Abstract
Purpose
A comparatively high prevalence of co-morbidities among African-American/Blacks (AA/B) has been implicated in disparate survival in breast cancer. There is a scarcity of data, however, if this effect persists when accounting for the adverse triple-negative breast cancer (TNBC) subtype which occurs at three-fold the rate in AA/B compared to white breast cancer patients.
Methods
We reviewed charts of 214 white and 202 AA/B breast cancer patients in the NCI-SEER Connecticut Tumor Registry who were diagnosed in 2000-07. We employed the Charlson Co-Morbidity Index (CCI), a weighted 17-item tool to predict risk of death in cancer populations. Cox Survival Analyses estimated hazard ratios (HR) for all-cause mortality in relation to TNBC and CCI adjusting for clinicopathological factors.
Results
Among patients with SEER-Local Stage, TNBC increased the risk of death (HR=2.18, 95% CI 1.14-4.16), which was attenuated when the CCI score was added to the model (Adj. HR=1.50, 95% CI 0.74-3.01). Conversely, the adverse impact of the CCI score persisted when controlling for TNBC (Adj. HR=1.49, 95% CI 1.29-1.71; per one point increase). Similar patterns were observed in SEER-Regional Stage but estimated HRs were lower. AA/B patients with a CCI score of ≥3 had a significantly higher risk of death compared to AA/B patients without comorbidities (Adj. HR=5.65, 95% CI 2.90-11.02). A lower and non-significant effect was observed for whites with a CCI of ≥3 (Adj. HR=1.90, 95% CI 0.68-5.29).
Conclusions
Co-morbidities at diagnosis increase risk of death independent of TNBC, and AA/B patients may be disproportionately at risk.
Keywords: Breast Cancer, TNBC, Co-Morbidity, Cancer Survival Disparity, Charlson Co-Morbidity Index
Introduction
Despite recent improvements in mortality for both African-American/Black (AA/B) and white breast cancer patients, substantially poorer survival continues for AA/B patients.1 Numerous reports have linked this disparity to reduced access to health care2 or delays in treatment.2-4 On the other hand, Albain et al. (2009) reported that AA/B ethnicity was an independent predictor of breast cancer death even under structured conditions of common treatment in a large national, cooperative clinical trial.5 Hence, there remains a need to explore putative contributing factors such as underlying aggressive disease or unmeasured clinical factors.
Adverse tumor biology has been suggested as another explanatory factor in the survival gap.2 AA/B breast cancer patients tend to present at more advanced stages and at younger ages6, 7 lending support to the hypothesis about aggressive disease. Some have suggested that advanced stage at diagnosis might be unrelated to mammography utilization given recent reports of comparable rates of annual or bi-annual screening between whites and blacks.8 Additionally, survival disparities have been reported within each stage.6 A promising biologic focus in disparities research is the adverse triple negative breast cancer (TNBC) in which AA/B have a three-fold greater prevalence compared to whites.9, 10 Defined by the simultaneous lack of expression of estrogen, progesterone and HER2 receptors in breast cancer cells, TNBC has been found to confer a significantly worse prognosis compared with other subtypes.9, 11 In a recent population-based study, we found that, while TNBC status was predictive of reduced survival for the sample as a whole, AA/B patients with regionally advanced disease had significantly reduced survival compared to whites independent of TNBC status; no racial disparity in survival was found among those with local disease.10
Our prior findings of a survival disadvantage among AA/B patients with advanced disease, whether or not tumors express the aggressive TNBC phenotype, suggests to us that clinical factors could be elevating risk of death. In recent years, co-morbidities at diagnosis have been implicated in cancer outcome disparities including death.12 These are conditions unrelated to breast cancer, such as diabetes or heart disease, which also pose a threat to overall survival. Studies have shown that African-Americans tend to have greater prevalence of obesity, hypertension, diabetes and diabetes-related complications, and renal disease.12-15 Inadequate control of comorbidities has been shown to adversely affect cancer treatment, as exemplified in the Black white Cancer Survival Study.16 Given the disproportionately greater prevalence of both co-morbidities and TNBC in the AA/B population, we investigated if both factors for death retained independent prognostic importance when assessed together.
Methods
Study Population
We conducted a pilot medical chart review in a random sample of 432 female breast cancer patients derived from our parent study of 2264 patients diagnosed with primary breast cancer (ICD-O-3 C50.0-C50.9) between January 1, 2000 and December 31, 2007 in the State of Connecticut. Data were obtained from the Connecticut Tumor Registry (CTR), a participant site in the NCI-SEER program. The parent study includes all AA/B patients diagnosed during the study period and a comparably sized random sample of white patients as described elsewhere.10, 17 Reasons for exclusion were duplicate records or discrepant information about racial status. In statistical analyses, we excluded 15 patients for whom there were missing data on age, TNBC status, vital status, or SEER Summary Stage for a final sample size of 416 (white = 214, AA/B=202). Median follow-up time among those who were alive at the end of the study was slightly higher for AA/B patients than whites (7.6 yrs vs 7.0 yrs, p=0.01). Access to medical records was approved by the Institutional Review Boards at University of Connecticut Health Center, Yale Cancer Center, Hartford Hospital, and the Human Investigation Committee at the Connecticut Department of Public Health.
