Abstract
Contrary to the many whole genome duplication events recorded for angiosperms (flowering plants), whole genome duplications in gymnosperms (non-flowering seed plants) seem to be much rarer. Although ancient whole genome duplications have been reported for most gymnosperm lineages as well, some are still contested and need to be confirmed. For instance, data for ginkgo, but particularly cycads have remained inconclusive so far, likely due to the quality of the data available and flaws in the analysis. We extracted and sequenced RNA from both the cycad Encephalartos natalensis and Ginkgo biloba. This was followed by transcriptome assembly, after which these data were used to build paralog age distributions. Based on these distributions, we identified remnants of an ancient whole genome duplication in both cycads and ginkgo. The most parsimonious explanation would be that this whole genome duplication event was shared between both species and had occurred prior to their divergence, about 300 million years ago.
Introduction
Whole genome duplications (WGDs) have been prevalent during the evolutionary history of flowering plants, and have even been linked to their origin as well as their fast rise to ecological dominance [1–3]. Furthermore, although the duplication of entire genomes is mostly regarded as an evolutionary dead-end [4–7], it has been proposed that, in times of rapid environmental change, WGDs can confer an important evolutionary advantage [8–11]. This is, for instance, suggested by the fact that many angiosperm lineages show evidence for independent WGD events around the Cretaceous-Paleogene (K-Pg) extinction ~66 million years ago (Mya) [11, 12].
Contrary to the many WGD events recorded for angiosperms, the history of the non-flowering gymnosperms paints a very different picture. Although far fewer gymnosperm species exist today compared to the angiosperms, and as such many lineages containing evidence for WGD events could have been lost, polyploidy events, ancient or more recent, in these seed plants seem rare. Thus far, Welwitschia mirabilis is the only gymnosperm showing evidence for a relatively recent WGD event [13, 14], possibly also overlapping the K-Pg boundary. In any case, this event occurred more recently than the divergence of Welwitschia from its closest relative, Gnetum (135–110 Mya) [15–17], the genome of which shows no sign of a WGD [13]. Furthermore, Ephedra, the third Gnetales genus, also lacks evidence of WGD events [13], excluding very recent duplication events that resulted in the widespread polyploidy seen in extant species of this genus [18–20]. Li et al. [13] also provided evidence for independent ancient WGDs in the conifer lineage that may have coincided with the more ancient Permian-Triassic boundary, ~250 Mya. Similarly, as with the angiosperms, these conifer-specific WGDs might have contributed to the survival and success of the conifer lineage during periods of drastic environmental change [13]. The same study found evidence for an ancient WGD in the Ginkgo lineage, attributing it to the ancient WGD event proposedly shared by all seed plants [1]. Clear remnants of WGDs in cycads were not uncovered, likely due to the dearth of available public EST data [21], resulting in insufficient resolution to call an ancient WGD event in this lineage.
The cycads were widespread during the Jurassic–Cretaceous, reaching their greatest diversity ~200–65 Mya [22–24]. Today, however, only a mere 348 extant species in ten genera remain [25]. The dramatic decrease in diversity was likely due to challenges such as at least three mass extinction events, as well as the arrival of, and major competition from, the angiosperms. Although the lineage itself dates back ~270 million years, most extant cycad species originated much more recently, most likely within the past 65 million years [22, 26, 27]. Therefore, the popular referral to cycads as living fossils is not entirely accurate, as the lineage itself is ancient but most species originated relatively recently. Their continued survival is somewhat paradoxical, as they have particularly slow growth and cannot compete with the fast growing, rather short-lived angiosperms. Here, we confirm that cycads have undergone an ancient WGD and show that this event was likely shared with Ginkgo biloba, preceding the divergence of these lineages.
