Abstract
The determinants of immunological protection against Mycobacterium tuberculosis (M.tb) infection in humans are not known. Mycobacterial growth inhibition assays have potential utility as in vitro surrogates of in vivo immunological control of M.tb. We evaluated a whole blood growth inhibition assay in a setting with high burden of TB and aimed to identify immune responses that correlate with control of mycobacterial growth. We hypothesized that individuals with underlying M.tb infection will exhibit greater M.tb growth inhibition than uninfected individuals and that children aged 4 to 12 years, an age during which TB incidence is curiously low, will also exhibit greater M.tb growth inhibition than adolescents or adults. Neither M.tb infection status, age of the study participants, nor M.tb strain was associated with differential control of mycobacterial growth. Abundance and function of innate or T cell responses were also not associated with mycobacterial growth. Our data suggest that this assay does not provide a useful measure of age-associated differential host control of M.tb infection in a high TB burden setting. We propose that universally high levels of mycobacterial sensitization (through environmental non-tuberculous mycobacteria and/or universal BCG vaccination) in persons from high TB burden settings may impart broad inhibition of mycobacterial growth, irrespective of M.tb infection status. This sensitization may mask the augmentative effects of mycobacterial sensitization on M.tb growth inhibition that is typical in low burden settings.
Introduction
Almost a quarter of the world’s population is estimated to be infected with Mycobacterium tuberculosis (M.tb) [1], driving the most deadly epidemic due to an infectious agent. In 2015, approximately 10.4 million people developed active tuberculosis (TB) disease, resulting in 1.4 million deaths [2]. Several risk factors for developing TB disease following M.tb infection have been identified, including compromised immunity due to HIV co-infection, diabetes, gender and age [3]. Pre-adolescent children older than 4 years of age have much lower rates of progression to TB disease following infection than adolescents or adults [4–7]. Furthermore, in such children pulmonary TB typically manifests as a mild, pauci-bacillary disease, whereas adolescents and adults more commonly present with multi-bacillary disease, more pronounced lung infiltration with immunopathology and lung cavitation [6,7]. The low risk of TB in children within this “golden age of TB” presents an opportunity to study natural resistance and/or characteristics of successful immunity to M.tb in humans, which are not well understood.
The need for a TB vaccine that is more efficacious than Bacillus Calmette-Guérin (BCG) is urgent. Efforts to develop TB vaccines are hampered by the lack of reliable immunological correlates of protection or biomarkers that predict vaccine efficacy [8]. The adaptive immune response mediated by T cells is necessary for host control of M.tb infection [9]. As a consequence, frequencies of antigen-specific IFN-γ-producing and polyfunctional CD4 T cells co-expressing IFN-γ, TNF-α and IL-2 have been commonly measured markers of vaccine immunogenicity in preclinical and clinical testing of TB vaccine candidates. However, antigen-specific Th1 cells are not sufficient for complete protection in mouse models of TB [10,11]. In human infants, frequencies and cytokine co-expression profiles of BCG-specific CD4 and CD8 T cells did not correlate with risk of TB [12,13].
Efforts to screen and select the most promising novel vaccine candidates ideally require a functional assay that can measure immune-mediated control or killing of intracellular mycobacteria. A number of mycobacterial growth inhibition assays (MGIA) have been developed to measure functional inhibition of mycobacterial replication by blood leukocytes [14–21]. Animal studies in mice [22,23] and cattle [24] have shown that in vitro mycobacterial growth inhibition corresponded with control of in vivo mycobacterial replication, highlighting the potential utility of MGIAs as correlates of protection.
In humans, significantly lower mycobacterial growth in whole blood from BCG vaccinated individuals or those previously infected with M.tb, compared with blood from individuals not sensitized to mycobacteria, have been reported [17,19,20]. These studies were conducted with very small numbers of participants from settings with very low TB incidence. It is not known to which degree mycobacterial growth inhibition would be influenced by much higher levels of mycobacterial sensitization, as is typical in settings with high TB burden.
Here, we aimed to evaluate the utility of a whole blood based MGIA in a setting with a very high burden of TB and to identify functional and phenotypic characteristics of peripheral blood immune responses that correlate with mycobacterial growth inhibition.
We hypothesized that individuals with underlying M.tb infection or mycobacterial sensitization will exhibit greater M.tb growth inhibition than uninfected individuals. We also hypothesized that children in the golden age of TB, between 4 and 12 years, will exhibit greater M.tb growth inhibition than adolescents or adults.
Materials and methods
Ethics statement
This study was approved by the University of Cape Town Human Research Ethics Committee. Written informed consent was obtained from adults prior to enrollment. Children provided written informed assent while their legal guardians provided written informed consent prior to enrollment.
Study design and participants
This was a cross-sectional study comprising three cohorts of healthy, HIV-negative participants from the Worcester region in the Western Cape Province of South Africa, a setting with high burden of TB. Individuals with any acute or chronic disease, those taking immunos^#ressive medication, with a previous diagnosis of active TB disease or who currently or previously participated in a TB vaccine trial, were excluded. Women who were pregnant or lactating were also excluded from participation. We aimed to enroll adults aged 19 to 51 years into the 1st cohort; young adults aged 18 years into the 2nd cohort; and 8 year old children into a 3rd cohort, with equal numbers of M.tb-infected and uninfected participants into each cohort. M.tb-infection status was assessed using the QuantiFERON-TB Gold In-Tube Assay (QFT) (QIAgen), according to the manufacturer’s instructions. HIV infection was diagnosed by rapid HIV-antibody test. Some aspects of the adult cohort have been described before [25].
Mycobacterial strains
Mycobacterium bovis BCG (Danish 1331, Statens Serum Institute), M.tb H37Rv (donated by Christopher Sassetti, University of Massachusetts), M.tb HN878 and M.tb CDC1551 were grown in Middlebrook 7H9 liquid medium (Sigma-Aldrich). For each batch of mycobacterial stock, the mycobacterial inoculation volume was established by titration to achieve a time-to-positivity (TTP) of 6.5 days in mycobacterial growth indicator tubes (MGIT) in a BACTEC MGIT 960 machine (BD Biosciences), using a 3-parameter exponential decay curve, as previously optimized for immunological MGIA assays [19]. These volumes equated to inocula between 8.5x103 colony forming units [CFU]/mL and 2.4x104 CFU/mL.
Blood sample collection and whole blood mycobacterial growth inhibition assay
Venous blood was collected from participants directly into QFT tubes, and into sodium heparin tubes to be used for mycobacterial growth inhibition assays, whole blood stimulations and flow cytometry analysis.
MGIA were performed in duplicate in Sarstedt tubes containing 300μl fresh whole blood and 300μl RPMI, inoculated with each individual mycobacterial strain (average TTP standard deviation of 0.09 days). Tubes were incubated for 96 hours at 37°C with slow constant rotation. The host cells in each duplicate tube were then lysed with 1mL sterile water and bacilli sedimented at 15,300 x g for 10 minutes before being resuspended in 500μl Middlebrook 7H9 broth and the entire volume inoculated into BACTEC MGIT (MGIT, all from Becton Dickinson). MGIT tubes were incubated in a BACTEC MGIT 960 until growth was detected. For each run, a “direct-to-MGIT” positive control, comprising direct mycobacterial inoculation of MGIT tubes, and incubated in parallel with the blood was performed to control for minor variability in inoculum. The log change in viability in each whole blood culture was calculated as log (final)–log (initial), where initial and final are the apparent volumes of the inoculum and the completed culture based on their respective TTP values, calculated using the titration curve. Results are reported as log change per day of whole blood culture. Data management, standard curves, interpolations, and calculation of change in mycobacterial growth were done using an Excel macro provided by one of the co-authors (R.W), as previously described [21].