Clinicopathological Data
Information in the CTR database includes: ER, PR, age at diagnosis, SEER Summary Stage (local, regional, and distant), ICD-O-3 histologic subtypes, tumor grade, number of positive axillary lymph nodes, and tumor size (cm). Local stage is defined in SEER as invasive cancer confined to the breast; and, regional and distant stages are defined, respectively, as cancer detected to have spread to the axillary lymph nodes or contiguous tissue, and disease that has spread to other organs. Information about first-course of chemotherapy is available in the CTR database, although in recent years SEER no longer makes this information available in the public dataset due to substantial missing data and unreliability of the information.18 TNBC status was derived from both the CTR database (i.e., ER, PR) and abstraction of summary pathology reports (i.e., HER2) at the registry as described in our previous investigations using this study sample.10, 17
Co-Morbidity Information
Medical conditions were abstracted using the validated Charlson Co-Morbidity Index (CII), a weighted list of 17 items developed in 198719 and a prominent tool in cancer research.20 The CCI includes: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular disease, dementia, chronic lung disease, rheumatologic disease, peptic ulcer disease, mild liver disease, diabetes without complications, diabetes with complications, hemiplegia, neoplasia, moderate/severe liver disease, metastatic disease, human immunodeficiency virus, and renal disease. A cumulative score is calculated based on a no (0) or yes (1) for each condition, and weighted according to a specific protocol.19 Briefly, the weight applied to a particular condition reflects the associated hazard ratio of death within one-year of cancer diagnosis. Due to emerging evidence of the prognostic importance of hypertension in distinguishing mortality risk in cancer survival disparities,21 we followed an approach used in Braithwaite21 and Tammemagi12 to adapt the CCI by including high blood pressure (CCI+HBP) as a co-morbidity by assigning an additional point. Scores for the CCI and CCI+HBP indices were employed in statistical analyses as either as a continuous or categorical variable (0, 1-2, ≥ 3). Lastly, we report the raw number of co-morbid conditions.
Statistical Analyses
Descriptive analyses comparing clinicopathological characteristics between AA/B and white patients were evaluated using a χ2 test for the categorical variables, such as the TNBC subtype, tumor grade, histologic type, SEER summary stage (local, regional, distant), smoking history (never, current, former), first-course chemotherapy status (yes, no), CCI and CCI+HBP categorical levels (0, 1-2, ≥ 3), and vital status. Independent t-Tests were used to assess mean differences between groups such as: weighted CCI score, weighted CCI+HBP score, number of co-morbidities using both the CCI and CCI+HBP indices, follow-up time among patients still alive at the end of the study, age, body mass index (BMI), tumor size, and number of positive axillary lymph nodes among patients with regional disease. Multivariate-adjusted hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated using Cox proportional hazards regression. Predictors were TNBC status (TNBC vs Non-TNBC), Race (W, AA/B), weighted CCI score or weighted CCI+HBP score (continuous or categorical) and the following covariates: age at diagnosis, SEER Summary Stage (for analyses of the full sample only), tumor size, and number of positive axillary lymph nodes. Due to substantial missing data on smoking history (n=121) and receipt of first-course chemotherapy (n=60), we included those variables in statistical models in sensitivity analyses to explore impact on HR estimates. Outcome was defined as any cause of death consistent with examining the impact of co-morbidities on overall mortality. SPSS Ver. 21.0 (© Copyright IBM Corporation) was used in all analyses.
Results
Clinicopathological Characteristics
Tumors from AA/B patients (Table 1) were more likely to express the TNBC phenotype than those from white patients (25.7% vs 16.4%, respectively, P<0.01). AA/B patients were more likely to be diagnosed at later stages (P=0.04) and less likely to be alive at the end of the study period than whites (65.8% vs 77.6%, respectively, P<0.01). More AA/B patients died from breast cancer than did whites (50.7% vs 43.8%, respectively) but this difference was not found to be statistically significant (P=0.81). We observed variation in receipt of first-course systemic therapy by stage and race, but differences did not reach statistical significance. white patients tended to have a slightly greater prevalence of the more favorable purely lobular histology than their AA/B counterparts (11.2% vs 5.9%, P=0.04).
Table 1. Clinicopathological Characteristics of Breast Cancer Patients (n=416) in the NCI-SEER Connecticut Tumor Registry (2000-07).
