Abstract
The hepatitis C virus (HCV) infects more than 180 million people worldwide, with long-term consequences including liver failure and hepatocellular carcinoma. Quercetin bioflavonoids can decrease HCV production in tissue culture, in part through inhibition of heat shock proteins. If quercetin demonstrates safety and antiviral activity in patients, then it could be developed into an inexpensive HCV treatment for third world countries or other affected populations that lack financial means to cover the cost of mainstream antivirals. A phase 1 dose escalation study was performed to evaluate the safety of quercetin in 30 untreated patients with chronic HCV infection and to preliminarily characterize quercetin’s potential in suppressing viral load and/or liver injury. Quercetin displayed safety in all trial participants. Additionally, 8 patients showed a “clinically meaningful” 0.41-log viral load decrease. There was a positive correlation (r = 0.41, p = 0.03) indicating a tendency for HCV decrease in patients with a lower ratio of plasma quercetin relative to dose. No significant changes in aspartate transaminase and alanine transaminase were detected. In conclusion, quercetin exhibited safety (up to 5g daily) and there was a potential for antiviral activity in some hepatitis C patients.
Keywords: bioflavonoids, dose escalation, hepatitis C, phase I, phytomedicine, quercetin
INTRODUCTION
The hepatitis C virus (HCV) infects 180 million people worldwide and accounts for more than 12 000 deaths in the US each year(Alter, 1997; Armstrong et al., 2006; Wasley et al., 2008). Although some HCV infections clear spontaneously, most patients with HCV become chronic carriers and risk serious complications, including cirrhosis and hepatocellular carcinoma (HCC) (Hoofnagle, 2002).
Curative antivirals are the most effective secondary prevention of HCV-associated chronic liver diseases. Previously, the standard HCV treatment was pegylated interferon combined with ribavirin (RBV). Unfortunately, adverse events or contra-indication excludes 70–80% of HCV patients from interferon-based therapy (Falck-Ytter et al., 2002). Ledipasvir-sofosbuvir (Harvoni; Gilead, Foster City, CA) was recently introduced as a new interferon-free/RBV-free option that prevents serious complications in patients (Afdhal et al., 2014a, 2014b; Kowdley et al., 2014). But the price of this medication limits access, as cost-benefit analysis estimates a 12 week ledipasvir-sofosbuvir treatment to cost $94 500 per patient (Younossi et al., 2015). Data suggest that a longer 24-week treatment, costing up to $189000, is needed in certain HCV-infected populations, such as patients with liver cirrhosis or previous antiviral exposure (Younossi et al., 2015).
The dietary supplement quercetin has the potential to be developed into an affordable and natural alternative to expensive interferon-free/RBV-free treatments. Quercetin is a bioflavonoid found in many plants, tea, and wine (Romano et al., 2013; Srinivas, 2015). It has several biological benefits including: suppression of respiratory tract infections, decrease in pancreatic cancer risk in male smokers, improvement in early cadaveric renal transplantation outcomes, and prevention of anaphylactic reactions (Fanning et al., 1983; Shoskes et al., 2005; Valentova et al., 2007; Bobe et al., 2008). Previous pharmacokinetic studies have elucidated quercetin’s metabolism (Harwood et al., 2007). Specifically, extensive first pass metabolism of quercetin in the liver and kidneys results in more conjugated quercetin and a lowered plasma concentration of free unconjugated quercetin. Bioavailability studies have reported quercetin’s elimination half-life to range from 31 to 50 h (Walle et al., 2001).
Our previous laboratory efforts determined that quercetin exerts in vitro antiviral properties in human HCC tissue culture. We demonstrated that quercetin can indirectly inhibit HCV replication through the suppression of host heat shock proteins (HSP). This effect could be related to the complexes that HSPs form with nonstructural protein 5A (NS5A), an essential HCV protein that is associated with viral production and blocking host antiviral responses (Gonzalez et al., 2009). Additional studies suggest that quercetin can also directly inhibit viral protease nonstructural protein 3 (NS3) (Bachmetov et al., 2012) and NS5A (Bhattacharya et al., 2012). Quercetin’s antiviral mechanism is of particular interest in antiviral research because this multi-level approach (direct and indirect) against HCV could maintain selective pressure against viral relapse (Prussia et al., 2011).
Given quercetin’s affordability and tolerability, with doses up to 1 g/day demonstrating safety in numerous trials, we sought to determine its safety in patients with HCV at higher doses (up to 5 g daily) (Nieman et al., 2007a, 2007b; McAnulty et al., 2008; Heinz et al., 2010a, 2010b; Shanely et al., 2010). Here we report the results of the first phase 1 clinical trial using quercetin in HCV patients with mild liver dysfunction. In addition, we report preliminary results on viral load to characterize possible antiviral activity. We also investigate quercetin’s anti-hepatotoxic effects as measured by the change in levels of aspartate transaminase (AST) and alanine transaminase (ALT), two liver enzymes associated with hepatocyte injury and inflammation (Bala et al., 2012).
