Abstract
Background
Current therapeutic strategies to effectively treat antibody-mediated rejection (AMR) are insufficient. Thus, we aimed to determine the benefit of a therapeutic protocol using bortezomib for refractory C4d+ AMR in pediatric kidney transplant patients.
Methods
We examined 7 patients with treatment refractory C4d+ AMR. Immunosuppression included antithymocyte globulin or anti-CD25 monoclonal antibody for induction therapy with maintenance corticosteroids, calcineurin inhibitor, and antimetabolite. Estimated glomerular filtration rate (eGFR) calculated by the Schwartz equation, biopsy findings assessed by 2013 Banff criteria, and human leukocyte antigen (HLA) donor specific antibodies (DSA) performed using the Luminex single antigen bead assay were monitored pre- and post- bortezomib therapy.
Results
Seven patients (86% male, 86% with ≥6/8 HLA mismatch, and 14% with pre-formed DSA) age 5 to 19 (median 15) years developed refractory C4d + AMR between 1–145 (median 65) months post-transplantation. All patients tolerated bortezomib. One patient had allograft loss. Of the six patients with surviving grafts (86%), the mean pre-bortezomib eGFR was 42 mls/min/1.73 m2 and the mean 1 year post-bortezomib eGFR was 53 mls/min/1.73m2. Five of 7 (71%) had improvement of histological findings of AMR, C4d staining, and/or acute cellular rejection. Reduction in HLA DSAs was more effective for Class I than Class II.
Conclusions
Bortezomib appears safe and may correlate with stabilization of eGFR in pediatric kidney transplant patients with refractory C4d+ AMR.
Keywords: bortezomib, proteasome inhibitor, antibody-mediated rejection, pediatric, transplant, kidney, nonadherence
Introduction
Antibody-mediated rejection (AMR) is a significant barrier to improving long-term kidney allograft survival [1–3]. Furthermore, AMR is the leading cause of allograft failure in pediatric kidney transplant patients, accounting for 35.6% of allograft losses with a mean time of approximately 3 years from diagnosis to failure [3–5]. Allograft failure from AMR increases sensitization which is associated with an increased risk of rejection and failure in subsequent allografts [3, 6], higher mortality [7] and longer transplant wait times [8]. Pediatric patients are particularly vulnerable to these complications given most will require re-transplantation in their lifetime.
Treatments for AMR include plasmapheresis, intravenous immunoglobulin (IVIG), and rituximab. These have varying efficacy for reducing human leukocyte antigen (HLA) Class I and II donor specific antibodies (DSA) [9–11]. Plasmapheresis has been shown to be beneficial in one randomized controlled trial [12] while two other randomized controlled trials found no benefit [13, 14]. Furthermore, there are no randomized controlled trials that support the benefits of IVIG for AMR despite its frequent use [15]. Additionally, rituximab has been shown in 2 small randomized controlled trials to improve short term estimated glomerular filtration rate (eGFR) 1 year post-treatment [16, 17]. These therapies, however, may be insufficient to treat AMR [18–22].
Bortezomib, a proteasome inhibitor, has been used with varying success [21, 23–34] to treat AMR, with the rationale that inhibition of plasma cells may more effectively decrease antibody production allowing recovery from AMR. Notably, patients treated for AMR with bortezomib have shown improvement in eGFR despite the persistence of DSAs [25, 26]. This suggests that bortezomib has additional immunomodulatory effects that may impact renal recovery from AMR [35–37]. In this case series, we aimed to report the safety and utility of bortezomib for stabilizing renal function, improving biopsy findings, and reducing DSA burden in 7 pediatric kidney transplant patients with treatment refractory C4d+AMR.
Materials and Methods
Clinical Protocols
All recipients of pediatric kidney transplants at UCLA undergo protocol biopsies at 6, 12, and 24 months posttransplantation or for clinical cause. Additionally, all patients are tested for DSA monthly for the first year, quarterly for the second year and biannually thereafter, for any change in clinical status, and /or for suspicion of medication non-adherence (MNA). All patients with de novo DSA undergo renal biopsies (MFI>1000 are considered positive) [38]. For DSAs alone without biopsy changes, treatment includes closer monitoring and high dose IVIG (2g/kg given over 2 days) for MFI > 2000. For DSA with biopsy changes (either histological evidence and/or C4d+), methylprednisolone pulse (10mg/kg/dose × 3 days), high dose IVIG, and rituximab (375mg/m2) are given. For high MFI DSA (>5000) and creatinine increasing by > 50%, plasmapheresis 1x volume exchange with albumin replacement 3 times a week is initiated. Antithymocyte globulin (ATG) is given for concurrent acute cellular rejection (ACR) 1B or higher. Our bortezomib protocol is based on the protocol of the START collaborative [39]. Plasmapheresis is done on days 1,4,8,15,17,19, and 22 immediately prior to bortezomib administration. Rituximab 375 mg/m2 (day 1), 4 doses of bortezomib 1.3 mg/m2/dose (days 1,4, 8,15), and IVIG 1g/kg on the last day of treatment (day 22) are given with each round of treatment. Rituximab is primarily given with the first round of therapy and is not readministered in patients who have received it previously. Each dose of bortezomib is preceded by methylprednisolone, acetaminophen, and diphenhydramine premedications. Valganciclovir and sulfamethoxazole/trimethoprim prophylaxis in addition to viral monitoring for BK virus, cytomegalovirus, and Epstein-Barr virus with PCR was initiated and continued for 1 year post-bortezomib protocol.
