Abstract
Dietary intervention and genetic fat-1 mice are two models for the investigation of effects associated with omega-3 polyunsaturated fatty acids (n3-PUFA). In order to assess their power to modulate the fatty acid and oxylipin pattern, we thoroughly compared fat-1 and wild-type C57BL/6 mice on a sunflower oil diet with wild-type mice on the same diet enriched with 1% EPA and 1% DHA for 0, 7, 14, 30 and 45 days. Feeding led after 14–30 days to a high steady state of n3-PUFA in all tissues at the expense of n6-PUFAs. Levels of n3-PUFA achieved by feeding were higher compared to fat-1 mice, particularly for EPA (max. 1.7% in whole blood of fat-1 vs. 7.8% following feeding). Changes in PUFAs were reflected in most oxylipins in plasma, brain and colon: Compared to wild-type mice on a standard diet, arachidonic acid metabolites were overall decreased while EPA and DHA oxylipins increased with feeding more than in fat-1 mice. In plasma of n3-PUFA fed animals, EPA and DHA metabolites from the lipoxygenase and cytochrome P450 pathways dominated over ARA derived counterparts.Fat-1 mice show n3-PUFA level which can be reached by dietary interventions, supporting the applicability of this model in n3-PUFA research. However, for specific questions, e.g. the role of EPA derived mediators or concentration dependent effects of (individual) PUFA, feeding studies are necessary.
Introduction
It has long been suggested that dietary intake of long-chain omega-3 polyunsaturated fatty acids (n3-PUFA), especially of eicosapentaenoic acid (C20:5 n3, EPA) and docosahexaenoic acid (C22:6 n3, DHA) is associated with beneficial health effects [1, 2]. Strong evidence exists for an improvement of cardiometabolic health by lowering blood trigylceride levels and cardiovascular outcomes, such as sudden cardiac death [2]. Furthermore, anti-inflammatory [1, 2] and anti-angiogenic [3, 4] effects have been described. Part of the effects might be explained by direct physiological actions of n3-PUFA. They have been shown to act directly on membrane ion channels, or to reduce expression of inflammatory genes via nuclear factor-kappaB (NFκB), e.g. by interacting with peroxisome proliferator-activated receptor gamma (PPARγ) [1, 2]. Moreover, n3-PUFA serve as substrates in the arachidonic acid (C20:4 n6, ARA) cascade. In this signaling cascade, ARA is converted via three enzymatic pathways and autoxidation to oxidative metabolites, called oxylipins, several of which are biologically highly active: (I) Cyclooxygenase (COX) conversion of ARA yields series-2 prostanoids, like the potent prostaglandin (PG) E2 which is involved in the regulation of pain, fever and inflammation or thromboxane (Tx) A2 which is involved in platelet aggregation [5–7]. (II) Lipoxygenase (LOX) action on ARA leads to multiple biologically active classes of lipid mediators via hydroperoxy intermediates, such as leukotrienes (LT), e.g. LTB4, involved in the chemotaxis of neutrophils, lipoxins with anti-inflammatory properties or hydroxy-FA (OH-FA) [5–7]. (III) Finally, cytochrome P450 (CYP) enzymes can convert ARA to OH- and epoxy-FA (Ep-FA). For instance, ω-hydrolase activity of CYP enzymes can yield the vasoconstrictory 20-hydroxyeicosatetraenoic acid (20-HETE) formed by members of the CYP4A or 4F family [5, 7, 8]. Conversion of ARA by CYP2C and 2J, e.g., leads to vasodilatory, anti-inflammatory, analgesic and angiogenic acting Ep-FA [5, 7–10] which are further metabolized to less potent dihydroxy-FA (DiH-FA) by the soluble epoxide hydrolase [5, 7, 11]. The effect of n3-PUFA on this important signaling cascade is multifaceted. On the one hand, by competing with ARA for conversion, the formation of potent ARA derived mediators, such as pro-inflammatory PGE2 and LTB4 is reduced, while their EPA derived counterparts, PGE3 and LTB5 have been shown to be less potent [1, 7]. On the other hand, enzymatic conversion of EPA and DHA can yield highly potent lipid mediators: CYP catalyzed epoxidation leads e.g. to anti-arrhythmic acting 17(18)-epoxy eicosatetraenoic acid (EpETE) from EPA and 19(20)-epoxy docosapentaenoic acid (EpDPE) from DHA [12]. Interestingly, while these Ep-FA share the anti-inflammatory action of the corresponding ARA oxylipins [3], 19(20)-EpDPE has been shown to inhibit angiogenesis in contrast to ARA derived Ep-FA [4]. Moreover, multiple hydroxylation leads to highly potent, specialized pro-resolving lipid mediators (SPM) such as resolvins and protectins [13, 14]. A comprehensive overview about the ARA cascade can be found in recent reviews, e.g. [5, 7, 15].
Regarding the clinical relevance of n3-PUFA in different diseases the results of epidemiological and intervention studies are conflicting [1, 2, 16]. Moreover, molecular modes of action for individual effects of n3-PUFA have not been fully unveiled, and dose dependencies remain largely unclear [1, 2]. In order to address these questions, appropriate experimental models allowing a well-defined modulation of the endogenous n3-PUFA and oxylipin profile are required.
In humans and other mammals EPA and DHA can be synthesized endogenously by combined elongation, desaturation and β-oxidation reactions from the essential n3-PUFA alpha linolenic acid (C18:3 n3, ALA) [17, 18]. However, conversion rates are low on a diet rich in linoleic acid (C18:2 n6, LA), as it is the case for a typical western diet (soy, corn and sunflower oil based) [17]. In humans and other mammals, endogenous supply of EPA and DHA thus relies on the dietary intake. In in vivo studies, animal diets are often enriched with EPA and DHA containing oils to modulate the endogenous n3-PUFA profile. This approach has been used in different disease models for the investigation of n3-PUFA associated biology, e.g. in inflammatory diseases, such as colon inflammation [19, 20], arthritis [21, 22], hypertension [23], liver injury [24] or Parkinson’s Disease [25].
Another approach to investigate physiological effects of elevated endogenous n3-PUFA concentrations is the use of the fat-1 transgenic mouse model. The DNA of these mice has been edited to carry the fat-1 gene of the nematode Caenorhabditis elegans encoding an n3 fatty acid desaturase catalyzing the conversion of n6 to n3 fatty acids [26]. This leads to a decreased endogenous n6/n3-ratio in fat-1 mice fed with a standard n6-PUFA rich diet compared to wild type (WT) animals, e.g. from 46.6 (WT) to 2.9 (fat-1) in erythrocytes [26]. Therefore, fat-1 mice have been used in many studies for the investigation of n3-PUFA associated effects, e.g. in inflammation, including colitis [27, 28], hepatitis [29], pancreatitis [30], different types of cancer, such as liver [31], colitis-associated colon cancer [32, 33] and melanoma [34] as well as Parkinson’s Disease [35] or chemically induced diabetes [36].
In most studies using fat-1 mice, selected FA and/or (variations of) the n6/n3-PUFA ratio in tissues are used to describe of the endogenous n3-PUFA status [27–37]. Only little attention has been paid to the modulation of n3- and n6-PUFA oxylipins in fat-1 compared to wild type mice. In disease models a focus was set on selected oxylipins, such as PGE and/or PGD from ARA and/or EPA [27, 28, 32, 34, 36], SPMs [28], precursor thereof [31] and few others [28, 32, 36]. A comprehensive set of free oxylipins in fat-1 mice has only been described in plasma [38] and total (free and esterified) OH-FA have been described in plasma and tissues [37]. Almost no data is available on the differences in fatty acids and oxylipins in fat-1 versus WT mice after dietary supplementation with n3-PUFA. The only available study compares the effects of nine weeks of feeding on selected oxylipins and fatty acids in kidney tissue [39]. Therefore, in the present study we thoroughly investigated the modulation of both, the total fatty acid and oxylipin profile in fat-1 vs WT mice on a standard, sunflower oil based diet and the same diet enriched with n3-PUFA (1% EPA and 1% DHA). Not only is a comprehensive set of tissues and blood (including plasma and blood cells) analyzed, we also show the time course of effects occurring on a diet enriched with n3-PUFA over a feeding period of 7–45 days. This study provides fundamental insights on the breadth of effects on the lipidome caused by the insertion of the fat-1 gene into the murine DNA in the context of a diet high in n6-PUFA compared to an n3-PUFA dietary intervention as well as the time dependency of nutrition induced changes.