METHODS
Patients
We recruited patients from August 2010 to June 2014 with the assistance of hepatologists at the University of California Los Angeles (UCLA) Pfleger Liver Institute. All patients provided written informed consent and the study was approved by the UCLA Office of the Human Research Protection Program. This study was conducted in accordance with the World Medical Association Declaration of Helsinki. Patients were informed of the newly FDA approved antivirals, but chose to enroll in this study because of financial reasons or preference for natural compounds. Eligibility criteria included: 18–65 years of age, either treatment-naive or not currently taking any antiviral therapy, and contraindications to standard HCV therapies. Additional requirements were the following: detectable HCV RNA in the serum; stable viral load within the previous year (no fluctuation > 2-log scale); compensated liver disease (total serum bilirubin < 1.5 g/dL; INR < 1.5; albumin > 3.4 g dL, platelet count > 125 000/mm3, no evidence of hepatic encephalopathy or ascites); acceptable liver enzymes (AST/ALT < 1.5 × upper limit of normal); acceptable hematological indices (hemoglobin > 13 g/dL for men and > 12 g/dL for women; neutrophil > 1500 mm3; AFP < 50 ng/mL); and acceptable renal function (creatine < 1.5 mg/dL). Exclusion criteria were the following: any other hepatitis etiologies or coinfection with HBV and/or HIV, HCC, a history of non-compliance, current alcohol use, pregnancy, breastfeeding, renal impairment, or renal transplant. As this is the first clinical trial testing quercetin’s safety in patients with HCV who are known to have various degrees of liver dysfunction and given the liver is one of quercetin’s primary sites of metabolism, we specifically excluded patients with more severe liver damage (AST/ALT > 1.5 × upper limit of normal, total serum bilirubin > 1.5 g/dL, platelet count < 125 000/mm3, abnormal coagulation studies). These eligibility criteria were established to avoid toxicities attributable to impaired clearance of potential quercetin toxic metabolites.
Outcomes
The main objectives were to determine the safety, tolerability, and the maximum tolerated dose (MTD) of quercetin. As secondary outcomes, we examined the change in patient viral load and liver enzyme levels (AST and ALT) between baseline and follow-up. A viral load decrease of 0.41-log (2.6-fold) compared with patient baseline was defined as a “clinically meaningful” decrease (Terrault et al., 2005). A change in AST and ALT exceeding 2 standard deviations (SDs), calculated based on the patient sample at baseline, was considered a notable increase or decrease. The ratio of quercetin plasma concentration (μg/L) to dose level (mg), denoted as pq/d, was calculated as an indicator of the amount of the original dose retained in the plasma.
Follow up
At patient’s study entry, we obtained a medical history, complete physical exam, and baseline laboratory assessment. Laboratory measurements (UCLA Laboratory, Los Angeles, CA) included complete blood count with differentials, chemistry (Na, K, Cl, HCO3, BUN, creatinine, Mg, and Phos), liver function tests (AST, ALT, Alk Phos, Tbilirubin, Dbilirubin, and PT/PTT/INR), hepatitis C viral load, and cholesterol panel (LDL, HDL, Tchol, and triglycerides). These laboratory measures were repeated at clinic visits during weeks 2 and 4 after start of study treatment. At clinic visits, we also monitored patients’ vital signs, weight, and adverse events. We defined adverse events according to an adapted NCI-Common Terminology Criteria for Adverse Events (NCI-CTCAE v4.0). Medical compliance was determined by patient attendance to clinic visits and the completion of symptom surveys. An additional follow-up clinic visit with laboratory assessment was obtained 1–2 weeks after dose completion, to assess the duration of viral load change observed while on quercetin (n = 22 patients). Plasma quercetin analysis of baseline and week 4 blood samples was performed at Appalachian State University-North Carolina Research Campus, according to protocol as previously described (Nieman et al., 2007a, 2007b).
Dosing
This study used a dose escalation with overdose control (EWOC) design, a Bayesian adaptive Phase I design in which dosing starts at a predetermined level, doses for subsequent patients are chosen using information on adverse effects experienced by previous patients, and dose escalation is slowed and stopped as the MTD is reached (Rogatko and Xu, 2005; Tighiouart et al., 2005). The EWOC algorithm generates dose trees based on initial dose (250 mg), patient cohort size (3), maximum dose increment (500 mg), and maximum dose. The trial was initially designed with a maximum dose of 2000 mg and then amended for a maximum of 5000 mg. This resulted in the utilization of 11 different dose levels. Patients were instructed to take the quercetin chews orally for 28 days alongside juice or water, preferably at the same time each day.
Statistical analysis
The EWOC method was planned to be used to estimate the MTD (Rogatko and Xu, 2005; Tighiouart et al., 2005); however, the MTD was not reached during the trial. Local regression, implemented with the lowess command in Stata 13 (Cleveland, 1979), was used to obtain nonparametric estimates of the relationship between quercetin plasma concentration and dose level and between viral load change and quercetin plasma/dose ratio. Since these relationships were approximately linear, we also summarized the strength of these associations using Pearson correlation coefficients.
RESULTS
Patient characteristics
Baseline clinical and demographic characteristics of the 30 study patients are shown in Table 1. Individuals with HCV genotypes determined through previous sequencing analysis were predominantly genotype 1a or 1b. Most patients had a white ethnic origin and were California residents. Fib-4 scores (a noninvasive index to assess liver scarring) for our patient population (Table 2) ranged from 0.38 to 4.15 with a median of 1.90 (Table 1), a score associated with an 80% chance of minimal to moderate liver fibrosis (Sterling et al., 2006).
Table 1.
