Summary
Haploinsufficiency (HI) is the best characterized mechanism through which dominant mutations exert their effect and cause disease. Non-haploinsufficiency (NHI) mechanisms, such as gain-of-function and dominant-negative mechanisms, are often characterized by the spatial clustering of mutations, thereby affecting only particular regions or base pairs of a gene. Variants leading to haploinsufficency might occasionally cluster as well, for example in critical domains, but such clustering is on the whole less pronounced with mutations often spread throughout the gene. Here we exploit this property and develop a method to specifically identify genes with significant spatial clustering patterns of de novo mutations in large cohorts. We apply our method to a dataset of 4,061 de novo missense mutations from published exome studies of trios with intellectual disability and developmental disorders (ID/DD) and successfully identify 15 genes with clustering mutations, including 12 genes for which mutations are known to cause neurodevelopmental disorders. For 11 out of these 12, NHI mutation mechanisms have been reported. Additionally, we identify three candidate ID/DD-associated genes of which two have an established role in neuronal processes. We further observe a higher intolerance to normal genetic variation of the identified genes compared to known genes for which mutations lead to HI. Finally, 3D modeling of these mutations on their protein structures shows that 81% of the observed mutations are unlikely to affect the overall structural integrity and that they therefore most likely act through a mechanism other than HI.
Main Text
De novo mutations affecting protein-coding genes are a major cause of intellectual disability (ID) and other developmental disorders (DDs).1, 2 Several whole exome sequencing (WES) studies have identified ID syndromes molecularly characterized by very specific spatial clustering of de novo missense mutations.3, 4, 5, 6 Similarly, large-scale WES studies of individuals affected by ID/DD have recently leveraged this phenomenon as supporting evidence of the involvement of a gene in disease.7, 8 This spatial clustering of de novo mutations (DNMs) is typical for missense mutations in genes without clear, or limited numbers of, truncating mutations subsequently degraded by nonsense-mediated mRNA decay, suggesting that these clustered mutations act through a different mechanism than haploinsufficiency (HI).9 Alternative pathophysiological mechanisms that might underlie (de novo) mutation clustering are gain-of-function or dominant-negative effects, resulting in the alteration or impairment of specific protein function.10, 11 We note that while spatial clustering is commonly taken to indicate a mechanism different from loss-of-function,12 this is not an absolute rule, and a loss-of-function mechanism cannot be excluded without functional evidence.13 Here, we developed a method to identify genes with spatially clustered DNMs and applied this to DNMs identified in a large cohort of individuals with ID/DD.14
We downloaded all DNMs occurring in individuals with ID/DD from de novo-db version 1.314 identified through WES and whole genome sequencing which were then re-annotated with our in-house variant annotation pipeline. The de novo mutations included in the analysis were previously validated by a second independent method or showed a high validation rate for a subset of de novo mutations. In addition, we added 1,183 de novo variants identified in the exomes of an in-house ID cohort that was previously published.8 To further reduce the risk of including sequencing artifacts and/or genotyping errors, we excluded all de novo variants that were present more than once in the ExAC dataset (Table S1).15 These efforts resulted in 6,495 protein coding DNMs, including 4,061 missense mutations, in 5,302 individuals with ID/DD (Table S2).
We set out to determine for any gene whether the observed de novo missense mutations cluster more than expected compared to random permutations. Hereto, we selected for each the longest representative transcript (i.e., part of the GENCODE basic set)16 and calculated the geometric mean distance over all missense DNMs on cDNA. was calculated by taking the mean distance normalized for transcript length l over all (M) combinations of and of the missense DNMs (Equation 1), where represents the position for mutation and respectively. Statistical significance was determined by performing 1.00E+08 (or N) permutations and calculating for each permuted geometric mean distance how many times this resulted in the same or smaller geometric mean distance as observed (Equation 2) Permutation p values were corrected for multiple testing via Bonferroni procedure based on the 19,280 genes of the Agilent SureSelect v5 exome enrichment kit.
| 1 |
| 2 |
We first validated our method on a dataset of DNMs identified in 2,448 unaffected siblings and healthy control studies14, 17, 18, 19, 20, 21, 22 (Table S3). In this cohort, we failed to identify genes for which clustering of de novo missense mutations reached statistical significance (Table S4). However, application of our method to the dataset of 4,061 DNMs, containing 583 genes with more than one de novo missense mutation, revealed 15 genes with significant clustering7, 8, 23, 24, 25 (Table 1, Figure 1, Figures S1–S15). In these genes, a total of 107 de novo missense mutations contributed to mutation clustering, ranging from three to 20 mutations per gene with an average distance ranging from 0 to 354 bp. To exclude a correlation between the extent of clustering and the total number of de novo missense mutations analyzed, we applied our method to a cohort of 6,154 de novo missense variants present in de novo-db excluding the five studies incorporated in the ID/DD cohort, and found no such correlation (Figure S16). To examine whether this set of 15 genes is relevant in the context of ID/DD, we compared these genes to a list of 1,541 genes for which mutations are known to cause ID/DD (Table S5). This list of genes was a compilation of two manually curated lists of disease-associated genes including “confirmed” unique genes from DDG2P (n = 1,098; see Web Resources) and 1,034 genes offered for diagnostic testing in individuals with ID/DD by our in-house diagnostic facility (see Web Resources). Among the 15 identified genes with mutation clustering, we find 12 genes for which mutations have previously been implicated in ID/DD, constituting a significant enrichment (p = 3.09e-03; Fisher’s exact test; Tables S6 and S7), and confirming that our method is valid for its purpose. The inclusion of exome data of two large DDD-studies in both the DDG2P gene list and the ID/DD cohort of this study could introduce a potential bias.1, 7 To exclude such bias, we repeated this analysis while excluding the DDD-specific genes identified in the two exome studies yielding a significant enrichment (p = 3.68E-02; Table S7A–S7C).
