Abstract
In food deprivation assays, several different responses have been observed in crustaceans. However, studying energy reserves utilization among more than one species during the same starvation period has not yet been performed, particularly to discern whether the responses are due to intrinsic and/or environmental factors. We hypothesize that decapod species with similar feeding habits have the same strategies in the use of energetic reserves during starvation, even though they inhabit different environments. The aim of this study was to compare the energy reserves mobilization of three decapods species (Cherax quadricarinatus, Palaemon argentinus and Munida gregaria) with similar feeding habits, exposed to similar food deprivation conditions. The crayfish, shrimp and squat-lobster were experimentally kept at continuous feeding or continuous starvation throughout 15 days. Every 3rd day, the midgut gland index (MGI), and the glycogen, lipid and protein contents were measured in the midgut gland (MG) and pleon muscle. Palaemon argentinus mobilized more reserves during starvation, followed by C. quadricarinatus, and the last M. gregaria. The starved shrimps presented low MGI, whereas MG showed a reduction in glycogen (from day 6 to 15), lipid (from day 3 to 15), and protein levels (at day 9 and 15) while in their muscle, lipid reserves decreased at days 3 and 6. In C. quadricarinatus, the most affected parameters in the MG were MGI, glycogen (from day 6 to 15), and lipids (at day 12 and 15). In the MG of M. gregaria only the glycogen was reduced during fasting from 3 to 15 days. Even though the three studied species have similar feeding habitats, we found that their energetic profile utilization is different and it could be explained by the habitat, life span, temperature, organ/tissue, and metabolism of the species. Our results may be useful to understand the several different responses of crustaceans during starvation.
Introduction
In their natural habitats, crustaceans have to overcome and tolerate the scarcity or the total absence of food for short or long periods for different reasons: molting; seasonal environmental changes, along with community structure changes; or pollution [1, 2]. Physiological responses to starvation are further interpreted through ecological modulators. In natural habitats, for instance, it is important to understand physiological responses to fasting, as they could indicate whether organisms had reduced their feeding due to food availability (quantity and quality), and/or the cessation of feeding to reduce a predation risk. Therefore, measures of starvation are important for assessing the state of a population [3]. During the intermoult period of crustaceans, starvation is responsible for the re-allocation of energy resources for tissue maintenance, and survival, regardless of metabolic costs [4]. Induced experimental starvation can reveal which macromolecules (glycogen, lipid and protein) are used, and in what sequence the different energy compartments are depleted. In this context, the midgut gland and the muscle of crustaceans are key body parts because they hold the greatest amount of energy reserves [5], which can be mobilized during non-feeding periods.
In crustaceans, several different responses occur during food deprivation assays, such as dissimilar sequence of glycogen, lipid and protein mobilization, which drop or increase enzyme activities. This is the result of the vast diversity of environments that they inhabit and their long evolutionary history [5]. Other factors that contribute to the observed diversity of interspecific variability of energetic reserves mobilization are the dissimilar biochemical methods and distinct experimental starvation periods utilized by the different authors [6]. However, both conjectures, diversity of environments and/or different experimental protocols, have not yet been demonstrated. Therefore, although there are numerous studies dealing with nutrition of decapod crustaceans and several comparisons were made across species, none has previously compared the effect of starvation on different species with the same methodology. This type of study can compare species from different habitats, and with different phylogeny, life spans, life cycle, feeding habits, etc. In addition, it might also be possible to find a relationship between different species and their physiological features using the same experimental design and biochemical analysis. We hypothesize that, as the three decapod species present similar feeding habits, their strategies in the use of energetic reserves will be similar during starvation, even though they inhabit different environments.
The studied decapod species were Cherax quadricarinatus, Palaemonetes argentinus and Munida gregaria, which belong to the same suborder Pleocyemata, and different Infraorders: Astacidea, Caridea, and Anomura, respectively. These species are good biological models because, among other characteristics, they are phylogenetically dissimilar, they can be maintained under laboratory conditions, can accept a commercial diet, are easily caught in nature, and have starvation resistance [7–11].
Considering the phylogenetic relationships among the infraorders within the decapod Reptantia clade, the estimated divergence time for Astacidea, Caridea, and Anomura linages are 278, 263, and 309 million years ago, respectively [12]. We understand that interspecific comparisons must take into account the phylogenetic relationships among species due to their lack of statistical independence owing to a shared ancestry [13, 14]. However, our three-studied decapod species are evolutionarily distant; therefore, from an evolutionary point of view we considered them as independent units for the purpose of the present study.
In general, the three studied species present similar feeding habits, as omnivores and deposit feeders. As omnivores, they feed on benthic invertebrates and algae, and as deposit feeders, they ingest detritus. Specifically, the redclaw crayfish, Cherax quadricarinatus (Decapoda, Parastacidae), is a freshwater crustacean, native from northern Australia and southeast Papua New Guinea. In natural ecosystems, crayfishes have polytrophic feeding habits and have been described as predators, omnivores and/or detritivores, consuming a variety of macrophytes, benthic invertebrates, algae and detritus [15] and references therein. The shrimp Palaemon argentinus (Decapoda, Caridea), inhabits shallow lakes and streams of South America (in Argentina, Paraguay, Uruguay and southern Brazil) [16, 17]. Palaemon argentinus is omnivorous and detritivorous, and consumes algae, benthic macroinvertebrates and zooplankton [18]. The squat-lobster Munida gregaria (Decapoda, Anomura), is distributed on the continental shelves off southern South America, New Zealand and southern Australia [19]. This species is an omnivorous generalist and has two different and simultaneous feeding habits: as omnivore it feeds on crustaceans, polychaets and macroalgae, and as a deposit feeder on particulate organic matter [20].
This study was aimed to compare the physiological responses of Cherax quadricarinatus, Palaemon argentinus and Munida gregaria exposed to similar food deprivation conditions. Specifically, analytical biochemical parameters were determined through a midgut gland index and energy reserves quantification. This information may be useful to understand the different responses of crustaceans during food deprivation conditions.
Materials and methods
Animals
Redclaw crayfish were hatched from a reproductive female stock supplied by Centro Nacional de Desarrollo Acuícola (CENADAC), Corrientes, Argentina. Each ovigerous female (59.8 ± 3.2 g mean body weight) was maintained in an individual glass aquarium (60x40x30 cm, width x length x height). Each aquarium contained 30L of dechlorinated tap water and was continually aerated. The temperature was maintained at 27±1°C with ALTMAN water heaters (100W, precision ± 1°C), and the photoperiod cycle was 10 h light: 14 h dark. Each aquarium was provided with a PVC tube cave (10 cm of diameter and 25 cm long) [21]. Females were fed daily ad libitum with both Elodea sp. and commercial TetraColor granules TETRA® (47.5% crude protein, 6.5% crude fat, 2.0% crude fiber, 6.0% moisture, 1.5% phosphorus, and 100 mg ascorbic acid/kg) according to Bugnot (2009) [22] and De Bock (2010) [23]. Juveniles became independent at stage 3 [24], and then, they were separated from their mothers. To reach the desired experimental weight, they were pooled and maintained under conditions described in previous studies [25, 26].
Shrimps Palaemon argentinus were obtained from the Nahuel Rucá pond, Buenos Aires, Argentina, considered as an unpolluted area [27]. The animals were collected with a hand net and transported to the laboratory. Shrimps were maintained in 70 L aquaria (60x35x25 cm width x length x height) with continually aerated freshwater. The temperature was maintained at 23±1°C by ALTMAN water heaters, and a photoperiod cycle of 10 h light: 14 h dark.
Squat-lobsters were captured in the Beagle Channel, Tierra del Fuego, Argentina, by trawling for 10 minutes using an epibenthic trawl with 1.8 m and 1.2 m of horizontal and vertical openings, and 10 mm of mesh size [28]. Animals were transported to the laboratory and kept in polypropylene aquaria tanks (65×50×30 cm, width x length x height) with a chilled seawater recirculation system at 6–8°C and a photoperiod cycle of 10 h light: 14 h dark. Water quality was maintained with mechanical (50 μm) and biological filters and a UV-sterilizer.