Patient characteristics (n = 30)
| Patient characteristics | |
|---|---|
| Age, years | |
| Range | 25–65 |
| Sex, N (%) | |
| Male | 20 (66.7) |
| Female | 10 (33.3) |
| Ethnicity, N (%) | |
| Hispanic or Latino | 3 (10.0) |
| Black or African American | 2 (6.7) |
| White | 25 (83.3) |
| Geographic region, N (%) | |
| California | 28 (93.3) |
| Michigan | 1 (3.3) |
| Arizona | 1 (3.3) |
| HCV genotype, N (%) | |
| 1 (a/b) | 18 (60.0) |
| 2 (a/b) | 2 (6.7) |
| 3 (a/b) | 5 (16.7) |
| Unknown | 5 (16.7) |
| Baseline viral load, IU/mLa | 1.0 × 105 − 6.9 × 107 (8.3 × 106 ± 1.2 × 107) |
| Baseline AST, U/La | 20−243 (67.7±46.6) |
| Baseline ALT, U/La | 24−276 (91.9±58.4) |
| Baseline Fib-4b | 0.38−4.15 (1.90) |
Baseline viral load, AST, and ALT values presented as range (Mean ± SD). Fib-4 value presented as range (median).
AST: aspartate transaminase, ALT: alanine transaminase, N: sample size, HCV: hepatitis C virus, lU/mL: international units per milliliter, U/L: units per liter.
Baseline viral load, AST, and ALT values presented as range (mean ± SD).
Baseline Fib-4 value presented as range (median).
Table 2.
Patient data overview
| Dose group (mg) | Patient no. | Fib-4 baseline | Plasma quercetin baseline (μg/L) | Plasma quercetin week 4 (μg/L) | % Change (baseline vs week 4)
|
|||
|---|---|---|---|---|---|---|---|---|
| Plasma quercetin | ALT | AST | Viral load | |||||
| 250 | 1a | 2.36 | 37.13 | 136.54 | 268% | −16% | −26% | −84% |
| 2a | 2.34 | 123.14 | 131.76 | 7% | 4% | −5% | −69% | |
| 3 | 2.52 | 32.98 | 232.34 | 604% | −10% | −29% | −15% | |
| 500 | 4 | 1.89 | 121.57 | 861.78 | 609% | −13% | −9% | 3% |
| 5 | 2.65 | 25.07 | 966.67 | 3756% | −4% | −58% | 78% | |
| 6 | 1.56 | 33.54 | 1515.81 | 4419% | −17% | −11% | −8% | |
| 1000 | 7 | 4.15 | 62.53 | 2895.74 | 4531% | 41% | 36% | 59% |
| 8 | 1.31 | 51.26 | 405.52 | 691% | 7% | 7% | −39% | |
| 9a | 1.71 | 57.79 | 864.05 | 1395% | −9% | −5% | −31% | |
| 1400 | 10 | 1.88 | 76.16 | 559.79 | 635% | −16% | −11% | 16% |
| 11 | 2.17 | 68.28 | 830.56 | 1116% | 7% | 4% | 16% | |
| 12 | 1.19 | 100.66 | 4216.33 | 4089% | 12% | 4% | 24% | |
| 2000 | 13 | 2.96 | 17.83 | 4030.97 | 22508% | −11% | −14% | −52% |
| 14 | 1.92 | 20.05 | 1243.43 | 6102% | −13% | −16% | 77% | |
| 2500 | 15 | 2.38 | 37.84 | 2871.57 | 7488% | 90% | 7% | 645% |
| 16 | 2.75 | 4.3 | 3769.9 | 87474% | −35% | −29% | −3% | |
| 17a | 1.12 | 32.72 | 1778.88 | 5337% | 5% | 9% | −85% | |
| 3000 | 18 | 2.83 | 31.9 | 4856.34 | 15124% | −26% | −9% | 32% |
| 19 | 0.82 | 31.09 | 2505.54 | 7958% | −15% | −10% | 28% | |
| 3500 | 20a | 0.38 | 64.06 | 822.76 | 1184% | −19% | −20% | −86% |
| 21 | 0.73 | 179.39 | 620.32 | 246% | 4% | −5% | −41% | |
| 22 | 1.32 | 8.94 | 4010.93 | 44785% | 2% | −11% | 31% | |
| 4000 | 23 | 1.94 | 3.79 | 3875.33 | 102036% | −8% | −13% | 53% |
| 24 | 2.61 | 19.76 | 3296.24 | 16581% | −9% | −10% | −35% | |
| 4500 | 25 | 0.83 | 2.34 | 3308.53 | 141366% | −11% | −15% | −61% |
| 26ab | 1.90 | 22.54 | 3096.78 | 13639% | 20% | 3% | −87% | |
| 27 | 0.90 | 20.13 | 4362.27 | 21567% | 8% | 11% | −16% | |
| 5000 | 28 | 1.89 | 11.83 | 5467.32 | 46101% | −8% | 6% | 155% |
| 29b | 1.04 | 9.77 | 2010.24 | 20469% | 10% | 13% | 26% | |
| 30b | 1.95 | 5.65 | 2132.26 | 37626% | −80% | −73% | −64% | |
0.41-log antiviral response at week 4.
0.41-log antiviral response at week 2.
AST: aspartate transaminase, ALT: alanine transaminase, Fib-4: Fibrosis-4 Score, mg: milligram, no.: number, μg/L: microgram per liter.
Patient adherence
Out of the 34 patients recruited, four withdrew from the study, and a substitute was found for one of the withdrawals, resulting in 30 evaluable patients included in analyses. One patient withdrew because of perceived low urine output but had normal urine and renal studies; one patient who was taking acetaminophen withdrew because of high AST and ALT levels, which came down after acetaminophen and quercetin were discontinued (the AST/ALT levels did not exceed the limit for adverse event reporting), and two patients withdrew because of distance. Compliance was high among the remaining 30 patients, with no adherence issues for any of these remaining patients.