Materials and methods
Chemicals
Acetic acid and methanol (Optima LC/MS Grade) as well as acetonitrile (HPLC-MS grade) were obtained from Fisher Scientific (Schwerte, Germany) and ammonium acetate (p.a.) was purchased from Merck (Darmstadt, Germany). Methyl tert-butyl ether and n-hexane (HPLC grade) were obtained from Carl Roth (Karlsruhe, Germany). Methyl tricosanoate (FAME C23:0) was obtained from Santa Cruz Biotechnology (Heidelberg, Germany). Oxylipin and deuterated oxylipin standards were purchased from Cayman Chemicals (local distributor: Biomol, Hamburg, Germany). Further oxylipin standards (Epoxy octadecadienoic acids (EpODEs) and dihydroxy octadecadienoic acids (DiHODEs)) were a kind gift from the laboratory of Bruce Hammock (UC Davis, CA, USA). Ethyl acetate, methyl formate and all other chemicals were purchased from Sigma Aldrich (Taufkirchen, Germany).
Feeding experiment
Animals were cared for in accordance with the institution’s guidelines for experimental animals based on EU Directive 2010/63/EU. All experiments were carried out and use of the animals was registered and approved by the Landesamt für Gesundheit und Soziales Berlin (Reg No.: T0025/13). Pellets for the feeding experiment were based on a standard experimental diet from ssniff (product number: E15051; ssniff Spezialdiäten GmbH, Soest, Germany) with 10% fat. The fat used for the standard diet (STD) was refined sunflower oil (Henry Lamotte Oils, Bremen, Germany) enriched with 0.2% (w/w) tocopherol mix (Covi-Ox T 70 EU, BASF, Ludwigshafen). The n3-PUFA rich diet (STD+n3) was the same diet containing 1% EPA and 1% DHA as ethyl esters (10% each in fat). Ethyl ester are generated during the purification and concentration of fish oil and thus represent together with the re-esterified triglycerides the most important class of supplementation products [40]. The fat content, peroxide value and the fatty acid composition of the experimental diets can be found in the supplementary information (S1 Table).
Heterozygous transgenic fat-1 mice were generated as described [26] and phenotyping (ratio of n6/n3-PUFA) from tails was carried out using gas chromatography. For the feeding experiment female C57BL/6 WT and fat-1 mice of 9–10 weeks of age were used (n = 6 per feeding group). Before the experiment, mice were kept on a diet with 3.3% fat (1.8% LA, 0.23% ALA in the diet, S2 Table) with water and food supply ad libitum. The chow was stored in plastic bags containing an oxygen absorber at -20°C. During the whole feeding experiment fresh chow was provided every 2–3 days. WT mice were kept on the experimental diets for 7, 14, 30 and 45 days. In order to compare the fatty acid profile in fat-1 mice to WT mice fed with an n3-PUFA enriched diet (maximum modulation of the fatty acid profile in blood and tissues following 30 days of feeding; see below), fat-1 mice were kept on the standard sunflower diet for 30 days. One group of WT and fat-1 animals was sacrificed on day 0. Animals were killed by cervical dislocation and organs (liver, kidney, spleen, brain and colon) as well as blood (by cardiac puncture) were collected. 10 μL of whole blood were directly diluted with 50 μL of deionized water. For plasma and blood cell generation, blood was directly centrifuged (800 x g, 10 min, 4°C). Plasma was collected and blood cells were washed once with phosphate buffered saline (containing 1.5 mg/mL ethylenediaminetetraacetic acid (EDTA)) and reconstituted to the original blood volume in phosphate buffered saline. All samples were stored at -80°C until further analysis.
Fatty acid analysis
Fatty acid composition was analyzed in all collected blood fractions (60 μL diluted whole blood, 50 μL plasma and 100 μL reconstituted blood cells) and tissues (30–35 mg) as described [41]. Briefly, blood and tissues were extracted with methanol/ methyl tert-butyl ether (1:2, v/v) and derivatized to fatty acid methyl esters (FAME) with methanolic hydrogen chloride (acetylchloride in methanol (1:10, v/v)) before analysis was carried out using gas chromatography with flame ionization detection (GC-FID). For the calculation of the relative pattern and absolute fatty acid concentrations response factors were used [41, 42]. Results are presented as mean ± standard error of the mean (SEM).
Oxylipin analysis
Extraction and analysis of oxylipins from plasma (200 μL) and colon (50±5 mg) was carried out as described [43]. Brain (50±5 mg) was homogenized following addition of internal standards and antioxidant solution [43, 44] in 750 μL ethyl acetate and 500 μL water (pH 6) in a ball mill using two 3 mm metal beads (25 Hz, 5 min, Retsch, Haan, Germany). Following homogenization and centrifugation (20 000 x g, 5 min, 4°C), the organic phase was collected and the sample extracted with another 750 μL ethyl acetate. The combined organic phases were evaporated using a vacuum centrifuge (Christ, Osterode am Harz, Germany) and the dried lipid extract was reconstituted in 300 μL methanol. All samples were diluted to 6 mL with water and acidified with acetic acid (to pH 3) directly before extraction on C18 cartridges (500 mg, Macherey-Nagel, Düren, Germany). Methyl formate was used for elution. Oxylipins were quantified by liquid chromatography-mass spectrometry (LC-MS) as described [43, 44]. Hemolytic plasma samples and samples with high TxB2 and 12-HETE—indicating improper anticoagulation—were excluded from analysis. Results are presented as mean ± SEM.
Statistical analysis
Statistical analyses were performed as indicated using GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.
Results
Behavior and bodyweight
During the experiment, no differences in animal behavior were observed and similar body weights between the feeding groups (17.9–21.7 g, supplementary information, S1 Fig) indicated no differences in feeding behavior.
Fatty acid profile
Fig 1 and Table 1 show the relative pattern of selected FA as well as the FA profile grouped as saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and n6- and n3-polyunsaturated fatty acids (PUFA) during the course of the feeding time. The full FA profile of blood and tissues can be found in the SI (S3A+S3B Table).
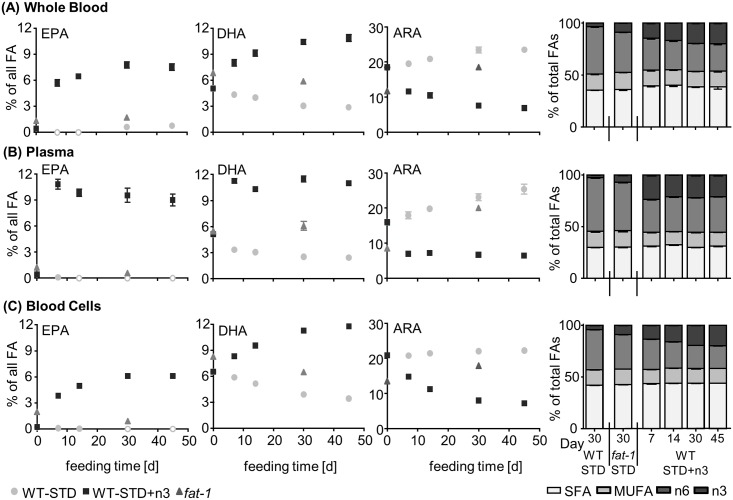
Fig 1. Fatty acid profile in blood.
Shown are relative amounts of EPA, DHA and ARA as well as the relative distribution of n3- and n6-PUFA, MUFA and SFA in transgenic fat-1 mice and wild type animals (WT-STD) on a sunflower oil based diet, as well as in wild type mice on the same diet enriched with EPA and DHA (WT-STD+n3) during the course of the feeding period (45 days) in (A) whole blood, (B) plasma and (C) blood cells. Analytes that were below the limit of quantification are marked with a white filling in the diagram. Results of the statistical analyses for selected fatty acids during the course of the feeding with the n3-PUFA enriched diet as well as for WT and fat-1 mice after 30 days on the experimental diets are shown in S6+S7 Tables.