No specific permissions were required for the locations used to capture Palaemon argentinus and Munida gregaria, because these zones are not protected or private areas. The three studied species are not endangered or protected species.
Experimental design
A total of eighty eight crayfish (5.26±1.46 g mean body weight in intermoult stage) were placed in individual glass containers (1500 mL) with 1400 mL of filtered water under continuous aeration. In order to maintain the temperature constant at 27±1°C, the containers were placed in aquaria (53x40x12 cm, width x length x height) with water heaters [9]. During the trial, the photoperiod cycle was set at 10 h light: 14 h dark and pH was set in a range of 7.3–8.4.
Five hundred twenty eight shrimps (0.16±0.04g mean body weight in intermoult stage) were placed in individual plastic containers (500 mL) with 400 mL of filtered water under continuous aeration. In order to maintain the temperature constant at 23±1°C, the containers were placed into aquaria (60x35x25 cm, width x length x height) with water heaters. During the trial, the photoperiod cycle was set at 10 h light: 14 h dark, and pH was set in a range of 7.9–8.9.
Eighty eight squat lobsters in intermoult stage (6.93±1.51 g mean body weight) were placed in individual plastic containers (700 mL) with seawater under continuous aeration. In order to maintain the temperature constant at 6±1°C, the containers were placed in aquaria tanks (25x37x10 cm, width x length x height) with chilled seawater. During the trial, the photoperiod cycle was set at 10 h light: 14 h dark, and pH was set in a range of 7.2–7.6.
The three species were fed daily ad libitum with TetraColor granules (TETRA®) and acclimated to the experimental conditions above described during 1 week before the experimental onset. Specifically, nutrient content in the diet is common for the three crustacean species; therefore, food is not a source of variability. The first day of the assay (time 0 (T0), initial control), 8 crayfish, 48 shrimps and 8 squat lobsters were weighed (precision 0.1 mg) and dissected, the midgut gland and pleon muscle extracted, and frozen at -80°C.
The remaining animals of Cherax quadricarinatus (n = 80), Palaemon argentinus (n = 480) and Munida gregaria (n = 80) were randomly and equitably distributed into two groups: half of each species on the fed group and the other half in the starved group. The fed group was daily fed ad libitum with TetraColor® granules throughout the 15 days of the entire experimental period. The starved animals were not fed until day 15. During the experimental period, all containers were cleaned and water was renewed twice a week. Animals were sampled at every three days (T3, T6, T9, T12 and T15) when 8 crayfishes, 48 shrimps and 8 squat lobsters of the two groups, fed and starved, were weighed. Each midgut gland and pleon muscle was dissected and frozen at -80°C. Considering the small size of shrimps (and the subsequent small volume of the obtained organ and muscular tissue) 6 midgut glands and pleon muscles from the same experimental group and time were pooled (8 pools with 6 organs or tissues each), to ensure enough material for all analysis.
The wet midgut gland index (MGI) was calculated to assess the potential mobilization of metabolic reserves during the assay. This index was calculated according to Jones et al. (2000) [29] as MGI (%) = (midgut gland weight/whole body weight) x 100. At T0 and for each macromolecule (glycogen, lipid and protein), a ratio between the macromolecule in the MG and in the muscle pleon was also calculated.
Energetic reserves of midgut gland and pleon muscle
Total lipids were extracted following Folch´s protocol [30]. Lipids were extracted by homogenizing pre-weighed samples of the midgut gland or muscle with a mixture of chloroform: methanol (2:1 v/v). The homogenate was filtered through a funnel with a filter paper to recover the liquid phase. Subsequently, liquid samples were washed with a NaCl solution (0.9%) to obtain two layers. Total lipids were determined by the sulfophospho-vanillin method [31]. This method consists of oxidizing cellular lipids to small fragments after a chemical digestion with hot concentrated sulfuric acid. After the addition of a solution of vanillin and phosphoric acid, a fuchsia complex was formed and its absorbance was read at 530 nm on a CINTRA 10e CBC spectrophotometer. The standard solution was prepared with commercial extra virgin olive oil (Cocinero, Molinos Río de la Plata S.A.).
Glycogen concentration in both the midgut gland and muscle was measured based on Lo´s protocol [32]. In a glass tube, 1 mL of 30% KOH saturated with Na2SO4 was added to the pre-weighed sample. Tubes with their screw cap were put in a boiling water bath for 1 h, and then cooled in ice. One milliliter of ethanol 96° was added to precipitate the glycogen. The samples were placed in ice for 30 min and then were centrifuged (ROLCO 2036) at 4500 rpm for 10 minutes. The glycogen precipitates were next dissolved in 1 mL of distilled water. An aliquot of 300 μL of the above glycogen solution was brought to a sample volume of 1 mL by the addition of distilled water, 1 mL of 8% phenol solution added, and 5 mL of H2SO4 was added rapidly. Subsequently, the tubes were allowed to stand for 10 minutes, then shaken and placed for 10–20 minutes in a water bath at 25–30°C, before readings were taken. The absorption spectrum was read at 490 nm and the standard solution was prepared with rabbit glycogen (Sigma G0885).
Finally, total soluble protein was evaluated with the Coomassie blue dye method according to Bradford´s protocol [33] using serum bovine albumin as the standard (Sigma A6003).
Statistical analysis
All data are expressed as mean ± standard error. The statistical analyses were performed comparing the three treatments fed, starved and the initial control group (T0). All statistical analysis was performed with the same sample size in each studied species at each time (Cherax quadricarinatus: N = 8; Palaemon argentinus: N = 8 (8 pools of 6 animals/organ or tissues each); and Munida gregaria: N = 8). Data from the midgut gland index, glycogen and total lipids reserves, and soluble protein were analyzed by Generalized Linear Mixed Models (GLMMs) using InfoStat software (2015) [34]. The heterogeneous variance structure was modeled and the most parsimonious model was selected by comparison using the Akaike Information Criterion (AIC), and graphical inspection of their residue distribution. Post-hoc comparisons were performed using Fisher’s LSD test. For all analyses, residuals were analyzed for normal distribution via statistic of the Shapiro-Wilk test. The significance level was set at 0.05.
Results
In starving condition, MGI changed in the crayfish and shrimp (Fig 1). From T6 to T15, starved crayfish had lower MGI values than fed animals and the initial control (Fig 1A). In starved shrimps, the MGI had lower values than T0 and feed animals at all sampling times (Fig 1B). On the other hand, MGI remained unchanged in Munida gregaria between treatments during the whole experiment (Fig 1C).
Fig 1.