Whole blood intracellular cytokine staining assays
Whole blood intracellular cytokine staining (ICS) assays to measure T cell and innate responses were performed using a qualified assay as previously described [25–27], with slight modifications. Briefly, 0.5 mL of heparinized whole blood was stimulated with antigens (see below) at 37°C for either 7 or 3 hours (see below). Brefeldin A (10 μg/mL; Sigma-Aldrich) was then added and the blood further incubated for another 5 or 3 hours. Stimulated whole blood was treated with 2 mM EDTA (Sigma-Aldrich), red blood cells were then lysed and white blood cells fixed using FACS Lysing solution (BD Biosciences). Fixed cells were either stained for immediate flow cytometric analysis or cryopreserved for batch analysis. TruCount beads (BD Biosciences) were included to determine the absolute number of each cell subset per milliliter of whole blood.
12 hr T cell whole blood assay
Blood was incubated in the presence of co-stimulatory antibodies, anti-CD28 and anti-CD49d (at 1 μg/mL each; BD Biosciences, San Jose, CA) at 37°C for 7 hours with either lyophilized BCG Danish 1331 (1.2 X106 CFU/mL, resuspended from the vaccine vial in RMPI; Statens Serum Institute), 15-mer peptide pool of ESAT-6 and CFP-10 overlapping by 10 amino acids (1 μg/mL) or phytohemagglutinin (PHA; 5 μg/mL; Sigma-Aldrich) or was left unstimulated. Brefeldin A was then added, and the blood was incubated for a further 5 hours.
6 hr innate cell whole blood assay
Blood was incubated at 37°C for 3 hours with either lyophilized BCG (Danish strain 1331; 2 X106 colony forming units [CFU]/mL) or recombinant BCG expressing green fluorescent protein (BCG-GFP, Pasteur strain; 3.5 X105 colony forming units [CFU]/mL; donated by Muazzam Jacobs, University of Cape Town), Lipopolysaccharide (LPS; 100 ng/mL; Sigma-Aldrich) or was left unstimulated. Brefeldin A was then added, and the blood was incubated for a further 3 hours.
Whole blood intracellular cytokine staining (ICS) and flow cytometry
The following monoclonal antibodies were used for surface and intracellular staining for flow cytometry.
T cell panel
anti-CD3-BV421 (300434, from Biolegend), anti-CD4-QDot605 (Q10008, from Life Technologies), anti-CD8-PerCP-Cy5.5 (341050), anti-γδTCR-APC (55718), anti-IFN-γ-AF700 (557995), anti-IL-2-FITC (340448) all from BD Biosciences, anti-TNFα-PE-Cy7 (25-7349-82) and anti-IL-17 (CAP17)-PE (12–7178) both from eBiosciences.
Innate cell panel
anti-CD14-BV570 (301831, Biolegend), anti-CD11c-PerCP-Cy5.5 (337210), anti-HLA-DR-AlexaFlour700 (307626) all from Biolegend, anti-CD66a/c/e-QDot605 (342302, Biolegend, with in-house conjugation to QDot605, Q-22001), anti-CD16-PE-Cy5 (555408, from BD Biosciences), anti-IL-12-PE (554575), anti-TNF-α-PE-Cy7 (25-7349-82) both from eBioscience, anti-IL-10-PE (506804), anti-IL-6-APC (501112), anti-CD3-BV421 (300434), anti-CD19-BV421 (302233) and anti-CD335-BV421 (331913) all from Biolegend.
Cryopreserved, fixed white cells were washed in PBS and stained for 1 hour at 4°C with surface marker antibodies. For intracellular staining, cells were permeabilized with Perm/Wash buffer (BD Biosciences) and incubated for 1 hour at 4°C with antibodies for intracellular markers. Fixed cells were then acquired on a LSR II flow cytometer (BD Biosciences).
Data analysis
Flow cytometry data was analyzed using FlowJo software (v9.7.2; Tree Star). When appropriate, background subtraction for ICS data was performed in Pestle, and data sorting and visualization was done in SPICE [28]. Statistical analyses and graphing were performed using PRISM (GraphPad Software v6, San Diego, Calif.). Nonparametric unpaired statistical analysis (Mann-Whitney U test) was used to determine differences between participant groups. Spearman’s rank correlation coefficients were calculated for correlation analyses. A p-value of less than 0.05 was considered significant.
Results
Study participants
In total, 161 participants were enrolled into the three cohorts of this study, 55 adults aged 19 to 51 years, 58 young adults aged 18 years and 48 children aged 8 years. Participants in each age group were approximately equally stratified according to M.tb infection status by QuantiFERON, which shows excellent agreement with TST in this setting [29,30]. All participants had a history of BCG vaccination. The demographic characteristics of the study participants are summarized in Table 1.
Table 1. Demographic characteristics of enrolled participants.
| Adults (19–51 years) | Young adults | Children | ||||
|---|---|---|---|---|---|---|
| QFT+ | QFT- | QFT+ | QFT- | QFT+ | QFT- | |
| Participants, n | 29 | 26 | 37 | 21 | 20 | 28 |
| Female, n (%) | 19 (66) | 19 (73) | 21 (57) | 10 (48) | 9 (45) | 15 (54) |
| Mean age, years | 30 | 32 | 18 | 18 | 8 | 8 |
| Ethnicity, n (%) | ||||||
| Black African | 10 (35) | 4 (14) | 17 (46) | 6 (28) | 0 (0) | 3 (11) |
| Mixed race | 17 (59) | 13 (50) | 20 (54) | 14 (67) | 20 (100) | 25 (89) |
| Caucasian | 1 (3) | 9 (36) | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Indian | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Influence of M.tb infection status on mycobacterial growth
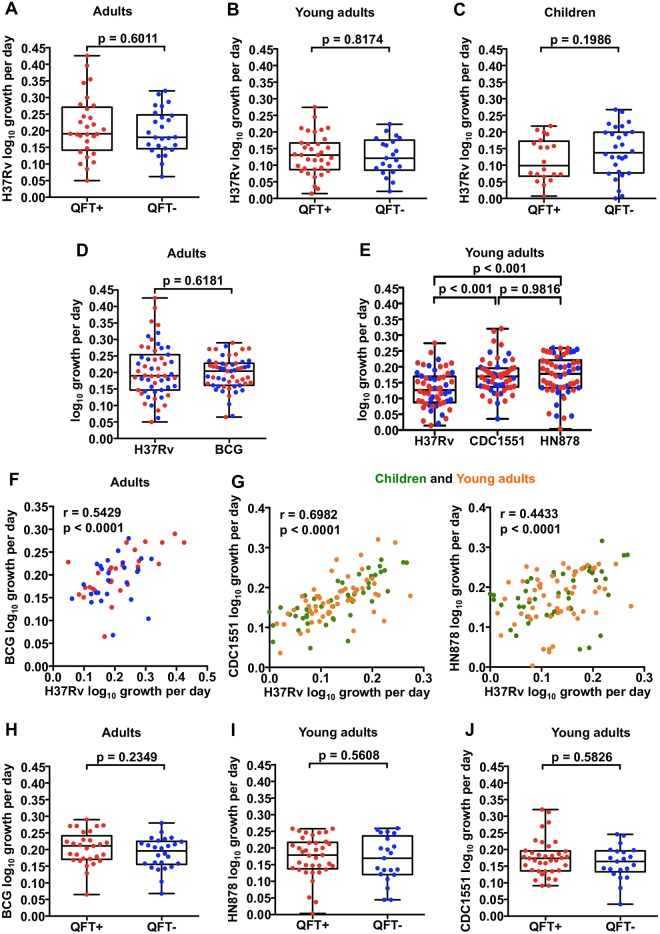
We sought to assess the utility of the whole blood MGIA to measure functional inhibition of mycobacterial replication by blood leukocytes in healthy individuals with or without M.tb infection. Since individuals with M.tb infection typically have higher, more activated and/or differentiated mycobacteria-specific T cell responses [25], we hypothesized that QFT+ individuals would control the growth of M.tb to a greater degree than uninfected individuals. However, no significant difference in M.tb H37Rv growth was observed between 26 QFT- and 29 QFT+ adults (Fig 1A). Similarly, M.tb H37Rv growth was also not different between the 21 QFT- and 37 QFT+ young adults (Fig 1B) or 28 QFT- and 20 QFT+ children (Fig 1C), and comparable to previous results in a non-endemic setting [21].
