s lifetime as a reservoir for any and all specificities of memory T cells. Thus, for example, upon viral infection a representative memory T cell for a specific viral epitope may migrate and reside within the meninges to protect the CNS, via its barriers, from future encounters with that pathogen. Not yet clear in this scenario, however, is knowledge of the mechanism that would maintain those cells within the meninges or how their activity might affect brain function.
The current working model probably leans more toward the first scenario, where the establishment and maintenance of the meningeal T cell repertoire can conceivably be perceived and mechanistically understood. Preliminary findings suggest that meningeal immunity is prone to anti–self-antigen specificity (50, 76). Ultimately, the way to address the question of antigenic specificity of meningeal T cells is likely to be via single-cell sequencing of the TCR, reconstruction of the transgenic TCR and its examination for recognition of self-antigens, and examination of cognitive function in mice transgenic for different TCRs retrieved from meningeal T cells.
Although we are far from a complete understanding of how meningeal immunity shapes CNS function, it is plausible to suggest that the CNS has pushed its immune activity to its borders (i.e., meningeal linings) to allow neural cells to function undisturbed. Therefore, another major challenge is to gain a more global understanding of how meningeal immunity affects the brain. So far, several cytokines are known to affect certain brain functions. A more comprehensive mapping of the presence and absence of different cytokines and immune cell subtypes, and how they affect signaling within the CNS, will be crucial. The use of reporter mice, combined with neuronal activation techniques such as optogenetics (103) or magnetogenetics (104), may allow mapping of neural ensembles that respond to the presence or absence of a particular molecular (immune-derived) player.
As our understanding of the interactions between these two complex systems advances, more questions will undoubtedly emerge. The field of neuroimmunology can be likened to an iceberg: We can perceive certain aspects (e.g., neuroinflammatory conditions) as a threat, but the huge undersurface (in this case, its therapeutic potential) has not been sufficiently explored. As we enter a new era of neuroimmunological research, equipped with an astonishing array of tools and technologies from the fields of neuroscience, immunology, genomics, and bioinformatics, we can look forward to some fascinating revelations and discoveries.
Science has made great progress in the fight against infectious agents, autoimmune diseases, and some types of cancers. However, apart from the fact that the U.S. Food and Drug Administration has approved 13 therapeutic modalities for MS, the treatment of neurological disorders is lagging behind. Perhaps the harnessing and targeting of the immune system as a therapy for neurological disorders should be moved to the frontlines as a crucial focus for the current and next generation of scientists.
Acknowledgments
I thank S. Smith for editing the manuscript, A. Impagliazzo for the artwork, and all members of my lab for valuable comments during multiple discussions of this work. This work was supported by NIH grants AG034113 and NS096967.
REFERENCES AND NOTES
- 1.Medawar PB. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 2.Hašek M, Chutna J, Sládecek M, Lodin Z. Nature. 1977;268:68–69. doi: 10.1038/268068a0. [DOI] [PubMed] [Google Scholar]
- 3.Wekerle H. Curr Top Microbiol Immunol. 2006;305:25–50. doi: 10.1007/3-540-29714-6_2. [DOI] [PubMed] [Google Scholar]
- 4.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Río-Hortega P. In: Cytology and Cellular Pathology of the Nervous System. Penfield W, editor. Paul B. Hoebaer; New York: 1932. pp. 483–534. [Google Scholar]
- 6.Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Proc Natl Acad Sci USA. 2000;97:2846–2851. doi: 10.1073/pnas.050569397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ader R, Cohen N. Science. 1982;215:1534–1536. doi: 10.1126/science.7063864. [DOI] [PubMed] [Google Scholar]
- 8.Ader R, Cohen N. Psychosom Med. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Steinman L. J Exp Med. 2003;197:1065–1071. doi: 10.1084/jem.20030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamvil SS, et al. J Exp Med. 1985;162:2107–2124. doi: 10.1084/jem.162.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamvil S, et al. Nature. 1985;317:355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 12.Steinman L, et al. Nature. 1982;299:738–740. doi: 10.1038/299738a0. [DOI] [PubMed] [Google Scholar]
- 13.Yednock TA, et al. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 14.Kuchroo VK, et al. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 16.Chabas D, et al. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 17.Lider O, Reshef T, Beraud E, Ben-Nun A, Cohen IR. Science. 1988;239:181–183. doi: 10.1126/science.2447648. [DOI] [PubMed] [Google Scholar]
- 18.Farez MF, et al. Cell. 2015;162:1338–1352. doi: 10.1016/j.cell.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayo L, et al. Nat Med. 2014;20:1147–1156. doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascanfroni ID, et al. Nat Immunol. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matzinger P. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 22.Cohen IR. Immunol Today. 1992;13:490–494. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]
- 23.Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moalem G, et al. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 25.Kuchroo VK, et al. Annu Rev Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- 26.Steinman L. Annu Rev Immunol. 2014;32:257–281. doi: 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- 27.Weiner HL. Ann Neurol. 2009;65:239–248. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- 28.Gourraud PA, et al. Ann Neurol. 2014;76:633–642. doi: 10.1002/ana.24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croxford AL, Spath S, Becher B. Trends Immunol. 2015;36:651–662. doi: 10.1016/j.it.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Simmons SB, Pierson ER, Lee SY, Goverman JM. Trends Immunol. 2013;34:410–422. doi: 10.1016/j.it.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pikor N, Gommerman JL. Mult Scler Relat Disord. 2012;1:123–130. doi: 10.1016/j.msard.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Petrovich E, et al. FASEB J. 2016;30:1767–1778. doi: 10.1096/fj.201500046. [DOI] [PubMed] [Google Scholar]