Results and discussion
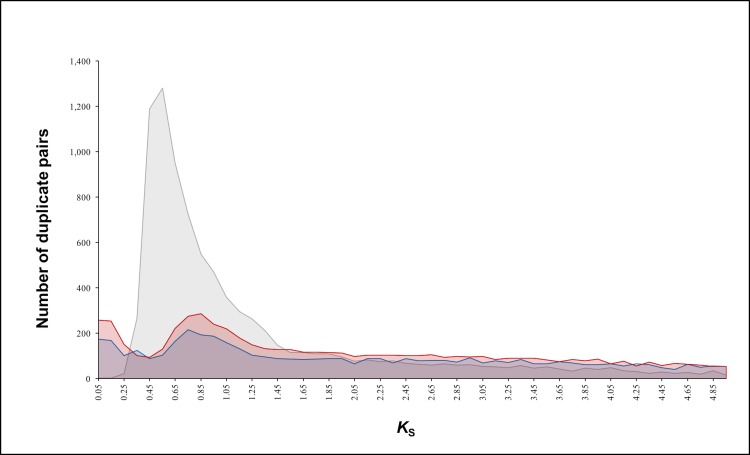
We sequenced transcriptome data from two tissues (see Materials and Methods) of representatives of both Encephalartos natalensis (a native cycad species from the Kwazulu-Natal province of South Africa) and Ginkgo biloba, and assembled high quality low-redundancy transcriptomes [28]. The E. natalensis and G. biloba assemblies contained 22,204 and 23,845 transcripts with average sequence lengths of 1,097 and 1,259 bases and average GC contents of 44.23 and 42.52%, respectively. Based on age distributions of paralogs inferred from synonymous substitutions per synonymous site, or so-called KS distributions [29], a distinct peak with a median KS of ~0.8 was identified for E. natalensis (Fig 1), a clear signature of an ancient WGD event. G. biloba showed a similar KS distribution, and also contained a peak at a KS of ~0.8 (Fig 1). This distribution is consistent with data reporting the presence of a WGD event in the evolutionary history of ginkgo [13]. Since the KS peaks for both E. natalensis and G. biloba were at similar KS values (Fig 1), this could suggest either a similar timeframe for both WGDs or a shared WGD event in the ancestor of the two lineages.
Fig 1. KS age distributions for Ginkgo biloba and Encephalartos natalensis transcriptomes.
The red graph represents the age distribution of duplicates (in the transcriptome) of G. biloba, while the blue graph represents the age distribution of duplicates of E. natalensis. The graph in grey denotes the KS distribution for one-to-one orthologs of G. biloba and E. natalensis.
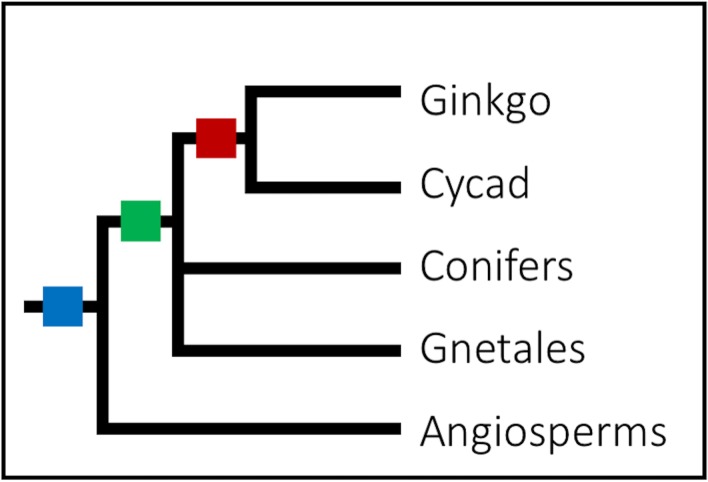
We built a KS age distribution of one-to-one orthologs between E. natalensis and G. biloba to investigate whether the duplication peaks identified in both indicated a shared WGD. Assuming similar substitution rates in the two lineages [30], the time of speciation seems to be slightly younger than the polyploidy events in both G. biloba and E. natalensis (Fig 1). Cycad and ginkgo are thought to have diverged from one another, and from the other gymnosperms, ~330–270 Mya [16, 17, 27, 31, 32]. Although several studies suggest that cycads diverged earliest during gymnosperm evolution, with the ginkgo lineage following not long thereafter [16, 26, 31–33], others show cycads and ginkgo as having diverged from a common ancestor [17, 34–37] (Fig 2). In either case, the more parsimonious explanation would be to assume that the duplication peaks observed in both lineages represent a shared event, rather than independent ancient duplications that have occurred early in the evolution of both lineages. However, the correct phylogenetic placement of this WGD event is uncertain. The WGD might represent the ancient seed-plant-specific WGD assumed to have occurred ~340 Mya [1], but could also be gymnosperm-specific or nested within the gymnosperms (Fig 2) [38].
