Abstract
Background
The relation between arrhythmias and stress is known. The aim of our current study was to elucidate whether plasma levels of previously described stress parameters are altered in highly symptomatic patients with atrial fibrillation (AF) per se and in patients undergoing ablation therapy by pulmonary vein isolation (PVI).
Methods
96 patients with AF undergoing PVI were recruited. Plasma levels of Endothelin-1 (ET-1), MCP-1 and Chromogranin-A (CGA) were measured before and three months after ablation completed with clinical follow-up with respect to AF recurrence. Additionally, we examined 40 healthy age- and sex-matched volunteers as a reference.
Results
Symptomatic AF patients showed increased levels of ET-1 compared to healthy controls (2.62pg/ml vs. 1.57pg/ml; p<0.01). Baseline levels of ET-1 were higher in patients presenting with AF after PVI (2.96pg/ml vs. 2.57pg/ml;p = 0.02). The temporal comparison revealed decreased ET-1 levels in patients without (2.57pg/ml vs. 2.33pg/ml; p<0.01) and unchanged ET-1 levels in patients with AF after PVI. Baseline MCP-1 was increased in AF patients vs. controls (268pg/ml vs. 227 pg/ml; p = 0.03). Both groups, with and without AF after PVI, showed an increase of MCP-1 compared to baseline (268pg/ml vs. 349pg/ml;p<0.01; 281pg/ml vs. 355pg/ml;p = 0.03). CGA was lower in AF patients compared to healthy controls (13.8ng/ml vs. 25.6ng/ml;p<0.01). Over time patients without AF after PVI showed an increase of CGA (14.2ng/ml vs. 20.7ng/ml;p<0.01). No change was observed in patients with AF after PVI.
Conclusion
Our study demonstrated dysregulated levels of ET-1, MCP-1 and CGA in symptomatic AF patients. We could demonstrate an association between ET-1 to presence or absence of AF. Furthermore, we could show that a decrease of ET-1 as well as an increase of CGA after PVI, representing a trend towards control cohort levels, were both associated with restoration of sinus rhythm. These results provide new insights into the role of stress-related biomarkers in AF and AF treatment by ablation therapy.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice affecting more than six million Europeans [1,2]. AF is associated with increased morbidity and mortality since it is a major risk factor for thromboembolism [2]. Treatment of AF includes pharmacological (antiarrhythmic drugs) and interventional (pulmonary vein isolation, PVI) strategies but still remains challenging. The pathophysiological background of AF is complex and still incompletely understood [3,4]. Recently, it has been shown that physical or emotional stress can provoke AF and vice versa [5–10]. Stress cannot be measured directly, but several substances in the blood can serve as surrogate parameters for stress [11]. In this study we sought to investigate a potential relationship and pathophysiological role of three stress surrogate markers (Endothelin-1, monocyte chemotactic protein-1 and chromogranin A) in AF.
Endothelin-1 (ET-1) is produced by endothelial cells, smooth muscle cells, monocytes and macrophages after stimulation by catecholamines, cortisol, hypoxia, or interleukins [12]. Increased levels of Endothelin-1 were described in patients with different cardiovascular diseases [12]. Furthermore, Endothelin-1 has been reported as a biomarker for stress associated acute cardiovascular diseases. Recently, we could demonstrate that patients having an acute cardiovascular event in context of emotional stress showed significantly higher serum levels of Endothelin-1 compared to patients with acute cardiovascular events not having experienced a kind of emotional stress [10,11].
Similar findings have been described for monocyte chemotactic protein-1 (MCP-1) [10,11]. MCP-1 is a chemokine synthesized mainly in monocytes and macrophages Several factors affect synthesis of MCP-1 e.g. TNFα, Interferon-γ, PDGF and Angiotensin that are inducing synthesis of MCP-1 via NF-kB [13], which are also known to play a role in AF pathophysiology [4].
Chromogranin A (CGA) is an essential part of secretory vesicle in endocrine cells, neurons and neuroendocrine cells. As a part of secretory vesicles CGA is released with the content of the vesicle [14,15]. The majority of circulating CGA in healthy people is derived from adrenal medulla. There is also evidence that increased levels of CGA are associated with stress as we could previously show high levels of CGA after physical stress after extreme exercise [16].
The aim of the present study was to evaluate a potential association of the circulating Endothelin-1, MCP-1, and CGA with AF in highly symptomatic patients as well as to investigate changes of these markers in AF patients undergoing ablation therapy with respect to AF recurrence.
To test this, we measured the plasma levels of Endothelin-1, MCP-1, and CGA in AF patients undergoing PVI before and three months after ablation and compared these to healthy controls.
Methods
Patients and clinical follow up
96 consecutive patients undergoing PVI in the University Hospital Grosshadern were analysed in our study. To compare baseline levels, 40 age and sex matched patients without any history of atrial fibrillation or cardiovascular disease were selected as “healthy volunteers”. The analysed specimens were collected in a general biobanking effort that was continuously approved by the local ethics committee of the University of Munich and the Ethics Commission of the State Chamber of Medicine in Bavaria, Germany over the last years (reference numbers of the ethics approvals: most recent 494–16; 240–12; 04154; 166/01).