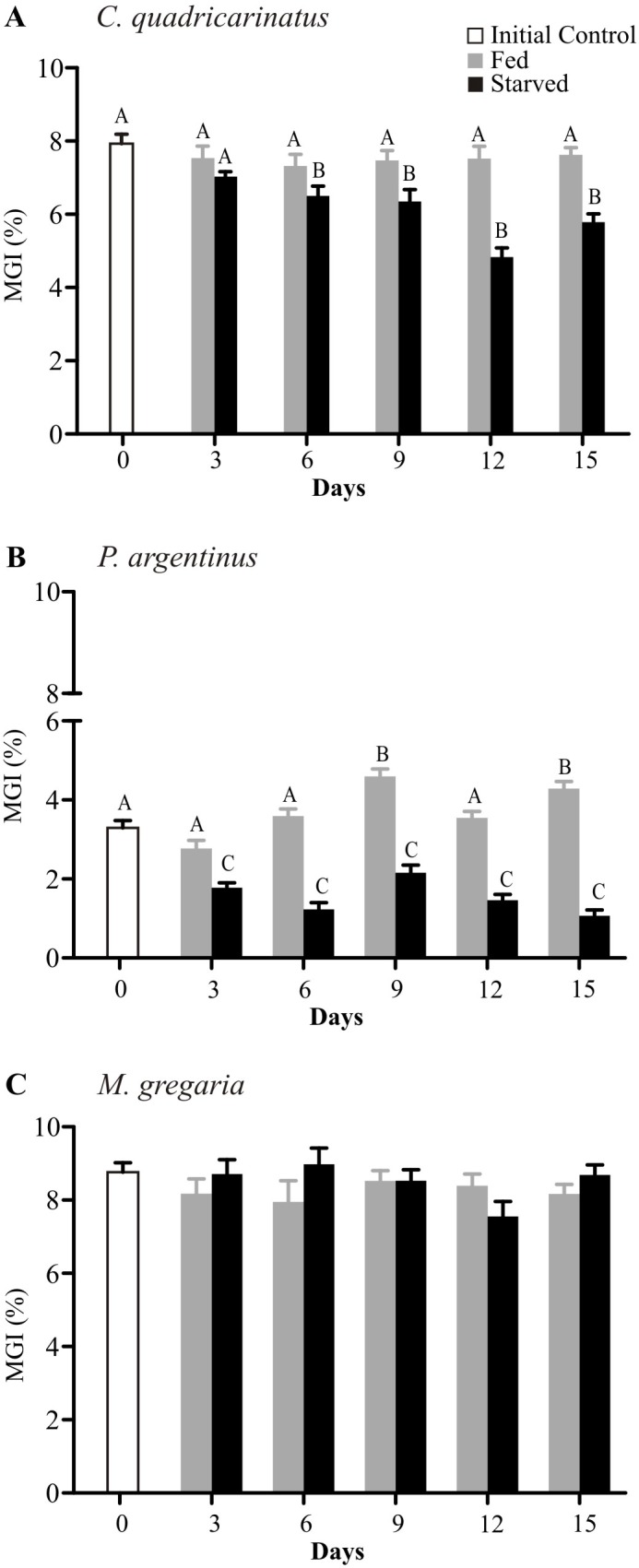
Midgut gland index of Cherax quadricarinatus (A), Palaemon argentinus (B) and Munida gregaria (C) after starvation. Different letters indicate statistical differences (p<0.05).
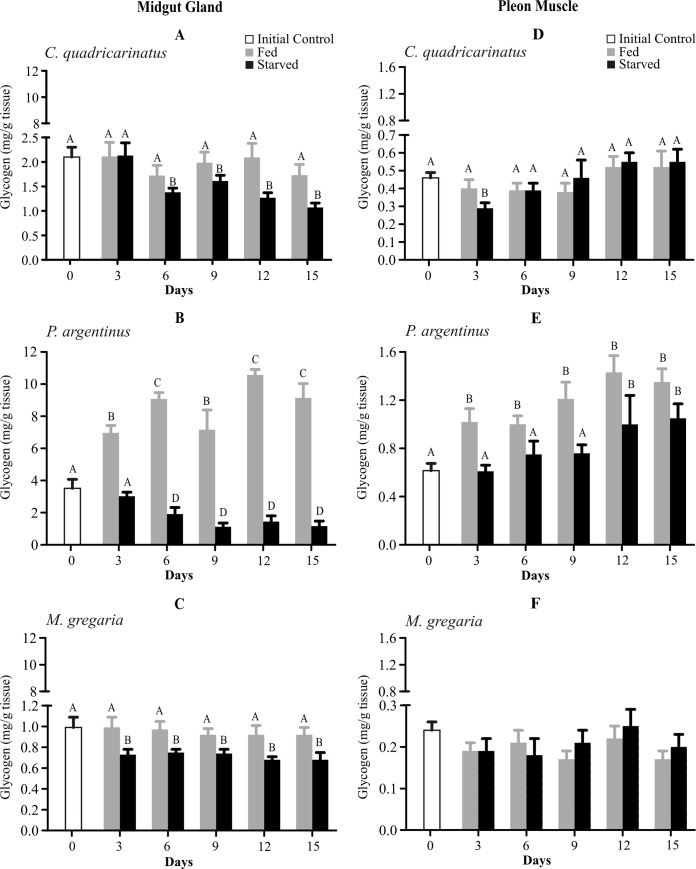
Crayfish started to consume midgut gland glycogen after day 6 of fasting (Fig 2A). Starved Palaemon argentinus reduced their glycogen reserves from T6 to T15 days compared to T0 and fed shrimps. In addition, in fed shrimps the MG glycogen concentration was higher than the control and throughout the experiment (Fig 2B). In starved Munida gregaria MG glycogen levels decreased earlier than in the other two species, after 3 days of starvation (Fig 2C). In the pleon muscle of both Cherax quadricarinatus and Munida gregaria, the glycogen reserves remained unchanged between treatments (Fig 2D and 2F). In Palaemon argentinus the glycogen concentration of the pleon muscle increased in both treatments: in fed animals at day 3, but later in starved animals, at day 12 (Fig 2E).
Fig 2.
Glycogen levels of midgut gland (left panel) and pleon muscle (right panel) of Cherax quadricarinatus (A, D), Palaemon argentinus (B, E) and Munida gregaria (C, F) after 15 days of starvation. Different letters indicate statistical differences (p<0.05).
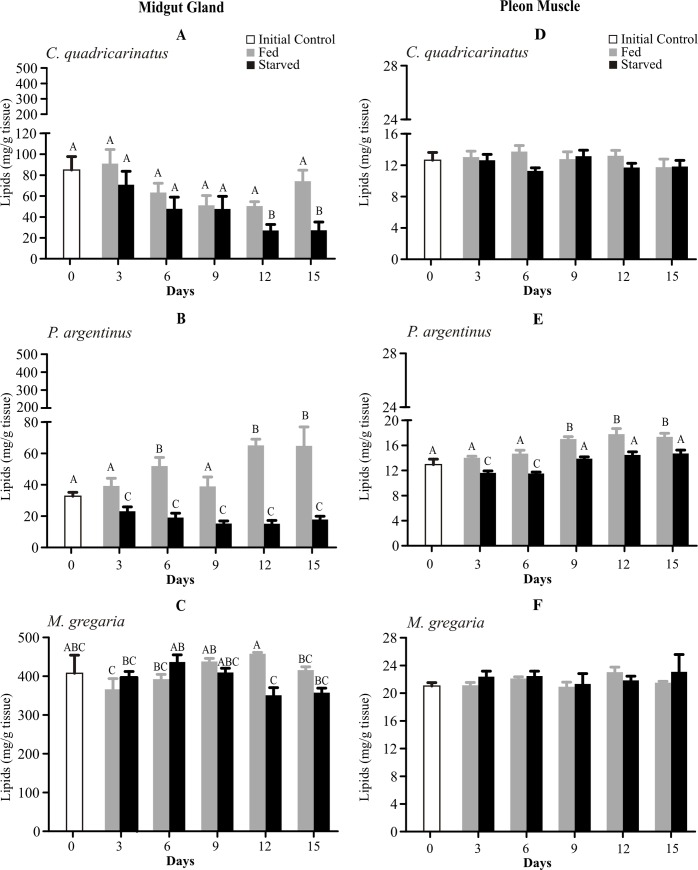
Midgut gland lipids changed differently in the three species (Fig 3). In starved crayfish, MG lipid concentration was lower at T12 and T15 than in fed animals and the initial control (Fig 3A). Similarly, in starved squat lobsters MG lipids decreased only at T12 (Fig 3C). On the contrary, in the shrimp MG lipid concentration was lower in the starved condition during all the experiment, whereas fed animals showed an increment on lipid values at T6, T12 and T15 (Fig 3B).
Fig 3.
Lipid levels of midgut gland (left panel) and pleon muscle (right panel) of Cherax quadricarinatus (A, D), Palaemon argentinus (B, E) and Munida gregaria (C, F) after 15 days of starvation. Different letters indicate statistical differences (p<0.05).
Our results show that crayfish, shrimp and squat lobster did not use their muscle lipids during the 15 days of starving conditions (Fig 3). In particular, we found that just at T3 and T6, starved shrimps presented lower lipid levels than the fed ones. Also, fed animals accumulated lipid reserves from T9 (Fig 3E).