Safety
Quercetin doses ranged from 250 to 5000 mg/day (Table 2). There were three patients per dose group, with the exception of three dose levels (2000, 3000, and 4000 mg/day), which had only two patients per group because of withdrawal from the study. High doses were well tolerated, with no adverse events or signs of toxicity. The MTD was not reached during the trial. There was no notable change from baseline measurement to week 2 and 4 measurements for complete blood count, complete metabolic panel, cholesterol panel, and coagulation study.
Liver enzyme response
While we observed several patients with notable AST or ALT decrease, there was no discernible pattern of liver enzyme change among the trial participants. Most patients’ AST and ALT remained within 2 SD at week 4. However, there were a few patients with liver enzyme changes that exceeded this threshold (Fig. 1a and 1b). For ALT level changes beyond 2 SD, one patient decreased and one patient increased. For AST level changes beyond 2 SD, two patients decreased and one patient increased.
Figure 1.
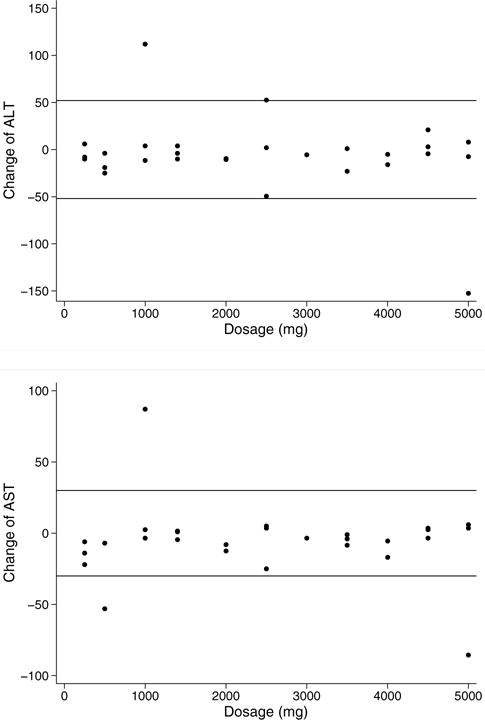
(A) ALT change (week 4). Change in ALT (week 4 minus baseline) by dose. Reference line: ±2 SD. (B) AST Change (week 4). Change in AST (week 4 minus baseline) by dose. Reference line: ±2 SD. This figure is available in colour online at wileyonlinelibrary.com/journal/ptr.
Viral load response
Fig. 2a and 2b present viral load change at weeks 2 and 4, respectively. There were 8 patients who exhibited a “clinically meaningful” decrease in HCV (2.6-fold or 0.41-log change) at week 2 and/or week 4. Two individuals had a meaningful decrease only at week 2. Three other patients decreased only at week 4. An additional three patients had decreases at both weeks 2 and 4. Two patients had a viral load increase over threefold. The HCV genotypes of the patients with 0.41-log viral decrease were similar to those among the total patient sample.
Figure 2.
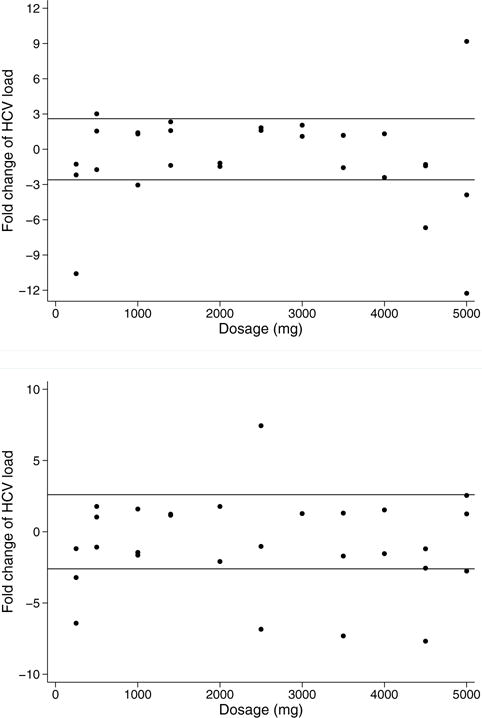
(A) Viral load change (Week 2). Fold change in HCV load (week 2 compared with baseline) by dose. Reference lines: ±2.6 fold (±0.41-log). (B) Viral load change (Week 4). Fold change in HCV load (week 4 compared with baseline) by dose. Reference lines: ±2.6 fold (±0.41-log). This figure is available in colour online at wileyonlinelibrary.com/journal/ptr.
Plasma quercetin measurements
The mean plasma quercetin level among patients at baseline was 44 μg/L (SD 42 μg/L) and ranged from 2 to 179 μg/L. The mean plasma level at week 4 was 2256 μg/L (SD 1595 μg/L). There was a positive association between quercetin dose and plasma level at week 4 (Fig. 3), with Pearson correlation coefficient r = 0.60 (p < 0.05). Change in plasma quercetin concentration from baseline to week 4 was not associated with age, weight, sex, or HCV genotype (all p > 0.05).
Figure 3.