Clinical evaluation of every patient was performed before and 3 months after PVI including medical history, EHRA score, physical examination, blood collection, echocardiography, ECG, and Holter ECG. AF freedom was assessed by 24 hour/7 day Holter ECG or clinical AF-related symptoms (e.g. palpitations, tachyarrhythmia) three month after PVI. AF recurrence was defined as any atrial tachyarrhythmia lasting >30 seconds documented by ECG [17].
Blood collection
Blood was collected via a direct venous puncture into 9 ml EDTA tubes (Sarstedt Monovette) and was processed for plasma isolation within 4 hours of collection. Blood was processed by spinning at 4000 rpm for 20 minutes at room temperature. Plasma was carefully transferred to a fresh RNAse/DNAse free tube and stored at -80°C.
PVI procedure
Ablation was performed using a standardized clinical protocol (circumferential isolation of all pulmonary veins without additional ablation lines or CFAE ablation) as previously described [18]. In brief, all patients received sedation with a benzodiazepine and local anaesthesia. Via Seldinger technique three 8F sheaths were inserted into the femoral vein (coronary sinus (CS) catheter, ablation catheter and lasso catheter). After placement of all catheters and transseptal puncture anatomic visualisation was performed by angiography and CARTO 3D Mapping. PV potentials were measured by inserting the lasso catheter in each pulmonary vein. Then electrical PVI was performed using circumferential radiofrequency energy point-by-point ablation at the ostial site of each PV until achieving the procedural endpoint of electrical isolation of the PVs documented by entrance block. Electrical entrance block between the PV and left atrium and exit block if applicable was re-evaluated after a 30min waiting period confirming the procedural success.
Biochemical assay
The plasma concentrations of Endothelin-1, MCP-1, and CGA were measured by a duoset enzyme linked immunosorbent assay (ELISA) (ET 1 and MCP: R&D System GmbH, Wiesbaden, Germany; CGA: IBL International, Hamburg, Germany).
Statistical analysis
Statistical analysis was performed with SPSS 21. For not normally distributed variables we performed Wilcoxon Test for paired variables and Mann-Whitney-U test for non-paired variables. For dichotomous variables we used Fisher-Yates test. All values mentioned in the results section are medians unless otherwise listed. In addition, linear regression analysis adjusting for age, sex, diabetes, creatinine, hypertension and coronary artery disease was performed for Entothelin-1 baseline levels. Results are presented using box-plots showing median as well as interquartile ranges. A p value lower than 0.05 was considered statistically significant.
Ethics statement
The study was performed on blood samples that were collected in a general biobanking effort that was continuously approved by the hospital’s ethics committee of the University of Munich (LMU) and the Ethics Commission of the State Chamber of Medicine in Bavaria (BLAEK), Germany over the last years (reference numbers of the ethics approvals: most recent 494–16; 240–12; 04154; 166/01). All procedures were performed according to the 1975 Declaration of Helsinki. Every participant provided written informed consent before participation, all samples were anonymized and processed according to the guidelines the local ethics committee of the University of Munich (LMU), Germany.
Results
Patient characteristics
In total, we included 96 AF patients in our study (Table 1). Within the AF population mean age was 61.8±10.9 years and 60.4% of the patients were male. 71.9% of the patients had paroxysmal AF, 28.1% had persistent AF. 81.2% of the patients treated by ablation showed no signs for AF recurrence three months after PVI. In addition, 40 age- and sex-matched healthy volunteers were included for baseline comparison of blood levels.
Table 1. Patient characteristics.
| AF study population | Healthy volunteers | p value | |
|---|---|---|---|
| Number of patients | 96 | 40 | |
| Demographics | |||
| age (years) | 61.8±10.9 | 60.2±12.58 | 0.52 |
| male sex (n, %) | 58 (60.4%) | 21 (52.5%) | 0.45 |
| Type of AF | |||
| paroxysmal AF (n, %) | 69 (71.9%) | ||
| persistent AF (n, %) | 27 (28.1%) | ||
| Cardiovascular Risk Factors | |||
| Hypertension (n, %) | 64 (66.7%) | ||
| Diabetes mellitus (n, %) | 6 (6.3%) | ||
| Hypercholesterolemia (n, %) | 15 (15.6%) | ||
| Concomittant diseases | |||
| Coronary Artery Disease (n, %) | 19 (19.8%) | ||
| Renal impairment (GFR<60ml/min; n, %) | 24 (25%) | ||
| Previous antiarrhythmic treatment | |||
| Ablation | 10 (10.4%) | ||
| Drug Treatment | 96 (100%) | ||
| Class I antiarrhythmic drugs | 36 (37.5%) | ||
| Class III antiarrhythmic drugs | 20 (20.8%) | ||
| Echocardiographic parameters | |||
| LA diameter (PLAX, mm) | 42±6.5 | ||
| Ejection fraction (%) | 61.5±7.7 |
For a further analysis we separated our AF patient cohort into two groups: 1) patients with recurrence of AF and 2) patients without recurrence of AF three months after ablation (Table 2). Both groups did not differ significantly regarding age (p = 0.95), sex (p = 1.00), type of AF (p = 0.95), previous ablation (p = 0.92), CAD (p = 0.7), or pre-treatment with antiarrhythmic drugs (p = 1.00).