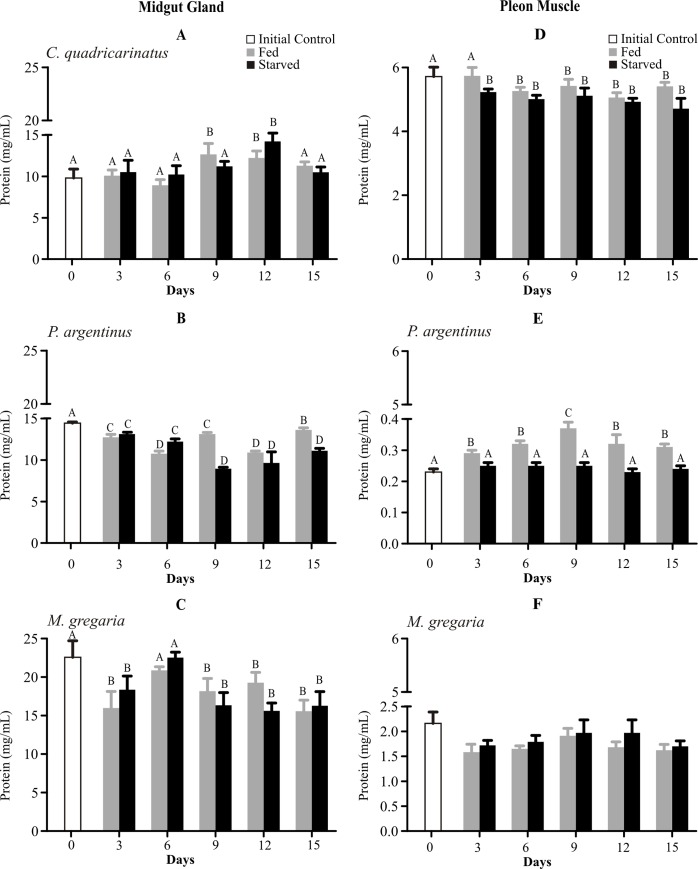
Protein levels of midgut gland remained unchanged in starved Cherax quadricarinatus, but decreased in Palaemon argentinus and Munida gregaria in both treatments (Fig 4). In fed C. quadricarinatus, proteins increased at T9 and T12, whereas in starved crayfishes proteins only increased at T12. Fed and starved shrimps showed lower protein values than initial controls at all times, and also were different at T6, T9 and T15 (Fig 4B). Similar results were observed in squat lobsters, where the midgut gland of fed and starved animals presented lower protein levels than T0, except at T6 (Fig 4C).
Fig 4.
Protein levels of midgut gland (left panel) and pleon muscle (right panel) of Cherax quadricarinatus (A, D), Palaemon argentinus (B, E) and Munida gregaria (C, F) after 15 days of starvation. Different letters indicate statistical differences (p<0.05).
In starving condition, muscle protein levels remained unchanged in shrimps and squat lobsters, but in crayfish proteins were lower after 3 days (Fig 4). In fed Cherax quadricarinatus protein concentration was lower than T0 after 6 days (Fig 4D). On the other hand, during the whole experimental time, protein levels were higher in fed than in starved shrimps and T0 (Fig 4E).
At T0 the midgut gland had higher levels of all macromolecules than the muscle. In Cherax quadricarinatus, Palaemon argentinus and Munida gregaria respectively, the ratio MG:muscle for each macromolecule were: 4.5, 5.7 and 4 for glycogen; 6.7, 2.5 and 19.4 for lipid; and 1.7, 62.8 and 10 for protein.
Discussion
Our results demonstrate that the three crustacean species present dissimilar physiological profiles and strategies in the utilization of energetic reserves during food deprivation despite having similar feeding habits. Specifically, Palaemon argentinus was the species that mobilized more kind of reserves during the 15 days of starvation, followed by Cherax quadricarinatus and Munida gregaria.
The midgut gland of starved shrimps presented low MGI, and a reduction in glycogen, lipid and protein levels; while in their muscle, lipid reserves decreased at days 3 and 6. In Cherax quadricarinatus, the most affected parameters in MG were MGI, glycogen and lipids. In the midgut gland of Munida gregaria only the glycogen concentration was reduced during fasting (Table 1).
Table 1. Summarized results of differences in the use of energy reserves of Cherax quadricarinatus, Palaemon argentinus and Munida gregaria after starvation.
| Parameter | C. quadricarinatus | P. argentinus | M. gregaria | |
|---|---|---|---|---|
| Midgut gland | MGI | T6 –T15 | T3 –T15 | unchanged |
| Glycogen | T6 –T15 | T6 –T15 | T3 –T15 | |
| Lipid | T12, T15 | T3 –T15 | non utilized | |
| Protein | non utilized | T9, T15 | non utilized | |
| Muscle | Glycogen | T3 | non utilized | non utilized |
| Lipid | non utilized | T3, T6 | non utilized | |
| Protein | non utilized | non utilized | non utilized |
In general, as a response to starving the reduction in the midgut gland weight is due to macro-molecule changes (e.g. glycogen, lipid and protein). In Cherax quadricarinatus and Palaemon argentinus, such a drop in MGI is coincident with a decline in glycogen in crayfish, as well as glycogen and lipids in shrimp. On the contrary, during starvation, the midgut gland weight in Munida gregaria remained unchanged, despite its glycogen reserves depletion (Fig 2C).
In shrimps, lobsters and crayfish, glycogen stores are quickly depleted and likely converted to glucose to obtain energy e.g. [29, 35–40]. Under starvation, in Munida gregaria and Palaemon argentinus, the decrease of MG glycogen was fast and reached basal levels at day 3 (ca. 0.74 mg/g tissue) or day 6 (ca. 1.2 mg/g tissue), respectively. Cherax quadricarinatus showed a stronger starvation resistance, since during our 15 day starvation period the MG glycogen decreased, probably reaching its basal levels at day 15, thereafter remaining constant [9, 11, 41].
The present study suggests that the three studied crustaceans did not mobilize glycogen reserves from the tail muscle during the non-feeding period. The preservation of glycogen tail muscle may reflect its utility as a fuel in searching for food and/or tail-flip escape reaction. Nevertheless, in brachyuran crabs such as Neohelice granulata (Varunidae) or Ocypode quadrata (Ocypodidae) the muscle glycogen levels gradually decrease after 7 or 15 days of starvation, in order to maintain its energy requirements [42, 43].
The decline in MG lipid levels in Cherax quadricarinatus and Palaemon argentinus suggest that a lipolysis is taking place in the midgut gland as an energetic source. In the shrimp lipids probably have reached basal levels at day 9 (15.3 mg/g tissue) of starvation. Other species have a faster rate of lipid depletion, e.g. Penaeus vannamei (Penaeidae) reaches the lowest lipid levels after 4 days of starvation [38], and Palaemon argentinus uses 66% of the initial lipids during the first day of starvation [8]. Instead, in our work, Palaemon argentinus utilized ~ 30% of their lipid reserves throughout 3 starvation days. Lipid reduction, as a response to a different period of food deprivation (6–28 days), was also reported in other crustaceans such as the copepod Acanthodiaptomus denticorni (Diaptomidae); and the Penaeidae shrimps: Penaeus vannamei, Penaeus duorarum, Penaeus semisulcatus, Penaeus monodon, Penaeus japonicus, and Penaeus esculentus [35, 37, 38, 44–47].
There is no clear pattern that Munida gregaria utilizes its MG lipid reserves during the 15 days of food deprivation. However, this species has 5 and 13 times more lipids than Cherax quadricarinatus and Palaemon argentinus, respectively. The preservation of lipidic reserves could be related to the subantartic water temperature (5–10°C) in which Munida gregaria thrives, and hence preventing a possible cellular damage. Even though there are few studies about the relationship between unsaturated fatty acids and membrane fluidity in marine organisms [48], ectothermic animals increase membrane content of unsaturated fatty acids as a response to cold conditions to maintain its fluidity [48, 49].