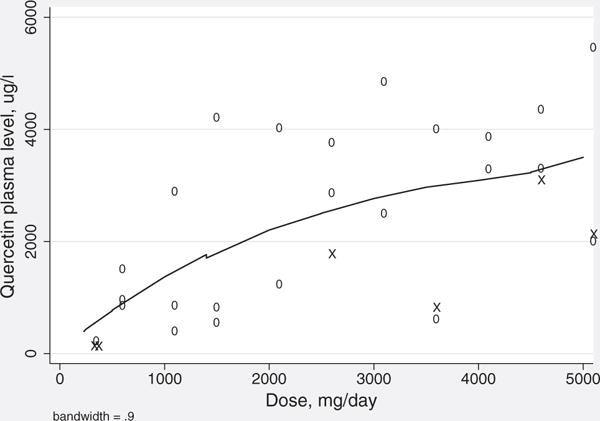
Quercetin dose curve. Scatterplot of quercetin plasma level at week 4 and quercetin dose with local regression line. X = patients at week 4 who displayed at least a 0.41-log (2.6 fold) viral load decrease. This figure is available in colour online at wileyonlinelibrary.com/journal/ptr.
Liver enzyme (AST and ALT) changes did not have a discernible relationship with week 4 plasma quercetin across the different doses. The correlations were r = −0.03 (p = 0.86) between AST change and dose and r = −0.17 (p = 0.38) between ALT change and dose. The majority of patients showed changes within 20% of their baseline value.
There was no clear association between plasma quercetin and relative viral load change (Table 2), with Pearson correlation coefficient r = 0.02 (p = 0.92). One outlier with a large increase in viral load was observed.
Patients with at least a 0.41-log viral load decrease tended to have the lowest levels of plasma quercetin when compared with other patients in their dose groups (Table 2). Based on this observation, we further investigated the relationship between viral load change and a measure of quercetin retained in the plasma, defined as the ratio of plasma quercetin concentration at week 4 to dose level (pq/d). There was significant correlation (r = 0.41, p = 0.03) between percent viral load change and pq/d (Fig. 4), indicating a tendency for viral load decrease in patients with less quercetin from their original dose detected in the plasma (lower pq/d). Conversely, individuals who showed an increase in viral load tended to have more quercetin in the plasma relative to their original dose (higher pq/d).
Figure 4.
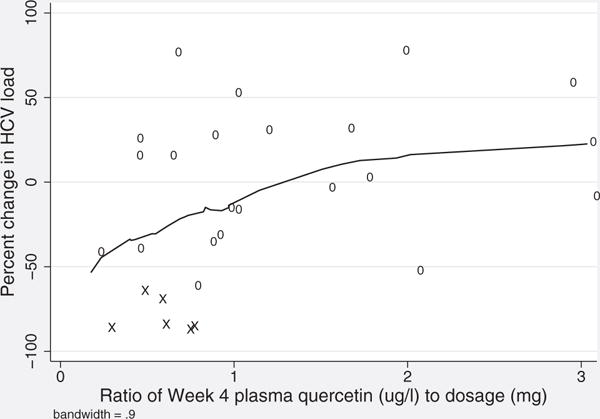
Viral load and plasma quercetin relative to dose. Scatterplot of percent increase in HCV load and quercetin ratio, defined as ratio of week 4 plasma quercetin (μg/L) to dose (mg), with local regression line. Two patients with outlying values omitted (Patients 17 and 32). X = patients at week 4 who displayed at least a 0.41-log (2.6 fold) viral load decrease.
DISCUSSION
We report a clinical trial administering more than 1 g of quercetin daily as an oral supplement, which is the highest dose level examined in clinical trials. Our primary goal of assessing safety was achieved with results indicating that chronically infected hepatitis C patients can tolerate this supplement up to 5000 mg daily for 28 days without adverse events or abnormal laboratory measurements. Due to its excellent tolerability it may prove to be safe for long-term use in other affected populations, such as individuals with HCV-associated liver dysfunction. Furthermore, viral load results suggested that quercetin could potentially have some antiviral activity in some patients. Individuals with a decrease in HCV had a lower level of plasma quercetin in comparison to other patients in the same dose group. This lower plasma quercetin level suggests that the compound may have been removed more efficiently from the bloodstream by the liver (and/or kidneys). However, because of the preliminary nature of this dose escalation clinical trial, we cannot conclude that quercetin has antiviral activity without further investigation. Since this phase I study has affirmed quercetin’s safety, future trials may now be conducted to more specifically investigate quercetin’s potential as an antiviral compound.
Limitations
One limitation was the strict AST/ALT criteria used to assess patient eligibility. Enrolling HCV patients with less severe liver enzyme dysfunction (AST/ALT < 1.5 of ULN) potentially limits our study towards patients without a large enough AST/ALT baseline elevation to demonstrate a significant decrease in these liver enzyme levels. In addition, there may have been variation in the timing of quercetin ingestion. The time when patients took their daily quercetin may not have been uniform (pre/post meal). Similarly, laboratory tests could have been drawn at different time points in relation to a patient’s most recent dose. This could lead to extra variation in AST/ALT, viral load, and plasma quercetin measurements that obscured associations.
The short duration of quercetin administration, 28 days, is another feature of the study that must be considered when interpreting the measurements for viral load and liver enzymes AST/ALT. The 4-week drug phase was selected because the primary objective was to assess safety and the MTD of quercetin in chronic HCV patients. However, antiviral studies are typically designed to be at least 12weeks in length. Thus, the short duration of this study may not have captured quercetin’s potential as an antiviral compound. The shorter time frame also may not have been adequate to detect changes in AST and ALT. Future studies with a primary goal of evaluating quercetin’s antiviral capabilities would require a longer drug phase, of at least 12 weeks or even longer (24 weeks) in the case of HCV relapse. These standard trial durations are 3–6 times longer than our study.