Fig 2. Possible locations for the whole genome duplication uncovered for Encephalartos natalensis and Ginkgo biloba.
Cycad and ginkgo share a common ancestor and are sister to the ancestor of Gnetales and conifers [17, 30, 34, 35, 37, 39]: the inferred polyploidy event could have occurred in the common ancestor of the cycad and gingko lineages (red square), the gymnosperm ancestor (green square), or in the seed plant ancestor (blue square). Note that the position of Gnetales remains uncertain (see text for details).
Due to the slow substitution rates of most of the gymnosperm taxa [13, 31, 39], these ancient WGD events in the lineages of cycad and ginkgo (Fig 1), and conifers [13] are still discernible as distinct peaks in their KS distributions (Fig 1, Suppl. Fig S3 in [13]). Slow substitution rates result in gradual genomic change, conserving the remnants of ancient events in the genomes of these species. Because these lineages separated from one another hundreds of millions of years ago, absolute dating of these WGD events is challenging, but they likely indicate a shared event in a common ancestor (Fig 2).
It should be noted that recently a draft genome for G. biloba was presented, in which the authors claimed to have found evidence for two WGD events based on the detection of two different peaks in a KS distribution of paralogs [40]. However, we think their KS distributions should be interpreted with caution, since the youngest peak, corresponding to a WGD estimated between 147 and 74 Mya [40], is likely artificial and the result of thresholds used to consider genes as duplicates rather than as identical. Furthermore, the inferred WGD dates as obtained by Guan et al. [40] should be considered unrealistic. For instance, the authors assume the older genome duplication to be between 735–515 Mya, which would predate the origin of land plants. As we have shown in the current study, the WGD in ginkgo is probably only slightly older (0.2 KS) than the divergence between cycads and ginkgo (Fig 1).
If the WGD detected here did not occur in the ancestor of the cycads and ginkgo, but earlier in the gymnosperm evolution (green or blue squares in Fig 2), we would expect to see remnants of this event in the Gnetales. Yet the complete absence of an ancient peak in the Gnetales remains difficult to explain. One reason why such evidence is lacking might be the faster rate of evolution in Gnetales, compared to other gymnosperms [39, 41, 42], resulting in any traces of ancient polyploidies to be lost. On the other hand, the placement of the Gnetales within the gymnosperms remains elusive. While different molecular markers have placed them within the conifer lineage (the ‘Gnepine’ and ‘Gnecup’ hypotheses), sister to the conifers (the ‘Gnetifer’ hypothesis), or sister to all other gymnosperms (the ‘Gnetales-sister’ hypothesis) [30, 34, 36, 43–45], certain morphological traits even place them closer to the angiosperms, or as a basal seed plant [46, 47]. Therefore, if the WGD event detected here indeed represents an ancient gymnosperm or seed-plant WGD (see [38]), the lack of evidence for WGDs in the Gnetales, except the more recent one in Welwitschia, and the difficulty in resolving their exact phylogenetic placement, could suggest an evolutionary history that is different from the other gymnosperms. In conclusion, despite the fact that early seed plant evolution remains problematic with respect to both phylogenetic relationships and relative and absolute dating of WGD events, we here provide conclusive evidence that cycads have also undergone an ancient WGD.
Materials and methods
Materials from Encephalartos natalensis and Ginkgo biloba were obtained from plants grown at the Manie van der Schijff Botanical Garden at the University of Pretoria, South Africa. Leaflets and rachis samples were collected separately from E. natalensis, while mature leaf and stem tissue were sampled from three male G. biloba trees. RNA was extracted using a standard CTAB RNA extraction method [48], followed by a clean-up step using the Qiagen RNeasy Plus Mini Kit. RNA was sent for sequencing at Novogene, China, generating 150-bp libraries.