Fig 1. In vitro mycobacterial growth inhibition in M.tb-infected (QFT+) and uninfected (QFT-) individuals.
Inhibition of M.tb H37Rv growth by whole blood from QFT+ and QFT- adults (A), young adults (B) and children (C) assessed using a mycobacterial growth inhibition assay (MGIA). The growths of M. bovis BCG, M.tb H37Rv, M.tb isolate CDC1551 and M.tb isolate HN878 in the whole blood of adults (D) and young adults (E), respectively were compared. Spearman’s correlation analyses of the growth of different mycobacterial strains (F and G). The growth of BCG (H), HN878 and CDC1551 (I and J), was measured in the whole blood of adults and young adults, respectively. The red and blue circles represent QFT+ and QFT- individuals, respectively while the green and orange circles represent children and young adults respectively. The horizontal line represents the median, the boxes represent the interquartile range, and the whiskers represent the range. Differences in mycobacterial growth inhibition between both groups of individuals were evaluated with the Mann-Whitney test (shown P values).
We also explored if the magnitude of the M.tb-specific IFN-γ response measured by the QFT assay, a test for ESAT-6- and CFP-10-specific CD4 T cells [25], was associated with mycobacterial growth in vitro. No correlation between IFN-γ QFT response (a marker of M.tb infection) and inhibition of M.tb H37Rv growth was observed (S1 Fig).
Growth inhibition of different mycobacterial strains
To determine the importance of the inoculum strain in outcome of the mycobacterial growth inhibition assay, we performed parallel MGIAs using M.tb H37Rv and Mycobacterium bovis BCG in the adult cohort, or parallel assays using H37Rv, the virulent W/Beijing M.tb strain HN878, and the less virulent Euro-American M.tb strain, CDC1551. We hypothesized that growth of BCG, and M.tb CDC1551 would be inhibited to a greater degree than the virulent M.tb HN878 [31,32]. In the adult cohort, growth of H37Rv and BCG were not different (Fig 1D). In young adults, growth of H37Rv was significantly lower compared to CDC1551 and HN878 (Fig 1E). Nevertheless, growth of H37Rv correlated significantly with that of CDC1551 (r = 0.6982, p < 0.0001) and HN878 (r = 0.4433, p < 0.0001) in young adults and children (Fig 1G), and with BCG in adults (r = 0.5429, p < 0.0001) (Fig 1F). In QFT- and QFT+ participants, growth of BCG was similar in adults (Fig 1H), as was growth of HN878 and CDC1551 in young adults (Fig 1I and 1J). These data suggest that the inoculum strain is not a major determinant in mycobacterial growth inhibition assays.
Influence of age on mycobacterial growth
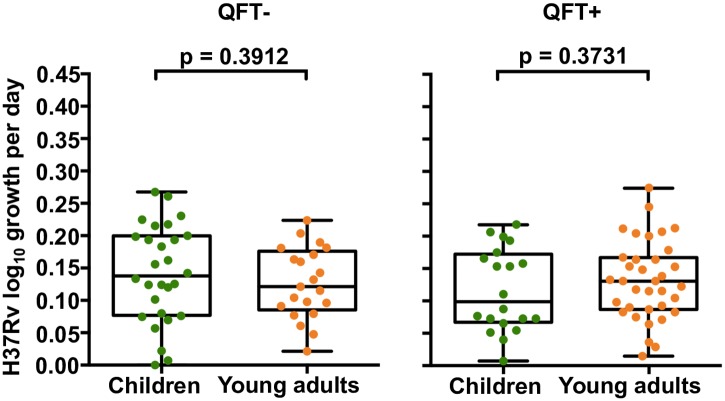
Next, we aimed to determine if inhibition of mycobacterial growth is different in children aged between 4 and 12 years, compared with young adults. In light of the low risk of TB disease in children within the Golden Age, we hypothesized that in vitro inhibition of M.tb H37Rv growth would be greater in 8 year old children compared to 18 year old young adults. However, no difference in M.tb H37Rv growth was observed between whole blood cultures from QFT- or QFT+ 8 and 18 year olds (Fig 2). M.tb CDC1551 and HN878 growth were also not different between QFT- 8 and 18 year olds (p = 0.413 and p = 0.328, respectively) and QFT+ 8 and 18 year olds (p = 0.260 and p = 0.297, respectively; data not shown).
Fig 2. In vitro mycobacterial growth inhibition in children and young adults.
Inhibition of M.tb H37Rv growth by whole blood from QFT- and QFT+ 8 and 18 year olds. Green and orange circles represent children and young adults, respectively. The horizontal line represents the median, the boxes represent the interquartile range, and the whiskers represent the range. Differences in mycobacterial growth inhibition between both groups of individuals were evaluated with the Mann-Whitney test (shown P values).
Innate cell function and control of mycobacterial growth
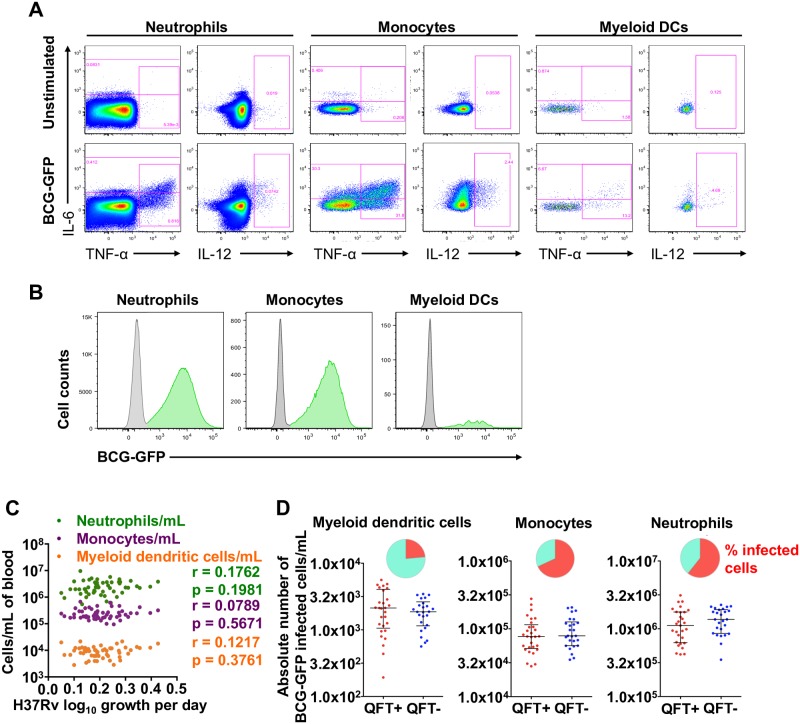
We hypothesized that whole blood growth inhibition would be mediated by innate cell responses to mycobacteria and sought to determine if frequencies and functions of innate cells were associated with mycobacterial growth. Neutrophil, monocyte and myeloid dendritic cell (mDC) numbers were enumerated, uptake of green fluorescent protein (GFP)-expressing BCG was measured and intracellular cytokine expression was measured by flow cytometry (Fig 3 and S2A Fig). No association was found between absolute counts of neutrophils, monocytes or myeloid dendritic cells and M.tb H37Rv growth in whole blood from adult participants (Fig 3C). Similarly, neither absolute counts nor relative proportions of GFP-positive innate cells were different between QFT+ and QFT- adults (Fig 3D) and did not correlate with M.tb H37Rv growth inhibition in whole blood (data not shown).