Safety and tolerability
All patients tolerated the oral quercetin supplement (250–5000 mg daily) without significant adverse events. Some patients reported that taking quercetin without food resulted in mild stomach discomfort, which was relieved if quercetin was taken after a meal.
Published clinical trials have established the safety and nutritional benefits of up to 1 g of daily quercetin (Nieman et al., 2007a, 2007b; McAnulty et al., 2008; Heinz et al., 2010a, 2010b; Shanely et al., 2010). Our study confirms that quercetin is safe up to 5 g daily. The MTD was not reached during this trial, implying that the MTD of quercetin is higher than 5 g. If higher bioavailability is required, utilization of quercetin above 5 g is both reasonable and feasible.
Liver enzymes
The absence of a consistent AST/ALT decrease in our patient sample suggests that quercetin lacked a significant effect on these liver enzymes over the short duration of the trial. Nevertheless, our results also indicate that quercetin did not exacerbate liver enzymes (AST/ALT levels below grade 3/4 adverse event reporting) in these patients with chronic HCV. This suggests that quercetin could be well tolerated for future trials in patients with more severe liver dysfunction.
Viral load
The majority of patients with a 0.41-log viral load decrease were in dose groups of 2500–5000 mg, which could be taken as encouragement that quercetin may have some inhibitory effect on HCV. Quercetin’s antiviral effect was previously demonstrated in vitro, through the inhibition of HSP (Gonzalez et al., 2009). Although quercetin’s in vitro antiviral activity is stronger than the results seen in our trial, some indications of a viral decrease are encouraging and suggest that quercetin’s mechanism of action merits further investigation.
Hypothesized antiviral mechanism
We found a correlation between plasma quercetin/dose (pq/d) ratio and viral load change, such that a smaller ratio was associated with larger viral load decreases. The pattern of patients with lower plasma quercetin/dose ratio experiencing a larger viral decrease may seem counterintuitive at first. However, because the site of quercetin’s antiviral activity against HCV is likely in the liver, this ratio may reveal differences in quercetin uptake. Bieger et al. describe how quercetin accumulates in the liver and kidneys where it is further metabolized and excreted. In pigs, quercetin can reach levels at least fourfold higher in the liver than in the plasma (Bieger et al., 2008). Research in liver cells (HepG2) also demonstrates that human hepatocytes are capable of concentrating quercetin intracellularly (Boulton et al., 1999). Various studies support that quercetin absorption is mediated through active uptake by sodium-dependent glucose transporters (SGLT1) (Gee et al., 2000; Ader et al., 2001) or the deglycosylation at the cell surface by lactase phlorizin hydrolase and subsequent passive diffusion (Day et al., 2000; Sesink et al., 2003). It is hypothesized that this deglycosylated metabolite is responsible for quercetin’s biological activity (Ishisaka et al., 2013).
Clinical trial “responders” with lower pq/d may have higher levels of quercetin in the liver, possibly providing an explanation for decreased viral loads. Specifically, variations in the liver’s uptake of quercetin could be responsible for differential levels of plasma quercetin (Fig. 5). In individuals with a low pq/d, more quercetin may accumulate in the liver, resulting in a lower level detected in the plasma. Subsequently, quercetin levels in liver tissue may be high enough to exert antiviral activity. Alternatively responders may generate higher levels of specific quercetin metabolites that are responsible for antiviral activity, regardless of quercetin liver uptake.
Figure 5.
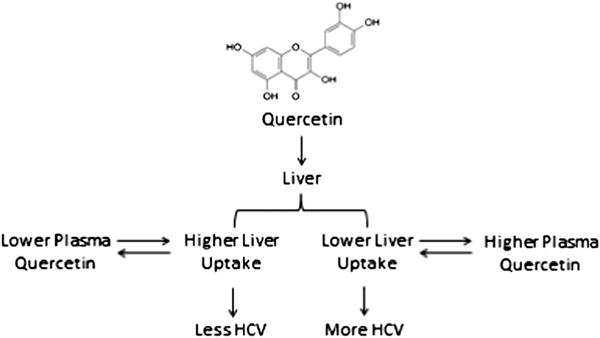
Hypothesized antiviral mechanism.
This hypothesis is preliminary and exploratory and further investigation is required to clarify quercetin’s antiviral mechanism. Future clinical trials could better elucidate quercetin antivirals by examining liver tissue in order to directly gauge quercetin liver uptake instead of indirectly measuring these values through plasma concentration. Similarly, additional research on quercetin’s metabolomic profile in patients with and without significant viral change would better characterize the relationship between specific quercetin metabolites and possible antiviral activity.
Clinical applications
Quercetin’s antiviral capabilities have yet to be confirmed. However, accessible natural compounds could have an application in the landscape of current HCV treatment options. Unlike standard HCV drugs that directly target the virus, quercetin may exert antiviral pressure both directly (inhibiting viral proteins) and indirectly (inhibiting host proteins). Potentially, antiviral bioflavonoids could be beneficial in combination with other HCV treatments. An antiviral with multi-level activity could discourage HCV recurrence in patients treated with standard compounds. Although interferon-free direct antiviral agents (DAAs) have not been in clinical practice long enough to fully gauge relapse rates, viral resistance has continued to be an obstacle throughout the history of HCV treatment. Recent studies show that post-treatment viral relapse is a prominent issue in a subset of patients with HCV treated with a combination of interferon, RBV, and DAAs (McHutchison et al., 2009; Fried et al., 2013; Kowdley et al., 2013). Therefore, HCV treatment strategies could consider applying accessible bioflavonoid compounds to maintain antiviral pressure post-treatment to prevent relapse, preserve DAA efficacy, and optimize the antiviral strategy in lowering the risk of associated liver complications.