Our results suggest that none of the three studied species utilize the energy from protein catabolism along the 15 days food deprivation period. During the experiment, soluble protein levels in midgut gland and muscle showed different fluctuation patterns, but in any case proteins were not spent. Starved prawns, shrimps or crabs can however, obtain energy through the catabolism of amino acids [37, 45, 50], but a prudent utilization of protein in short starvation periods may be an adaptive strategy to avoid the usage of high costly macromolecules, which could represent an energetic saving in case of prolonged periods without food [38]. For example, in the shrimp Penaeus vannamei soluble protein concentration remains relatively constant during 5 days of starvation [38]. Moreover, the lobster Nephrops norvegicus (Nephropidae) fasted through 12 weeks and 6 months, does not reduce protein levels in their tail muscle [51]. We hypothesize that the catabolism of protein and free amino acid pool might provoke the enzyme proteolysis and the lack of amino acid for the enzyme de novo synthesis, which could be essential in different metabolic processes for obtaining energy through the Krebs cycle, glycolysis, and fatty acid β-oxidation. Some authors suggest that in crustaceans the most important energy reserve compartments are the midgut gland and muscle [5]. However, our results in comparing the concentration of three macromolecules at T0 confirm that the midgut gland is the major organ of reserve storage, and that the tail muscle does not mobilize energetic resources due to the starvation stress in the same degree as the midgut gland.
Another interesting result of our research was that fed Palaemon argentinus increased lipid and glycogen levels in MG and lipid, glycogen and protein levels in pleon muscle, although the stored concentration is much higher in the midgut gland than in the muscle. This result confirms that this species has the capacity to accumulate more reserves, also illustrating the plasticity of this organ. The histological analysis of the midgut gland will be necessary to evaluate possible histopathological effects.
We are confident that our results are unbiased, even in the comparison of a permanent cultured species such as Cherax quadricarinatus with the other wild the species. Juveniles of crayfish were obtained in laboratory conditions from cultured individuals, with a regular feeding regime. Palaemon argentinus and Munida gregaria were captured from the wild, and therefore they might already have been subjected to starvation and could have a physiological advantage. Cherax quadricarinatus can recover from both short and long fasting periods. For example, in a regime of 4d of feeding and 4 d of starvation, energetic reserves remain unchanged [52]. After 50 days of starvation and 30 days of re-feeding, this crayfish recovers levels of glycogen, lipids, digestive enzyme activity, hepatopancreas structure, and regains its molting frequency [11]. Moreover, such a long fasting period can promote growth at low culture temperatures [53].
On the other hand, as the tested species thrive in different environments, i.e. marine or freshwater, they likely present different nutritional requirements. Wild animals used in the present study, Munida gregaria and Palaemon argentinus, can grab, manipulate, ingest, and metabolize the food they were offered at the beginning of the experiments. Several studies have demonstrated that wild crustaceans can be fed with TetraColor®. For example, the squat lobster fed exclusively with TetraColor® can develop its ovary in a 4-month experiment [7] and successfully incubate embryos during their whole development [54]. Palaemon argentinus shrimp feeding on these commercial pellets can grow its midgut gland (Fig 1), and molt regularly (pers. obs.). Therefore, these results indicate that even though the diet may not be optimal for the three species, it is adequate for the animals to efficiently accomplish physiological processes with high energetic requirements, such as reproduction or molting. Furthermore, it is unknown whether feeding habits or food itself, could be under natural selection pressure, affecting directly the physiological fitness of these species.
Our results demonstrate that the species with the shortest life-span has mobilized more reserves during starvation: Palaemon argentinus (life-span ~1.5 yr) mobilized more reserves than Cherax quadricarinatus (life-span >3 yr), followed by Munida gregaria (life-span >5 yr) (Tables 1 and 2). Among decapods life-span varies considerably [55]. We did a literature review on the energetic mobilization of decapods during starvation in relation to their habitat and life-span (Table 2). The percentage of mobilization indicates the proportion of reserve reduction (considering the reserve concentration of fed animals as 100%) during the starvation period (Table 2). A comparison of the mobilization percentage among 21 decapod species shows a range from 28% to 99%. This mobilization is relatively low in species with longer life-span (>3 years) relativized to starvation days. Nevertheless, Lithodes santolla, Munida gregaria and Penaeus esculentus mobilize ~ 32% reserves during 60, 15 and 14 days of starving respectively; whereas, Homarus americanus, Nephrops norvegicus, Procambarus clarkii, Cherax destructor, and Cherax quadricarinatus mobilize more reserves (>60%) but during a longer period (102, 84, 210, 150, 154, and 80 days respectively) (Table 2). Therefore, we hypothesize that decapod species with longer life spans present lower proportion of reserve mobilization than species with short life-span, at similar starvation period. This pattern is also common to our studied species. Another approach shown in the literature is that marine decapods likely utilize glycogen reserves as an energy source during starvation independently of their life-span. By contrast, the starved freshwater decapods usually tend to utilize the three macromolecules: glycogen, lipids and protein (Table 2). In general, freshwater habitat is more variable than marine habitat with respect to temperature, current (lentic or lotic), nutrients (eutrophic or oligotrophic), and other factors. We further hypothesize that freshwater decapods present more plasticity to utilize more than one type of reserves due to variable habitat than marine decapods. Finally, the amount of different energetic reserves used by species could be related to the molt rate (as a proxy to the metabolic rate, given the numerous catabolic and anabolic processes involved in molting [56]; namely, at low molt rates the types of mobilized macromolecules will be few during starvation and vice versa (e.g Lithodes santolla, Munida gregaria, Nephrops norvegicus) (Table 2). This could be because a high molt rate would imply a high metabolism and, therefore, greater energy requirements would be needed during starvation to maintain homeostasis. In our three studied species molting frequency is variable: 40 d-1 [57], 45–50 d-1 (Sacristan unpublished data), and 2 yr-1 [28] in Palaemon argentinus, Cherax quadricarinatus and Munida gregaria respectively, and therefore this coincides with the type of reserve mobilized during starvation for the three species.
Table 2. Decapod preferential energy reserves mobilization according to habitat, life span, percentage of mobilization and starvation days in midgut gland and muscle.