Table 1. Fatty acid profile in tissues.
Shown are relative amounts of EPA, DHA and ARA as well as the sum of n6-PUFA, n3-PUFA, MUFA and SFA in liver, kidney, spleen, colon and brain tissue in WT (WT-STD) and fat-1 mice after 30 days on a standard sunflower oil based diet and in WT mice on the same diet enriched with EPA and DHA (WT-STD+n3) during the course of the feeding period (day 7–45). Results of the statistical analyses for selected fatty acids during the course of the feeding with the n3-PUFA enriched diet as well as for WT and fat-1 mice after 30 days on the experimental diets are shown in S6+S7 Tables.
| EPA | DHA | ARA | n6-PUFA | n3-PUFA | MUFA | SFA | ||
|---|---|---|---|---|---|---|---|---|
| Liver | ||||||||
| WT-STD | Day 30 | <LOQ | 3.7 ± 0.2 | 15.9 ± 0.9 | 39.5 ± 0.8 | 3.8 ± 0.2 | 24 ± 2 | 33 ±1 |
| fat-1 | Day 30 | 0.16 ± 0.02 | 6.8 ± 0.5 | 13.2 ± 0.7 | 36.7 ± 0.6 | 7.2 ± 0.5 | 24 ± 2 | 32 ± 1 |
| WT-STD+n3 | Day 7 | 6.1 ± 0.4 | 14.7 ± 0.3 | 7.8 ± 0.2 | 26.8 ± 0.6 | 22.3 ± 0.6 | 13.5 ± 0.5 | 37.4 ± 0.3 |
| Day 14 | 4.4 ± 0.2 | 11.1 ± 0.4 | 5.9 ± 0.4 | 27 ± 1 | 17.0 ± 0.6 | 21 ± 1 | 35.1 ± 0.8 | |
| Day 30 | 5.4 ± 0.4 | 14.1 ± 0.8 | 5.1 ± 0.2 | 26.3 ± 0.5 | 21 ± 1 | 19 ± 2 | 34.7 ± 0.5 | |
| Day 45 | 4.2 ± 0.5 | 12.5 ± 0.5 | 6.1 ± 0.2 | 29 ± 1 | 18.1 ± 0.9 | 18.9 ± 0.6 | 34.4 ± 0.5 | |
| Kidney | ||||||||
| WT-STD | Day 30 | 0.015 ± 0.002 | 7.4 ± 0.6 | 22 ± 2 | 38.8 ± 0.7 | 7.7 ± 0.6 | 16 ± 2 | 38.0 ± 0.6 |
| fat-1 | Day 30 | 1.01 ± 0.07 | 12.1 ± 0.3 | 20.3 ± 0.6 | 35.5 ± 0.5 | 13.9 ± 0.4 | 12.1 ± 0.5 | 38.5 ± 0.3 |
| WT-STD+n3 | Day 7 | 4.6 ± 0.1 | 14.9 ± 0.7 | 12.9 ± 0.8 | 27.8 ± 0.3 | 20.6 ± 0.8 | 13 ± 1 | 39.1 ± 0.3 |
| Day 14 | 5.2 ± 0.2 | 16.3 ± 0.7 | 10.9 ± 0.6 | 25.7 ± 0.2 | 22.6 ± 0.8 | 13 ± 1 | 38.7 ± 0.2 | |
| Day 30 | 6.3 ± 0.3 | 18.0 ± 0.5 | 10.3 ± 0.4 | 24.6 ± 0.2 | 25.5 ± 0.6 | 11.0 ± 0.7 | 39.0 ± 0.2 | |
| Day 45 | 6.2 ± 0.2 | 16.5 ± 0.7 | 9.5 ± 0.5 | 25.2 ± 0.3 | 23.9 ± 0.9 | 13 ± 1 | 38.1 ± 0.4 | |
| Spleen | ||||||||
| WT-STD | Day 30 | 0.035 ± 0.007 | 2.6 ± 0.1 | 20.4 ± 0.8 | 39 ± 1 | 3.0 ± 0.1 | 16 ± 2 | 42.8 ± 0.5 |
| fat-1 | Day 30 | 1.6 ± 0.2 | 4.9 ± 0.6 | 14 ± 1 | 31.2 ± 0.2 | 9 ± 1 | 18 ± 3 | 41 ± 1 |
| WT-STD+n3 | Day 7 | 3.58 ± 0.07 | 9.8 ± 0.3 | 10.5 ± 0.5 | 25.7 ± 0.4 | 17.8 ± 0.4 | 13.3 ± 0.9 | 43.2 ± 0.2 |
| Day 14 | 4.0 ± 0.1 | 10.3 ± 0.2 | 8.3 ± 0.4 | 23.4 ± 0.4 | 18.7 ± 0.4 | 14.8 ± 0.7 | 43.0 ± 0.3 | |
| Day 30 | 4.3 ± 0.1 | 11.2 ± 0.2 | 7.7 ± 0.2 | 23.2 ± 0.2 | 20.1 ± 0.5 | 13.1 ± 0.7 | 43.6 ± 0.3 | |
| Day 45 | 3.8 ± 0.2 | 10.5 ± 0.6 | 6.9 ± 0.5 | 23.6 ± 0.2 | 19 ± 1 | 16 ± 2 | 42.1 ± 0.9 | |
| Brain | ||||||||
| WT-STD | Day 30 | <LOQ | 17.1 ± 0.3 | 11.3 ± 0.2 | 15.5 ± 0.3 | 17.2 ± 0.3 | 20.5 ± 0.6 | 46.8 ± 0.2 |
| fat-1 | Day 30 | 0.066 ± 0.005 | 16.3 ± 0.9 | 9.8 ± 0.8 | 14.0 ± 0.9 | 16.5 ± 0.9 | 25 ± 3 | 45 ± 1 |
| WT-STD+n3 | Day 7 | 0.15 ± 0.02 | 16.7 ± 0.4 | 10.3 ± 0.3 | 14.3 ± 0.4 | 17.1 ± 0.4 | 23 ± 1 | 46.0 ± 0.4 |
| Day 14 | 0.14 ± 0.01 | 17.4 ± 0.3 | 10.6 ± 0.2 | 14.6 ± 0.3 | 17.9 ± 0.3 | 20.9 ± 0.7 | 46.6 ± 0.3 | |
| Day 30 | 0.18 ± 0.01 | 19.0 ± 0.3 | 9.5 ± 0.2 | 13.2 ± 0.2 | 19.6 ± 0.3 | 21.2 ± 0.5 | 46.0 ± 0.2 | |
| Day 45 | 0.16 ± 0.01 | 17.4 ± 0.5 | 8.8 ± 0.2 | 15.8 ± 0.4 | 15.8 ± 0.6 | 20 ± 1 | 48.7 ± 0.3 | |
| Colon | ||||||||
| WT-STD | Day 30 | 0.048 ± 0.010 | 0.73 ± 0.13 | 5.7 ± 1.0 | 35.5 ± 0.8 | 0.96 ± 0.15 | 35± 1 | 29.0 ± 0.8 |
| fat-1 | Day 30 | 0.65 ± 0.22 | 1.5 ± 0.5 | 4.7 ± 1.4 | 32 ± 1 | 2.9 ± 0.8 | 35 ± 2 | 31 ± 1 |
| WT-STD+n3 | Day 7 | 1.8 ± 0.3 | 3.5 ± 0.4 | 2.8 ± 0.8 | 28.8 ± 0.6 | 6.7 ± 0.7 | 32 ± 2 | 32.9 ± 0.8 |
| Day 14 | 2.8 ± 0.4 | 5.0 ± 0.5 | 3.6 ± 0.6 | 26.0 ± 0.6 | 9.2 ± 0.8 | 30 ± 1 | 34.6 ± 0.7 | |
| Day 30 | 2.0 ± 0.2 | 4.5 ± 0.3 | 1.8 ± 0.4 | 27.5 ± 0.6 | 7.7 ± 0.5 | 30.5 ± 0.9 | 34.2 ± 0.6 | |
| Day 45 | 3.0 ± 0.4 | 5.7 ± 0.2 | 3.5 ± 0.4 | 27.4 ± 0.5 | 10.2 ± 0.6 | 27.4 ± 0.8 | 35.0 ± 0.3 |
Feeding of a diet enriched in EPA and DHA (1% EPA and 1% DHA as ethyl ester, WT-STD+n3) led to a time dependent increase in the relative and absolute concentrations of n3-PUFA, particularly EPA, DHA and n3 docosapentaenoic acid (22:5n3, n3-DPA), in blood and all investigated tissues (Fig 1, Table 1, S2 Fig, S3 Table). The feeding time necessary to reach a maximal increase was tissue dependent; however, 14–30 days were sufficient to reach maximum level in all tissues and blood (as % of total FA, Fig 1, Table 1, S3 Table). The overall FA pattern was changed least in brain (Table 1, S2D Fig).