Table 1.
List of Identified Genes with Clustering de Novo Missense Mutations
| Gene name | Transcript ID | # de novo missense | Median distance (bp) | p value | Adj. p value |
|---|---|---|---|---|---|
| ACTL6Ba | ENST00000160382 | 3 | 0 | 5.70E-07 | 1.10E-02 |
| ALG13 | ENST00000394780 | 3 | 0 | 1.50E-07 | 2.89E-03 |
| CDK13 | ENST00000181839 | 12 | 273 | <1.00E-08 | <1.93E-04 |
| COL4A3BP | ENST00000380494 | 6 | 18 | 2.60E-07 | 5.01E-03 |
| GABBR2a | ENST00000259455 | 3 | 0 | 9.00E-08 | 1.74E-03 |
| GRIN2B | ENST00000609686 | 11 | 354 | 1.57E-06 | 3.03E-02 |
| KCNH1 | ENST00000271751 | 7 | 65 | 1.00E-07 | 1.93E-03 |
| KCNQ2 | ENST00000354587 | 20 | 301 | 5.00E-08 | 9.64E-04 |
| KIF5C | ENST00000435030 | 3 | 0 | 1.40E-07 | 2.70E-03 |
| PACS1 | ENST00000320580 | 9 | 0 | <1.00E-08 | <1.93E-04 |
| PACS2a | ENST00000458164 | 3 | 0 | 1.50E-07 | 2.89E-03 |
| PCGF2 | ENST00000360797 | 3 | 0 | 1.11E-06 | 2.14E-02 |
| PPP2R1A | ENST00000322088 | 4 | 5 | 4.60E-07 | 8.87E-03 |
| PPP2R5D | ENST00000485511 | 16 | 10 | <1.00E-08 | <1.93E-04 |
| SMAD4 | ENST00000398417 | 4 | 6 | 1.60E-07 | 3.08E-03 |
P values are based on a permutation test (N = 1.00E+08). Adj. p values are corrected by Bonferroni correction. The three identified genes that have not yet been implicated in ID/DD are indicated by an a.
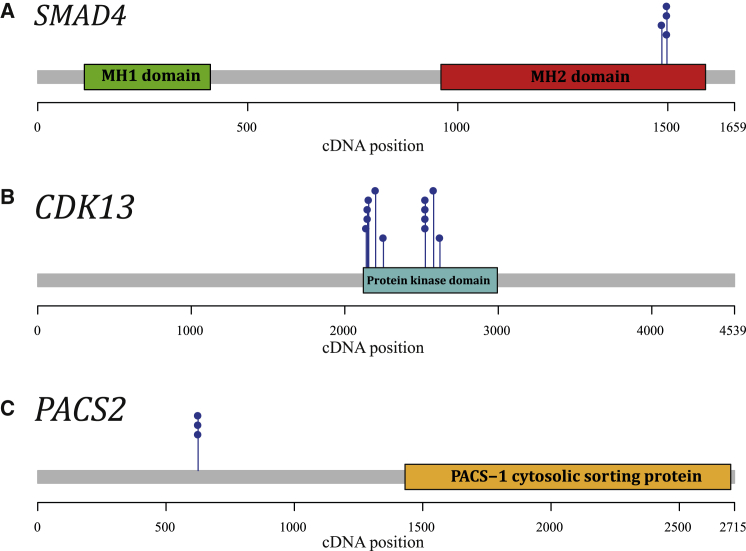
Figure 1.
Examples of Identified Genes with Clustering Mutations
Protein domains are annotated based on Pfam HMM search.26 cDNA locations of de novo missense mutations are depicted by blue pins. Genes shown here are as follows: SMAD4 (A), CDK13 (B), PACS2 (C). Figures visualizing the clustering of de novo missense mutations in the other 12 genes are provided in Figures S1–S15.