Patient Selection and Evaluation
This is a single center retrospective review of 7 pediatric kidney transplant patients with refractory C4d+ AMR treated with bortezomib at Mattel Children’s Hospital, UCLA between November 2011 and January 2014. This retrospective chart review was performed in accordance with the UCLA institutional review board (IRB #11-003592) and formal patient consent is not required. Additionally, this report conforms with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The overall prevalence of biopsy proven C4d+ AMR in our patient population over the report period was approximately 12%. Patients were selected for treatment with the bortezomib protocol if their pre-bortezomib renal biopsy demonstrated C4d+ AMR and they were refractory to AMR treatment based on meeting 2 of 3 of the following criteria: 1) persistent or worsening biopsy findings, 2) persistent or increasing DSAs that did not decrease by 30% for MFI <10,000 or by 50% for MFI >10,000 or 3) declining graft function for 1 month despite treatment with the above described protocols. Persistent or worsening biopsy findings included progression from no rejection to AMR, progression from ACR to AMR, or persistence of AMR on biopsy.
The standard UCLA immunosuppressive regimen for pediatric transplant recipients included induction with either ATG for high risk patients (cPRA > 30% or delayed graft function) or anti-CD 25 monoclonal antibody for low risk patients cPRA<30%. Maintenance immunosuppression included steroid based immunosuppression, a calcineurin inhibitor, and an anti-metabolite. AMR at time of bortezomib treatment was considered early if it occurred < 6 months post-transplant and late AMR was defined as occurring > 6 months post-transplant [29]. In addition to the protocol surveillance biopsies mentioned above, patients underwent biopsies for cause based on positive DSAs with or without declining graft function. All biopsies were evaluated based on the 2013 Banff Criteria [40].
Patients underwent biopsies at the following time points: prior to any treatment for AMR, within 3 months prior to bortezomib treatment, and within 1 month after bortezomib protocol completion. The eGFR is reported as a measure of renal function and was calculated using the updated Schwartz equation [41]. Non-adherence was defined as physician and/or clinic staff documentation of patient report MNA, undetectable drug levels, or missed appointments [42]. We examined DSA and eGFR pre-bortezomib, at the time of post-bortezomib biopsy, and at 1 year following bortezomib treatment completion.
HLA Antibody Testing
HLA typing of recipient and donor was performed using molecular methods for HLA-A, -B, -DRB1,-DRB3/4/5, and - DQB1 loci by LABType SSO at intermediate resolution according to the manufacturer’s specifications and the results were analyzed using HLA Visual software (One Lambda, Canoga Park, CA). Donors were further typed for HLA-Cw, DQA1, DPB1, or at high resolution (LABType SSO DNA typing) to define additional DSA or allele specific DSA. The detection and characterization of HLA antibodies were performed using a Luminex single antigen bead (SAB) assay (One Lambda Inc, Canoga Park, CA) and quantified by mean fluorescence intensity (MFI). Antibodies were considered positive when MFI was ≥1000 for HLA-A, -B, -DR, -DQ, and ≥2000 for HLA-C and –DP [38]. The immunodominant DSA (iDSA) for Class I and Class II was defined as the DSA with the highest MFI. A significant change in iDSA was defined as a 30% decrease for MFIs ≤ 10,000 and a 50% decrease for MFIs >10,000 to account for any variability in MFI secondary to bead saturation [43].
Results
Demographic and clinical information relevant to the initiation of bortezomib are summarized in Table 1 and Table 2. Five of 7 patients (71%) were deceased donor transplant recipients and 7 of 8 (86%) had poor HLA matching (≥6/8 HLA mismatch) (Table 1). Patient 1, a 5 year old patient, was treated for early AMR 1 month post-transplant. His post-transplant course was complicated by delayed graft function and delayed tacrolimus initiation due to concerns for worsening acute tubular necrosis (ATN). Bortezomib was initiated for a combination of increasing DSA MFI and progression from ATN to C4d+ AMR on biopsy (Table 2 and Table 3). The other six patients (86%) were adolescents with a median age of 16 (range 14–19) years and late AMR (Table 1). These patients had a median time of progression from our center’s standard AMR treatment to the bortezomib protocol of 6.5 (range 3–20) months. In these patients, bortezomib was initiated at a median time of 65 (range 16–145) months post-transplant. Patient 2 was treated for AMR prior to bortezomib based on rapidly increasing MFI of DSA in association with previous ACR on biopsy (Table 2 and Table 3). Notably patient 6, was transplanted through preformed DSA and was successfully treated for AMR that occurred early post-transplant. However, her AMR recurred >6 months post-transplant after an extended period without follow up due to non-adherence, and she was treated with bortezomib. In contrast to the other 6 patients, she received rituximab with the bortezomib protocol despite having received it previously because of a dramatic reduction of her eGFR and attempt at rescue therapy (Table 2). The remainder of the patients (3–5, 7) had C4d+ AMR continuously from time of any treatment for AMR to time bortezomib was given (Table 2 and Table 3).