| Reserve mobilization organ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference number | Crustaceans | Infraorder/Suborder | Family | Habitat | Life-span(years) | Midgut gland | Muscle | Mobilization (%) | Starvation (days) | Reference |
| 1 | Crangon crangon | Caridea | Crangonidae | marine | 3.3 | glycogen | – | – | 28 | [58] |
| 2 | Homarus americanus | Astacidea | Nephropidae | marine | >31 | glycogen | – | 60 | 102 | [59] |
| 3 | Lithodes santolla | Anomura | Lithodidae | marine | ~14 | glycogen | lipid | 35 | 60 | Sacristan et al unpublished |
| 4 | Munida gregaria | Anomura | Munididae | marine | >5 | glycogen | non utilized | 32 | 15 | presente study |
| 5 | Nephrops norvegicus | Astacidea | Nephropidae | marine | 15 | glycogen | glycogen | 87 | 210 | [39] |
| 6 | Ocypode quadrata | Brachyura | Ocypodidae | marine | 3 | lipid | glycogen | 53 | 15 | [43] |
| 7 | Penaeus vannamei | Dendrobranchiata | Penaeidae | marine | 1.5–2 | glycogen and lipid | – | 80 and 84 | 5 | [38] |
| 8 | Penaeus duorarum | Dendrobranchiata | Penaeidae | marine | 1.3–1.6 | glycogen | – | – | – | [35] |
| 9 | Penaeus esculentus | Dendrobranchiata | Penaeidae | marine | 2.4 | – | protein and lipid | 28 and 33 | 14 | [45] |
| 10 | Penaeus japonicus | Dendrobranchiata | Penaeidae | marine | ~1.3 | glycogen | lipid | 72 | 28 | [37] |
| 11 | Portunus pelagicus | Brachyura | Portunidae | marine | 3 | glycogen | – | 99 | 6 | [60] |
| 12 | Hemigrapsus nudus | Brachyura | Varunidae | marine | – | protein | – | – | 23 | [61] |
| 13 | Neohelice granulata | Brachyura | Varunidae | brackish | 3 | glycogen | – | 80 | 7 | [42] |
| 14 | Orconectes limosus | Astacidea | Cambaridae | freshwater | >1.5 | lipid | – | 28 | 41 | [62] |
| 15 | Orconectes virilis | Astacidea | Cambaridae | freshwater | >1.5 | protein and carbohydrates | protein and carbohydrates | 42 and 37 | 14 | [63] |
| 16 | Procambarus clarkii | Astacidea | Cambaridae | freshwater | 3.5–6.5 | glycogen, lipid and protein | glycogen, lipid and protein | 80, 80 and 89 | 150 | [64] |
| 17 | Procambarus zonangulus | Astacidea | Cambaridae | freshwater | >1.5 | glycogen, lipid and protein | glycogen, lipid and protein | 87, 60 and 60 | 150 | [64] |
| 18 | Macrobrachium rosenbergii | Caridea | Palaemonidae | freshwater | – | glycogen | – | 74 | 4 | [65] |
| 19 | Palaemon argentinus | Caridea | Palaemonidae | freshwater | 1.3 | glycogen, lipid and protein | lipid | 66, 45 and 38 | 15 | present study |
| 20 | Cherax destructor | Astacidea | Parastacidae | freshwater | >3 | carbohydrates, lipid and protein | – | 77, 98 and 61 | 154 | [29] |
| 21 | Cherax quadricarinatus | Astacidea | Parastacidae | freshwater | >3 | glycogen and lipid | non utilized | 49 and 68; 72 and 95 | 15; 80 | present study; [11] |
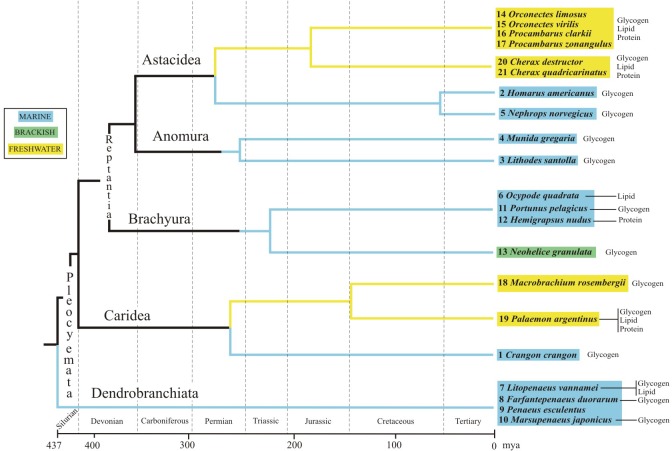
Crustacean decapods display stronger relationships between the type of mobilized reserve and habitat, than phylogenetic distance among species (Fig 5). Specifically, infraorder Astacidea contains (short phylogenetic distance): astacids and nephropids, each one a freshwater or a marine lineage respectively. Freshwater crayfishes likely utilize the three kind of reserves as an energy source during starvation. Instead, marine clawed lobsters consume glycogen reserves independently of their phylogenetic relationship within the Astacidea. In the Caridea lineage, similar responses are observed between freshwater (Palaemonidae) and marine (Crangonidae) species (Fig 5). Moreover, marine species usually tend to utilize glycogen reserves during fasting periods, e.g. infraorders Anomura, Brachyura, and the suborder Dendrobranchiata (penaeid shrimps and their relatives) (Fig 5). Therefore, glycogen reserves consumption as a preferential energy source in the midgut gland could be considered as an ancestral character in decapods.
Fig 5. Energetic reserves mobilization of midgut gland, environments, and phylogenetic relationship among decapods crustaceans.
Phylogenetic tree adapted from Porter et al. (2005) [12]. Numbers on left to the species names are the reference numbers of Table 2. Different color boxes (light blue, green and yellow) indicate the habitat of species.
The sea realm is the ancestral environment of the Decapoda, with conquest of freshwater habitats being a later adaptation [66, 67]. In the freshwater prawn of genus Macrobrachium, and in lineages that split later, several adaptations associated with the transition to fresh water habitat or “freshwaterization” process were observed, such as reproductive adaptations, larval development and starvation resistance [68] [67] and references therein. In this sense, the ability to catabolize different compounds under short fasting periods may have emerged as an adaptive advantage to colonize more unstable environments such as freshwater. This capacity could have arisen more recently in the evolution of the organisms that inhabit freshwater habitats, and could have appeared more than once, since it can be observed in Caridea and Reptantia (Fig 5). Therefore, the physiological responses perceived the present study and previous works could indicate that freshwater decapods have acquired the ability to mobilize more than one kind of energetic reserve as part of the freshwaterization process. We hypothesize that in the evolutionary history of decapod species could have arisen a certain character that allowed to develop different mechanisms in response to starvation. Nevertheless, more comparative research between related species from different habitats and dissimilar phylogenetic distance are needed to test this hypothesis.
Considerable research has been focused on nutrition of decapod crustaceans and many comparisons have been made across species. This is the first study, which uses the same methodology and compares three decapod species with similar feeding habits living in different environments, and with patterns of energetic mobilization that are comparable across other decapod species. Nevertheless, for future works we propose the use of biochemical methods instead of proximal composition analyses for the study of decapod starvation response in order to test our hypothesis, and thus avoid the variability factor associated to the methodology employed. Specifically, reserve mobilization may be explained through intrinsic factors, such as life span and molt rate, as well as modulating environmental factors (e.g. temperature), habitat and phylogenetic relationships. Therefore, the present study shows that decapod crustaceans display a vast diversity of reserve mobilization strategies to deal with starvation, and suggests that these strategies are not related to the type of food. Finally, according to our results, the literature reviewed, and the hypothesis suggested above, presumably a variety of shared trends takes place in the physiological responses of decapod crustaceans during starvation. However, we could not confirm experimentally this assumption as it is necessary to include a number of species per habitat in the same study along with the use of an identical methodological approach.
Acknowledgments
The authors would like to thank Dr. Mariano Diez and Sr. Marcelo Pérez for field assistance with Munida gregaria. We also thank Lic. Amir Dyzenchauz for language revision. Funds for this research were provided by the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012–01333 to LSLG; PICT 2012–0554 to GAL, 2012–0137 to AVFG, and 2015–2968 to HJS). Additional funds were provided by the Universidad Nacional de Mar del Plata, EXA 773/16.
Data Availability
All relevant data are available from the Figshare Repository at the following DOI: 10.6084/m9.figshare.5357896 and URL: https://figshare.com/s/3e41d4a9e0916cbe3a2a.