| White n =214 | AA/B n=202 | P-Value1 | |
|---|---|---|---|
|
| |||
| TNBC | |||
| ER- PR- HER2- | 35 (16.4) | 52 (25.7) | |
| Non-TNBC | <0.01 | ||
| ER- PR- HER2+ | 6 (2.8) | 20 (9.9) | |
| ER/PR+ HER2- | 143 (66.8) | 117 (57.9) | |
| ER/PR+ HER2+ | 30 (14.0) | 13 (6.4) | |
|
| |||
| Vital Status | |||
| Alive | 166 (77.6) | 133 (65.8) | <0.01 |
| Dead | 48 (22.4) | 69 (34.2) | |
| Median follow-up time (Among Alive)3 | 9.7 yrs | 10.3 yrs | 0.22 |
|
| |||
| Cause of Death | |||
| Breast Cancer | 21 (43.8) | 35 (50.7) | |
| Other Cancer | 8 (16.7) | 8 (11.6) | 0.81 |
| CVD | 6 (12.5) | 7 (10.1) | |
| Other | 13 (27.1) | 19 (27.5) | |
|
| |||
| Histological Sub-Type | |||
| Ductal | 124 (57.9) | 127 (62.9) | |
| Lobular | 24 (11.2) | 12 (5.9) | 0.04 |
| Mixed Ductal/Lobular | 52 (24.3) | 37 (18.3) | |
| Other | 14 (6.5) | 26 (12.9) | |
|
| |||
| Tumor Grade | |||
| I | 25 (12.1) | 22 (11.5) | |
| II | 93 (45.1) | 73 (38.2) | 0.04 |
| III/IV | 88 (42.7) | 96 (50.3) | |
|
| |||
| Tumor Size, Mean (SD) | 2.07 cm (1.5) | 2.26 cm (1.6) | 0.22 |
|
| |||
| Positive Axillary Nodes,4 Mean (SD) | 3.35 (3.9) | 6.67 (16.3) | 0.11 |
|
| |||
| SEER Summary Stage | |||
| Local | 145 (67.8) | 119 (58.9) | |
| Regional | 66 (30.8) | 73 (36.1) | 0.04 |
| Distant | 3 (1.4) | 10 (5.0) | |
|
| |||
| First-Course Chemotherapy5 (Yes) | |||
| Local | 42 (32.3) | 41 (39.4) | 0.27 |
| Regional | 47 (75.8) | 50 (73.5) | 0.84 |
Chi-square test for categorical data and independent t-test for continuous variables
n (%) except where noted
Non-parametric Test of Medians
Among patients with Regional Summary Stage (n=63 White; n=66 AA/B)
Too few patients with Distant disease (n=12) to perform reliable analyses
Prevalence of Co-Morbidity
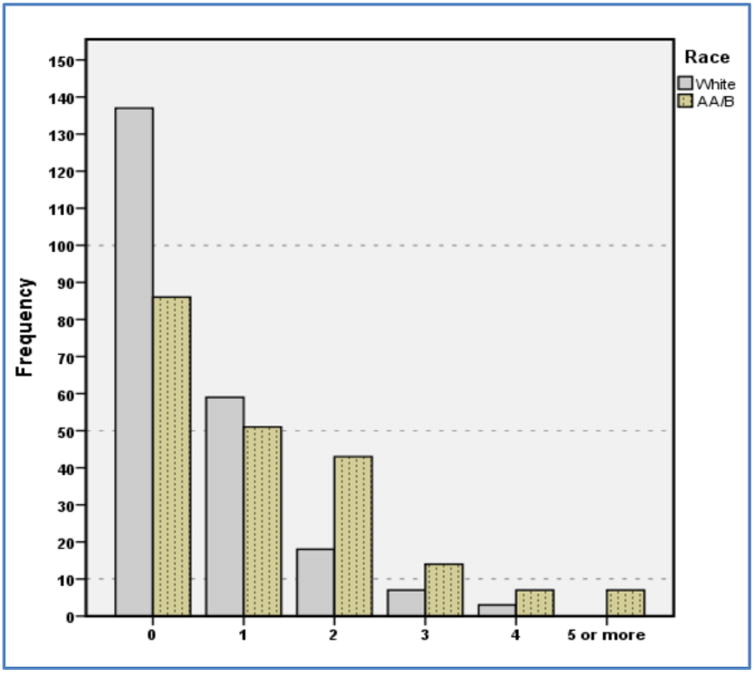
AA/B breast cancer patients exhibited a significantly higher mean CCI score (Table 2) than whites (1.38 vs 0.53, respectively, P < 0.0001), as well as the CCI+HBP score (1.90 vs 0.86, respectively, P<0.0001), even though they tended to be significantly younger at diagnosis (54.8 yr vs 58.4 yr, respectively, P=0.007). When measured with the CCI, a greater proportion of white patients had no co-morbidities at breast cancer diagnosis relative to AA/B patients (82.8 % vs 58.9%, respectively P=0.0001). When a diagnosis of hypertension was added to the CCI (CCI+HBP), the number of patients with a CCI+HBP score of 0 decreased in both groups and the disparity persisted (60.9 % vs. 39.1%, respectively P=0.0001). The distribution of the number of co-morbidities including HBP by race is depicted in Fig. 1. Regarding specific co-morbidities, AA/B patients were more likely than whites to be diagnosed with hypertension (47.5% vs 30.8%, P=0.001) and Type II Diabetes (23.3% vs 4.2%, P<0.001). While AA/B patients also were more likely to have a history of several other individual conditions, the absolute number of patients is small.(Table 2) Mean body mass index was higher in AA/B patients compared to whites (31.9 vs 26.4, respectively, P<0.0001), which represents a clinically relevant shift from overweight (25 to < 30) to obese (≥ 30).