Table 2. Clinical characteristics of patients with and without AF recurrence.
| AF recurrence | No AF recurrence | p-value | |
|---|---|---|---|
| Number of patients | 18 (18.8%) | 78 (81.2%) | |
| Demographics | |||
| age (years) | 62.6±10.3 | 61.8±11.1 | p = 0.95 |
| male sex (n, %) | 11 (61.1%) | 47 (60.3%) | p = 1.00 |
| Type of AF | |||
| paroxysmal AF (n, %) | 13 (72.2%) | 57 (73.1%) | p = 0.95 |
| persistent AF (n, %) | 5 (26.8%) | 21 (26.9%) | p = 0.95 |
| Cardiovascular Risk Factors | |||
| Hypertension (n, %) | 14 (77.8%) | 50 (64.1%) | p = 0.22 |
| Diabetes mellitus (n, %) | 1 (5.6%) | 5 (6.4%) | p = 0.89 |
| Hypercholesterolemia (n, %) | 3 (16.7%) | 12 (15.4%) | p = 0.89 |
| Concomitant diseases | |||
| Coronary Artery Disease (n, %) | 3 (16.7%) | 16 (20.5%) | p = 0.7 |
| Renal impairment (GFR<60ml/min; n, %) | 6 (33.3%) | 18 (23.1%) | p = 0.4 |
| Previous antiarrhythmic treatment | |||
| Ablation | 2 (11.1%) | 8 (10.2%) | p = 0.92 |
| Drug Treatment | 18 (100%) | 78 (100%) | p = 1.00 |
| Class I antiarrhythmic drugs | 5 (27.8%) | 31 (39.7%) | p = 0.32 |
| Class III antiarrhythmic drugs | 4 (22.2%) | 16 (20.5%) | p = 0.87 |
| Echocardiographic parameters | |||
| LA diameter (PLAX, mm) | 42±7 | 43±5 | p = 0.34 |
| Ejection fraction (%) | 58±12.7 | 62±6 | p = 0.37 |
Endothelin-1 (ET-1) plasma levels
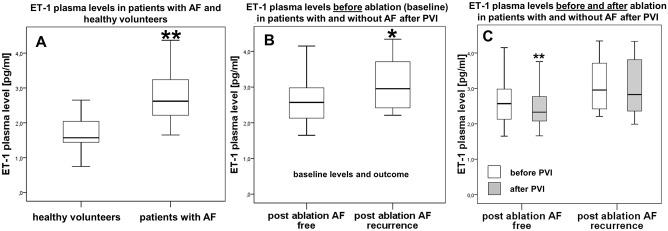
To evaluate Endothelin-1 (ET-1) as a potential biomarker for AF we measured ET-1 plasma levels at baseline in patients with AF in comparison to healthy volunteers without AF. Patients with AF showed significantly higher levels of ET-1 compared to age- and sex-matched healthy volunteers without history of AF (2.62 pg/ml vs. 1.57 pg/ml; p<0.001; Fig 1-A).
Fig 1. Plasma levels of Endothelin-1 [pg/ml].
A, Healthy reference group without AF (left) vs. all AF study patients (right). B, Baseline Endothelin-1 levels in patients without (left) and with AF recurrence (right) three months after ablation. C, Endothelin-1 plasma levels before and after PVI in patients without (left) and patients with AF recurrence (right). Baseline levels presented in white bars, levels three months after ablation presented in grey bars. * p<0.05, ** p<0.01.
Further analysis within the AF ablation cohort with respect to AF recurrence revealed that a significantly lower level of ET-1 prior ablation was associated with freedom of AF in the follow up period of 3 month (2.57 pg/ml vs. 2.96 pg/ml; p = 0.02; Fig 1-B). A regression analysis adjusting for age, sex, diabetes, creatinine, hypertension and coronary artery disease could confirm this statistically significant difference (p = 0.029). Patients without AF recurrence demonstrated a further decrease of ET-1 levels three months after ablation getting closer to the level of the healthy volunteers (2.33 pg/ml vs. 2.57 pg/ml; p<0.01) whereas ET-1 levels in patients with AF recurrence remained unchanged at an elevated level (2.83 pg/ml vs. 2.96 pg/ml; p = 0.09; Fig 1-C).