After 30 days on the STD+n3 diet, EPA ranged in blood and tissues from 0.18–9.5% (brain/plasma [min./max.]) and DHA levels ranged from 4.5–19% (colon/brain, Fig 1, Table 1). While the absolute increase in EPA and DHA compared to baseline was in a similar range for both FA (S3B Table), individual relative differences, expressed as mean %difference [(c(FA)WT-STD+n3, D30—c(FA)WT, D0)/c(FA)WT, D0*100], were remarkably higher for EPA compared to DHA due to low baseline levels of EPA (S3 Fig). In all groups (WT mice on the standard, sunflower oil based diet (WT-STD), WT-STD+n3, and fat-1) EPA level in blood and tissues were lower as compared to DHA. It should be noted, that in response to n3-PUFA feeding, SFA and MUFA changed only slightly (however, significantly for MUFA in many tissues and blood), while n6-PUFA levels, particularly ARA, were significantly decreased (Fig 1, Table 1, S2 and S3 Figs, S6 Table).
Fat-1 mice fed 30 days with a standard, sunflower oil based diet showed higher levels of EPA, n3-DPA and DHA in comparison to WT animals on the same diet (Fig 1, Table 1, S3B Table) reaching statistical significance for EPA and DHA in many tissues (S7 Table). However, compared to WT-STD+n3 mice, level of EPA and DHA were significantly lower in fat-1 mice (p<0.0001 for all tissues and blood, except DHA in brain, Fig 1, Table 1, S7 Table). Particularly, levels of EPA were low (0.066–1.7%; brain/whole blood; Fig 1, Table 1) which is also reflected in the high %difference of EPA between both groups (S4 Fig). Relative differences in DHA and n3-DPA were more moderate in most tissues and blood between WT-STD+n3 and fat-1 mice (S4 Fig).
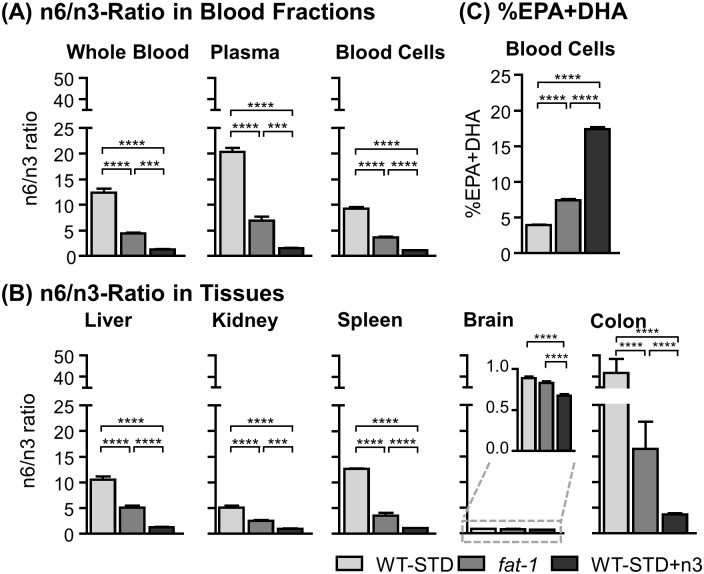
The n6/n3 ratio in blood and tissues as well as the sum of %EPA and %DHA (%EPA+DHA) in blood cells, a modification of the omega-3 index [45], as marker for the endogenous n3-PUFA status are presented in Fig 2 for the different groups after 30 days on the experimental diets. Data on blood and tissues in all groups can be found in the SI (S5 Fig, S4 Table). The n6/n3 ratio in WT-STD+n3 mice was below 2 in all tissues and blood (except colon with 3.6) and 2.5–6.9 in fat-1 mice (except brain and colon with 0.83 and 16 respectively), being significantly lower than in WT-STD mice with n6/n3 ratios of 5.1–20 (except brain and colon with 0.89 and 41, Fig 2A+2B). %EPA+DHA in blood cells was significantly higher (p<0.0001) in WT-STD+n3 (17.4±0.2%) and fat-1 (7.4±0.2%) in comparison to WT-STD (3.9±0.1%, Fig 2C).
Fig 2. n6/n3 ratio in blood as well as tissues and %EPA+DHA in blood cells.
Shown is the n6/n3 ratio in blood (A) and in tissues (B), as well as %EPA+DHA in blood cells (C) in transgenic fat-1 mice and wild type animals (WT-STD) on a standard sunflower oil based diet, as well as in wild type mice on the same diet enriched with EPA and DHA (WT-STD+n3) after 30 days of feeding. The n6/n3 ratio was calculated as Ʃ%(C18:2 n6, C18:3 n6, C20:3 n6, C20:4 n6, C22:4n6)/ Ʃ%(C18:3 n3, C20:5 n3, C22:5 n3, C22:6 n3). Statistical differences were determined using one-way ANOVA followed by Tukey’s post test (*** p<0.001, **** p<0.0001).
Oxylipin pattern
Oxylipins were analyzed in selected tissues, i.e. plasma, colon and brain. Concentrations of all oxylipins covered by the LC-MS method in all feeding groups in plasma, colon and brain are presented in S5 Table. Since a steady state in the nutrition induced changes in fatty acids (see above) was reached after 30 days, the differences between the groups are highlighted for this time point in Fig 3 for selected oxylipins from EPA, DHA and ARA.
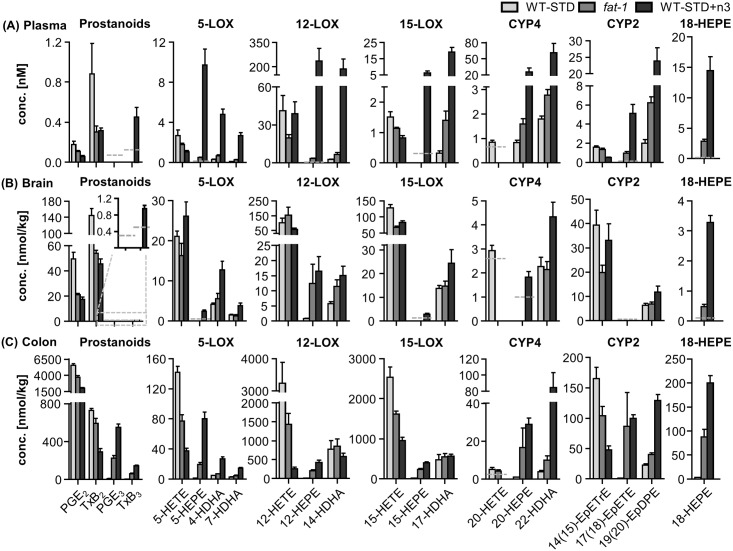
Fig 3. Concentrations of oxylipins.