Table 2. Co-Morbidities among Breast Cancer Patients (n=416) in the NCI-SEER Connecticut Tumor Registry (2000-07).
| White n =214 | AA/B n=202 | P-Value1 | |
|---|---|---|---|
|
| |||
| CCI Score3 | |||
| 0 | 173 (80.8)2 | 118 (58.4) | |
| 1-2 | 28 (13.1) | 52 (25.7) | <0.0001 |
| ≥3 | 13 (6.1) | 32 (15.8) | |
| Mean (SD) | 0.56 (1.6) | 1.41 (2.7) | <0.0001 |
| Range | 0-8 | 0-15 | |
| No. of Conditions | |||
| Mean (SD) | 0.26 (0.63) | .71 (1.17) | <0.0001 |
| Range | 0-3 | 0-8 | |
|
| |||
| CCI+HBP Score4 | |||
| 0 | 130 (60.7)2 | 84 (41.6) | |
| 1-2 | 63 (29.4) | 77 (38.1) | <0.0001 |
| ≥3 | 21 (9.8) | 41 (20.3) | |
| Mean (SD) | 0.86 (1.7) | 1.88 (2.8) | <0.0001 |
| Range | 0-9 | 0-17 | |
| No. of Conditions | |||
| Mean (SD) | 0.57 (0.88) | 1.19 (1.41) | <0.0001 |
| Range | 0-4 | 0-9 | |
|
| |||
| Selected Co-Morbidities (Yes)6 | |||
| Hypertension | 66 (30.8) | 96 (47.5) | 0.001 |
| Type II Diabetes (any severity) | 9 (4.2) | 47 (23.3) | <0.001 |
| Kidney Disease (any severity) | 2 (0.9) | 10 (5.0) | 0.02 |
| Congestive Heart Disease | 2 (0.9) | 7 (3.5) | 0.09 |
| Hx Myocardial Infarction | 3 (1.4) | 7 (3.5) | 0.21 |
| Liver Disease | 2 (0.9) | 5 (2.5) | 0.27 |
| Hx Cerebrovascular Event | 5 (2.3) | 9 (4.5) | 0.28 |
| Connective Tissue Disorders | 4 (1.9) | 7 (3.5) | 0.37 |
| Pulmonary Disease (any severity) | 6 (2.8) | 4 (2.0) | 0.75 |
| Peripheral Vascular Disease | 3 (1.4) | 3 (1.5) | 0.99 |
|
| |||
| Age | |||
| < 40 years | 8 (3.7) | 23 (11.4) | 0.003 |
| Mean (SD) | 58.4 yrs (13.7) | 54.8 yrs (13.9) | 0.007 |
| Median | 56 yrs | 54 yrs | 0.09 |
|
| |||
| Body Mass Index (kg/m2)5 | |||
| <18.5 | 4 (3.1) | 3 (2.5) | |
| ≥18.5 to <25 | 60 (45.8) | 17 (14.0) | <0.0001 |
| ≥25 to <30 | 35 (26.7) | 40 (33.1) | |
| ≥30 | 32 (24.4) | 61 (50.4) | |
| Mean (SD) | 26.4 (5.5) | 31.9 (8.2) | <0.0001 |
|
| |||
| Smoking History5 | |||
| Never | 103 (55.4) | 107 (62.9) | |
| Former | 56 (30.1) | 33 (19.4) | 0.07 |
| Current | 27 (14.5) | 30 (17.6) | |
Chi-square test for categorical data and independent t-test for continuous variables.
n (%) except where noted.
Weighted Charlson Co-Morbidity Index score
Weighted Charlson Co-Morbidity Index with one point added to score for diagnosis of High Blood Pressure.
Missing data (n=for 121 Body Mass Index; n=60 for Smoking History)
Fisher's Exact Test
Independent Samples Median Test
Fig. 1. Distribution of No. of Co-Morbidities including HBP in 224 White and 208 AA/B Breast Cancer Patients diagnosed in Connecticut (2000-07).
Mortality Risks for TNBC and Co-Morbidity
Table 3 presents adjusted HRs (95% CIs) for the prognostic roles of TNBC and co-morbidity at diagnosis in relation to overall mortality. Four models were evaluated: 1) age-adjusted; 2) basic multivariate model; 3) inclusion of CCI into the model; and, 4) inclusion of CCI+HBP into the model. For all stages combined (n=416), TNBC status conferred an increased risk for death, compared to a combined non-TNBC category, in both age-adjusted and basic multivariate analyses (HR=1.92, 95% CI 1.29-2.93, and, HR=1.89, 95% CI 1.24-2.86, respectively). HRs were attenuated when either the continuous CCI or CCI+HBP scores were added to the model (HR=1.64, 95% CI 1.06-2.53, and, HR=1.65, 95% CI 1.07-2.55, respectively). Based on statistically significant interactions between SEER Stage and the two CCI scores detected in the multivariate model (Table 2), we repeated analyses by stage. Among patients with local disease (n=264), TNBC signifcantly predicted death employing the basic multivariate model (HR=2.18 95% CI 1.14-4.16). HR estimates, however, were attenuated and no longer statistically significant when the CCI score and CCI+HBP score were entered into the model (HR=1.50, 95% CI 0.74-3.01, and, HR=1.46, 95% CI 0.73-2.91, respectively). For patients with regional disease (n=139), the HR estimate of the prognostic impact of TNBC was non-significant in the basic model (HR=1.64, 95% CI 0.85-3.16) as were HRs in both the CII and CCI+HBP adjusted models (HR=1.29, 95% CI 0.63-2.64, and, HR=1.31, 95% CI 0.64-2.67, respectively).