MCP-1 plasma levels
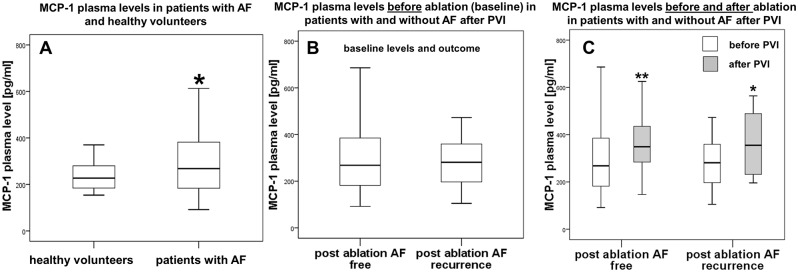
Next, we measured MCP-1 and found significantly increased plasma levels in AF patients compared to healthy controls (268 pg/ml vs. 227 pg/ml; p = 0.03; Fig 2-A). However, MCP-1 baseline levels did not differ between patients with or without AF recurrence three months after PVI (281 pg/ml vs. 268 pg/ml; p = 0.86; Fig 2-B). In both groups MCP-1 plasma levels increased significantly after ablation independent form AF recurrence (patients without recurrence: 268 pg/ml vs. 349 pg/ml; Δ 81 pg/ml; p<0.01; patients with recurrence: 281 pg/ml vs. 355 pg/ml; Δ74 pg/ml; p = 0.03; Fig 2-C).
Fig 2. Plasma levels of MCP-1 [pg/ml].
A, Healthy reference group without AF (left) vs. all AF study patients (right). B, Baseline MCP-1 levels in patients without (left) and with AF recurrence (right) three months after ablation. C, MCP-1 plasma levels before and after PVI in patients without (left) and patients with AF recurrence (right). Baseline levels presented in white bars, levels three months after ablation presented in grey bars. * p<0.05, ** p<0.01.
Chromogranin A (CGA) plasma levels
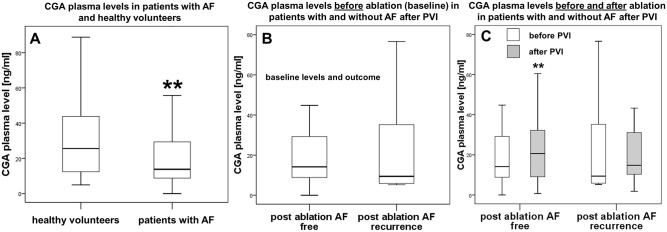
Patients with AF showed significantly lower plasma levels of CGA compared to our reference group (13.8 ng/ml vs. 25.6 ng/ml; p<0.01; Fig 3-A). We did not observe significant differences at baseline between patients with or without later AF recurrence (14.2 ng/ml vs. 9.4 ng/ml; p = 0.42; Fig 3-B). After three months CGA levels increased significantly only in patients without recurrence towards the level of healthy controls (14.2 ng/ml vs. 20.7 ng/ml; p = 0.008) but not in patients with recurrence (9.4 ng/ml vs. 14.8 ng/ml; p = 0.25; Fig 3-C).
Fig 3. Plasma levels of CGA [ng/ml].
A, Healthy reference group without AF (left) vs. all AF study patients (right). B, Baseline CGA levels in patients without (left) and with AF recurrence (right) three months after ablation. C, CGA plasma levels before and after PVI in patients without (left) and patients with AF recurrence (right). Baseline levels presented in white bars, levels three months after ablation presented in grey bars. * p<0.05, ** p<0.01.
Discussion
In our current study we evaluated several blood markers of stress as potential biomarkers for atrial fibrillation and the association to AF burden/recurrence after an ablation therapy. The major findings of the present study were: (1) Patients with AF show marked differences with increased levels of ET-1 and MCP-1 and reduced levels of CGA compared to healthy controls; (2) in AF patients higher ET-1 levels were associated with AF recurrence three months after PVI; (3) freedom from AF after ablation was associated with a significant decrease of ET-1 and a significant increase of CGA three months after ablation compared to baseline.
Endothelin-1
Our study shows that patients with AF have higher levels of Endothelin-1 compared to healthy controls without AF. This finding supports our hypothesis of a stress associated arrhythmia. However, previous reports about the relationship between ET-1 and AF are inconsistent. Some studies demonstrated elevated levels of ET-1 and proposed ET-1 being involved in remodelling processes and atrial dilatation [1,19]. In contrast, some clinical studies failed to show altered levels of ET-1 in patients with AF [20,21]. An examination of patients with AF receiving maze procedure could show that these patients show an increased content of ET-1 in left atrial appendage tissue correlating, among others, with atrial size and AF persistence [19]. Our findings suggest ET-1 as a predictive biomarker for the occurrence of AF after the ablation therapy. This is further supported by two reports by Nakazawa et al. and Wang et al. [1,22]. However, none of these studies showed a decrease of ET-1 after restoration of sinus rhythm as it is demonstrated by our study.