The high cost of new FDA approved DAAs creates a need to find alternative antiviral strategies. If quercetin shows anti-HCV activity in future trials, this compound could be an alternative option for patients who cannot access expensive mainstream drugs. This approach has the potential to reach the most affected HCV demographics in a cost-effective manner.
In conclusion, this phase I study demonstrates that quercetin is well tolerated and results suggest that quercetin may have some inhibitory effect on HCV. Future trials should further investigate this natural compound’s mechanism and its potential to become a safe and affordable antiviral for chronic hepatitis C patients.
Acknowledgments
We thank Tom Lines (Quercegen Pharmaceutical LLC) for providing us with custom made Quercetin chews for our study. Additionally, we thank Roxanna Khosravi for her editorial assistance. The trial (clinicaltrial.gov NCT01438320) was approved by the UCLA Institutional Review Board and was conducted at the UCLA Pfleger Liver Institute. This study was funded in part by Tower Cancer Research Foundation, Landon Foundation-American Association for Cancer Research Innovator Award For Cancer Prevention Research, and NIH National Institution of Diabetes, Digestive and Kidney Disease grant number R01DK090794-SWF. Crespi was also partially supported by NIH National Cancer Institute CA16042. The quercetin chew was contributed by Quercegen Pharmaceutical LLC, Sudbury, MA.
APPENDICES
HCV: hepatitis C virus
HCC: hepatocellular carcinoma
AST: aspartate transaminase
ALT: alanine transaminase
PEG-IFN: pegylated interferon
RBV: ribavirin
HSP: heat shock protein
NS3: nonstructural protein 3
NS5A: nonstructural protein 5A
UCLA: University of California, Los Angeles
RNA: ribonucleic acid
HBV: hepatitis B
HIV: human immunodeficiency virus
MTD: maximum tolerated dose
SD: standard deviation
Na: sodium
K: potassium
Cl: chloride
HCO3: bicarbonate
BUN: blood urea nitrogen
Mg: magnesium
Phos: phosphorus
Alk Phos: alkaline Phosphatase
Tbilirubin: total bilirubin
Dbilirubin: direct bilirubin
PT: prothrombin Time
PTT: partial thromboplastin Time
INR: international normalized ratio
AFP: alpha-fetoprotein
LDL: low-density lipoprotein
HDL: high-density lipoprotein
Tchol: total cholesterol
NCI-CTCAE v4.0: NCI-Common Terminology Criteria for Adverse Events Version 4.0
n: sample size
ASU-NCRC: Appalachian State University-North Carolina Research Campus
EWOC: escalation with overdose control
pq/d: ratio of plasma quercetin concentration at week 4 to dose level
SGLT1: sodium dependent glucose transporters
LPH: lactase phlorizin hydrolase
DAAs: direct antiviral agents
FDA: Food and Drug Administration
LLC: limited liability company
TCRF: Tower Cancer Research Foundation
AACR: American Association for Cancer Research
NIDDK: National Institution of Diabetes, Digestive and Kidney Disease
ULN: Upper limit of normal
dL: deciliter
mm3: cubic meter
L: liter
g: gram
mg: milligram
μg: microgram
g/dL: gram per liter
mg/dL: milligram per liter
μg/L: microgram per liter
p: p-value
r: Pearson correlation coefficient
IU/mL: international units per milliliter
U/L: units per liter
no.: number
UCLA-OHRPP: University of California Los Angeles Office of the Human Research Protection Program
WMA: World Medical Association
Fib-4: Fibrosis-4
Footnotes
Conflict of Interest
All authors have nothing to disclose.