Table 3. Adjusted HRs (95% CIs) for Any Cause of Death among Breast Cancer Patients (n=416) in the NCI-SEER Connecticut Tumor Registry (2000-07).
| n | Deaths | Age-Adjusted | Multivariate Model1 | Multivariate Model with CCI Score3 | Multivariate Model with CCI+HBP Score4 | |
|---|---|---|---|---|---|---|
|
| ||||||
| ALL PATIENTS | 416 | 1176 | ||||
|
| ||||||
| TNBC | ||||||
| Non-TNBC | 1.00 | 1.00 | 1.00 | 1.00 | ||
| TNBC2 | 1.92 (1.29-2.93) | 1.89 (1.24-2.86) | 1.64 (1.06-2.53) | 1.65 (1.07-2.55) | ||
| CCI Score | 1.23 (1.22-1.33) | - - - | 1.17 (1.10-1.24) | - - - | ||
| CCI+HBP Score | 1.26 (1.21-1.32) | - - - | - - - | 1.16 (1.10-1.50) | ||
|
| ||||||
| LOCAL STAGE5 | 264 | 53 | ||||
|
| ||||||
| TNBC | ||||||
| Non-TNBC | 1.00 | 1.00 | 1.00 | 1.00 | ||
| TNBC | 2.20 (1.16-4.16) | 2.18 (1.14-4.16) | 1.50 (0.74-3.01) | 1.46 (0.73-2.91) | ||
| CCI Score | 1.55 (1.37-1.76) | - - - | 1.49 (1.29-1.71) | - - - | ||
| CCI+HBP Score | 1.52 (1.33-1.73) | - - - | - - - | 1.47 (1.27-1.69) | ||
|
| ||||||
| REGIONAL STAGE5 | 139 | 53 | ||||
|
| ||||||
| TNBC | ||||||
| Non-TNBC | 1.00 | 1.00 | 1.00 | 1.00 | ||
| TNBC | 1.60 (0.87-2.94) | 1.64 (0.85-3.16) | 1.29 (0.63-2.64) | 1.31 (0.64-2.67) | ||
| CCI Score | 1.18 (1.09-1.27) | - - - | 1.13 (1.04-1.24) | - - - | ||
| CCI+HBP Score | 1.17 (1.09-1.26) | - - - | - - - | 1.13 (1.04-1.23) | ||
Cox Proportional Hazards Survival Analyses simultaneously adjusted for age, race, TNBC status and SEER Summary Stage for all patients (n=416); age, race, TNBC status and tumor size for patients with Local or Regional disease.
Triple-Negative Breast Cancer.
Weighted Charlson Co-Morbidity Index score included as a continuous variable.
Weighted Charlson Co-Morbidity Index with one point added to continuous CCI score given a diagnosis of High Blood Pressure
Stratification based on interaction of SEER Stage * CCI Score (Wald = 14.2, P=0.001); and, SEER Stage * CCI Score+HBP (Wald = 14.6, P=0.001) in basic model.
Includes distant stage.
When assessing the prognostic impact of co-morbidity at diagnosis controlling for TNBC status, a single point elevation in the CCI or CCI+HBP scores conferred a significantly increased risk of death (HR=1.17, 95% CI 1.10 -1.24, and, HR=1.16, 95% CI 1.10-1.50, respectively). We observed a variation of this effect by stage: adjusted HR estimates among those with local breast cancer for the CCI and CCI+HBP were statistically significant (HR=1.49, 95% CI 1.29-1.71, and, HR=1.47, 95% CI 1.27-1.69, respectively) whereas corresponding estimates were lower among those with regional stage disease at diagnosis (HR=1.13, 95% CI 1.04-1.24, and, HR=1.13 95% CI 1.04-1.23, respectively).
Kaplan-Meier curves (Fig. 2) depict the survival experience of patients according to levels of CCI+HBP score (0, 1-2, ≥ 3). While the Log-Rank tests are significant for both white and (χ2=22.9, P<0.0001) and AA/B patients (χ2=64.7, P<0.0001 Log-Rank Test), the variation in mortality risk among whites appears to be limited to the comparison between the highest and lowest strata. We present the multivariate-adjusted HRs for all-cause mortality for the CCI and CCI+HBP strata stratified by race (Fig.2). The HR for AA/B patients with a CCI score of ≥ 3 (n=32) compared to patients with a score of 0 (n=118) was statistically significant (HR=5.65, 95% CI 2.90-11.09 but for patients with a CCI score of 1-2 (n=52), the effect was not statistically significant (HR=1.54, 95% CI 0.82-2.91). For whites, the HR estimates among patients with a CCI score of ≥ 3 (n=13) or 1-2 (n=28) were not statistically significant when compared to referent group without co-morbidities (n=178).
Fig. 2. Kaplan-Meier Survival Curves: Charlson Co-Morbidity Index Plus HBP (CCI+HBP) and Death from Any Cause.