Presented are concentrations of selected prostanoids, 5-LOX, 12-LOX, 15-LOX, CYP4 and CYP2 products of ARA, EPA and DHA as well as 18-HEPE in (A) plasma, (B) brain and (C) colon in transgenic fat-1 mice and wild type animals (WT-STD) on a sunflower oil based diet, as well as in wild type mice on the same diet enriched with EPA and DHA (WT-STD+n3) after 30 days of feeding. The lower limit of quantification (LLOQ) for the analyte is indicated in case it was not exceeded in >50% of the samples per group. Results of the statistical analyses for the comparison of oxylipins between the feeding groups after 30 days on the experimental diets are shown in S8 Table.
Feeding of a diet enriched with n3-PUFA (1% EPA and 1% DHA as ethyl ester) led to high changes in the oxylipin pattern of plasma, brain and colon, especially in the first seven days of feeding (S5 Table). Due to low basal concentrations of EPA metabolites, their relative increase was overall higher compared to DHA metabolites (exemplary shown for 30 days of feeding in SI S6 Fig) while absolute increases in EPA and DHA metabolites were similar (except in brain, S5 Table). Reductions of the circulating ARA eicosanoids were less consistent compared to trends in the FA while ARA eicosanoids in colon and brain were uniformly decreased (S6 Fig).
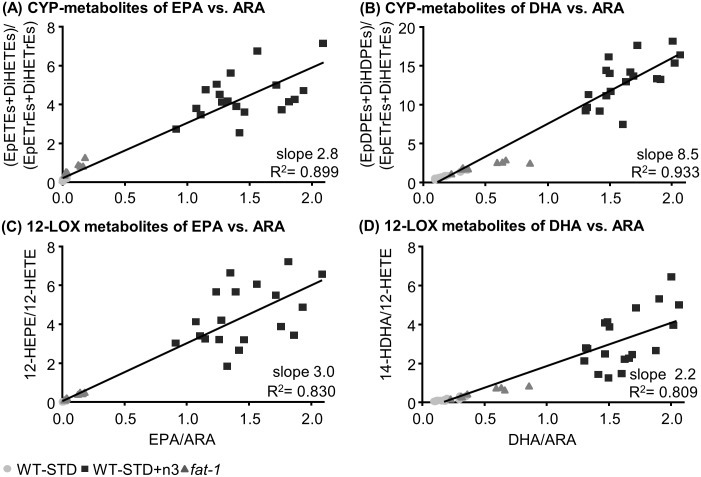
After 30 days on the standard sunflower oil based diet, concentrations of EPA and DHA metabolites in plasma of fat-1 mice were elevated, yet not significantly, compared to WT-STD mice (Fig 3, S8A Table). However, reflecting changes in FA, concentrations of EPA and DHA oxylipins in fat-1 mice were significantly lower compared to WT-STD+n3 (Fig 3, S8A Table). Particularly, concentrations of oxylipins formed in the LOX and CYP pathway were high, dominating the oxylipin profile in plasma of WT-STD+n3 mice, while COX metabolites were barely altered. Interestingly, the sum of plasma oxylipins was highly elevated in WT-STD+n3 compared to fat-1 and WT-STD (S7I Fig). Comparing the ratio of precursor PUFA and their oxidative products in all feeding groups, an almost linear correlation (R2>0.8) resulted for CYP and LOX metabolites (Fig 4). The slope of >2 indicates that moderate changes in the concentrations of EPA or DHA led to a more pronounced change in the concentrations of their oxylipins.
Fig 4. Correlation between plasma n3-PUFA/n6-PUFA oxylipins and the ratio of their precursor fatty acids.
The ratio of CYP metabolites (sum of epoxy-FA and dihydroxy-FA) from EPA (A) and DHA (B) to the respective ARA metabolites are plotted against the ratio of their precursor PUFA. In panel C-D the same correlation is shown for selected 12-LOX metabolites (12-HETE and 14-HDHA). The slope of the linear regression and the correlation coefficient were calculated based on all feeding groups of the experiment.
In colon and brain tissue both, fat-1 and WT-STD+n3 mice showed a shift in the absolute and relative pattern towards n3-PUFA derived oxylipins after 30 days on the experimental diets in comparison to WT-STD mice (Fig 3, S7II Fig). As in plasma, WT-STD+n3 mice showed higher concentrations of n3-PUFA oxylipins compared to fat-1. However, differences between both groups were less pronounced, yet statistically significant in colon for many analytes, compared to plasma (Fig 3, S7I and S8 Figs, S8B+S8C Table). Additionally, in colon and brain tissue the sum of all oxylipins was decreased (S7I Fig). Although DHA in brain tissue showed minor differences between the feeding groups after 30 days on the experimental diets, DHA derived docosanoids were uniformly higher in WT-STD+n3 and fat-1 mice as compared to WT-STD mice (Fig 3B), reaching statistical significance for some HDHAs (S8C Table). As for EPA, concentrations of EPA-eicosanoids in brain were low.
The increase in n3-PUFAs observed in plasma, brain and colon of WT-STD+n3 and fat-1 compared to WT-STD was accompanied by a decrease in most ARA derived eicosanoids (Fig 3, S7I Fig, S5 Table). Similar to the higher level of n3-PUFA oxylipins in WT-STD+n3 compared to fat-1, concentrations of ARA metabolites in plasma and colon were lower in WT-STD+n3 than in fat-1, reaching statistical significance for many eicosanoids in colon. In brain, ARA eicosanoid levels in both groups were similar.
Discussion
Feeding of n3-PUFAs EPA and DHA with the diet led to significantly higher concentrations of respective fatty acids in blood and tissues compared to WT mice on a standard sunflower diet as reported earlier in rodents [12, 19–22]. In men, similar observations were made following EPA+DHA supplementation, however, changes were more moderate caused by lower supplementation levels of n3-PUFA [46–52]. After 14–30 days of feeding a sunflower oil based diet enriched with 1% EPA and 1% DHA, levels of both FA reached a maximum steady state. This data suggests that studies aiming to investigate the effect of n3-PUFA need to implement a pre-feeding period of at least 14–30 days in order to maximally modulate the fatty acid profile. Fat-1 mice showed significantly lower concentrations of n3-PUFA as compared to supplemented WT mice which was most pronounced for EPA, being 2.7–34 fold lower in blood and tissues, while DHA concentrations were at most threefold lower at maximum steady state at 30 days and thereafter (Fig 1, Table 1). This can be explained by the genetic background of these animals. In the fat-1 mouse model the fat-1 gene of the roundworm C. elegans was introduced into the DNA of C57BL/6 wildtype mice. As a consequence, these animals are able to biosynthesize n3- from n6-PUFA [26]. In C. elegans, different n6-PUFA, such as LA, C20:3 n6 and ARA are converted by the n3 desaturase, encoded by the fat-1 gene, resulting in ALA, C20:4 n3 and EPA, respectively [53]. The roundworm lacks further elongase activity. Therefore, the biosynthetic fatty acid pathway stops at EPA, being the most abundant PUFA in the worm [53]. By contrast, in mammals EPA can be further elongated to n3-DPA which in turn can be converted via C24:5 n3 to DHA [17, 18]. These reactions occur at high rates, e.g. 63% (EPA to n3-DPA) and 37% (n3-DPA to DHA) in humans [54]. EPA biosynthesis in mammals from the essential ALA, however, is low—caused by the rate limiting desaturation of ALA to C18:4 n3 in combination with high dietary LA and low ALA consumption [19, 20]. This results in low endogenous EPA levels compared to DHA, being <0.05% in WT mice on a standard sunflower oil based diet in most tissues and blood (Fig 1, Table 1) or two- to tenfold lower than DHA in blood of non-supplemented humans [46, 47, 49–52].
Due to n3 desaturase activity in fat-1 mice, endogenous EPA formation is higher compared to WT mice. However, further metabolism by mammalian enzymes again results in high DHA concentrations compared to EPA, e.g. 6.8% (DHA) vs. 0.16% (EPA) in liver, which is consistent with previous results [29]. Thus, n3 desaturase activity led to high DHA levels in fat-1 while EPA levels were in the low range.