Fig 3. Detection of GFP-expressing BCG by innate cells and association between absolute numbers of innate cells and mycobacterial growth inhibition.
(A) Representative flow cytometry plot of IL-6, IL-12 and TNF-α cytokine expression by myeloid dentritic cells (mDCs), monocytes and neutrophils, measured in whole blood stimulated for 6 hours with BCG, BCG-GFP (shown) or LPS, relative to an unstimulated control sample. (B) Representative histograms indicating proportions of innate cells that phagocytosed BCG-GFP (green). (C) Absolute numbers of innate cell subsets per milliliter of unstimulated whole blood plotted against M.tb H37Rv growth. R and p values were calculated using Spearman’s correlation. (D) Absolute numbers of BCG-GFP-positive mDCs, monocytes or neutrophils per mL of whole blood in adult individuals, stratified by QFT status. The inclusion of TruCount beads during the cell staining steps of the innate whole blood assay allowed determination of the absolute number of each subset of cells per mL of whole blood. The red and blue circles represent QFT+ and QFT- adults, respectively. Horizontal lines represent medians and whiskers, the interquartile range. Differences in absolute counts of BCG-GFP-positive innate cells between the groups were evaluated with the Mann-Whitney test (shown P values). The pie charts show relative proportions of BCG-GFP-positive cells among each innate cell subset.
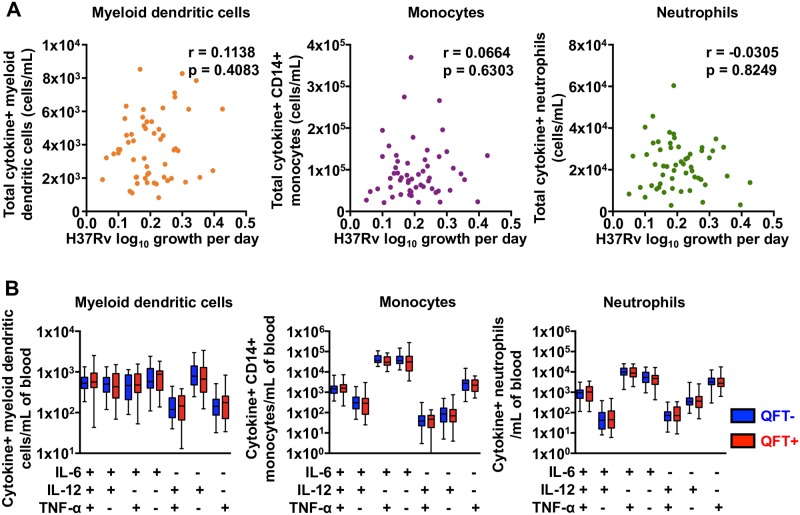
Finally, we determined if absolute counts of BCG-GFP-specific innate cells co-expressing IL-6, IL-12 and/or TNF-α were associated with control of M.tb growth. Again, no correlation between absolute numbers of total cytokine expressing innate cells and control of mycobacterial growth was observed (Fig 4A). Further, there was no difference in numbers of mDCs, monocytes and neutrophils expressing specific patterns of innate cytokines between QFT+ and QFT- adults (Fig 4B).
Fig 4. Mycobacteria-specific cytokine expression by innate cells in whole blood from adults and mycobacterial growth inhibition.
(A) Absolute numbers of total cytokine expressing innate cells per mL of whole blood plotted against M.tb H37Rv growth. R and p values were calculated using Spearman’s correlation analysis. (B) Numbers of BCG-specific mDCs, monocytes and neutrophils co-expressing IL-6, IL-12 and/or TNF-α in whole blood from QFT+ (red) and QFT- (blue) adults. Medians are represented by the horizontal line, interquartile ranges by the boxes, and ranges by the whiskers. The Mann-Whitney test was used to assess differences between QFT+ and QFT- adults and none were found to be different.
Antigen-specific T cell function and control of mycobacterial growth
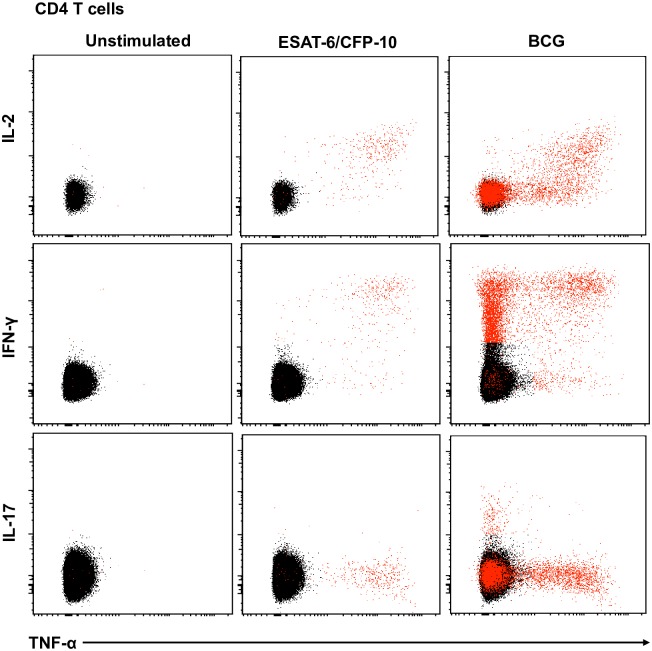
To explore if mycobacteria-specific CD4, CD8 or γδ T cell responses are associated with control of mycobacterial growth, whole blood from adults was stimulated with BCG or ESAT-6/CFP-10 peptide pools for 12 hours. Intracellular expression of IL-2, IFN-γ, TNF-α, and/or IL-17 was assessed by flow cytometry. The gating strategy and representative plots are shown in S2B Fig and Fig 5.
Fig 5. Representative flow cytometry plots of cytokine expression by CD4 T cells.
CD4 T cells expressing IFN-γ, IL-2, TNF-α and/or IL-17 upon stimulation with BCG or ESAT-6/CFP-10 peptide pools for 12 hours, compared to an unstimulated control sample. The plots represent cytokine-positive cells (red) overlayed onto the background of the entire CD4 T cell parent population (black). Similar assessment was also performed for CD8 and γδ T cells (data not shown).