RNA-seq libraries from these tissues were used to construct de novo transcriptome assemblies for E. natalensis (66,583,315 paired end reads; 20 Gbp) and G. biloba (98,762,732 paired end reads; 29.6 Gbp). The de novo assemblies were constructed with Velvet v1.2.10 [49] followed by Oases v0.2.08 [50] with a k-mer size of 101, retaining only contigs larger than 200 bases. The redundancy of the assemblies was removed with the Evidential gene pipeline and only the primary transcript of each loci was used in further analyses [51].
TransDecoder v.3.0.0 was used to predict coding regions in the transcriptomes of E. natalensis and G. biloba [52], after which the 19,991 and 23,845 longest coding and peptide sequence transcripts were selected for the species, respectively. KS age distributions for E. natalensis and G. biloba were constructed as described previously [29]. Briefly, to construct the paranome an all-against-all BLASTP search was performed of all the longest transcripts with an E-value cutoff of 1 × 10−10, followed by gene family construction and prediction using the mclblastline pipeline (v10-201, http://micans.org/mcl) [53]. Each gene family was aligned using MUSCLE (v3.8.31). To obtain KS estimates for all pairwise comparisons in gene families, maximum likelihood estimation was performed using the CODEML program of the PAML package (v4.4c) [54, 55]. Gene families were then subdivided into subfamilies for which KS estimates between members did not exceed a value of 5. To correct for the redundancy of KS values (a gene family of n members produces n(n–1)/2 pairwise KS estimates for n–1 retained duplication events), a phylogenetic tree was constructed for each subfamily using PhyML [56] under default settings. For each duplication node in the resulting phylogenetic tree, all m KS estimates between the two child clades were added to the KS distribution with a weight of 1/m (where m is the number of KS estimates for a duplication event), so that the weights of all KS estimates for a single duplication event sum up to one. One-to-one orthologous pairs between E. natalensis and G. biloba were created by performing a reciprocal best hit BLASTP analysis of the longest translated transcripts from one species against the other. Valid orthologous pairs were then identified as having at least 30% identity over 150 amino acids.
Accession numbers
Raw reads of both transcriptomes are available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/) under the submission number SUB2337915.
Acknowledgments
The authors thank Jason Samson, Philip Rousseau and the Manie van der Schijff Botanical Gardens of the University of Pretoria for plant material and assisting with sampling. EM acknowledges the UP Research Development Programme (RDP) and the Genomics Research Institute (GRI) for support. YVdP acknowledges the Multidisciplinary Research Partnership ‘Bioinformatics: from nucleotides to networks’ Project (no. 01MR0310W) of Ghent University, and funding from the European Union Seventh Framework Programme (FP7/2007-2013) under European Research Council Advanced Grant Agreement 322739 –DOUBLEUP.
In memory of Philip Rousseau.
Data Availability
Raw reads of both transcriptomes are available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/) under the submission number SUB2337915.