Table 1.
Patient Demographics
| Pt # | Donor Type | Age at Bortezomib Protocol | Sex | HLA Mismatch A, B, DR (DQ) | Tx# | cPRA% HLA Class I/Class II | Etiology of ESRD | MNA |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | DD | 5y | M | 2,1,2 (2) | 1 | 0/0 | Hypoxia | No |
| 2 | DD | 17y | M | 2,2,2 (2) | 1 | 0/0 | ANCA GN | Yes |
| 3 | DD | 15y | M | 1,2,2 (2) | 1 | 0/0 | Indomethacin Toxicity | Yes |
| 4 | LRD | 14y | M | 1,1,1 (1) | 1 | 0/0 | Congenital Nephrotic Syndrome | Yes |
| 5 | LRD | 14y | M | 1,2,1 (2) | 1 | 0/0 | Congenital Nephrotic Syndrome | Yes |
| 6 | DD | 18y | F | 2,2,1 (1) | 2 | 84/43 | Neurogenic Bladder | Yes |
| 7 | DD | 19y | M | 1,2,2 (2) | 1 | 0/0 | Dysplasia | Yes |
Pt patient, DD deceased donor, LRD living related donor, y year, M male, F female, HLA human leukocyte antigen, Tx transplant, cPRA calculated panel reactive antibody, ESRD end stage renal disease, ANCA GN anti-neutrophil cytoplasmic antibody glomerulonephritis, MNA medication nonadherence
Table 2.
Immunosuppression, Timing of AMR Treatment and Bortezomib Protocol, and Indication for Bortezomib Protocol
| Pt # | Induction IS | Maintenance IS at time of Bortezomib | Time Post-Tx AMR Treatment Initiated | Time Post-Tx of Previous AMR Treatments | Time Treated for AMR prior to Bortezomib Protocol | Time Post-Tx of Bortezomib Protocol | Indication for Bortezomib Protocol | Rounds of Bortezomib Protocol |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | ATG | FK MMF Steroid |
0m | MP (0m) IVIG (0m) ATG (0m) |
1m | 1m | ↑ DSA ATN→AMR |
1 |
| 2 | Basiliximab | FK MMF Steroid |
13m | MP (13m) IVIG (13m, 15m) |
3m | 16m | ↑ DSA ACR→AMR |
3 |
| 3 | Daclizumab | FK Leflunomide Steroid |
65m | MP (65m) Rituximab (65m) IVIG (65m–70m) |
5m | 70m | ↓ eGFR Persistent DSA Persistent AMR |
2 |
| 4 | Daclizumab | FK MMF Steroid |
134m | MP (134m), Rituximab (134m), IVIG (134m, 138m) |
6m | 140m | ↑ DSA Persistent AMR |
2 |
| 5 | Daclizumab | FK MMF Steroid |
138m | MP(138m), Rituximab (138m) IVIG (138m & 142m) |
7m | 145m | ↑ DSA Persistent AMR |
2 |
| 6 | ATG | FK MMF Steroid |
0m | IVIG (0m–15m) PP (0m–15m) Rituximab (1m) MP (8m) ATG (8m) |
19m | 19m | ↓ eGFR ↑ DSA AMR Recurrence |
2 |
| 7 | Daclizumab | CYA MMF Steroid |
45m | MP (45m), Rituximab (45m) IVIG (45m, 51m, 63m–65m) PP (63m–65m) |
20m | 65m | ↑ DSA Persistent AMR |
2 |
Pt patient, IS immunosuppression, FK tacrolimus, MMF mycophenolate mofetil, y year, m months, MP methylprednisolone pulse, IVIG intravenous immunoglobulin, PP plasmapheresis, ATG antithymocyte globulin, DSA donor specific antibody, eGFR estimated glomerular filtration rate, ATN acute tubular necrosis, AMR antibody-mediated rejection, ACR acute cellular rejection. Ranges reflect multiple doses/treatments during a given time period.
Table 3.