References
- Ader P, Block M, Pietzsch S, Wolffram S. Interaction of quercetin glucosides with the intestinal sodium/glucose co-transporter (SGLT-1) Cancer Lett. 2001;162(2):175–180. doi: 10.1016/s0304-3835(00)00645-5. [DOI] [PubMed] [Google Scholar]
- Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014a;370(16):1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014b;370(20):1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(3 Suppl 1):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- Bachmetov L, Gal-Tanamy M, Shapira A, et al. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat. 2012;19(2):e81–e88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- Bala S, Petrasek J, Mundkur S, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56(5):1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Ansari IH, Mehle A, Striker R. Fluorescence resonance energy transfer-based intracellular assay for the conformation of hepatitis C virus drug target NS5A. J Virol. 2012;86(15):8277–8286. doi: 10.1128/JVI.00645-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieger J, Cermak R, Blank R, et al. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J Nutr. 2008;138(8):1417–1420. doi: 10.1093/jn/138.8.1417. [DOI] [PubMed] [Google Scholar]
- Bobe G, Weinstein SJ, Albanes D, et al. Flavonoid intake and risk of pancreatic cancer in male smokers (Finland) Cancer Epidemiol Biomarkers Prev. 2008;17(3):553–562. doi: 10.1158/1055-9965.EPI-07-2523. [DOI] [PubMed] [Google Scholar]
- Boulton DW, Walle UK, Walle T. Fate of the flavonoid quercetin in human cell lines: chemical instability and metabolism. J Pharm Pharmacol. 1999;51(3):353–359. doi: 10.1211/0022357991772367. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. [Google Scholar]
- Day AJ, Bao Y, Morgan MR, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic Biol Med. 2000;29(12):1234–1243. doi: 10.1016/s0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136(4):288–292. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- Fanning MJ, Macander P, Drzewiecki G, Middleton E., Jr Quercetin inhibits anaphylactic contraction of guinea pig ileum smooth muscle. Int Arch Allergy Appl Immunol. 1983;71(4):371–373. doi: 10.1159/000233423. [DOI] [PubMed] [Google Scholar]
- Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918–1929. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, DuPont MS, Day AJ, Plumb GW, Williamson G, Johnson IT. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J Nutr. 2000;130(11):2765–2771. doi: 10.1093/jn/130.11.2765. [DOI] [PubMed] [Google Scholar]
- Gonzalez O, Fontanes V, Raychaudhuri S, et al. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology. 2009;50(6):1756–1764. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45(11):2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Heinz SA, Henson DA, Austin MD, Jin F, Nieman DC. Quercetin supplementation and upper respiratory tract infection: a randomized community clinical trial. Pharmacol Res. 2010a;62(3):237–242. doi: 10.1016/j.phrs.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz SA, Henson DA, Nieman DC, Austin MD, Jin F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br J Nutr. 2010b;104(6):849–857. doi: 10.1017/S000711451000156X. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36(5 Suppl 1):S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- Ishisaka A, Kawabata K, Miki S, et al. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS One. 2013;8(11):e80843. doi: 10.1371/journal.pone.0080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowdley KV, Lawitz E, Crespo I, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381(9883):2100–2107. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- McAnulty SR, McAnulty LS, Nieman DC, et al. Chronic quercetin ingestion and exercise-induced oxidative damage and inflammation. Appl Physiol Nutr Metab. 2008;33(2):254–262. doi: 10.1139/H07-177. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361(6):580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Davis JM, et al. Quercetin’s influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol. 2007a;103(5):1728–1735. doi: 10.1152/japplphysiol.00707.2007. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Gross SJ, et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med Sci Sports Exerc. 2007b;39(9):1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- Prussia A, Thepchatri P, Snyder JP, Plemper RK. Systematic approaches towards the development of host-directed antiviral therapeutics. Int J Mol Sci. 2011;12(6):4027–4052. doi: 10.3390/ijms12064027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatko A, Xu TM. EWOC User’s Guide Version 2.0. 2005 Available from http://www.sph.emory.edu/BRI-WCI/ewoc.html.
- Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids. Phytother Res. 2013;27(11):1588–1596. doi: 10.1002/ptr.5023. [DOI] [PubMed] [Google Scholar]
- Sesink AL, Arts IC, Faassen-Peters M, Hollman PC. Intestinal uptake of quercetin-3-glucoside in rats involves hydrolysis by lactase phlorizin hydrolase. J Nutr. 2003;133(3):773–776. doi: 10.1093/jn/133.3.773. [DOI] [PubMed] [Google Scholar]
- Shanely RA, Knab AM, Nieman DC, Jin F, McAnulty SR, Landram MJ. Quercetin supplementation does not alter antioxidant status in humans. Free Radic Res. 2010;44(2):224–231. doi: 10.3109/10715760903407293. [DOI] [PubMed] [Google Scholar]
- Shoskes D, Lapierre C, Cruz-Correa M, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation. 2005;80(11):1556–1559. doi: 10.1097/01.tp.0000183290.64309.21. [DOI] [PubMed] [Google Scholar]
- Srinivas NR. Recent trends in preclinical drug-drug interaction studies of flavonoids — review of case studies, issues and perspectives. Phytother Res. 2015;29(11):1679–1691. doi: 10.1002/ptr.5447. [DOI] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- Terrault NA, Pawlotsky JM, McHutchison J, et al. Clinical utility of viral load measurements in individuals with chronic hepatitis C infection on antiviral therapy. J Viral Hepat. 2005;12(5):465–472. doi: 10.1111/j.1365-2893.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- Tighiouart M, Rogatko A, Babb JS. Flexible Bayesian methods for cancer phase I clinical trials. Dose escalation with overdose control. Stat Med. 2005;24(14):2183–2196. doi: 10.1002/sim.2106. [DOI] [PubMed] [Google Scholar]
- Valentova K, Stejskal D, Bednar P, et al. Biosafety, antioxidant status, and metabolites in urine after consumption of dried cranberry juice in healthy women: a pilot double-blind placebo-controlled trial. J Agric Food Chem. 2007;55(8):3217–3224. doi: 10.1021/jf0636014. [DOI] [PubMed] [Google Scholar]
- Walle T, Walle UK, Halushka PV. Carbon dioxide is the major metabolite of quercetin in humans. J Nutr. 2001;131(10):2648–2652. doi: 10.1093/jn/131.10.2648. [DOI] [PubMed] [Google Scholar]
- Wasley A, Grytdal S, Gallagher K, Centers for Disease and Prevention Surveillance for acute viral hepatitis—United States, 2006. MMWR Surveill Summ. 2008;57(2):1–24. [PubMed] [Google Scholar]
- Younossi ZM, Park H, Saab S, Ahmed A, Dieterich D, Gordon SC. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment PharmacolTher. 2015;41(6):544–563. doi: 10.1111/apt.13081. [DOI] [PubMed] [Google Scholar]