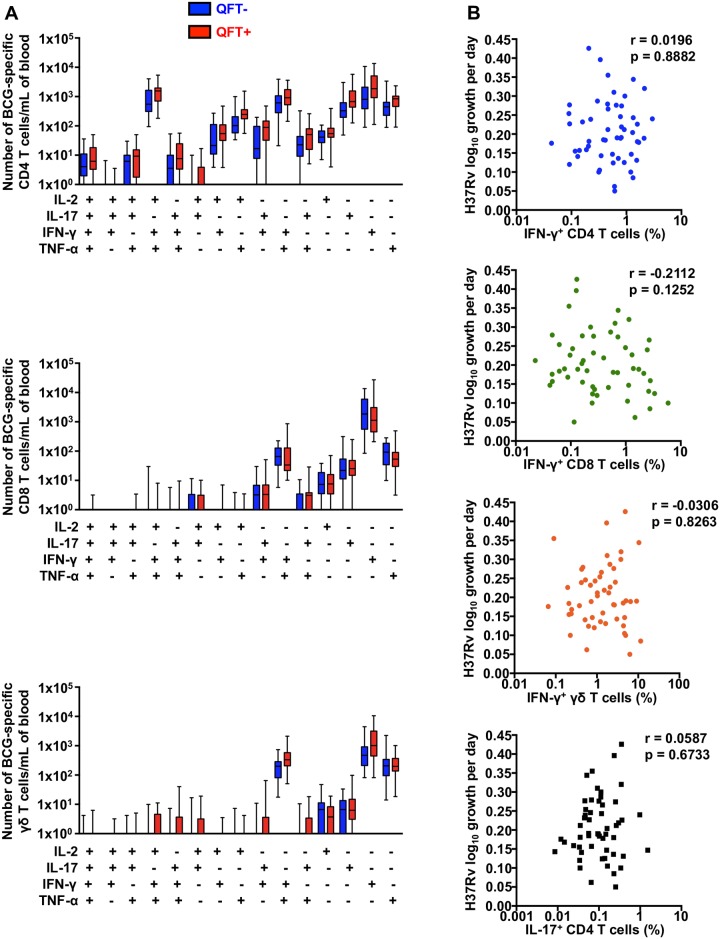
T cells are critical for control of M.tb growth in animal challenge models [33–38]. Because we did not observe differences in mycobacterial growth inhibition between M.tb-infected and uninfected individuals, we compared numbers of BCG-specific CD4, CD8 and γδ T cells between M.tb-infected and uninfected adults. None of the cytokine-expressing subsets of BCG-reactive CD4, CD8 and γδ T cells were found to be significantly different in abundance in blood from M.tb-infected and uninfected adults (Fig 6A).
Fig 6. Mycobacteria-specific T cells in whole blood from adults and mycobacterial growth inhibition.
(A) Absolute numbers of BCG-specific CD4, CD8 and γδ T cell subsets co-expressing IL-2, IFN-γ, TNF-α and/or IL-17, in whole bood from QFT+ (red) and QFT- (blue) adults. Medians are represented by the horizontal lines, interquartile ranges by the boxes, and ranges by the whiskers. The Mann-Whitney test was used to assess differences between QFT+ and QFT- adults and none were found to be different. (B) M.tb H37Rv growth plotted against frequencies of BCG-specific CD4, CD8 or γδ T cells expressing IFN-γ, or CD4 T cells expressing IL-17 in adults. R and p values were calculated using Spearman’s correlation analysis.
BCG-specific CD4 T cells expressed IFN-γ alone or co-expressed IFN-γ with TNF and IL-2. We also detected an IL-17 expressing population of BCG-specific CD4 T cells (Fig 6B). By contrast, BCG-stimulated CD8 and γδ T cells predominantly expressed only IFN-γ.
Frequencies of BCG-specific CD4 T cells, CD8 T cells, or γδ T cells expressing the Th1 cytokine IFN-γ did not correlate with inhibition of M.tb H37Rv growth in whole blood (Fig 6B). Similarly, IL-17 expression by BCG-specific CD4 T cells also was not correlated with growth inhibition of M.tb H37Rv.
Discussion
Twenty-three percent of the global population is currently infected with M.tb, yet only 3–10% of these individuals develop active TB disease within their lifetimes [1], providing compelling evidence that host immunity can successfully control bacterial growth [39]. This is further supported by a meta-analysis of 18 studies which estimated that individuals with latent M.tb infection had a 79% reduction in risk of TB disease after reinfection, than uninfected individuals [40]. It follows that M.tb infected persons must possess anti-mycobacterial immune responses that can control mycobacterial growth to a greater degree than uninfected persons. Consistent with this, studies from low TB burden settings showed that in vitro mycobacterial growth was significantly inhibited in whole blood from tuberculin-positive (M.tb-infected/previously exposed) individuals compared to tuberculin-negative individuals [17,19,20]. We determined whether M.tb infection would confer greater control of mycobacterial growth in persons from a high TB burden setting. Our results revealed that the capacity of whole blood to inhibit mycobacterial growth was not different between M.tb-infected and uninfected adults. Since prevalence of TB is typically about two-fold higher in males than females [2] we also investigated if gender was associated with in vitro mycobacterial growth, but observed no association. This finding was replicated in 2 other age groups comprising 8 year old children and 18 year old young adults.
The reasons for this result are not definitive but are likely important to understand. We showed that mycobacteria-specific T cell and innate cell responses were not significantly different between M.tb-infected and uninfected individuals, suggesting universally high immunological sensitization in the study population. Such high levels of immune responses to mycobacteria may derive from near universal BCG vaccination at birth, exposure to environmental mycobacteria and high levels of exposure to M.tb [30]. We postulate that this immunological sensitization may explain the equivalent growth inhibition observed in M.tb-infected and uninfected persons. A similar masking mechanism has been suggested to underlie the poor efficacy of BCG in settings with immunological sensitization to environmental mycobacteria [41].
We also observed no difference in mycobacterial growth inhibition in 8 year old children and young adults, despite the consistent epidemiological finding that pre-adolescent children above the age of 4 years are at significantly lower risk of TB compared with adolescents and adults [4,5]. This result may imply that factors other than immunity to mycobacteria underlie the age-associated risk of TB. However, in light of the broad dynamic range exhibited by the MGIA but limited ability of whole blood to restrict growth under these conditions, and the lack of differences between infected and uninfected persons, this interpretation is not strongly supported by our data. In fact, our finding that growth inhibition of M. bovis BCG and the M.tb strains H37Rv, HN878 and CDC1551, which exhibit a diverse range of virulence in animal models [31,32], correlated significantly, further questions the utility of the MGIA in our setting.
We report no association between IFN-γ QFT response and mycobacterial growth inhibition and did not identify any immunological outcomes that were associated with mycobacterial growth inhibition. This is consistent with findings from other human MGIA studies which reported no correlation between IFN-γ and mycobacterial growth inhibition [21,42,43]. IFN-γ is known to be essential for immunity against TB [44–46]. However, some murine studies also report no correlation between IFN-γ and protection against M.tb [10,11]. In other MGIA studies, IFN-γ was found to be essential in inhibiting mycobacterial growth in the murine model [23] and in the bovine model [24]. In addition to IFN-γ, previous studies have suggested an essential role for TNF-α [47–49] and IL-17 [50–52] in the anti-mycobacterial immune response. Vaccine induced protection in M.tb-infected mice correlated with IL-2 expressing CD4 T cells with a central memory phenotype [53,54].