Biopsy Findings Before AMR Treatment, Before Bortezomib Protocol, and After Treatment with Bortezomib Protocol and Graft Outcome
| Pt # | Pre-AMR Treatment Biopsy | Pre-Bortezomib Biopsy | Post-Bortezomib Biopsy | Graft Loss |
|---|---|---|---|---|
| 1 | No AMR or ACR, No IFTA, Severe ATN | C4d+ AMR, ACR IIA | No AMR, Borderline ACR | No |
| 2 | No AMR, ACR1A, No IFTA | C4d+ AMR, ACR1A, No IFTA | C4d+ AMR, Borderline ACR, No IFTA | No |
| 3 | C4d+ AMR, No ACR, Moderate IFTA | C4d+ AMR, No ACR, No IFTA | C4d+ AMR, No ACR, No IFTA | No |
| 4 | C4d+ AMR, No ACR, TG, No IFTA | C4d + AMR, No ACR, No IFTA | C4d+ AMR, No ACR, Mild IFTA | No |
| 5 | C4d+ AMR, No ACR, No IFTA | C4d+ AMR, Borderline ACR, No IFTA | C4d+ AMR, No ACR, Mild TG | No |
| 6 | C4d+ AMR, No ACR, No IFTA | C4d+ AMR, ACR1B, No IFTA | Decreased C4d, AMR, ACR 1B, Mild IFTA | Yes |
| 7 | C4d+ AMR, ACR1A, No IFTA | C4d+ AMR, No ACR, Mild IFTA | C4d- AMR, No ACR, No IFTA | No |
Pt patient, AMR antibody-mediated rejection, ACR acute cellular rejection, IFTA interstitial fibrosis tubular atrophy, ATN acute tubular necrosis, TG transplant glomerulopathy
All 7 patients had C4d+ AMR associated with either Class I or Class II DSA at the time of bortezomib protocol treatment. Three of 7 (43%) with diffusely positive C4d staining had either partial or complete resolution in their post-bortezomib biopsies. One of 7 (14%), the sole patient with early AMR (Patient 1), had complete resolution of his C4d staining and histological evidence of AMR (Table 3). Additionally, 4 of 7 (57%) patients had concomitant ACR on biopsy prior to treatment. Of these patients, 75% had improvement of Banff grade and 25% had complete resolution (Table 3).
Four of 7 (57%) patients treated with the bortezomib protocol had Class I DSA. Of those, 75% had a significant decrease in the Class I iDSA MFI after treatment with sustained resolution in 50% of patients 1 year post-treatment (Fig. 1a). All 7 patients treated with the bortezomib protocol had Class II DSA. Two (29%) had significant improvement of Class II iDSA after treatment. Only 1 patient (14%) had sustained resolution of Class II iDSA 1 year post-treatment (Patient 1) (Fig. 1b).
Fig. 1.
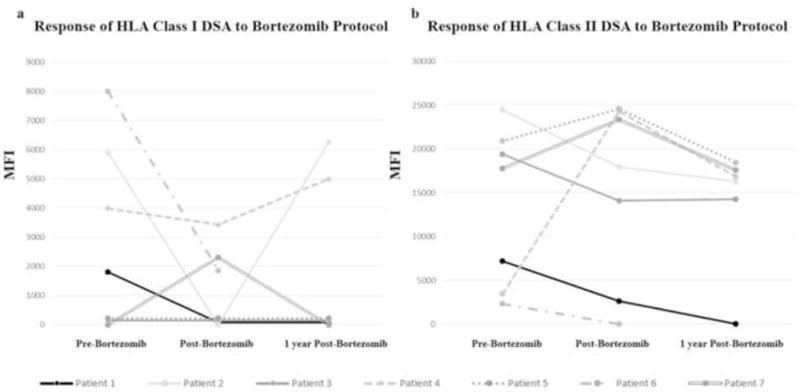
Change in immunodominant DSA (iDSA) over time in relation to treatment with bortezomib protocol. Data for all patients presented before and after treatment. Six patients with functioning grafts had one year follow up data available. (a) HLA Class I: Of 4 patients with Class I DSAs prior to treatment, 3 had a significant decrease in iDSA mean fluorescence intensity (MFI) after treatment. One patient developed Class I DSA after treatment. Two patients had rebound, 1 patient had resolution, and 3 patients were negative 1 year post-treatment. The iDSA was to the “A” locus in 3 patients and “Cw” in 2 patients. (b) HLA class II: All 7 patients had Class II DSAs and 2 had significant improvement of iDSA after treatment. One patient had resolution of Class II DSA and 5 patients had no significant change in iDSA 1 year post-treatment. The iDSA was to “DQ” in 5 patients and “DR” in 2 patients.