ET-1 has been shown to be associated with atrial structural [19] and electrical remodelling, e.g., by impairing calcium homeostasis [23]. Therefore, our findings could be in line with described effects of ET-1 in non-ischemic ventricular arrhythmia suggesting a direct pro-arrhythmic effect [24–26]. Nevertheless, in the context of our study it remains unclear if the altered ET-1 changes are actually a causal factor for AF or just a surrogate marker of a reduced stress level based on an arrhythmia free interval.
MCP-1
Clinical trials have shown increased serum levels of MCP-1 in stress related cardiovascular disease [11]. In our study, patients with AF had higher levels of MCP-1 than healthy controls supporting our hypothesis that AF and stress might be correlating factors. Based on this hypothesis, treated patients without AF recurrence in the follow up period should present reduced MCP-1 levels. However, in our study we could demonstrate an increase after ablation in all treated patients independent from AF recurrence. A potential explanation for this is the also known proinflammatory effect of MCP-1 and its role in wound healing [27–29]. The goal of a PVI is actually to generate a localized scarring to achieve an electrical isolation of the pulmonary veins. Increased levels of MCP-1 could therefore be an indicator for an inflammatory and pro-fibrotic signalling as a response to the ablation procedure itself. Taken the fact that MCP-1 increase was, in contrast to ET-1, independent from AF burden after ablation, it could be hypothesised that MCP-1 change is likely to be more driven by the inflammatory response rather than by changes in stress levels. Furthermore, taken the observation that MCP-1 levels are higher in AF patients compared to healthy controls but failure to show an association with AF after ablation might also be interpreted as MCP-1 and AF being both related to stress as a common underlying factor but are not associated to each other in direct relationship.
Chromogranin A
Circulating levels of CGA are influenced by cardiovascular diseases, which are also known risk factors of AF such as hypertension, heart failure, or acute coronary syndrome [4,30–32].
Nevertheless, no data is available on CGA in the context of AF. In our study we measured lower levels of CGA in patients with AF, a finding that does not directly support our hypothesis as AF being stress-related or vice versa. Given that the adrenergic system has huge impact on the development of AF [33], we expected higher levels of CGA as a surrogate for increased sympathoadrenergic tone. However, studies have also shown the importance of a balanced sympathetic/parasympathetic system since an increased vagal tone can also cause AF, that could at least partly explain the effects of ganglionated plexi ablation [34–39]. Low levels of CGA might be an indicator of a dysbalanced autonomic tone with an increased vagal tone and therefore an indicator or the causal fact of AF. There are data about patients treated with cryoballoon as well as radiofrequency ablation and additional ganglionic plexi modification (decreasing vagal tone) showing better rates of AF freedom [36,40–45]. This might be very speculative; however, this vagal modification resulting in a decreased tone of parasympathetic nervous system might thereby also translate in an increase of CGA (as a marker of sympathetic activity) after ablation. This would also explain the time course of CGA in our study. Patients without AF recurrence showed an increase of CGA (restoration of a balanced autonomic signalling) whereas patients with AF recurrence did not show a significant increase of CGA levels.
Clinical implications and future perspective
Current success rates for PVI are reported with 60–70% after one year [18,46]. General use of more complex ablation strategies like additional ablation lines or CFAE mapping failed to improve success rates [47]. As a consequence individually tailored approaches of ablation seem necessary. Individual mechanisms like stress-related AF, vagal AF or obesity have to be considered to choose the optimal treatment. Our study findings support ET-1, MCP-1 and CGA as promising novel biomarkers for AF. Thus, they may help guiding the individual therapy and improving success rates in addition to other variables like MRI, genetic analysis, etc. [48–50]. The results of our study are hypothesis generating suggesting ET-1 actually being a marker for AF, MCP-1 might be stress- but not AF-related, while CGA being inversely related with AF speculating a role of vagal tone. Nevertheless, further studies dealing with the detailed role of these biomarkers are required to elucidate their value in a clinical setting.
Limitations
A main limitation of our study is the short follow-up period of three months. In our study about 80% of our patients were still in sinus rhythm three months after ablation and a longer follow-up period might identify more patients with later AF recurrence. Our study results therefore only allow to draw conclusions regarding a short-term recurrence of AF or restoration of sinus rhythm but not a long-term ablation success.
To detect AF recurrence we used 24h Holter ECG recordings and patients’ reports on AF related symptoms as recommended by current guidelines. However, using those assessments we cannot detect AF recurrence in 100%, especially in case of asymptomatic episodes. The only alternative are implantable loop recorders that cannot be implanted routinely in all patients at the moment. Nevertheless, we think that this approach is acceptable for the purpose of our study, to evaluate stress biomarkers in the context of AF symptoms.
Furthermore, our study evaluated a heterogeneous population including patients with paroxysmal as well as persistent AF. Due to small sample sizes (especially in the persistent AF group) no separate analysis was possible. As a consequence we cannot rule out different biomarker patterns between these two entities of AF.