Funding Statement
The authors acknowledge the UP Research Development Programme (RDP), the Genomics Research Institute (GRI), Multidisciplinary Research Partnership ‘Bioinformatics: from nucleotides to networks’ Project (no. 01MR0310W) of Ghent University, and funding from the European Union Seventh Framework Programme (FP7/2007-2013) under European Research Council Advanced Grant Agreement 322739 – DOUBLEUP for support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473(7345):97–100. doi: 10.1038/nature09916 [DOI] [PubMed] [Google Scholar]
- 2.De Bodt S, Maere S, Van de Peer Y. Genome duplication and the origin of angiosperms. Trends Ecology Evolution. 2005;20(11):591–7. doi: 10.1016/j.tree.2005.07.008 . [DOI] [PubMed] [Google Scholar]
- 3.Soltis DE, Bell CD, Kim S, Soltis PS. Origin and early evolution of angiosperms. Annals of the New York Academy of Sciences. 2008;1133:3–25. doi: 10.1196/annals.1438.005 . [DOI] [PubMed] [Google Scholar]
- 4.Mayrose I, Zhan SH, Rothfels CJ, Arrigo N, Barker MS, Rieseberg LH, et al. Methods for studying polyploid diversification and the dead end hypothesis: a reply to Soltis et al. (2014). New Phytol. 2015;206(1):27–35. doi: 10.1111/nph.13192 . [DOI] [PubMed] [Google Scholar]
- 5.Vanneste K, Maere S, Van de Peer Y. Tangled up in two: a burst of genome duplications at the end of the Cretaceous and the consequences for plant evolution. Phil Trans R Soc B. 2014;369(1648):20130353 doi: 10.1098/rstb.2013.0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrigo N, Barker MS. Rarely successful polyploids and their legacy in plant genomes. Curr Opin Plant Biol. 2012;15(2):140–6. doi: 10.1016/j.pbi.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 7.Mayrose I, Zhan SH, Rothfels CJ, Magnuson-Ford K, Barker MS, Rieseberg LH, et al. Recently formed polyploid plants diversify at lower rates. Science. 2011;333(6047):1257–. doi: 10.1126/science.1207205 [DOI] [PubMed] [Google Scholar]
- 8.Comai L. The advantages and disadvantages of being polyploid. Nat Rev Genet. 2005;6(11):836–46. doi: 10.1038/nrg1711 [DOI] [PubMed] [Google Scholar]
- 9.Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng C, et al. Polyploidy and angiosperm diversification. American Journal of Botany. 2009;96(1):336–48. doi: 10.3732/ajb.0800079 . [DOI] [PubMed] [Google Scholar]
- 10.Madlung A. Polyploidy and its effect on evolutionary success: old questions revisited with new tools. Heredity (Edinb). 2013;110(2):99–104. doi: 10.1038/hdy.2012.79 ; PubMed Central PMCID: PMCPMC3554449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van de Peer Y, Mizrachi E, Marchal K. The evolutionary conundrum of polyploidy. Nat Rev Genet. 2017. doi: 10.1038/nrg.2017.26 [DOI] [PubMed] [Google Scholar]
- 12.Vanneste K, Baele G, Maere S, Van de Peer Y. Analysis of 41 plant genomes supports a wave of successful genome duplications in association with the Cretaceous–Paleogene boundary. Genome Research. 2014;24(8):1334–47. doi: 10.1101/gr.168997.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Baniaga AE, Sessa EB, Scascitelli M, Graham SW, Rieseberg LH, et al. Early genome duplications in conifers and other seed plants. Science Advances. 2015;1(10):e1501084 doi: 10.1126/sciadv.1501084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui L, Wall PK, Leebens-Mack JH, Lindsay BG, Soltis DE, Doyle JJ, et al. Widespread genome duplications throughout the history of flowering plants. Genome Research. 2006;16(6):738–49. doi: 10.1101/gr.4825606 ; PubMed Central PMCID: PMCPMC1479859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rydin C, Pedersen KR, Friis EM. On the evolutionary history of Ephedra: Cretaceous fossils and extant molecules. P Natl Acad Sci USA. 2004;101(47):16571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallon S, Lupia R. Ferns diversified in the shadow of angiosperms. Nature. 2004;428(6982):553–7. doi: 10.1038/nature02361 . [DOI] [PubMed] [Google Scholar]
- 17.Burleigh JG, Barbazuk WB, Davis JM, Morse AM, Soltis PS. Exploring diversification and genome size evolution in extant gymnosperms through phylogenetic synthesis. Journal of Botany. 2012;2012. [Google Scholar]
- 18.Wu H, Ma Z, Wang MM, Qin AL, Ran JH, Wang XQ. A high frequency of allopolyploid speciation in the gymnospermous genus Ephedra and its possible association with some biological and ecological features. Molecular Ecology. 2016;25(5):1192–210. doi: 10.1111/mec.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Giannasi DE, Price RA. Phylogenetic relationships in Ephedra (Ephedraceae) inferred from chloroplast and nuclear DNA sequences. Molecular Phylogenetics and Evolution. 2005;35(1):48–59. doi: 10.1016/j.ympev.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 20.Khoshoo T. Polyploidy in gymnosperms. Evolution. 1959:24–39. [Google Scholar]
- 21.Brenner ED, Stevenson DW, McCombie RW, Katari MS, Rudd SA, Mayer KF, et al. Expressed sequence tag analysis in Cycas, the most primitive living seed plant. Genome Biology. 2003;4(12):R781. doi: 10.1186/gb-2003-4-12-r78 ; PubMed Central PMCID: PMCPMC329417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S. Recent synchronous radiation of a living fossil. Science. 2011;334(6057):796–9. doi: 10.1126/science.1209926 . [DOI] [PubMed] [Google Scholar]
- 23.Jones D. Cycads of the world: Ancient plant in today’s landscape. 2002. Epub 2.