The bortezomib protocol was potentially associated with stabilization of the eGFR immediately post-treatment for 6 of 7 (86%) patients with refractory C4d+ AMR (Fig. 2). One of 7 (17%) with severe impairment of eGFR prior to treatment and MNA suffered allograft loss and returned to dialysis immediately following treatment (Patient 6). The remaining 5 patients with late AMR and the 1 patient with early AMR continued to have relatively stable eGFR 1 year post-treatment. The sole patient with early AMR (Patient 1) had significant recovery of eGFR from dialysis dependence to 73 ml/min/1.73m2. There were no additional allograft failures (Fig. 2).
Fig. 2.
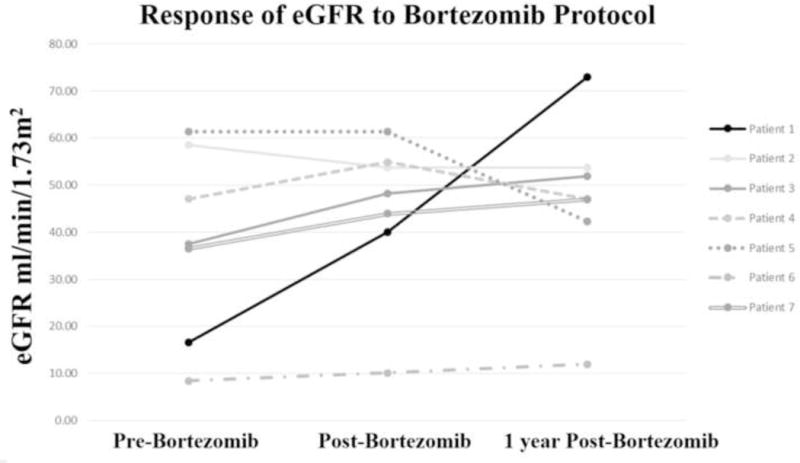
Estimated glomerular filtration rate (eGFR) over time in relation to treatment with bortezomib protocol.
Overall, treatment with bortezomib was well tolerated. No patients had peripheral neuropathy, neutropenia, or thrombocytopenia associated with treatment. One patient (Patient 1) had a major infection, coagulase negative staphylococcus bacteremia, which responded to antibiotic therapy. Of note, this patient had also received ATG. Two patients (Patients 1 and 6) had diarrhea concurrent with bortezomib treatment. One patient (Patient 3) had pre-existing BK Viremia, which remained stable throughout treatment with a peak serum BK level of 1,474 copies/mL and was not associated with BK nephropathy. One patient (Patient 7) had a mild sinus infection treated successfully with oral azithromycin.
Discussion
Our report examined the safety and effectiveness of using a bortezomib protocol for treatment refractory C4d+ AMR in a group of pediatric kidney transplant patients composed primarily of adolescents with late AMR. Bortezomib was well tolerated in our patients. The sole patient with early AMR in our cohort had dramatic recovery with the use of bortezomib as rescue therapy, consistent with previous studies [19, 20, 29]. This included resolution of DSAs, improvement in biopsy findings, and recovery of renal function.
Notably, the addition of bortezomib may have been associated with stabilization of allograft function in 6 of the 7 patients with refractory C4d+ AMR, the majority having late AMR. Late AMR has been recognized as a clinically distinct entity from early AMR that is resistant to treatment and associated with poor allograft survival [18–22]. Previous authors have noted stabilization or improvement of late AMR with bortezomib [21, 26, 28, 29], but our case series is the first to report this finding in a cohort of mainly high risk adolescents. This potential effect was maintained 1 year after treatment, without any additional allograft losses in the year following treatment. Bortezomib was futile in one patient with late AMR, severely compromised graft function at the time of treatment initiation, and overt defiant non-adherence with refusal to participate in clinic visits and laboratory monitoring. This is consistent with previous case reports [27, 44]. Our protocol may have correlated with a stabilization in renal function in patients with late AMR and eGFR > 30 ml/min/1.73m2 at the time of treatment initiation. Therefore, we suggest that intensive DSA monitoring allowing for early detection and treatment of late AMR may maximize the benefit of bortezomib by allowing for treatment prior to significant allograft dysfunction.
Potential stabilization of eGFR in patients with refractory C4d+ AMR appeared to be independent of DSA response. Consistent with previous studies [45–47], reduction in iDSA with bortezomib treatment was more effective for Class I antibodies than Class II. In vitro studies have shown that bortezomib treated lymphocytes [47] and multiple myeloma cells [48] preferentially reduce HLA Class I expression and decrease stimulation for continued Class I antibody production [47]. The effect of bortezomib on cell surface expression of HLA Class I but not Class II may explain why this therapy has not produced dramatic recovery of renal function or biopsy findings in many patients with late AMR associated with HLA Class II DSAs [22, 49]. Interestingly, patients treated with bortezomib have shown modest improvement in eGFR despite the persistence of DSAs, which is consistent with our findings [25, 26]. This suggests that the immunomodulatory actions of bortezomib, such as modifying cell surface antigen expression and T-cell function, may contribute to its value as a therapy for AMR beyond its effect on the plasma cell and DSA production [47].