- 33.Aubé B, et al. J Immunol. 2014;193:2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- 34.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Colonna M, Wang Y. Nat Rev Neurosci. 2016;17:201–207. doi: 10.1038/nrn.2016.7. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, et al. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heppner FL, Ransohoff RM, Becher B. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 38.Jay TR, et al. J Exp Med. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh SE, et al. Proc Natl Acad Sci USA. 2016;113:E1316–E1325. doi: 10.1073/pnas.1525466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kipnis J, et al. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kipnis J, et al. Proc Natl Acad Sci USA. 2000;97:7446–7451. doi: 10.1073/pnas.97.13.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baruch K, et al. Nat Med. 2016;22:135–137. doi: 10.1038/nm.4022. [DOI] [PubMed] [Google Scholar]
- 43.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. Proc Natl Acad Sci USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazeldine J, Lord JM, Belli A. Front Neurol. 2015;6:235. doi: 10.3389/fneur.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh JT, et al. J Clin Invest. 2015;125:699–714. doi: 10.1172/JCI76210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Walsh JT, et al. J Immunol. 2014;193:5013–5022. doi: 10.4049/jimmunol.1302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth TL, et al. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo MV, McGavern DB. Trends Immunol. 2015;36:637–650. doi: 10.1016/j.it.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gadani SP, Walsh JT, Lukens JR, Kipnis J. Neuron. 2015;87:47–62. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitz J, et al. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Kipnis J, et al. Proc Natl Acad Sci USA. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. Proc Natl Acad Sci USA. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kipnis J, Gadani S, Derecki NC. Nat Rev Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derecki NC, et al. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kipnis J, Derecki NC, Yang C, Scrable H. Trends Immunol. 2008;29:455–463. doi: 10.1016/j.it.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Brynskikh A, Warren T, Zhu J, Kipnis J. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Radjavi A, Smirnov I, Kipnis J. Brain Behav Immun. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radjavi A, Smirnov I, Derecki N, Kipnis J. Mol Psychiatry. 2014;19:531–533. doi: 10.1038/mp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schläger C, et al. Nature. 2016;530:349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 61.Kivisäkk P, et al. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kivisäkk P, et al. Proc Natl Acad Sci USA. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shechter R, London A, Schwartz M. Nat Rev Immunol. 2013;13:206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- 64.Lécuyer MA, Kebir H, Prat A. Biochim Biophys Acta. 2016;1862:472–482. doi: 10.1016/j.bbadis.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Broux B, Gowing E, Prat A. Semin Immunopathol. 2015;37:577–590. doi: 10.1007/s00281-015-0516-2. [DOI] [PubMed] [Google Scholar]
- 66.Engelhardt B, Ransohoff RM. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Larochelle C, et al. Ann Neurol. 2015;78:39–53. doi: 10.1002/ana.24415. [DOI] [PubMed] [Google Scholar]
- 68.Nedergaard M. Science. 2013;340:1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie L, et al. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weller RO, Djuanda E, Yow HY, Carare RO. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 71.Goldmann J, et al. J Leukoc Biol. 2006;80:797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 72.Harris MG, et al. Sci Rep. 2014;4:4422. doi: 10.1038/srep04422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louveau A, et al. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aspelund A, et al. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andres KH, von Düring M, Muszynski K, Schmidt RF. Anat Embryol. 1987;175:289–301. doi: 10.1007/BF00309843. [DOI] [PubMed] [Google Scholar]
- 76.Prineas JW. Science. 1979;203:1123–1125. doi: 10.1126/science.424741. [DOI] [PubMed] [Google Scholar]
- 77.Varga L, Piukovich I, Zoltán OT, Gábor M, Földi M. Acta Med Acad Sci Hung. 1966;22:15–23. [PubMed] [Google Scholar]
- 78.Alitalo K. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 79.Yang Y, Oliver G. J Clin Invest. 2014;124:888–897. doi: 10.1172/JCI71609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aspelund A, et al. J Clin Invest. 2014;124:3975–3986. doi: 10.1172/JCI75395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peters A, et al. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magliozzi R, et al. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 83.Stephan AH, Barres BA, Stevens B. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 84.Vasek MJ, et al. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong S, et al. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paolicelli RC, et al. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 87.Lui H, et al. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stevens B, et al. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 89.von Hehn CA, Baron R, Woolf CJ. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosas-Ballina M, et al. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Talbot S, et al. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiu IM, et al. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tracey KJ. Cell. 2016;164:343–344. doi: 10.1016/j.cell.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 94.Pavlov VA, Tracey KJ. Immunol Res. 2015;63:38–57. doi: 10.1007/s12026-015-8718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shatz CJ. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee H, et al. Nature. 2014;509:195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Datwani A, et al. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atwal JK, et al. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 99.Kioussis D, Pachnis V. Immunity. 2009;31:705–710. doi: 10.1016/j.immuni.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 100.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ingram WM, Goodrich LM, Robey EA, Eisen MB. PLOS ONE. 2013;8:e75246. doi: 10.1371/journal.pone.0075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Filiano AJ, et al. Nature. 2016;535:425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rajasethupathy P, Ferenczi E, Deisseroth K. Cell. 2016;165:524–534. doi: 10.1016/j.cell.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wheeler MA, et al. Nat Neurosci. 2016;19:756–761. doi: 10.1038/nn.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]