Further studies are necessary to put our findings in the context of a long-term follow-up and to explain the underlying pathophysiology. As an independent replication of our findings in a larger cohort is needed, our current study has to be called hypothesis generating potentially guiding strategies for future studies.
Nevertheless, to the best of our knowledge our study is the first to provide insights into the relationship and the absolute values of these stress markers to the presence or absence of AF before and after an ablation therapy as well as in comparison to a healthy control cohort.
Conclusion
In this study we could provide evidence that established stress-related biomarkers ET-1, MCP-1, CGA were differentially regulated among patients with AF compared to healthy controls. Furthermore, the investigated biomarker levels before and three months after an ablation therapy revealed a direct association of ET-1 levels with respect to presence or absence of AF, and an inverse relationship of CGA levels regarding the presence of AF. These data provide new insight for the role of stress-related biomarkers in AF pathophysiology as well as potential biomarker for AF ablation therapy. However, larger, randomized, controlled clinical trials are necessary to finally prove clinical significance of those biomarkers and potentially allow to identify subgroups of patients at higher risk that might benefit from alternative ablation strategies like additional ganglionated plexi modification.
Supporting information
GFR, glomerular filtration rate; LA, left atrium.
(XLSX)
Acknowledgments
Dr. Clauss was supported by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme (PIOF-GA-2012-328352). Dres. Clauss, Wakili and Kääb were supported by the German Centre for Cardiovascular Research (DZHK; 81X2600210, 81X2600204). Dres. Wakili and Kääb received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 633193 (CATCH ME).
Abbreviations
- AF
atrial fibrillation
- CAD
Coronary Artery Disease
- CFAE
complex fractionated atrial electrograms
- CGA
Chromogranin A
- ET-1
Endothelin 1
- GFR
glomerular filtration rate
- MCP-1
monocyte chemotactic protein-1
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B-cells
- PLAX
parasternal long axis
- PVI
pulmonary vein isolation
- TNFα
tumor necrosis factor alpha
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Dr. Clauss was supported by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme (PIOF-GA-2012-328352). Dres. Clauss, Wakili and Kääb were supported by the German Centre for Cardiovascular Research (DZHK; 81X2600210, 81X2600204). Dres. Wakili and Kääb received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 633193 (CATCH ME). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wang H, Liu J, Fang P, Lei S, Li X, Hou Y, et al. (2012) Big endothelin-1 as a predictor of atrial fibrillation recurrence after primary ablation only in patients with paroxysmal atrial fibrillation. Herz 37: 919–925. doi: 10.1007/s00059-012-3626-9 [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33: 2719–2747. doi: 10.1093/eurheartj/ehs253 [DOI] [PubMed] [Google Scholar]
- 3.Nattel S (2002) New ideas about atrial fibrillation 50 years on. Nature 415: 219–226. doi: 10.1038/415219a [DOI] [PubMed] [Google Scholar]
- 4.Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S (2011) Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 121: 2955–2968. doi: 10.1172/JCI46315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burg MM, Soufer A, Lampert R, Collins D, Soufer R (2011) Autonomic contribution to endothelin-1 increase during laboratory anger-recall stress in patients with coronary artery disease. Mol Med 17: 495–501. doi: 10.2119/molmed.2010.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson A, Madsen-Hardig B, Olsson SB (2004) Arrhythmia-provoking factors and symptoms at the onset of paroxysmal atrial fibrillation: a study based on interviews with 100 patients seeking hospital assistance. BMC Cardiovasc Disord 4: 13 doi: 10.1186/1471-2261-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maryniak A, Walczak F, Bodalski R, Szumowski L, Derejko P, Urbanek P, et al. (2006) Atrial fibrillation onset circumstances and their relation to patients' quality of life. Kardiol Pol 64: 1102–1108; discussion 1109. [PubMed] [Google Scholar]
- 8.Mattioli AV, Bonatti S, Zennaro M, Mattioli G (2005) The relationship between personality, socio-economic factors, acute life stress and the development, spontaneous conversion and recurrences of acute lone atrial fibrillation. Europace 7: 211–220. doi: 10.1016/j.eupc.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 9.Schönfelder A RP, Zerm T (2006) Ursachen und medikamentöse Therapie von Vorhofflimmern und Vorhofflattern. Schweiz Med Forum: 45–153. [Google Scholar]