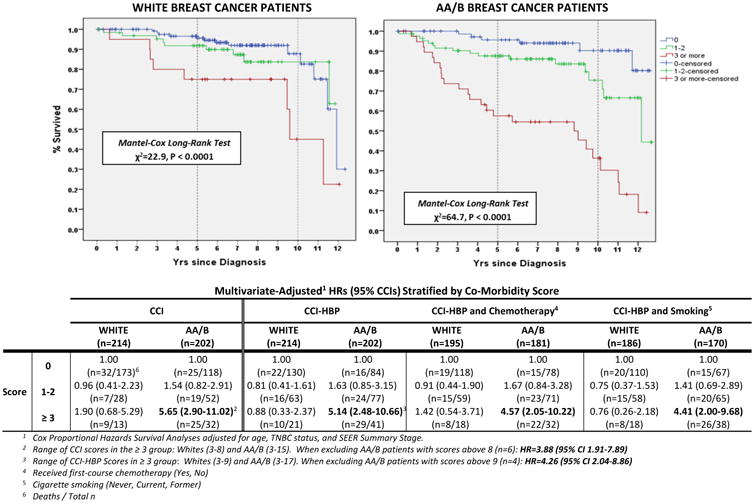
We repeated these analyses using the CCI+HBP score (Fig. 2). Similar to what was observed in the CCI score, AA/B patients with a CCI+HBP score of ≥3 (n=41) had a large and significant increased risk of death from any cause (HR=5.14, 95% CI 2.48-10.66) compared to AA/B patients with a CCI+HBP score of 0 (n=84). HR estimates for AA/B patients with a CCI+HBP score of 1-2 (n=77) were not statistically significant. Among whites, HR estimates were not significant for patients with either a CCI+HBP score ≥3 or 1-2. Lastly, we conducted exploratory analyses adding, separately, receipt of chemotherapy and smoking status into the multivariate model. Trends for both AA/B and white patients were comparable to HR estimates observed in the CCI and CCI+HBP multivariate models.
Sensitivity analyses of degree of co-morbidity score. As the upper ranges of the CCI and CCI+HBP scores were higher in AA/B patients compared to whites, we assessed if the effect of co-morbidities on mortality risk was dominated by extreme scores. Therefore, we restricted the analysis to AA/B patients with scores of <=8 (CCI) and <=9 (CCI+HBP) so as to have the same upper limit as whites (Fig. 2). Modified HR estimates for AA/B patients in the top co-morbidity strata were somewhat attenuated but remained statistically significant (HR=3.88, 95% CI 1.91-7.89 for CCI model; and, HR=4.26, 95% CI 2.04-8.86 in CCI+HBP model) than in analyses including extreme scores.
Discussion
We report evidence from an NCI-SEER based study sample that co-morbidities at diagnosis exert an independent risk for overall mortality among breast cancer patients, and we add to the literature by demonstrating that this effect persisted after controlling for the aggressive TNBC subtype. We further found that the prognostic effect of TNBC, itself, was attenuated when information about co-morbidities was included in multivariate models. This is not to imply that the prognostic value of TNBC is a matter of statistical confounding by co-morbidities but, rather, we suggest that TNBC and co-morbidities are both explanatory factors in the complex multi-determined problem of continuing survival disparity in breast cancer. The impact of co-morbidities appears to be stronger in local versus regional disease presumably due to the added risk of death from greater anatomic burden in the more advanced stage. While a differential effect by stage also was observed for TNBC, consistent with our prior study,10 estimates became non-significant after co-morbidities were introduced into models. We cannot, however, rule out loss of statistical power in analyses of local disease given the suggestive HR point estimates.
Our findings also suggest that survival disparity may be related to not only a greater prevalence of comorbidities at diagnosis, but, also, to a possible disproportionate impact from these conditions among AA/B patients. Specifically, while AA/B patients with a CCI score of ≥3 were at substantially increased risk of death compared to AA/B patients with a score of 0, the increased HR for white patients was not statistically significant. When evaluating the CC+HBP score, a similar pattern was observed by race but in these analyses, notably, CIs did not overlap between AA/B and white patients (HR= 5.14, 95% CI 2.48-10.66 versus HR=0.88, 95% CI 0.33-2.37, respectively). Further, a differential impact on mortality was maintained in a sensitivity analysis when we excluded those AA/B patients in the ≥ 3 score stratum with comorbidity scores higher than the upper limit in white patients, although the modified HRs were somewhat attenuated and CIs overlapped (Fig. 2). We interpret the persistence of statistically significant HR estimates for AA/B patients with a comorbidity score of ≥ 3 or more to suggest that even when the range of scores is the same - risk of death was greater in AA/B compared to white patients. Nonetheless, due to the small sample sizes in the stratified analyses, these trends must considered with great caution and future, larger studies are recommended.