- 24.Taylor EL, Taylor TN, Krings M. Paleobotany: the biology and evolution of fossil plants. 2009.
- 25.Calonje M, Stevenson DW, Stanberg L. The World List of Cycads. 2013. Epub Online edition [Internet].
- 26.Crisp MD, Cook LG. Cenozoic extinctions account for the low diversity of extant gymnosperms compared with angiosperms. New Phytol. 2011;192(4):997–1009. doi: 10.1111/j.1469-8137.2011.03862.x [DOI] [PubMed] [Google Scholar]
- 27.Condamine FL, Nagalingum NS, Marshall CR, Morlon H. Origin and diversification of living cycads: a cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evolutionary Biology. 2015;15(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanneste K, Sterck L, Myburg Z, Van de Peer Y, Mizrachi E. Horsetails Are Ancient Polyploids: Evidence from Equisetum giganteum. The Plant Cell. 2015;27(6):1567–78. doi: 10.1105/tpc.15.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanneste K, Van de Peer Y, Maere S. Inference of genome duplications from age distributions revisited. Mol Biol Evol. 2013;30(1):177–90. doi: 10.1093/molbev/mss214 . [DOI] [PubMed] [Google Scholar]
- 30.Li Z, De la Torre AR, Sterck L, Canovas FM, Avila C, Sierra IM, et al. Single-copy genes as molecular markers for phylogenomic studies in seed plants. Genome Biology and Evolution. 2017;9(5):1130–47. doi: 10.1093/gbe/evx070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magallón SA, Hilu KW, Quandt D. Land plant evolutionary timeline: gene effects are secondary to fossil constraints in relaxed clock estimation of age and substitution rates. American Journal of Botany. 2013;100(3):556–73. doi: 10.3732/ajb.1200416 [DOI] [PubMed] [Google Scholar]
- 32.de la Torre-Barcena JE, Kolokotronis SO, Lee EK, Stevenson DW, Brenner ED, Katari MS, et al. The impact of outgroup choice and missing data on major seed plant phylogenetics using genome-wide EST data. PLOS ONE. 2009;4(6):e5764 doi: 10.1371/journal.pone.0005764 ; PubMed Central PMCID: PMCPMC2685480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaw S, Parkinson CL, Cheng Y, Vincent TM, Palmer JD. Seed plant phylogeny inferred from all three plant genomes: monophyly of extant gymnosperms and origin of Gnetales from conifers. Proc Natl Acad Sci U S A. 2000;97(8):4086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickett NJ, Mirarab S, Nguyen N, Warnow T, Carpenter E, Matasci N, et al. Phylotranscriptomic analysis of the origin and early diversification of land plants. P Natl Acad Sci USA. 2014;111(45):E4859–E68. doi: 10.1073/pnas.1323926111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruhfel BR, Gitzendanner MA, Soltis PS, Soltis DE, Burleigh JG. From algae to angiosperms-inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evolutionary Biology. 2014;14:23 doi: 10.1186/1471-2148-14-23 ; PubMed Central PMCID: PMCPMC3933183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xi ZX, Rest JS, Davis CC. Phylogenomics and Coalescent Analyses Resolve Extant Seed Plant Relationships. PLOS ONE. 2013;8(11). doi: 10.1371/journal.pone.0080870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C-S, Chaw S-M, Huang Y-Y. Chloroplast phylogenomics indicates that Ginkgo biloba is sister to cycads. Genome Biology and Evolution. 2013;5(1):243–54. doi: 10.1093/gbe/evt001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruprecht C, Lohaus R, Vanneste K, Mutwil M, Nikoloski Z, Van de Peer Y, et al. Revisiting ancestral polyploidy in plants. Science Advances. 