- 10.Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Volker C, et al. (2008) Cardiovascular events during World Cup soccer. N Engl J Med 358: 475–483. doi: 10.1056/NEJMoa0707427 [DOI] [PubMed] [Google Scholar]
- 11.Wilbert-Lampen U, Nickel T, Leistner D, Guthlin D, Matis T, Volker C, et al. (2010) Modified serum profiles of inflammatory and vasoconstrictive factors in patients with emotional stress-induced acute coronary syndrome during World Cup Soccer 2006. J Am Coll Cardiol 55: 637–642. doi: 10.1016/j.jacc.2009.07.073 [DOI] [PubMed] [Google Scholar]
- 12.Barton M, Yanagisawa M (2008) Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol 86: 485–498. doi: 10.1139/Y08-059 [DOI] [PubMed] [Google Scholar]
- 13.Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL (2009) Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol 41: 998–1001. doi: 10.1016/j.biocel.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 14.Di Comite G, Morganti A (2011) Chromogranin A: a novel factor acting at the cross road between the neuroendocrine and the cardiovascular systems. J Hypertens 29: 409–414. doi: 10.1097/HJH.0b013e328341a429 [DOI] [PubMed] [Google Scholar]
- 15.Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M (2010) Chromogranin A—biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol 17: 2427–2443. doi: 10.1245/s10434-010-1006-3 [DOI] [PubMed] [Google Scholar]
- 16.Nickel T, Vogeser M, Emslander I, David R, Heilmeier B, Op den Winkel M, et al. (2012) Extreme exercise enhances chromogranin A levels correlating with stress levels but not with cardiac burden. Atherosclerosis 220: 219–222. doi: 10.1016/j.atherosclerosis.2011.09.036 [DOI] [PubMed] [Google Scholar]
- 17.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. (2012) 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 9: 632–696.e621. doi: 10.1016/j.hrthm.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 18.Wakili R, Clauss S, Schmidt V, Ulbrich M, Hahnefeld A, Schussler F, et al. (2014) Impact of real-time contact force and impedance measurement in pulmonary vein isolation procedures for treatment of atrial fibrillation. Clin Res Cardiol 103: 97–106. doi: 10.1007/s00392-013-0625-7 [DOI] [PubMed] [Google Scholar]
- 19.Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, et al. (2010) Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol 3: 369–379. doi: 10.1161/CIRCEP.109.924985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wozakowska-Kaplon B, Bartkowiak R, Janiszewska G, Grabowska U (2010) Does atrial fibrillation affect plasma endothelin level? Cardiol J 17: 471–476. [PubMed] [Google Scholar]
- 21.Lewicka E, Dudzinska-Gehrmann J, Dabrowska-Kugacka A, Zagozdzon P, Kozlowski D, Raczak G (2014) Neurohumoral factors as markers of recurrence of atrial fibrillation examined in patients with dual-chamber pacemaker. Int J Cardiol. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa Y, Ashihara T, Tsutamoto T, Ito M, Horie M (2009) Endothelin-1 as a predictor of atrial fibrillation recurrence after pulmonary vein isolation. Heart Rhythm 6: 725–730. doi: 10.1016/j.hrthm.2009.02.027 [DOI] [PubMed] [Google Scholar]
- 23.Endoh M, Fujita S, Yang HT, Talukder MA, Maruya J, Norota I (1998) Endothelin: receptor subtypes, signal transduction, regulation of Ca2+ transients and contractility in rabbit ventricular myocardium. Life Sci 62: 1485–1489. [DOI] [PubMed] [Google Scholar]
- 24.Yorikane R, Koike H, Miyake S (1991) Electrophysiological effects of endothelin-1 on canine myocardial cells. J Cardiovasc Pharmacol 17 Suppl 7: S159–162. [DOI] [PubMed] [Google Scholar]
- 25.Merkely B, Kiss O, Vago H, Zima E, Szabo T, Geller L (2000) Arrhythmogenic action of endothelin-1. Cardiovasc Res 48: 357–358. [DOI] [PubMed] [Google Scholar]
- 26.Merkely B, Szabo T, Geller L, Kiss O, Horkay F, Raschack M, et al. (2000) The selective endothelin-A-receptor antagonist LU 135.252 inhibits the direct arrhythmogenic action of endothelin-1. J Cardiovasc Pharmacol 36: S314–316. [DOI] [PubMed] [Google Scholar]
- 27.Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R (1998) Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 153: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trautmann A, Toksoy A, Engelhardt E, Brocker EB, Gillitzer R (2000) Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol 190: 100–106. doi: 10.1002/(SICI)1096-9896(200001)190:1<100::AID-PATH496>3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- 29.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, et al. (2001) Wound healing in MIP-1alpha(-/-) and MCP-1(-/-) mice. Am J Pathol 159: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, et al. (2002) Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J 23: 967–974. doi: 10.1053/euhj.2001.2977 [DOI] [PubMed] [Google Scholar]
- 31.Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, et al. (2007) Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J 28: 1117–1127. doi: 10.1093/eurheartj/ehm022 [DOI] [PubMed] [Google Scholar]