Levels of intermediary formed n3-DPA were in the same range as EPA levels in fat-1 mice, supporting higher conversion rates of EPA to n3-DPA than of n3-DPA to DHA as observed in humans [54]. This finding is also supported by levels of n3-DPA in STD fed WT mice, which were higher compared to EPA levels in all investigated tissues and blood cells. However, it should be noted, that n3-DPA might also be formed in the process of DHA retroconversion: Following a single dose of 3 mg [13C]22:6-triacylglycerol to male rats (300 g), retroconversion was found to be 9% of the total plasma [13C]22:6 n3 (estimated by [13C]22:5 n3+[13C]20:5 n3 in plasma lipids) [55].
The n6/n3 ratio, a frequently used marker to describe the endogenous n3-PUFA status [19, 25, 28, 29, 32, 34, 56] was significantly reduced in fat-1 compared to WT animals on a standard diet. Although calculated slightly different, the observed n6/n3 ratios were comparable, however, a little higher than ratios observed by Kang et al. [26]. Feeding of WT animals with a diet enriched with 1% EPA and 1% DHA led to significantly lower n6/n3 ratios. It remains to be determined if this is also associated with a higher degree of protection. In a model of Parkinson’s disease this seems to be the case: Fat-1 mice did not show a neuroprotective effect [35], while n3-PUFA supplementation did [25]. The lack of efficiency in the fat-1 mouse model might be due to a lower modulation of the endogenous n3 and n6 PUFA profile compared to supplementation, as discussed by Bousquet et al. [35].
The %EPA+DHA in blood cells for the description of the endogenous n3-PUFA status is a modification of the omega-3 index which is discussed as a risk factor for cardiovascular diseases in humans [45, 57]. In fat-1 mice, %EPA+DHA in blood cells was 7.4±0.2%. Translating from mouse to man, these levels were in the range of an omega-3 index that has been shown to be correlated with a lower cardiovascular risk, e.g. for mortality from coronary heart disease (omega-3 index >8%, [45, 57]). %EPA+DHA in blood cells of fat-1 mice was also comparable to the omega-3 index observed in healthy volunteers following supplementation (0.46–1.6 mg/d EPA and 0.38–1.1 g/d DHA for up to 12 weeks) which ranged from 8.4–11% (calculated from the means presented for EPA and DHA as %of total FA [46, 49, 50]). However, the ratio of DHA to EPA in fat-1 mice was 7 while in humans after supplementation this ratio was on average two [46, 49, 50]. Thus, individual level of EPA and DHA were differently modulated in fat-1 mice compared to n3-PUFA supplementation with almost equal amounts of EPA and DHA. Keeping in mind that EPA and DHA effects might be different (e.g. EpDPEs were more effective in reducing pain than EpETEs in a model of pain associated with inflammation [58]), care must be taken when directly transferring results obtained from the fat-1 mouse model to humans. In response to higher endogenous level of n3-PUFA the share of n6-PUFA, such as ARA and related FA, was decreased which is consistent with previous findings in n3-PUFA fed animals on a dietary background high in LA [12, 21, 22] and fat-1 mice [27–29, 31–36]. Thus, EPA and DHA supplementation directly led to a notable displacement of ARA, which is a common explanation for their anti-inflammatory action [1–3]. This theory is based on the assumption that most ARA derived oxylipins act pro-inflammatory and that n3-PUFA compete for conversion by the same enzymes yielding, e.g., less potent, EPA derived PGE3 or LTB5 [1, 7]. However, it should be noted, that many n3-PUFA derived oxylipins also possess anti-inflammatory properties [3, 7]. A pro-inflammatory phenotype might thus also result from a lack of n3-PUFA oxylipins. Nevertheless, our study supports a replacement of ARA by n3-PUFA on the level of oxylipins, reflecting the changes observed for PUFA (Fig 3): While oxylipin levels in WT animals on a standard diet—in line with the high ARA level—were dominated by ARA derived oxylipins, the pattern shifted to n3-PUFA derived ones in fat-1 and n3-PUFA fed animals. This is consistent with previous results, showing similar trends for free oxylipins [38] and esterified OH-FA [37] in fat-1 mice as well as for esterified CYP metabolites in plasma and tissues of rodents [12]. It should be noted that in general the modulation of the oxylipin pattern was more pronounced for n3-supplemented than for fat-1 mice compared to WT mice on the standard diet. As a result, n3-PUFA feeding led overall to higher concentrations of n3-PUFA derived oxylipins and lower ARA derived eicosanoids (except in brain) compared to fat-1 mice.
As shown in Fig 3 for exemplary oxylipins, the product patterns of the LOX (5, 12 and 15) and CYP (hydroxylation and epoxygenation) pathways in plasma after n3-PUFA feeding were dominated by EPA and DHA oxylipins. This is somewhat remarkable, since ARA remained a dominating PUFA (6.7% ARA vs. 9.5% EPA vs. 11% DHA in plasma of WT-STD+n3 mice, Fig 1) and indicates a preferred formation of n3-PUFA oxylipins over ARA derived ones. The ratio of substrates and products for CYP and LOX (Fig 4) found in this study suggests that a preference of the enzymes could explain part of the effect. However, the moderate preference of, e.g., epoxygenating and hydroxylating CYP enzymes for DHA or EPA over ARA (ARA:EPA:DHA 1:4:1.5 for CYP2J2) [12, 59] alone seems not to sufficiently explain the massive difference observed in the overall pattern of oxylipins in plasma. Interestingly, in tissues, the dominance of LOX and CYP derived n3-PUFA oxylipins was less pronounced and concentrations were mostly in a similar range as ARA derived eicosanoids which indicates a tissue specific regulation. Nevertheless, our findings once more show that a moderate shift in the fatty acid pattern causes a pronounced increase in their oxidation products.
In contrast, only a slight shift in ARA derived eicosanoids to n3-PUFA oxylipins was observed for COX products in plasma and tissues. While absolute concentrations of COX derived ARA metabolites were similarly decreased as LOX and CYP products, EPA metabolite concentrations in fat-1 and n3-PUFA fed mice were still very low compared to their ARA derived counterparts. This can be explained by the low conversion rate of n3-PUFA by COX [60] leading on the one hand to low EPA-product formation and on the other hand to inhibition of ARA conversion by COXs.
It is interesting that compared to plasma and brain the highest concentrations of oxylipins from all three branches of the ARA cascade were found in colon, although the share of EPA, DHA and ARA among all FA in colon was low compared to other tissues and blood. Particularly COX metabolites were found in high concentrations, indicating an important role of these lipid mediators in homeostasis. Distinct differences in the oxylipin pattern were found between fat-1 and WT mice on the STD diet in colon. While relative changes in ARA and DHA metabolites were moderate, EPA metabolites from all enzymatic pathways were massively increased in fat-1 compared to WT-STD mice. This high increase may in part explain the effectiveness of the fat-1 model in colitis and colitis-associated colon cancer [27, 28, 32, 33]. While specialized pro-resolving mediators (SPM) derived from EPA were not found in colon tissue of fat-1 mice, 18-HEPE for example, as anti-inflammatory pathway indicator and precursor for E series resolvins [61] with unclear formation pathway, was highly increased.
In brain, only slight modulations in the fatty acid profile were found between the groups. Differences in oxylipins, however, were pronounced. This may have resulted from residual blood in the tissue, although highest care was taken during sample preparation. Nonetheless, the pronounced effect on brain oxylipin levels by n3-PUFA warrants further investigation.