Funding Statement
Funds for this research were provided by the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012- 01333 to LSLG; PICT 2012-0554 to GAL, PICT 2012-0137 to AVFG, and 2015-2968 to HJS). Additional funds were provided by the Universidad Nacional de Mar del Plata, EXA 773/16 to AVFG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Albentosa M, Fernández-Reiriz MJ, Labarta U, Pérez-Camacho A. Response of two species of clams, Ruditapes decussatus and Venerupis pullastra, to starvation: physiological and biochemical parameters. Comp Biochem Physiol B. 2007; 146: 241–9. doi: 10.1016/j.cbpb.2006.10.109 [DOI] [PubMed] [Google Scholar]
- 2.Hu M, Wang Y, Tsang ST, Cheung SG, Shin PK. Effect of prolonged starvation on body weight and blood-chemistry in two horseshoe crab species: Tachypleus tridentatus and Carcinoscorpius rotundicauda (Chelicerata: Xiphosura). J Exp Mar Biol Ecol. 2010; 395: 112–9. [Google Scholar]
- 3.Watts AJ, McGill RA, Albalat A, Neil DM. Biophysical and biochemical changes occur in Nephrops norvegicus during starvation. J Exp Mar Biol Ecol. 2014; 457: 81–9. [Google Scholar]
- 4.Morales AE, Pérez-Jiménez A, Furné M, Guderley H. Starvation, energetics, and antioxidant defenses In: Abele D, Vázquez-Medina JP, Zenteno-Savín T, editors. Oxidative stress in aquatic ecosystems. 1st ed. Blackwell Publising, Oxford; 2012. pp. 281–294. [Google Scholar]
- 5.Sánchez-Paz A, García-Carreño F, Muhlia-Almazán A, Peregrino-Uriarte AB, Hernández-López J, Yepiz-Plascencia G. Usage of energy reserves in crustaceans during starvation: status and future directions. Insect Biochem Mol Biol. 2006; 36: 241–9. doi: 10.1016/j.ibmb.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 6.Oliveira GT, Rossi IC, Kucharski LC, Da Silva RS. Hepatopancreas gluconeogenesis and glycogen content during fasting in crabs previously maintained on a high-protein or carbohydrate-rich diet. Comp Biochem Physiol A. 2004; 137: 383–90. [DOI] [PubMed] [Google Scholar]
- 7.Romero M. Hábitos alimentarios y bioenergética de la langostilla Munida subrugosa (Crustacea: Decapoda) del Canal Beagle, Tierra del Fuego, Argentina. PhD Thesis. Univerisad Nacional de Córdoba. 2003.
- 8.Neves CA, Pastor MPS, Nery LEM, Santos EA. Effects of the parasite Probopyrus ringueleti (Isopoda) on glucose, glycogen and lipid concentration in starved Palaemonetes argentinus (Decapoda). Dis aquat org. 2004; 58: 209–13. doi: 10.3354/dao058209 [DOI] [PubMed] [Google Scholar]
- 9.Stumpf L, Calvo NS, Pietrokovsky S, Greco LSL. Nutritional vulnerability and compensatory growth in early juveniles of the “red claw” crayfish Cherax quadricarinatus. Aquaculture. 2010; 304: 34–41. [Google Scholar]
- 10.Calvo NS, Tropea C, Anger K, Greco LSL. Starvation resistance in juvenile freshwater crayfish. Aquatic Biol. 2012; 16: 287–97. [Google Scholar]
- 11.Sacristán HJ, Ansaldo M, Franco-Tadic LM, Gimenez AVF, Greco LSL. Long-Term starvation and posterior feeding effects on biochemical and physiological responses of midgut gland of Cherax quadricarinatus juveniles (Parastacidae). PloS One. 2016; 11:e0150854 doi: 10.1371/journal.pone.0150854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter ML, Pérez-Losada M, Crandall KA. Model-based multi-locus estimation of decapod phylogeny and divergence times. Mol Phylogenet Evol. 2005; 37: 355–69. doi: 10.1016/j.ympev.2005.06.021 [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein J. Phylogenies and the comparative method. Amer Nat. 1985; 125: 1–15. [Google Scholar]
- 14.Faria SC, Faleiros RO, Brayner FA, Alves LC, Bianchini A, Romero C, et al. Macroevolution of thermal tolerance in intertidal crabs from Neotropical provinces: A phylogenetic comparative evaluation of critical limits. Ecol Evol. 2017; 7: 3167–76. doi: 10.1002/ece3.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saoud IP, Ghanawi J, Thompson KR, Webster CD. A review of the culture and diseases of redclaw crayfish Cherax quadricarinatus (von Martens 1868). J World Aquacult Soc. 2013; 44: 1–29. [Google Scholar]
- 16.Boschi E. Decapoda Natantia.[Natant Decapod]. Fauna de agua dulce de la República Argentina. 1981. pp 61.
- 17.Morrone J, Lopretto E. Parsimony analysis of endemicity of freshwater Decapoda (Crustacea: Malacostraca) from southern South America. Neotropica. 1995; 41:3–8. [Google Scholar]
- 18.Collins PA. Feeding of Palaemonetes argentinus (Decapoda Palaemonidae) from am oxbow lake of the Parana River, Argentina. J Crust Biol. 1999; 19: 485–92. [Google Scholar]
- 19.Baba K, Schnabel KE, Rodrigues C, Nizinski M, Lin C-W, Cabezas P, et al. Catalogue of squat lobsters of the world (Crustacea: Decapoda: Anomura-Families Chriostylidae, Galatheidae and Kiwaidae). Magnolia Press; 2008. pp 220. [Google Scholar]
- 20.Romero MC, Lovrich GA, Tapella F, Thatje S. Feeding ecology of the crab Munida subrugosa (Decapoda: Anomura: Galatheidae) in the Beagle Channel, Argentina. J Mar Biol Assoc UK. 2004; 84: 359–65. [Google Scholar]
- 21.Jones CM. Production of juvenile redclaw crayfish, Cherax quadricarinatus (von Martens)(Decapoda, Parastacidae) II. Juvenile nutrition and habitat. Aquaculture. 1995; 138: 239–45. [Google Scholar]
- 22.Bugnot AB, Greco LSL. Sperm production in the red claw crayfish Cherax quadricarinatus (Decapoda, Parastacidae). Aquaculture. 2009; 295: 292–9. [Google Scholar]
- 23.De Bock MS, Greco LSL. Sex reversal and growth performance in juvenile females of the freshwater crayfish Cherax quadricarinatus (Parastacidae): effect of increasing temperature and androgenic gland extract in the diet. Aquacul Int. 2010; 18: 231–43. [Google Scholar]
- 24.Levi T, Barki A, Hulata G, Karplus I. Mother-offspring relationships in the red-claw crayfish Cherax quadricarinatus. J Crust Biol. 1999; 19: 477–84. [Google Scholar]
- 25.Tropea C, Piazza Y, Greco LSL. Effect of long-term exposure to high temperature on survival, growth and reproductive parameters of the “redclaw” crayfish Cherax quadricarinatus. Aquaculture. 2010; 302: 49–56. [Google Scholar]
- 26.Vazquez FJ, Tropea C, López Greco LS. Development of the female reproductive system in the freshwater crayfish Cherax quadricarinatus (Decapoda, Parastacidae). Invertebr Biol. 2008; 127: 433–43. [Google Scholar]
- 27.Boudet LC, Escalante A, Von Haeften G, Moreno V, Gerpe M. Assessment of heavy metal accumulation in two aquatic macrophytes: a field study. Ecotoxicol Environ Cont. 2011; 6. [Google Scholar]
- 28.Tapella F. Reproducción, crecimiento, distribución y abundancia de la langostilla Munida subrugosa (Anomura: Galatheidae) del canal Beagle, Tierra del Fuego, Argentina. PhD Thesis. Univerisad Nacional de Córdoba. 2002.