- 32.Rosjo H, Masson S, Latini R, Flyvbjerg A, Milani V, La Rovere MT, et al. (2010) Prognostic value of chromogranin A in chronic heart failure: data from the GISSI-Heart Failure trial. Eur J Heart Fail 12: 549–556. doi: 10.1093/eurjhf/hfq055 [DOI] [PubMed] [Google Scholar]
- 33.Workman AJ (2010) Cardiac adrenergic control and atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol 381: 235–249. doi: 10.1007/s00210-009-0474-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coumel P (1994) [Paroxysmal atrial fibrillation: role of autonomic nervous system]. Arch Mal Coeur Vaiss 87 Spec No 3: 55–62. [PubMed] [Google Scholar]
- 35.Coumel P (1994) Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J 15 Suppl A: 9–16. [DOI] [PubMed] [Google Scholar]
- 36.Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, et al. (2013) Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol 62: 2318–2325. doi: 10.1016/j.jacc.2013.06.053 [DOI] [PubMed] [Google Scholar]
- 37.Katritsis GD, Katritsis DG (2014) Cardiac Autonomic Denervation for Ablation of Atrial Fibrillation. Arrhythm Electrophysiol Rev 3: 113–115. doi: 10.15420/aer.2014.3.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurotobi T, Shimada Y, Kino N, Ito K, Tonomura D, Yano K, et al. (2015) Features of intrinsic ganglionated plexi in both atria after extensive pulmonary isolation and their clinical significance after catheter ablation in patients with atrial fibrillation. Heart Rhythm 12: 470–476. doi: 10.1016/j.hrthm.2014.11.033 [DOI] [PubMed] [Google Scholar]
- 39.Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ (2011) Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm 8: 672–678. doi: 10.1016/j.hrthm.2010.12.047 [DOI] [PubMed] [Google Scholar]
- 40.Sarah Worsnick P, Angela Naperkowski, RN, Danielle E., Donald C, Faiz Subzposh, MD, Gopi Dandamudi, MD, FHRS, and Pugazhendhi Vijayaraman M, FHRS. Geisinger Heart, Institute WB, PA, Geisinger Heart Institute, Wilkes-, Barre P, Geisinger Health System, Geisinger Wyoming Valley, Medical Center W-B, PA (2016) Cryoballon ablation of pulmonary veins: Ganglionic plexi modufication and AF recurrence Heart Rhythm. 5 ed. pp. S48.
- 41.Miyazaki S, Nakamura H, Taniguchi H, Hachiya H, Ichihara N, Takagi T, et al. (2016) Impact of the order of the targeted pulmonary vein on the vagal response during second-generation cryoballoon ablation. Heart Rhythm 13: 1010–1017. doi: 10.1016/j.hrthm.2015.12.031 [DOI] [PubMed] [Google Scholar]
- 42.Pugazhendhi Vijayaraman SAW, Danielle E Donald, Angela Naperkowski (2016) Acute effects of cryoballoon ablation on sinoatrial and atrioventricular node function: vagal modulation by anterior right ganglionic plexi ablation Journal of Interventional Cardiac Electrophysiology 45: 246. [Google Scholar]
- 43.Xu FQ, Yu RH, Guo JJ, Bai R, Liu N, An YI, et al. (2017) Catheter Ablation of Recurrent Paroxysmal Atrial Fibrillation: Is Gap-Closure Combining Ganglionated Plexi Ablation More Effective? Pacing Clin Electrophysiol. [DOI] [PubMed] [Google Scholar]
- 44.Afzal MR, Samanta A, Chatta J, Ansari B, Atherton S, Sabzwari S, et al. (2017) Adjunctive ablation strategies improve the efficacy of pulmonary vein isolation in non-paroxysmal atrial fibrillation: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther 15: 227–235. doi: 10.1080/14779072.2017.1294064 [DOI] [PubMed] [Google Scholar]
- 45.Qin M, Liu X, Wu SH, Zhang XD (2016) Atrial Substrate Modification in Atrial Fibrillation: Targeting GP or CFAE? Evidence from Meta-Analysis of Clinical Trials. PLoS One 11: e0164989 doi: 10.1371/journal.pone.0164989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakili R, Siebermair J, Fichtner S, Sinner MF, Klocker E, Olesch L, et al. (2016) One-year clinical outcome after ablation with a novel multipolar irrigated ablation catheter for treatment of atrial fibrillation: potential implications for clinical use. Europace. [DOI] [PubMed] [Google Scholar]
- 47.Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. (2015) Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372: 1812–1822. doi: 10.1056/NEJMoa1408288 [DOI] [PubMed] [Google Scholar]
- 48.Lau DH, Schotten U, Mahajan R, Antic NA, Hatem SN, Pathak RK, et al. (2016) Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J 37: 1573–1581. doi: 10.1093/eurheartj/ehv375 [DOI] [PubMed] [Google Scholar]
- 49.Fabritz L, Guasch E, Antoniades C, Bardinet I, Benninger G, Betts TR, et al. (2016) Expert consensus document: Defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol 13: 230–237. doi: 10.1038/nrcardio.2015.194 [DOI] [PubMed] [Google Scholar]
- 50.Lyman GH, Moses HL (2016) Biomarker Tests for Molecularly Targeted Therapies—The Key to Unlocking Precision Medicine. N Engl J Med 375: 4–6. doi: 10.1056/NEJMp1604033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GFR, glomerular filtration rate; LA, left atrium.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.