We also found that the addition of bortezomib may have been associated with an improvement in concomitant ACR in patients with refractory C4d+ AMR, which has been previously described [50]. Most of these patients had mild ACR (Ib or lower), however, which is generally not resistant to conventional therapy. More notably, the one patient with early AMR had concomitant ACR IIa which improved to borderline on follow up biopsy. This could represent a direct effect of bortezomib on T-cells. Bortezomib has been shown to suppress rapamycin resistant memory T cells while preserving the survival of T regulatory cells in vitro [35], and there is evidence that bortezomib reduces T lymphocyte numbers and the Th1/Th2 ratio in chemotherapy patients [36]. The observed effect on ACR may also be secondary to the use of corticosteroids as a premedication for bortezomib or the co-administration of ATG in some of our patients.
We recognize the limitations of our case series. One limitation is the wide range of timing and intensity of treatment for AMR prior to the initiation of bortezomib. Additionally, there is the possibility of observing effects from the multiple other treatments that were given prior to or concomitant with bortezomib in these results. Nonetheless, this is the largest reported pediatric case series describing the safety and potential benefits of a bortezomib protocol for refractory C4d+ AMR. Despite our small sample size, we were able to demonstrate that a bortezomib protocol can safely be used in the pediatric population, is potentially correlated with a stabilization of eGFR in adolescents with late AMR, and was effective rescue therapy for early AMR. Intensive DSA monitoring allowing for early detection and treatment may optimize the benefit of this protocol for late AMR by allowing for treatment prior to significant functional decline; however, our findings need validation in a larger randomized control trial.
Acknowledgments
Funding: We would like to thank The Casey Lee Ball Foundation and the UCLA Children’s Discovery and Innovation Institute and The Today’s and Tomorrow’s Children Fund for supporting this work.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 2.Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 3.North American Pediatric Renal Trials and Collaborative Studies. 2010 Annual Transplant Report 2010 [Google Scholar]
- 4.Wiebe C, Gibson IW, Blydt-Hansen TD, Pochinco D, Birk PE, Ho J, Karpinski M, Goldberg A, Storsley L, Rush DN, Nickerson PW. Rates and Determinants of Progression to Graft Failure in Kidney Allograft Recipients With De Novo Donor-Specific Antibody. Am J Transplant. 2015;15:2921–2930. doi: 10.1111/ajt.13347. [DOI] [PubMed] [Google Scholar]
- 5.Kim JJ, Balasubramanian R, Michaelides G, Wittenhagen P, Sebire NJ, Mamode N, Shaw O, Vaughan R, Marks SD. The clinical spectrum of de novo donor-specific antibodies in pediatric renal transplant recipients. Am J Transplant. 2014;14:2350–2358. doi: 10.1111/ajt.12859. [DOI] [PubMed] [Google Scholar]
- 6.Huber L, Lachmann N, Niemann M, Naik M, Liefeldt L, Glander P, Schmidt D, Halleck F, Waiser J, Brakemeier S, Neumayer HH, Schonemann C, Budde K. Pretransplant virtual PRA and long-term outcomes of kidney transplant recipients. Transpl Int. 2015;28:710–719. doi: 10.1111/tri.12533. [DOI] [PubMed] [Google Scholar]
- 7.Sapir-Pichhadze R, Tinckam KJ, Laupacis A, Logan AG, Beyene J, Kim SJ. Immune Sensitization and Mortality in Wait-Listed Kidney Transplant Candidates. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 2004–2012. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: United Network for Organ Sharing; Richmond, VA: University Renal Research and Education Association; Ann Arbor, MI: 2012. http://www.srtr.org/ [Google Scholar]
- 9.Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, Ratner LE, Cohen DJ, Radhakrishnan J. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23:2061–2071. doi: 10.1681/ASN.2012070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terasaki PI, Ozawa M, Castro R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408–415. doi: 10.1111/j.1600-6143.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 11.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant. 2009;9:1063–1071. doi: 10.1111/j.1600-6143.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonomini V, Vangelista A, Frasca GM, Di Felice A, Liviano D’Arcangelo G. Effects of plasmapheresis in renal transplant rejection. A controlled study. Trans Am Soc Artif Intern Organs. 1985;31:698–703. [PubMed] [Google Scholar]
- 13.Blake P, Sutton D, Cardella CJ. Plasma exchange in acute renal transplant rejection. Prog Clin Biol Res. 1990;337:249–252. [PubMed] [Google Scholar]