However, depletion of CD4 and/or CD8 T cells in human [19,20], murine [55] and bovine [56] studies resulted in reduced ability to inhibit M.tb growth. Boom et al. also postulated that γδ T-cells may play a role in protective immunity against M.tb [57]. Studies by Worku and Hoft indicated that γδ T cells mediate superior levels of M.tb control [16,58].
To the best of our knowledge, this is the first time that the whole blood MGIA has been assessed in a setting with high burden of TB. Our results suggest that whole blood growth inhibition assays do not provide a useful measure of age-associated differential host control of M.tb infection in a high TB burden setting. We also found no innate or T cell correlates of control of mycobacerial growth. We propose that universally high levels of mycobacterial sensitization in persons from high TB burden settings may impart broad inhibition of mycobacterial growth, irrespective of M.tb-infection status. Such sensitization may occur through BCG vaccination given to nearly every South African infant at birth, although we cannot exclude possible exposure to environmental non-tuberculous mycobacteria. This is consistent with findings by Fletcher et al. who reported improved control of mycobacterial growth in vitro, following primary but not secondary vaccination with BCG [21]. Mycobacterial sensitization may thus mask the augmentative effects of mycobacterial sensitization on M.tb growth inhibition that is typical in low burden settings.
Our study has some limitations. The mycobacterial growth inhibition assay employed whole blood, yet observations of the periphery may not be representative of the lung, the predominant site of M.tb infection. Moreover, we could not measure all other potentially protective factors. For example, Worku and Hoft have previously reported that FAS, FAS ligand, perforin, granulysin and granzyme A were associated with mycobacterial growth inhibition [58]. In addition, haemoglobin (Hb) and iron levels present a potential confounding factor which could contribute to variability in mycobacterial growth when using whole blood. Tanner et al. reported an association between mean corpuscular haemoglobin (MCH) levels and in vitro BCG growth in whole blood from humans [59], an association that was not observed when using PBMC. In our study, participant MCH and Hb levels were not recorded and therefore we could not assess effects of MCH or Hb levels on mycobacterial growth. In addition, other factors have been shown to influence mycobacterial growth inhibition, including neutrophil counts [60], antibody-mediated responses [61,62] and complement [63], which may contribute to growth inhibition in whole blood assays but are not present in PBMC.
Finally, while MGIAs represent a level of systemic immune control of M.tb that is measurable in blood, it is clear that control of bacterial replication is determined at the level of individual granulomas and/or tissue sites of infection, which are typically highly heterogeneous [64–67] Regardless, a recent study by Fletcher et al. showed enhanced control of BCG using a PBMC-based MGIA 4–8 weeks after BCG vaccination in previously unsensitized individuals [21]. Efforts to further optimize mycobacterial growth inhibition assays and standardize experimental protocols between different laboratories have yielded promising results [68]. These data suggest that early prioritization of TB vaccine candidates for further clinical testing may be possible using blood-based MGIA assays in individuals selected with a lack of prior mycobacterial sensitization and from low TB burden settings.
Supporting information
M.tb H37Rv growth was measured using whole blood MGIA and correlated against IFN-γ in supernatants from QuantiFERON-TB Gold In-Tube assay (QFT). P- and r- values were calculated using the Spearman rank correlation test. The dotted vertical line represents the QFT cut-off (0.35 IU/mL of IFN-γ) for diagnosis of M.tb infection.
(TIF)
(A) The innate cell gating strategy included 1) a time gate for consistency during acquisition; 2) a gate to exclude doublets; 3) a gate to exclude antibody aggregates; 4) neutrophils were gated on CD66+ population; and 5) CD66- cells were further gated out to exclude T cells (CD3+), B cells (CD19+) and NK cells (CD335+), all on the same fluorochrome (BV421) used as a dump channel; 6) CD14+ monocytes and 7) CD14-CD11c+HLA-DR+ myeloid dendritic cells were identified from the remaining cells. (B) The T cell gating strategy included 1) a time gate for consistency during acquisition; 2) a gate to exclude antibody aggregates; 3) a lymphocyte gate based on expression of CD3+ T cells; 4) a gate to exclude doublets; 5) γδ T cells were identified based on expression of γδ-T cell receptor; the remaining cells were used to identify 6) and 7) CD4-CD8+ T cells, and 8) and 9) CD8-CD4+ T cells.
(TIF)
Acknowledgments
Ms. Bernadette Pienaar passed away before the submission of the final version of this manuscript. Dr. Adam Penn-Nicholson accepts responsibility for the integrity and validity of the data collected and analyzed.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
An author of this publication (Scriba) received funding from the European and Developing Countries Clinical Trials Partnership (EDCTP) through a project entitled “Inflammatory determinants of risk of tuberculosis disease” (grant #TA.2011.40200.010). However, EDCTP cannot accept any responsibility for information or views expressed herein. This work was also supported by a grant from the US FDA (FDA grant #IU18FD004012-01, PI Brennan). Aeras provided support in the form of salaries for author MJB, but did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Houben RMGJ Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). WHO | Global tuberculosis report 2016 [Internet]. WHO. 2016. http://www.who.int/tb/publications/global_report/en/
- 3.Lawn SD, Zumla AI. Tuberculosis. Lancet [Internet]. 2011;378(9785):57–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21420161 doi: 10.1016/S0140-6736(10)62173-3 [DOI] [PubMed] [Google Scholar]
- 4.Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker LG. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One. 2011;6(10):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser N, Zahnd C, Hermans S, Salazar-Vizcaya L, Estill J, Morrow C, et al. Tuberculosis in Cape Town: An age-structured transmission model. Epidemics. 2016;14:54–61. doi: 10.1016/j.epidem.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marais BJ, Gie RP, Schaaf HS E A. The natural history of childhood intrathoracic tuberculosis—A critical review of the pre-chemotherapy literature. Int J Tuberc L Dis. 2004;8(4):392–402. [PubMed] [Google Scholar]
- 7.Marais BJ, Donald PR, Gie RP, Schaaf HS, Beyers N. Diversity of disease in childhood pulmonary tuberculosis. Ann Trop Paediatr [Internet]. 2005;25(2):79–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15949195 doi: 10.1179/146532805X45665 [DOI] [PubMed] [Google Scholar]
- 8.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol [Internet]. 2011;11(5):343–54. Available from: http://www.nature.com/nri/journal/v11/n5/full/nri2960.html%5Cn http://www.nature.com/nri/journal/v11/n5/pdf/nri2960.pdf%5Cn http://www.ncbi.nlm.nih.gov/pubmed/21475309 doi: 10.1038/nri2960 [DOI] [PubMed] [Google Scholar]
- 9.Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264(1):74–87. doi: 10.1111/imr.12274 [DOI] [PubMed] [Google Scholar]