Overall, the fatty acid and oxylipin pattern in fat-1 mice and n3 supplemented mice were modulated to higher concentrations of n3-PUFA and their metabolites in blood and tissues compared to WT mice on a standard sunflower diet. In general, the modulation in fat-1 mice was lower compared to n3-PUFA supplementation. The applicability of the fat-1 mouse model to investigate n3-PUFA associated effects, however, has been demonstrated in various disease models, e.g. colon inflammation or hepatitis [27–34, 36]. Since levels of EPA+DHA in blood cells of fat-1 mice were comparable to humans after supplementation, this model mimics n3-PUFA concentrations readily achievable with dietary supplementation. However, levels of the individual FA, particularly EPA were different, which might result in different physiological effects. An advantage of the fat-1 mouse model in comparison to feeding of n3-PUFA is the possibility of using one standard diet for the experimental groups. Therefore, confounding factors which might be introduced by the use of different experimental diets [56] or degradation of oxidation prone PUFA to potentially bioactive compounds [62] are reduced. However, for several questions, this model might not be suitable, particularly for the investigation of concentration dependent effects or the optimization of the dietary n6/n3 ratio needed for protection against diseases [63]. Here, feeding studies using defined concentrations of n3-PUFA in the diet in combination with an effective feeding regime are the most suitable approach. Another drawback of the fat-1 mouse model is that a discrimination between individual effects derived from DHA and EPA is not possible while the diet for feeding studies can be modulated accordingly.
Given the large amount of biologically relevant effects observed in studies using fat-1 mice, these results indicate that efficacy of n3-PUFA, and their derived oxylipins, might thus be found already in the context of rather low endogenous levels of n3-PUFA which could be easily achieved—and even surpassed—by dietary interventions. However, for some questions, e.g. the in depth and concentration dependent effects of (individual) n3-PUFA in vivo, feeding studies remain the model of choice.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(PDF)
(XLSX)
(XLSX)
(XLSX)
(PDF)
(PDF)
(PDF)
Abbreviations
- ALA
alpha linolenic acid
- ARA
arachidonic acid
- COX
cyclooxygenase
- CYP
cytochrom P450
- DHA
docosahexaenoic acid
- DiHDPE
dihydroxy docosapentaenoic acid
- DiH-FA
dihydroxy fatty acid
- EPA
eicosapentaenoic acid
- EpDPE
epoxy docosapentaenoic acid
- EpETE
epoxy eicosatetraenoic acid
- EpETrE
epoxy eicosatrienoic acid
- Ep-FA
epoxy fatty acid
- GC-FID
gas chromatography flame ionization detection
- HDHA
hydroxy docosahexaenoic acid
- HEPE
hydroxy eicosapentaenoic acid
- HETE
hydroxy eicosatetraenoic acid
- LA
linoleic acid
- LC-MS
liquid chromatography mass spectrometry
- LOQ
limit of quantification
- LOX
lipoxygenase
- LT
leukotriene
- MUFA
monounsaturated fatty acid
- n3-DPA
n3 docosapentaenoic acid
- OH-FA
hydroxy fatty acid
- PG
prostaglandin
- PUFA
polyunsaturated fatty acid
- Rv
resolvin
- SEM
standard error of the mean
- SFA
saturated fatty acid
- SPM
specialized pro-resolving lipid mediator
- Tx
thromboxane
- WT
wild type
Data Availability
All relevant data are included within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants of the German Research Foundation (DFG) to NHS (SCHE 1801) and KHW (WE 2908).
References
- 1.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et biophysica acta. 2015;1851(4):469–84. doi: 10.1016/j.bbalip.2014.08.010 . [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology. 2011;58(20):2047–67. doi: 10.1016/j.jacc.2011.06.063 . [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, et al. omega-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins & other lipid mediators. 2014;113–115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(16):6530–5. doi: 10.1073/pnas.1304321110 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. Journal of lipid research. 2009;50(6):1015–38. doi: 10.1194/jlr.R900004-JLR200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–5. doi: 10.1126/science.294.5548.1871 . [DOI] [PubMed] [Google Scholar]
- 7.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr. 2015;6(5):513–40. doi: 10.3945/an.114.007732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer metastasis reviews. 2011;30(3–4):525–40. doi: 10.1007/s10555-011-9315-y ; [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, et al. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):5093–7. doi: 10.1073/pnas.1101073108 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inceoglu B, Wagner KM, Yang J, Bettaieb A, Schebb NH, Hwang SH, et al. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11390–5. doi: 10.1073/pnas.1208708109 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annual review of pharmacology and toxicology. 2005;45:311–33. doi: 10.1146/annurev.pharmtox.45.120403.095920 . [DOI] [PubMed] [Google Scholar]
- 12.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. The Journal of biological chemistry. 2010;285(43):32720–33. doi: 10.1074/jbc.M110.118406 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation Omega-3 fatty acids and their resolvin/protectin mediators. Prostaglandins & other lipid mediators. 2012;97(3–4):73–82. doi: 10.1016/j.prostaglandins.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 15.Willenberg I, Ostermann AI, Schebb NH. Targeted metabolomics of the arachidonic acid cascade: current state and challenges of LC-MS analysis of oxylipins. Analytical and bioanalytical chemistry. 2015;407(10):2675–83. doi: 10.1007/s00216-014-8369-4 . [DOI] [PubMed] [Google Scholar]
- 16.Weylandt KH, Serini S, Chen YQ, Su HM, Lim K, Cittadini A, et al. Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. BioMed research international. 2015;2015:143109 doi: 10.1155/2015/143109 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduction, nutrition, development. 2005;45(5):581–97. doi: 10.1051/rnd:2005047 . [DOI] [PubMed] [Google Scholar]
- 18.Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Progress in lipid research. 2010;49(2):186–99. doi: 10.1016/j.plipres.2009.12.002 . [DOI] [PubMed] [Google Scholar]
- 19.Camuesco D, Galvez J, Nieto A, Comalada M, Rodriguez-Cabezas ME, Concha A, et al. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. The Journal of nutrition. 2005;135(4):687–94. . [DOI] [PubMed] [Google Scholar]
- 20.Nieto N, Torres MI, Rios A, Gil A. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. The Journal of nutrition. 2002;132(1):11–9. . [DOI] [PubMed] [Google Scholar]
- 21.Olson MV, Liu YC, Dangi B, Paul Zimmer J, Salem N Jr., Nauroth JM. Docosahexaenoic acid reduces inflammation and joint destruction in mice with collagen-induced arthritis. Inflamm Res. 2013;62(12):1003–13. doi: 10.1007/s00011-013-0658-4 . [DOI] [PubMed] [Google Scholar]
- 22.Volker DH, FitzGerald PE, Garg ML. The eicosapentaenoic to docosahexaenoic acid ratio of diets affects the pathogenesis of arthritis in Lew/SSN rats. The Journal of nutrition. 2000;130(3):559–65. . [DOI] [PubMed] [Google Scholar]
- 23.Ulu A, Harris TR, Morisseau C, Miyabe C, Inoue H, Schuster G, et al. Anti-inflammatory Effects of omega-3 Polyunsaturated Fatty Acids and Soluble Epoxide Hydrolase Inhibitors in Angiotensin-II-Dependent Hypertension. J Cardiovasc Pharm. 2013;62(3):285–97. doi: 10.1097/FJC.0b013e318298e460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris TR, Kodani S, Yang J, Imai DM, Hammock BD. An omega-3-enriched diet alone does not attenuate CCl4-induced hepatic fibrosis. The Journal of nutritional biochemistry. 2016;38:93–101. doi: 10.1016/j.jnutbio.2016.08.010 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bousquet M, Saint-Pierre M, Julien C, Salem N Jr., Cicchetti F, Calon F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson's disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22(4):1213–25. doi: 10.1096/fj.07-9677com . [DOI] [PubMed] [Google Scholar]
- 26.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427(6974):504 doi: 10.1038/427504a . [DOI] [PubMed] [Google Scholar]
- 27.Gravaghi C, La Perle KM, Ogrodwski P, Kang JX, Quimby F, Lipkin M, et al. Cox-2 expression, PGE(2) and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. The Journal of nutritional biochemistry. 2011;22(4):360–5. doi: 10.1016/j.jnutbio.2010.03.003 . [DOI] [PubMed] [Google Scholar]