- 14.Allen NH, Dyer P, Geoghegan T, Harris K, Lee HA, Slapak M. Plasma exchange in acute renal allograft rejection. A controlled trial. Transplantation. 1983;35:425–428. doi: 10.1097/00007890-198305000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation. 2012;94:775–783. doi: 10.1097/TP.0b013e31825d1587. [DOI] [PubMed] [Google Scholar]
- 16.Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant. 2008;8:2607–2617. doi: 10.1111/j.1600-6143.2008.02411.x. [DOI] [PubMed] [Google Scholar]
- 17.Vo AA, Choi J, Cisneros K, Reinsmoen N, Haas M, Ge S, Toyoda M, Kahwaji J, Peng A, Villicana R, Jordan SC. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation. 2014;98:312–319. doi: 10.1097/TP.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 18.Dorje C, Midtvedt K, Holdaas H, Naper C, Strom EH, Oyen O, Leivestad T, Aronsen T, Jenssen T, Flaa-Johnsen L, Lindahl JP, Hartmann A, Reisaeter AV. Early versus late acute antibody-mediated rejection in renal transplant recipients. Transplantation. 2013;96:79–84. doi: 10.1097/TP.0b013e31829434d4. [DOI] [PubMed] [Google Scholar]
- 19.Pefaur J, Diaz P, Panace R, Salinas P, Fiabane A, Quinteros N, Chea R, Naranjo E, Wurgaft A, Beltran E, Elgueta S, Wegmann ME, Gajardo JG, Contreras L. Early and late humoral rejection: a clinicopathologic entity in two times. Transplant Proc. 2008;40:3229–3236. doi: 10.1016/j.transproceed.2008.03.123. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, Liu ZH, Ji S, Chen J, Tang Z, Zeng C, Zheng C, Li LS. Late and early C4d-positive acute rejection: different clinico-histopathological subentities in renal transplantation. Kidney Int. 2006;70:377–383. doi: 10.1038/sj.ki.5001552. [DOI] [PubMed] [Google Scholar]
- 21.Gupta G, Abu Jawdeh BG, Racusen LC, Bhasin B, Arend LJ, Trollinger B, Kraus E, Rabb H, Zachary AA, Montgomery RA, Alachkar N. Late antibody-mediated rejection in renal allografts: outcome after conventional and novel therapies. Transplantation. 2014;97:1240–1246. doi: 10.1097/01.TP.0000442503.85766.91. [DOI] [PubMed] [Google Scholar]
- 22.Banasik M, Koscielska-Kasprzak K, Myszka M, Bartoszek D, Zabinska M, Boratynska M, Kaminska D, Mazanowska O, Zmonarski S, Krajewska M, Halon A, Dawiskiba T, Nowakowska B, Klinger M. A significant role for anti-human leukocyte antigen antibodies and antibody-mediated rejection in the biopsy-for-cause population. Transplant Proc. 2014;46:2613–2617. doi: 10.1016/j.transproceed.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Flechner SM, Fatica R, Askar M, Stephany BR, Poggio E, Koo A, Banning S, Chiesa-Vottero A, Srinivas T. The role of proteasome inhibition with bortezomib in the treatment of antibody-mediated rejection after kidney-only or kidney-combined organ transplantation. Transplantation. 2010;90:1486–1492. doi: 10.1097/TP.0b013e3181fdd9b0. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhuri A, Ozawa M, Everly MJ, Ettenger R, Dharnidharka V, Benfield M, Mathias R, Portale A, McDonald R, Harmon W, Kershaw D, Vehaskari VM, Kamil E, Baluarte HJ, Warady B, Li L, Sigdel TK, Hsieh SC, Dai H, Naesens M, Waskerwitz J, Salvatierra O, Jr, Terasaki PI, Sarwal MM. The clinical impact of humoral immunity in pediatric renal transplantation. J Am Soc Nephrol. 2013;24:655–664. doi: 10.1681/ASN.2012070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touzot M, Couvrat-Desvergnes G, Castagnet S, Cesbron A, Renaudin K, Cantarovich D, Giral M. Differential modulation of donor-specific antibodies after B-cell depleting therapies to cure chronic antibody mediated rejection. Transplantation. 2015;99:63–68. doi: 10.1097/TP.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 26.Waiser J, Budde K, Schutz M, Liefeldt L, Rudolph B, Schonemann C, Neumayer HH, Lachmann N. Comparison between bortezomib and rituximab in the treatment of antibody-mediated renal allograft rejection. Nephrol Dial Transplant. 2012;27:1246–1251. doi: 10.1093/ndt/gfr465. [DOI] [PubMed] [Google Scholar]
- 27.Ryckewaert A, Allain-Launay E, Moreau A, Blancho G, Cesbron A, Blin N, Roussey G. Failure of bortezomib to cure acute antibody-mediated rejection in a non-compliant renal transplant patient. Pediatr Transplant. 2013;17:E131–136. doi: 10.1111/petr.12113. [DOI] [PubMed] [Google Scholar]
- 28.Twombley K, Thach L, Ribeiro A, Joseph C, Seikaly M. Acute antibody-mediated rejection in pediatric kidney transplants: a single center experience. Pediatr Transplant. 2013;17:E149–155. doi: 10.1111/petr.12129. [DOI] [PubMed] [Google Scholar]