2017;3(7):e1603195 doi: 10.1126/sciadv.1603195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De La Torre AR, Li Z, Van de Peer Y, Ingvarsson PK. Contrasting rates of molecular evolution and patterns of selection among gymnosperms and flowering plants. Mol Biol Evol. 2017;34(6):1363–77. doi: 10.1093/molbev/msx069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan R, Zhao Y, Zhang H, Fan G, Liu X, Zhou W, et al. Draft genome of the living fossil Ginkgo biloba. Gigascience. 2016;5(49):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanderson MJ. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol Biol Evol. 2002;19(1):101–9. . [DOI] [PubMed] [Google Scholar]
- 42.Magallón SA, Sanderson MJ. Angiosperm divergence times: the effect of genes, codon positions, and time constraints. Evolution. 2005;59(8):1653–70. . [DOI] [PubMed] [Google Scholar]
- 43.Bowe LM, Coat G, dePamphilis CW. Phylogeny of seed plants based on all three genomic compartments: extant gymnosperms are monophyletic and Gnetales' closest relatives are conifers. Proceedings of the National Academy of Science of the United States of America. 2000;97(8):4092–7. ; PubMed Central PMCID: PMCPMC18159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaw S, Zharkikh A, Sung HM, Lau TC, Li WH. Molecular phylogeny of extant gymnosperms and seed plant evolution: analysis of nuclear 18S rRNA sequences. Mol Biol Evol. 1997;14(1):56–68. . [DOI] [PubMed] [Google Scholar]
- 45.Lee EK, Cibrian-Jaramillo A, Kolokotronis SO, Katari MS, Stamatakis A, Ott M, et al. A functional phylogenomic view of the seed plants. PLOS Genet. 2011;7(12):e1002411 doi: 10.1371/journal.pgen.1002411 ; PubMed Central PMCID: PMCPMC3240601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magallón SA, Sanderson MJ. Relationships among seed plants inferred from highly conserved genes: sorting conflicting phylogenetic signals among ancient lineages. American Journal of Botany. 2002;89(12):1991–2006. doi: 10.3732/ajb.89.12.1991 [DOI] [PubMed] [Google Scholar]
- 47.Doyle JA. Seed plant phylogeny and the relationships of Gnetales. International Journal of Plant Sciences. 1996:S3–S39. [Google Scholar]
- 48.Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter. 1993;11(2):113–6. [Google Scholar]
- 49.Zerbino DR, Birney E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Research. 2008;18(5):821–9. doi: 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulz MH, Zerbino DR, Vingron M, Birney E. Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics. 2012;28(8):1086–92. doi: 10.1093/bioinformatics/bts094 ; PubMed Central PMCID: PMCPMC3324515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert D. EvidentialGene: mRNA Transcript Assembly Software 7th annual arthropod genomics symposium. 2013:1. 10.7490/f1000research.1112594.1.
- 52.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nature Protocols. 2013;8(8):1494–512. doi: 10.1038/nprot.2013.084 ; PubMed Central PMCID: PMCPMC3875132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30(7):1575–84. ; PubMed Central PMCID: PMCPMC101833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol. 1994;11(5):725–36. . [DOI] [PubMed] [Google Scholar]
- 55.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24(8):1586–91. doi: 10.1093/molbev/msm088 . [DOI] [PubMed] [Google Scholar]
- 56.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 2010;59(3):307–21. doi: 10.1093/sysbio/syq010 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw reads of both transcriptomes are available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/) under the submission number SUB2337915.