In addition, we found that the inclusion of high blood pressure status into the CCI appeared to somewhat refine the predictive value of cumulative co-morbidities among AA/B patients. That is, unlike the CCI, the HR estimate for AA/B patients with a CCI+HBP score of 1-2 was higher and statistically significant (HRs 1.77 and 2.85, respectively). Among whites with ≥ 3 co-morbidities, however, the HR estimate was statistically significant in CCI analyses but became reduced and non-significant when analyzing CCI+HBP. We speculate that this inconsistent effect by race may be related to uncontrolled versus controlled hypertension, given its well-documented poor control in AA/B patients.22
A strength of our analyses is that data are derived from the NCI-SEER registry in Connecticut, a high-quality population-based resource. Consistent with prior studies, we reported that both TNBC10 and co-morbidities12 were more prevalent among AA/B compared to white patients. Other key clinicopathological characteristics of our study sample conform with many survival studies in breast cancer (e.g., lower age at diagnosis and differential histological subtype distribution of AA/B breast cancer patients compared to whites), suggesting the representativeness of our sample and findings. It should be noted, however, that median age of study participants is somewhat younger than national statistics about breast cancer patients. In a prior analysis of the parent study, we noted that younger patients (< 60 years) were more likely to have had HER2 testing patients compared to older patients and that roughly 66% of all tumors had been assayed.17 The American Society of Clinical Oncology recommendation for universal HER2 testing was released at the beginning of our study in 2001.23 Prior to more complete adoption in the ensuing years, it is possible that tumors from younger patients were tested at disproportionally higher rates in community settings due to clinical expectations of aggressive tumor biology and/or increased awareness among younger patients about the utility of HER2 testing. Another limitation of our study is that the sample size was inadequate to explore the impact of specific co-morbidities. Future studies are suggested to identify those conditions that might best explain survival disparity. Lastly, lack of treatment data is drawback given that a number of studies have shown that differential treatment patterns may be explanatory factors in survival disparities in breast cancer.4 Due to high rates of missing and incomplete treatment data in the SEER database, this information is no longer available in the SEER public database.18
There is emerging recognition of the importance of assessing co-morbidities in cancer research. A recent Annual Report to the Nation on the Status of Cancer by Edwards and colleagues (2014), for example, discussed the important influence of co-morbidities in patients with breast cancer, among other cancers, on overall survival rate and dying of other causes.24 The translational value of understanding diabetes or HBP, for example, would be manifest when determining anti-cancer as well as general medical treatment protocols. Research on cancer survivorship and disparities is ongoing.25 While the CCI has been employed widely in research and remains an effective tool,20 the list of clinical conditions in the index was developed and validated in cohorts almost exclusively consisting of patients of European descent. High blood pressure is not among the 17-item list, however, although it is established that HBP is found in higher rates of among African-Americans.22 In a study of 416 African-American and 838 white breast cancer patients in the Kaiser Permanente Northern California Medical Care Program, Braithwaite et al. (2009) found that inclusion of HBP into the CCI provided improved prediction of survival and accounted for approximately 30% of the survival disparity.21 Our analysis, however, did not find notable differences in risk estimates using the CCI+HBP versus the CCI. Yet, given the established links between HBP and mortality, and its greater prevalence in the AA/B population, it behooves us to suggest that larger studies are warranted to explore inclusion of HBP in co-morbidity indices. Another potential factor to explore in future studies is Sickle Cell Trait (SCT), a genotype which is far more prevalent in the AA/B population (8.5%) compared to whites (<0.01) but is understudied in cancer survival disparities. There is growing awareness of clinical complications in SCT,26, 27 and we recently published a multi-case review of major adverse events among cancer patients with Sickle Cell Trait/Disease undergoing systemic therapy.28
Additional recommendations for future studies include incorporation of other key prognostic factors in cancer, such as treatment specification and behavioral factors (e.g., obesity, smoking). While our exploratory analyses with chemotherapy receipt and smoking history added to multivariate analyses did not appreciably alter the primary HR estimates, there were a substantial number of patients with missing data which casts some doubt on the reliability of the exploratory findings. Lastly, it would be of interest to test breast cancer specific survival or recurrence-free survival to explore if co-morbidity may influence these outcomes via effects on treatment decisions, dosing and tolerability.
In the current study, presence of co-morbidities resulted in increased risk of death for all patients whether or not tumors expressed the TNBC phenotype. Yet the impact might be disproportionately greater among AA/B patients suggesting effects from recognized sub-optimal clinical control of hypertension,29 diabetes,30 and other chronic co-morbidities in this patient population, practice guidelines recommending consideration of co-morbidity status when individualizing cancer treatment and dosing,31 limited guidelines for follow-up care of breast cancer survivors to ameliorate co-morbid conditions32, and other unmeasured factors in the current investigation.