- 29.Jones P, Obst J. Effects of starvation and subsequent refeeding on the size and nutrient content of the hepatopancreas of Cherax destructor (Decapoda: Parastacidae). J Crus Biol. 2000; 20: 431–41. [Google Scholar]
- 30.Folch J, Lees M, Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957; 226: 497–509. [PubMed] [Google Scholar]
- 31.Frings CS, Dunn RT. A colorimetric method for determination of total serum lipids based on the sulfo-phospho-vanillin reaction. Am J Clin Path. 1970; 53: 89–91. [DOI] [PubMed] [Google Scholar]
- 32.Lo S, Russell JC, Taylor A. Determination of glycogen in small tissue samples. J App Physiol. 1970; 28: 234–6. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal biochem. 1976; 72: 248–54. [DOI] [PubMed] [Google Scholar]
- 34.Zuur AF, Ieno EN, Saveliev AA. Mixed Effects Models and Extensions in Ecology with R: Springer; 2009. [Google Scholar]
- 35.Schafer H. Storage materials utilized by starved pink shrimp, Penaeus duorarum Burkenroad. FAO Fish Rep. 1968; 57: 393–403. [Google Scholar]
- 36.Cuzon G, Ceccaldi H. Influence of the fasting stabulation on the metabolism of the shrimp Crangon crangon (L.). Comptes rendus des seances de la Societe de biologie et de ses filiales. 1972; 167: 66–9. [PubMed] [Google Scholar]
- 37.Cuzon G, Cahu C, Aldrin J, Messager J, Stephan G, Mevel M. Starvation effect on metabolism of Penaeus japonicus. J World Aquacul Soc. 1980; 11: 410–23. [Google Scholar]
- 38.Sánchez-Paz A, García-Carreño F, Hernández-López J, Muhlia-Almazán A, Yepiz-Plascencia G. Effect of short-term starvation on hepatopancreas and plasma energy reserves of the Pacific white shrimp (Litopenaeus vannamei). J Exp Mar Biol Ecol. 2007; 340: 184–93. [Google Scholar]
- 39.Baden S, Depledge M, Hagerman L. Glycogen depletion and altered copper and manganese handling in Nephrops norvegicus following starvation and exposure to hypoxia. Marine Ecology-Progress Series. 1994. [Google Scholar]
- 40.Auerswald L, Meyer B, Teschke M, Hagen W, Kawaguchi S. Physiological response of adult Antarctic krill, Euphausia superba, to long-term starvation. Polar Biol. 2015; 38: 763–80. [Google Scholar]
- 41.Calvo NS, Stumpf L, Pietrokovsky S, Greco LSL. Early and late effects of feed restriction on survival, growth and hepatopancreas structure in juveniles of the red claw crayfish Cherax quadricarinatus. Aquaculture. 2011; 319: 355–62. [Google Scholar]
- 42.Vinagre AS, Da Silva RS. Effects of starvation on the carbohydrate and lipid metabolism in crabs previously maintained on a high protein or carbohydrate-rich diet. Comp Biochem Physiol A. 1992; 102: 579–83. [Google Scholar]
- 43.Vinagre A, Chung JS. Effects of starvation on energy metabolism and crustacean hyperglycemic hormone (CHH) of the Atlantic ghost crab Ocypode quadrata (Fabricius, 1787). Mar Biol. 2016; 163: 1–11. [Google Scholar]
- 44.Stuck K, Watts S, Wang S. Biochemical responses during starvation and subsequent recovery in postlarval Pacific white shrimp, Penaeus vannamei. Mar Biol. 1996; 125: 33–45. [Google Scholar]
- 45.Barclay M, Dall W, Smith D. Changes in lipid and protein during starvation and the moulting cycle in the tiger prawn, Penaeus esculentus Haswell. J Exp Mar Biol Ecol. 1983; 68: 229–44. [Google Scholar]
- 46.Bourdier G, Amblard C. Lipids in Acanthodiaptomus denticomis during starvation and fed on three different algae. J Plankton Res. 1989; 11: 1201–12. [Google Scholar]
- 47.Moore L, Smith D, Loneragan N. Blood refractive index and whole-body lipid content as indicators of nutritional condition for penaeid prawns (Decapoda: Penaeidae). J Exp Mar Biol Ecology. 2000; 244: 131–43. [Google Scholar]
- 48.Parrish CC. Lipids in marine ecosystems. ISRN Oceanography. 2013. [Google Scholar]
- 49.Hazel JR. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol. 1995; 57: 19–42. doi: 10.1146/annurev.ph.57.030195.000315 [DOI] [PubMed] [Google Scholar]
- 50.Dall W, Smith D. Changes in protein-bound and free amino acids in the muscle of the tiger prawn Penaeus esculentus during starvation. Mar biol. 1987; 95: 509–20. [Google Scholar]
- 51.Parslow-Williams PJ. Nutritional limitation in populations of the Norway lobster, Nephrops norvegicus (L.) in the Firth of Clyde, Scotland: University of Glasgow; 1998. [Google Scholar]
- 52.Stumpf L, Díaz FC, Viau VE, Valenti WC, Greco LSL. Effect of food shortage on growth, energetic reserves mobilization, and water quality in juveniles of the redclaw crayfish, Cherax quadricarinatus, reared in groups. J Crus Biol. 2014; 34: 639–46. [Google Scholar]
- 53.Stumpf L, López Greco LS. Compensatory Growth in Juveniles of Freshwater Redclaw Crayfish Cherax quadricarinatus Reared at Three Different Temperatures: Hyperphagia and Food Efficiency as Primary Mechanisms. PLOS ONE. 2015; 10(9):e0139372 doi: 10.1371/journal.pone.0139372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pérez-Barros P, Thatje S, Calcagno JA, Lovrich GA. Larval development of the subantarctic squat lobster Munida subrugosa (White, 1847)(Anomura: Galatheidae), reared in the laboratory. J Exp Mar Biol Ecol. 2007; 352: 35–41. [Google Scholar]
- 55.Vogt G. Ageing and longevity in the Decapoda (Crustacea): a review. Zoologischer Anzeiger-A J of Comp Zool. 2012; 251: 1–25. [Google Scholar]
- 56.Chang ES. Physiological and biochemical changes during the molt cycle in decapod crustaceans: an overview. J Exp Mar Biol Ecol. 1995; 193: 1–14. [Google Scholar]
- 57.Díaz AC, Sousa LG, Cuartas EI, Petriella AM. Growth, molt and survival of Palaemonetes argentinus (Decapoda, Caridea) under different light-dark conditions. Iheringia Sér Zool. 2003; 93: 249–54. [Google Scholar]
- 58.Cuzon G, Ceccaldi H. Influence of the fasting stabulation on the metabolism of the shrimp Crangon crangon (L.). Comptes rendus des séances de la Société de biologie et de ses filiales. 1973; 167: 66 [PubMed] [Google Scholar]
- 59.Stewart JE, Horner G, Arie B. Effects of temperature, food, and starvation on several physiological parameters of the lobster Homarus americanus. J Fish Board Can. 1972; 29: 439–42. [Google Scholar]
- 60.Sugumar V, Vijayalakshmi G, Saranya K. Molt cycle related changes and effect of short term starvation on the biochemical constituents of the blue swimmer crab Portunus pelagicus. Saudi J Biol Sci. 2013; 93–103. doi: 10.1016/j.sjbs.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neiland KA, Scheer BT. The influence of fasting and of sinus gland removal on body composition of Hemigrapsus nudus. Physiol Comp Oecol. 1953; 2: 198–209. [Google Scholar]
- 62.Speck U, Urich K. Consumption of body constitutents during starvation in crayfish, Orconectes limosus. Zeitschrift fur vergleichende Physiologie. 1969; 410–414. [Google Scholar]
- 63.Hazlett B, Rubenstein D, Rittschof D. Starvation, energy reserves, and aggression in the crayfish Orconectes virilis (Hagen, 1870) (Decapoda, Cambaridae). Crustaceana. 1975;11–6. [Google Scholar]
- 64.Powell ML, Watts SA. Response to long‐term nutrient deprivation and recovery in the crayfishes Procambarus clarkii and Procambarus zonangulus (Crustacea, Decapoda): component and proximate analyses. J World Aquacul Soc. 2010; 41: 71–80. [Google Scholar]
- 65.Clifford HC, Brick RW. Nutritional physiology of the freshwater shrimp Macrobrachium rosenbergii (de Man)-I. Substrate metabolism in fasting juvenile shrimp. Comp Biochem Physiol A. 1983; 74: 561–8. [Google Scholar]
- 66.Schram FR. On the origin of Decapoda In: Martin JW, Crandall KA, Felder DL, editors. Decapod crustacean phylogenetics. Boca Raton, FL: CRC Press; 2009. pp 3–13. [Google Scholar]
- 67.Kawai T, Cumberlidge N. A Global Overview of the Conservation of Freshwater Decapod Crustaceans. 2016. pp. 430
- 68.Jalihal D, Sankolli K, Shenoy S. Evolution of larval developmental patterns and the process of freshwaterization in the prawn genus Macrobrachium Bate, 1868 (Decapoda, Palaemonidae). Crustaceana. 1993; 65: 365–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available from the Figshare Repository at the following DOI: 10.6084/m9.figshare.5357896 and URL: https://figshare.com/s/3e41d4a9e0916cbe3a2a.