- 28.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(30):11276–81. doi: 10.1073/pnas.0601280103 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmocker C, Weylandt KH, Kahlke L, Wang J, Lobeck H, Tiegs G, et al. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology. 2007;45(4):864–9. doi: 10.1002/hep.21626 . [DOI] [PubMed] [Google Scholar]
- 30.Weylandt KH, Nadolny A, Kahlke L, Kohnke T, Schmocker C, Wang J, et al. Reduction of inflammation and chronic tissue damage by omega-3 fatty acids in fat-1 transgenic mice with pancreatitis. Biochimica et biophysica acta. 2008;1782(11):634–41. doi: 10.1016/j.bbadis.2008.08.011 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weylandt KH, Krause LF, Gomolka B, Chiu CY, Bilal S, Nadolny A, et al. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-alpha. Carcinogenesis. 2011;32(6):897–903. doi: 10.1093/carcin/bgr049 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, et al. Reduced colitis-associated colon cancer in fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68(10):3985–91. doi: 10.1158/0008-5472.CAN-07-6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowak J, Weylandt KH, Habbel P, Wang J, Dignass A, Glickman JN, et al. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis. 2007;28(9):1991–5. doi: 10.1093/carcin/bgm166 [DOI] [PubMed] [Google Scholar]
- 34.Xia SH, Wang JD, He CW, Hong S, Serhan CN, Kang JX. Melanoma growth is reduced in fat-1 transgenic mice: Impact of omega-6/omega-3 essential fatty acids. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12499–504. doi: 10.1073/pnas.0605394103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bousquet M, Gue K, Emond V, Julien P, Kang JX, Cicchetti F, et al. Transgenic conversion of omega-6 into omega-3 fatty acids in a mouse model of Parkinson's disease. Journal of lipid research. 2011;52(2):263–71. doi: 10.1194/jlr.M011692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellenger J, Bellenger S, Bataille A, Massey KA, Nicolaou A, Rialland M, et al. High Pancreatic n-3 Fatty Acids Prevent STZ-Induced Diabetes in Fat-1 Mice: Inflammatory Pathway Inhibition. Diabetes. 2011;60(4):1090–9. doi: 10.2337/db10-0901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu CY, Smyl C, Dogan I, Rothe M, Weylandt KH. Quantitative Profiling of Hydroxy Lipid Metabolites in Mouse Organs Reveals Distinct Lipidomic Profiles and Modifications Due to Elevated n-3 Fatty Acid Levels. Biology. 2017;6(1). doi: 10.3390/biology6010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Astarita G, McKenzie JH, Wang B, Strassburg K, Doneanu A, Johnson J, et al. A protective lipidomic biosignature associated with a balanced omega-6/omega-3 ratio in fat-1 transgenic mice. PloS one. 2014;9(4):e96221 doi: 10.1371/journal.pone.0096221 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelton D, Lysecki C, Aukema H, Anderson B, Kang JX, Ma DWL. Endogenous synthesis of n-3 PUFA modifies fatty acid composition of kidney phospholipids and eicosanoid levels in the fat-1 mouse. Prostaglandins, leukotrienes, and essential fatty acids. 2013;89(4):169–77. doi: 10.1016/j.plefa.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 40.Kutzner L, Ostermann AI, Konrad T, Riegel D, Hellhake S, Schuchardt JP, et al. Lipid Class Specific Quantitative Analysis of n-3 Polyunsaturated Fatty Acids in Food Supplements. Journal of agricultural and food chemistry. 2017;65(1):139–47. doi: 10.1021/acs.jafc.6b03745 . [DOI] [PubMed] [Google Scholar]
- 41.Ostermann AI, Muller M, Willenberg I, Schebb NH. Determining the fatty acid composition in plasma and tissues as fatty acid methyl esters using gas chromatography—a comparison of different derivatization and extraction procedures. Prostaglandins, leukotrienes, and essential fatty acids. 2014;91(6):235–41. doi: 10.1016/j.plefa.2014.10.002 . [DOI] [PubMed] [Google Scholar]
- 42.Craske JD, Bannon CD. Letter to the Editor. J Am Oil Chem Soc. 1988;65(7):1190–1. [Google Scholar]
- 43.Willenberg I, Rund K, Rong S, Shushakova N, Gueler F, Schebb NH. Characterization of changes in plasma and tissue oxylipin levels in LPS and CLP induced murine sepsis. Inflamm Res. 2016;65(2):133–42. doi: 10.1007/s00011-015-0897-7 [DOI] [PubMed] [Google Scholar]
- 44.Ostermann AI, Willenberg I, Schebb NH. Comparison of sample preparation methods for the quantitative analysis of eicosanoids and other oxylipins in plasma by means of LC-MS/MS. Analytical and bioanalytical chemistry. 2015;407(5):1403–14. doi: 10.1007/s00216-014-8377-4 . [DOI] [PubMed] [Google Scholar]
- 45.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Preventive medicine. 2004;39(1):212–20. doi: 10.1016/j.ypmed.2004.02.030 . [DOI] [PubMed] [Google Scholar]
- 46.Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, et al. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. Journal of lipid research. 2014;55(6):1150–64. doi: 10.1194/jlr.M047357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. Journal of lipid research. 2012;53(8):1662–9. doi: 10.1194/jlr.P025577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostermann AI, Schebb NH. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food & function. 2017;8(7):2355–67. doi: 10.1039/c7fo00403f . [DOI] [PubMed] [Google Scholar]
- 49.Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins & other lipid mediators. 2014;113–115:21–9. doi: 10.1016/j.prostaglandins.2014.05.002 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuchardt JP, Schmidt S, Kressel G, Willenberg I, Hammock BD, Hahn A, et al. Modulation of blood oxylipin levels by long-chain omega-3 fatty acid supplementation in hyper- and normolipidemic men. Prostaglandins, leukotrienes, and essential fatty acids. 2014;90(2–3):27–37. doi: 10.1016/j.plefa.2013.12.008 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuchardt JP, Schneider I, Willenberg I, Yang J, Hammock BD, Hahn A, et al. Increase of EPA-derived hydroxy, epoxy and dihydroxy fatty acid levels in human plasma after a single dose of long-chain omega-3 PUFA. Prostaglandins & other lipid mediators. 2014;109–111:23–31. doi: 10.1016/j.prostaglandins.2014.03.001 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. Journal of lipid research. 2010;51(8):2074–81. doi: 10.1194/M900193-JLR200 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):5854–9. doi: 10.1073/pnas.092064799 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N Jr. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. Journal of lipid research. 2001;42(8):1257–65. . [PubMed] [Google Scholar]
- 55.Brossard N, Croset M, Pachiaudi C, Riou JP, Tayot JL, Lagarde M. Retroconversion and metabolism of [13C]22:6n-3 in humans and rats after intake of a single dose of [13C]22:6n-3-triacylglycerols. The American journal of clinical nutrition. 1996;64(4):577–86. . [DOI] [PubMed] [Google Scholar]
- 56.Kang JX. Fat-1 transgenic mice: A new model for omega-3 research. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77(5–6):263–7. doi: 10.1016/j.plefa.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Schacky C. The Omega-3 Index as a risk factor for cardiovascular diseases. Prostaglandins & other lipid mediators. 2011;96(1–4):94–8. doi: 10.1016/j.prostaglandins.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 58.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. Journal of lipid research. 2010;51(12):3481–90. doi: 10.1194/jlr.M006007 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westphal C, Konkel A, Schunck WH. Cytochrome p450 enzymes in the bioactivation of polyunsaturated Fatty acids and their role in cardiovascular disease. Advances in experimental medicine and biology. 2015;851:151–87. doi: 10.1007/978-3-319-16009-2_6 . [DOI] [PubMed] [Google Scholar]
- 60.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chemical reviews. 2011;111(10):5821–65. doi: 10.1021/cr2002992 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chemical reviews. 2011;111(10):5922–43. doi: 10.1021/cr100396c ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W, Yang H, Johnson D, Gensler C, Decker E, Zhang G. Chemistry and biology of omega-3 PUFA peroxidation-derived compounds. Prostaglandins & other lipid mediators. 2016. doi: 10.1016/j.prostaglandins.2016.12.004 . [DOI] [PubMed] [Google Scholar]
- 63.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2002;56(8):365–79. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(PDF)
(XLSX)
(XLSX)
(XLSX)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are included within the paper and its Supporting Information files.