Acknowledgments
This study was funded, in part, by the Connecticut Breast Health Initiative, Inc. 501 C) (3). Certain data used in this study were obtained from the Connecticut Tumor Registry (CTR) located in the Connecticut Department of Public Health. Authors assume full responsibility for analyses and interpretation of these data. The CTR is supported by a contract (HHSN2612013000191) between the National Cancer Institute and the Connecticut Department of Public Health. We gratefully acknowledge the comprehensive data processing and supervision provided by Cathryn E. Phillips, Certified Tumor Registrar and CTR Manager, and, the extensive data collection and project management services provided by Rajni Mehta, M.P.H., and staff, at the Rapid Case Ascertainment Shared Resource of the Yale Cancer Center.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–18. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Ademuyiwa FO, Edge SB, Erwin DO, Orom H, Ambrosone CB, Underwood W., 3rd Breast cancer racial disparities: unanswered questions. Cancer Res. 2011;71:640–4. doi: 10.1158/0008-5472.CAN-10-3021. [DOI] [PubMed] [Google Scholar]
- 3.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–60. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 4.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004-2006. J Clin Oncol. 2010;28:4135–41. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 5.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Facts & Figures for African Americans, 2011-12. 2013 [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 8.Sabatino SA, white MC, Thompson TD, Klabunde CN. Cancer screening test use - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:464–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, Flagg EW, O'Regan RM, Gabram SG, Eley JW. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113:357–70. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 10.Swede H, Gregorio DI, Tannenbaum SH, Brockmeyer JA, Ambrosone C, Wilson LL, Pensa MA, Gonsalves L, Stevens RG, Runowicz CD. Prevalence and prognostic role of triple-negative breast cancer by race: a surveillance study. Clin Breast Cancer. 2011;11:332–41. doi: 10.1016/j.clbc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakha EA, Ellis IO. Triple-negative/basal-like breast cancer: review. Pathology. 2009;41:40–7. doi: 10.1080/00313020802563510. [DOI] [PubMed] [Google Scholar]
- 12.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. Jama. 2005;294:1765–72. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 13.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–6. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 14.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 15.Schillinger D, Sarkar U. Numbers don't lie, but do they tell the whole story? Diabetes Care. 2009;32:1746–7. doi: 10.2337/dc09-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muss HB, Hunter CP, Wesley M, Correa P, Chen VW, Greenberg RS, Eley JW, Austin DF, Kurman R, Edwards BK. Treatment plans for black and white women with stage II node-positive breast cancer The National Cancer Institute Black/white Cancer Survival Study experience. Cancer. 1992;70:2460–7. doi: 10.1002/1097-0142(19921115)70:10<2460::aid-cncr2820701012>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Pensa M, Swede H, Brockmeyer JA, Gregorio DI. Patterns of HER2 testing in the management of primary breast cancer. Cancer Epidemiol. 2009;33:113–7. doi: 10.1016/j.canep.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL. Comparison of SEER Treatment Data With Medicare Claims. Med Care. 2014 doi: 10.1097/MLR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity a critical review of available methods. J Clin Epidemiol. 2003;56:221–9. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite D, Tammemagi CM, Moore DH, Ozanne EM, Hiatt RA, Belkora J, West DW, Satariano WA, Liebman M, Esserman L. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer. 2009;124:1213–9. doi: 10.1002/ijc.24054. [DOI] [PubMed] [Google Scholar]
- 22.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Jr, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 23.Bast RC, Jr, Ravdin P, Hayes DF, Bates S, Fritsche H, Jr, Jessup JM, Kemeny N, Locker GY, Mennel RG, Somerfield MR American Society of Clinical Oncology Tumor Markers Expert Panel. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–78. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [PubMed] [Google Scholar]
- 24.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: are we doing enough to address the root causes? J Clin Oncol. 2006;24:2170–8. doi: 10.1200/JCO.2005.05.4734. [DOI] [PubMed] [Google Scholar]
- 25.Cooper LA, Ortega AN, Ammerman AS, Buchwald D, Paskett ED, Powell LH, Thompson B, Tucker KL, Warnecke RB, McCarthy WJ, Viswanath KV, Henderson JA, et al. Calling for a Bold New Vision of Health Disparities Intervention Research. Am J Public Health. 2015:e1–3. doi: 10.2105/AJPH.2014.302386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldsmith JC, Bonham VL, Joiner CH, Kato GJ, Noonan AS, Steinberg MH. Framing the research agenda for sickle cell trait: building on the current understanding of clinical events and their potential implications. Am J Hematol. 2012;87:340–6. doi: 10.1002/ajh.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naik RP, Derebail VK, Grams ME, Franceschini N, Auer PL, Peloso GM, Young BA, Lettre G, Peralta CA, Katz R, Hyacinth HI, Quarells RC, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. Jama. 2014;312:2115–25. doi: 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swede H, Andemariam B, Gregorio DI, Jones BA, Braithwaite D, Rohan TE, Stevens RG. Adverse events in cancer patients with sickle cell trait or disease: case reports. Genet Med. 2015;17:237–41. doi: 10.1038/gim.2014.105. [DOI] [PubMed] [Google Scholar]
- 29.Flack JM, Ference BA, Levy P. Should African Americans with hypertension be treated differently than non-African Americans? Curr Hypertens Rep. 2014;16:409. doi: 10.1007/s11906-013-0409-5. 013-0409-5. [DOI] [PubMed] [Google Scholar]
- 30.Marzec LN, Maddox TM. Medication adherence in patients with diabetes and dyslipidemia: associated factors and strategies for improvement. Curr Cardiol Rep. 2013;15:418. doi: 10.1007/s11886-013-0418-7. 013-0418-7. [DOI] [PubMed] [Google Scholar]
- 31.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Forero A, Giordano SH, Goldstein LJ, Gradishar WJ, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 32.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66:43–73. doi: 10.3322/caac.21319. [DOI] [PubMed] [Google Scholar]