- 10.Elias D, Akuffo H, Britton S. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans R Soc Trop Med Hyg. 2005;99(5):363–8. doi: 10.1016/j.trstmh.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Mittrücker H-W, Steinhoff U, Köhler A, Krause M, Lazar D, Mex P, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A [Internet]. 2007;104(30):12434–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17640915 doi: 10.1073/pnas.0703510104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagina BMN, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guérin vaccination of newborns. Am J Respir Crit Care Med. 2010;182(8):1073–9. doi: 10.1164/rccm.201003-0334OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–8. doi: 10.1016/S0140-6736(13)60177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng SH, Walker L, Poole J, Aber VR, Walker KB, Mitchison D a, et al. Demonstration of increased anti-mycobacterial activity in peripheral blood monocytes after BCG vaccination in British school children. Clin Exp Immunol [Internet]. 1988;74(1):20–5. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1541699&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 15.Silver RF, Li Q, Boom WH, Ellner JJ. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol [Internet]. 1998;160(5):2408–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9498784 [PubMed] [Google Scholar]
- 16.Worku S, Hoft DF. In vitro measurement of protective mycobacterial immunity: antigen-specific expansion of T cells capable of inhibiting intracellular growth of bacille Calmette-Guérin. Clin Infect Dis. 2000;30 Suppl 3(Suppl 3):S257–61. [DOI] [PubMed] [Google Scholar]
- 17.Kampmann B, Gaora PO, Snewin V a, Gares MP, Young DB, Levin M. Evaluation of human antimycobacterial immunity using recombinant reporter mycobacteria. J Infect Dis. 2000;182(3):895–901. doi: 10.1086/315766 [DOI] [PubMed] [Google Scholar]
- 18.Wallis RS, Palaci M, Vinhas S, Hise AG, Ribeiro FC, Landen K, et al. A whole blood bactericidal assay for tuberculosis. J Infect Dis [Internet]. 2001;183(8):1300–3. Available from: http://jid.oxfordjournals.org/content/183/8/1300%5Cn http://jid.oxfordjournals.org/content/183/8/1300.full%5Cn http://jid.oxfordjournals.org/content/183/8/1300.full.pdf%5Cn http://www.ncbi.nlm.nih.gov/pubmed/11262217 doi: 10.1086/319679 [DOI] [PubMed] [Google Scholar]
- 19.Cheon S-H, Kampmann B, Hise AG, Phillips M, Song H-Y, Landen K, et al. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin Diagn Lab Immunol [Internet]. 2002;9(4):901–7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=120034&tool=pmcentrez&rendertype=abstract doi: 10.1128/CDLI.9.4.901-907.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tena GN, Young DB, Eley B, Henderson H, Nicol MP, Levin M, et al. Failure to control growth of mycobacteria in blood from children infected with human immunodeficiency virus and its relationship to T cell function. J Infect Dis. 2003;187(10):1544–51. doi: 10.1086/374799 [DOI] [PubMed] [Google Scholar]
- 21.Fletcher HA, Tanner R, Wallis RS, Meyer J, Manjaly ZR, Harris S, et al. Inhibition of mycobacterial growth in vitro following primary but not secondary vaccination with Mycobacterium bovis BCG. Clin Vaccine Immunol. 2013;20(11):1683–9. doi: 10.1128/CVI.00427-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra M, Yang AL, Lim J, Kolibab K, Derrick S, Cadieux N, et al. Development of a murine mycobacterial growth inhibition assay for evaluating vaccines against Mycobacterium tuberculosis. Clin Vaccine Immunol [Internet]. 2009;16(7):1025–32. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2708400&tool=pmcentrez&rendertype=abstract doi: 10.1128/CVI.00067-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsay L, Matsumiya M, Tanner R, Poyntz H, Griffiths KL, Stylianou E, et al. Mycobacterial growth inhibition in murine splenocytes as a surrogate for protection against Mycobacterium tuberculosis (M. tb). Tuberculosis. 2013;93(5):551–7. doi: 10.1016/j.tube.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 24.Carpenter E, Fray L, Gormley E. Cellular responses and Mycobacterium bovis BCG growth inhibition by bovine lymphocytes. Immunol Cell Biol. 1997;75(6):554–60. doi: 10.1038/icb.1997.86 [DOI] [PubMed] [Google Scholar]
- 25.Penn-Nicholson A, Nemes E, Hanekom WA, Hatherill M, Scriba TJ. Mycobacterium tuberculosis-specific CD4 T cells are the principal source of IFN-γ in QuantiFERON assays in healthy persons. Tuberculosis (Edinb) [Internet]. 2015. May 1 [cited 2017 Feb 9];95(3):350–1. Available from: http://www.tuberculosisjournal.com/article/S1472979214206238/fulltext [DOI] [PubMed] [Google Scholar]
- 26.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods. 2004;291(1–2):185–95. doi: 10.1016/j.jim.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 27.Kagina BM, Mansoor N, Kpamegan EP, Penn-Nicholson A, Nemes E, Smit E, et al. Qualification of a whole blood intracellular cytokine staining assay to measure mycobacteria-specific CD4 and CD8 T cell immunity by flow cytometry. J Immunol Methods. 2015;417:22–33. doi: 10.1016/j.jim.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roederer M, Nozzi JL, Nason MC. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytom Part A. 2011;79 A(2):167– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahomed H, Hawkridge T, Verver S, Geiter L, Hatherill M, Abrahams D, et al. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis [Internet]. 2011;15(3):331–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21333099 [PubMed] [Google Scholar]
- 30.Andrews JR, Hatherill M, Mahomed H, Hanekom WA, Campo M, Hawn TR, et al. The dynamics of QuantiFERON-TB Gold in-Tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med. 2015;191(5):584–91. doi: 10.1164/rccm.201409-1704OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subbian S, Tsenova L, Yang G, O’Brien P, Parsons S, Peixoto B, et al. Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol [Internet]. 2011;1(4):110016 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3352086&tool=pmcentrez&rendertype=abstract doi: 10.1098/rsob.110016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manca C, Tsenova L, Barry CE, Bergtold A, Freeman S, Haslett PAJ, et al. Mycobacterium tuberculosis CDC1551 Induces a More Vigorous Host Response In Vivo and In Vitro, But Is Not More Virulent Than Other Clinical Isolates. J Immunol [Internet]. 1999;162(11):6740–6. Available from: http://www.jimmunol.org/cgi/content/abstract/162/11/6740 [PubMed] [Google Scholar]
- 33.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992;89(24):12013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol [Internet]. 1999;162(9):5407–16. Available from: http://www.jimmunol.org/content/162/9/5407.full [PubMed] [Google Scholar]
- 35.Lawn SD, Myer L, Edwards D, Bekker L-G, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–25. doi: 10.1097/QAD.0b013e32832d3b6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green AM, Difazio R, Flynn JL. IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J Immunol [Internet]. 2013;190(1):270–7. Available from: http://www.jimmunol.org/content/190/1/270.full doi: 10.4049/jimmunol.1200061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, Klein E, et al. CD4 T Cell Depletion Exacerbates Acute Mycobacterium tuberculosis While Reactivation of Latent Infection Is Dependent on Severity of Tissue Depletion in Cynomolgus Macaques. AIDS Res Hum Retroviruses [Internet]. 2012;28(12):1693–702. Available from: http://online.liebertpub.com/doi/abs/10.1089/aid.2012.0028 doi: 10.1089/AID.2012.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao S, Huang D, Chen CY, Halliday L, Wang RC, Chen ZW. CD4+ T Cells Contain Early Extrapulmonary Tuberculosis (TB) Dissemination and Rapid TB Progression and Sustain Multieffector Functions of CD8+ T and CD3- Lymphocytes: Mechanisms of CD4+ T Cell Immunity. J Immunol [Internet]. 2014. March 1 [cited 2017 Feb 9];192(5):2120–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24489088 doi: 10.4049/jimmunol.1301373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philips J a., Ernst JD. Tuberculosis Pathogenesis and Immunity. Annu Rev Pathol Mech Dis. 2012;7(1):353–84. [DOI] [PubMed] [Google Scholar]
- 40.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54(6):784–91. doi: 10.1093/cid/cir951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine PEM, Carneiro I a M, Milstein JB, Clements CJ. Issues relating to the use of BCG in immunization programmes—A discussion document. 1999;1–45.