- 29.Walsh RC, Brailey P, Girnita A, Alloway RR, Shields AR, Wall GE, Sadaka BH, Cardi M, Tevar A, Govil A, Mogilishetty G, Roy-Chaudhury P, Woodle ES. Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation. 2011;91:1218–1226. doi: 10.1097/TP.0b013e318218e901. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen S, Gallay B, Butani L. Efficacy of bortezomib for reducing donor-specific antibodies in children and adolescents on a steroid minimization regimen. Pediatr Transplant. 2014;18:463–468. doi: 10.1111/petr.12274. [DOI] [PubMed] [Google Scholar]
- 31.Marks SD. Treatment strategies to treat antibody-mediated rejection and to reduce donor-specific antibodies. Pediatr Transplant. 2014;18:417–419. doi: 10.1111/petr.12284. [DOI] [PubMed] [Google Scholar]
- 32.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. doi: 10.1111/ajt.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claes DJ, Yin H, Goebel J. Protective immunity and use of bortezomib for antibody-mediated rejection in a pediatric kidney transplant recipient. Pediatr Transplant. 2014;18:E100–105. doi: 10.1111/petr.12256. [DOI] [PubMed] [Google Scholar]
- 34.Roberti I, Vyas S. Successful treatment of severe acute antibody-mediated rejection of renal allografts with bortezomib - a report of two pediatric cases. Pediatr Transplant. 2015 doi: 10.1111/petr.12612. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH, Cho HS, Yoon IH, Kim KH, Kim SJ, Park CG. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88:1349–1359. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Li Y, Huang B, Zheng D, Chen M, Zhou Z. Drug-induced modulation of T lymphocytes as a potential mechanism of susceptibility to infections in patients with multiple myeloma during bortezomib therapy. Cell Biochem Biophys. 2015;71:457–464. doi: 10.1007/s12013-014-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philogene MC, Sikorski P, Montgomery RA, Leffell MS, Zachary AA. Differential effect of bortezomib on HLA class I and class II antibody. Transplantation. 2014;98:660–665. doi: 10.1097/TP.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 38.Blumberg JM, Gritsch HA, Reed EF, Cecka JM, Lipshutz GS, Danovitch GM, McGuire S, Gjertson DW, Veale JL. Kidney paired donation in the presence of donor-specific antibodies. Kidney Int. 2013;84:1009–1016. doi: 10.1038/ki.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodle ES, Light J, Rubin M, Thielke J, Morrow WR, Mahle W, Franklin C, Gabardi S, Gill J, Roberti I, Shapiro R, Wall GE, Walsh RC, Zand M, Alloway RR. Proteasome Inhibitor Therapy for Antibody Mediated Rejection:Initial Report from a Multicenter Collaborative. Am J Transplant. 2010;10:83–84. [Google Scholar]
- 40.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing c Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 43.Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, Lunz J, Mohanakumar T, Nickerson P, Tambur AR, Zeevi A, Heeger PS, Gjertson D. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant. 2013;13:1859–1870. doi: 10.1111/ajt.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen S, Gallay B, Butani L. Efficacy of bortezomib for reducing donor-specific antibodies in children and adolescents on a steroid minimization regimen. Pediatr Transplant. 2014;18:463–468. doi: 10.1111/petr.12274. [DOI] [PubMed] [Google Scholar]
- 45.Kannabhiran D, Everly MJ, Walker-McDermott JK, Tiongko S, Friedlander R, Putheti P, Sharma V, Dadhania D. Changes in IgG subclasses of donor specific anti-HLA antibodies following bortezomib-based therapy for antibody mediated rejection. Clin Transpl. 2012:229–235. [PubMed] [Google Scholar]
- 46.Khuu T, Cadeiras M, Wisniewski N, Reed EF, Deng MC. Reduced HLA Class II antibody response to proteasome inhibition in heart transplantation. J Heart Lung Transplant. 2015;34:863–865. doi: 10.1016/j.healun.2015.01.982. [DOI] [PubMed] [Google Scholar]
- 47.Philogene MC, Sikorski P, Montgomery RA, Leffell MS, Zachary AA. Differential Effect of Bortezomib on HLA Class I and Class II Antibody. Transplantation. 2014;18:463–468. doi: 10.1097/TP.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 48.Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, Storrie B, Mulder A, Shaughnessy JD, Jr, Barlogie B, van Rhee F. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willicombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Galliford J, McLean A, Cook TH, Cairns T, Roufosse C, Taube D. De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation. 2012;94:172–177. doi: 10.1097/TP.0b013e3182543950. [DOI] [PubMed] [Google Scholar]
- 50.Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, Roy-Chaudhury P, Govil A, Mogilishetty G, Rike AH, Cardi M, Wadih G, Tevar A, Woodle ES. Bortezomib provides effective therapy for antibody-and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]