- 42.Hoft DF, Worku S, Kampmann B, Whalen CC, Ellner JJ, Hirsch CS, et al. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J Infect Dis. 2002;186(10):1448–57. doi: 10.1086/344359 [DOI] [PubMed] [Google Scholar]
- 43.Kampmann B, Tena GN, Mzazi S, Eley B, Young DB, Levin M. Novel human in vitro system for evaluating antimycobacterial vaccines. Infect Immun. 2004;72(11):6401–7. doi: 10.1128/IAI.72.11.6401-6407.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med [Internet]. 1993;178(6):2243–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8245795%5Cn http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2191280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-?? immunity. Vol. 26, Seminars in Immunology. 2014. p. 454–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity [Internet]. 1995;2(6):561–72. Available from: http://www.sciencedirect.com/science/article/pii/1074761395900012%5Cn http://www.ncbi.nlm.nih.gov/pubmed/7540941 [DOI] [PubMed] [Google Scholar]
- 48.Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62(2):340–50. doi: 10.1002/art.27271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha- neutralizing agent. N Engl J Med [Internet]. 2001;345(15):1098–104. Available from: m:%5CProject Team Management%5CPreclinical Programs%5CScientific Information%5CPDF Articles%5CNEJM345(15)1098.pdf doi: 10.1056/NEJMoa011110 [DOI] [PubMed] [Google Scholar]
- 50.Khader S a, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449 [DOI] [PubMed] [Google Scholar]
- 51.Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, et al. Interleukin-23 dependent IL-17 drives Th1 responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42(2):364–73. doi: 10.1002/eji.201141569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scriba TJ, Kalsdorf B, Abrahams D-A, Isaacs F, Hofmeister J, Black G, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol [Internet]. 2008;180(3):1962–70. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2219462&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindenstrom T, Knudsen NP, Agger EM, Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol [Internet]. 2013;190(12):6311–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23677471 doi: 10.4049/jimmunol.1300248 [DOI] [PubMed] [Google Scholar]
- 54.Woodworth JS, Aagaard CS, Hansen PR, Cassidy JP, Agger EM, Andersen P. Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation. J Immunol [Internet]. 2014;192(7):3247–58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24574499 doi: 10.4049/jimmunol.1300283 [DOI] [PubMed] [Google Scholar]
- 55.Cowley SC, Elkins KL. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J Immunol. 2003;171(9):4689–99. [DOI] [PubMed] [Google Scholar]
- 56.Denis M, Wedlock DN, Buddle BM. Ability of T cell subsets and their soluble mediators to modulate the replication of Mycobacterium bovis in bovine macrophages. Cell Immunol. 2004;232(1–2):1–8. doi: 10.1016/j.cellimm.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 57.Boom WH. Gammadelta T cells and Mycobacterium tuberculosis. Microbes Infect [Internet]. 1999;1(3):187–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10801229 [DOI] [PubMed] [Google Scholar]
- 58.Worku S, Hoft DF. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect Immun. 2003;71(4):1763–73. doi: 10.1128/IAI.71.4.1763-1773.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanner R, O’Shea MK, White AD, Müller J, Harrington-Kandt R, Matsumiya M, et al. The influence of haemoglobin and iron on in vitro mycobacterial growth inhibition assays. Sci Rep [Internet]. 2017;7(March):43478 Available from: http://www.nature.com/articles/srep43478%5Cn http://www.ncbi.nlm.nih.gov/pubmed/28256545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117(7):1988–94. doi: 10.1172/JCI31097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen T, Blanc C, Eder AZ, Prados-Rosales R, Souza ACO, Kim RS, et al. Association of Human Antibodies to Arabinomannan with Enhanced Mycobacterial Opsonophagocytosis and Intracellular Growth Reduction. J Infect Dis. 2016;214(2):300–10. doi: 10.1093/infdis/jiw141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prados-Rosales R, Carreño L, Cheng T, Blanc C, Weinrick B, Malek A, et al. Enhanced control of Mycobacterium tuberculosis extrapulmonary dissemination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog. 2017;13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol [Internet]. 1990. [cited 2017 Jul 28];144(7). Available from: http://www.jimmunol.org/content/144/7/2771.long [PubMed] [Google Scholar]
- 64.Gideon HP, Phuah JY, Myers AJ, Bryson BD, Rodgers MA, Coleman MT, et al. Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization. PLoS Pathog [Internet]. 2015;11(1):1–28. Available from: http://dx.doi.org/10.1371/journal.ppat.1004603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Via LE, England K, Weiner DM, Schimel D, Zimmerman MD, Dayao E, et al. A sterilizing tuberculosis treatment regimen is associated with faster clearance of bacteria in cavitary lesions in marmosets. Antimicrob Agents Chemother. 2015;59(7):4181–9. doi: 10.1128/AAC.00115-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Via LE, Schimel D, Weiner DM, Dartois V, Dayao E, Cai Y, et al. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [18F]2-fluoro-deoxy-D-glucose positron emission tomography and computed tomography. Antimicrob Agents Chemother. 2012;56(8):4391–402. doi: 10.1128/AAC.00531-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med [Internet]. 2014;20(1):75–9. Available from: http://www.nature.com/doifinder/10.1038/nm.3412 doi: 10.1038/nm.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zelmer A, Tanner R, Stylianou E, Damelang T, Morris S, Izzo A, et al. A new tool for tuberculosis vaccine screening: Ex vivo Mycobacterial Growth Inhibition Assay indicates BCG-mediated protection in a murine model of tuberculosis. BMC Infect Dis [Internet]. 2016. December 12 [cited 2017 May 25];16(1):412 Available from: http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-016-1751-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
M.tb H37Rv growth was measured using whole blood MGIA and correlated against IFN-γ in supernatants from QuantiFERON-TB Gold In-Tube assay (QFT). P- and r- values were calculated using the Spearman rank correlation test. The dotted vertical line represents the QFT cut-off (0.35 IU/mL of IFN-γ) for diagnosis of M.tb infection.
(TIF)
(A) The innate cell gating strategy included 1) a time gate for consistency during acquisition; 2) a gate to exclude doublets; 3) a gate to exclude antibody aggregates; 4) neutrophils were gated on CD66+ population; and 5) CD66- cells were further gated out to exclude T cells (CD3+), B cells (CD19+) and NK cells (CD335+), all on the same fluorochrome (BV421) used as a dump channel; 6) CD14+ monocytes and 7) CD14-CD11c+HLA-DR+ myeloid dendritic cells were identified from the remaining cells. (B) The T cell gating strategy included 1) a time gate for consistency during acquisition; 2) a gate to exclude antibody aggregates; 3) a lymphocyte gate based on expression of CD3+ T cells; 4) a gate to exclude doublets; 5) γδ T cells were identified based on expression of γδ-T cell receptor; the remaining cells were used to identify 6) and 7) CD4-CD8+ T cells, and 8) and 9) CD8-CD4+ T cells.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.