We also identified three genes with clustered de novo missense mutations that have not yet been implicated in ID/DD: ACTL6B (MIM:612458), GABBR2 (MIM:607340), and PACS2 (MIM:610423). None of these genes would have been identified based on an enrichment for de novo mutations in this cohort (Table S8). Further systematic evaluation of gene function supports a role in (neuro)development for two of these genes (Table 2 and Table S9). ACTL6B, encoding Actin-like 6B (also known as BAF53B), is a pivotal co-factor for the SWI/SNF neuron-specific chromatin remodeling complex nBAF, which is required for neural development and dendritic outgrowth.27, 28 Also, GABBR2, which is a component of the G protein-coupled GABA receptor, plays a critical role in the fine-tuning of inhibitory synaptic transmission,29, 30, 31 and other members of the GABA receptor family have already been conclusively linked to neurodevelopmental disorders.32, 33 GABBR2 was very recently also reported by others to show significant de novo mutation clustering in a neurodevelopmental cohort.6
Table 2.
Gene Function for Candidate Genes with Clustered Mutations
| Gene Name | Summary of Gene Function | Interactions |
|---|---|---|
| ACTL6B | Belongs to the neuron-specific chromatin remodeling complex (nBAF complex) and is required for postmitotic neural development and dendritic outgrowth. | Complex formation with ACTB, ARID1A, SMARCA2, SMARCA4, SMARCE1, SMARCC1, SMARCC2, SMARCD2, SMARCB1 |
| GABBR2 | Postsynaptic GABAB receptor activity regulates excitatory neuronal architecture and spatial memory. | Heterodimerization is required for the formation of a functional GABA-B receptor. |
| PACS2 | Multifunctional sorting protein, controling endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. | N/A |
Third column indicates whether the encoded protein has physical interactions with other proteins. See Table S9 for extended information.
Our method might potentially identify clustering based on identical mutations in multiple individuals only as a result of issues in the underlying cohort. It could for instance be that the same individual was included in multiple studies and therefore occurs twice in the cohort. For 99 out of 107 de novo missense mutations (92.5%) occurring in the 15 genes with clustering mutations, we could decisively conclude that they occurred as unique events in separate individuals based on a combination of the gender of the affected individual and the presence of additional de novo mutations (Table S10). Nevertheless, it might be possible that siblings of affected individuals were included who share a DNM due to parental gonadal mosaicism.34 Alternatively, DNMs might occur multiple times in disease cohorts as a consequence of a locally increased mutation rate. Examples of the latter might for instance incur a selective growth advantage (i.e., selfish mutations35) and thereby result in a pattern of mutational clustering such as known for FGFR2 (MIM 176943) mutations in Apert syndrome (MIM 101200).35 However, biological relevance for the mutations in the identified genes in the context of ID/DD is suggested by the fact that in our control cohort genes with significant clusters were absent, and that for the majority of our identified genes experimental evidence in literature supports a NHI mutational mechanism (Table S11).
We hypothesized that the clustering de novo missense mutations of the 15 genes might exert their effects through mechanisms other than haploinsufficiency. To validate this hypothesis, we compiled a set of 116 genes known for mutations that exert disease through non-haploinsufficient (NHI) mechanisms. Hereto, we selected for genes that have a “confirmed” status in the DDG2P list, or are present on both the Radboudumc ID/DD diagnostic testing and DDG2P lists (irrespective of the DDG2P status). Furthermore, genes were selected to be dominant (mono-allelic), with the pathophysiological mechanism being either “activating,” “all missense/in frame,” and/or “dominant negative” (Table S12). In addition, we generated a set of 183 haploinsufficient genes for which mutations are associated with ID/DD from the DDG2P gene list by selecting “loss-of-function” as the “mutation consequence” and “mono-allelic” for the “allelic requirement” in the DDG2P gene list (Table S13).
Interestingly, for eight of the 12 genes for which mutations are known to cause ID/DD and for which we identified mutation clustering, the disease mechanism on the constructed gene list was reported to be NHI. For these eight genes, it is either gain-of-function or dominant negative, thereby showing statistical enrichment for NHI mechanisms (p = 2.66E-03, Fisher’s exact test; Table S14 and S15). For two of the three remaining genes (GRIN2B [MIM 138252] and SMAD4 [MIM 600993]) both HI and NHI consequences have been reported,36, 37, 38, 39 suggesting that for mutations in these genes more complex genotype-phenotype relations might exist, where HI and NHI mechanisms cause clinically distinct ID/DD-related disorders. For KCNQ2 (MIM 602235), the reported mutational mechanism is HI although a literature search also revealed cases with dominant-negative effects.40 We also investigated the extent of the evidence for NHI mechanisms and found that extensive functional work of mutations supporting NHI mechanisms has been previously published for eight of the 12 known genes (Table S11).
Further we hypothesized that NHI genes should be depleted for truncating mutations in individuals with ID/DD, i.e., mutations resulting in premature translation termination, whereby the mRNA is targeted for nonsense mediated decay. In our initial analyses focusing on de novo missense mutations only, we excluded truncating mutations from our dataset. Retrospectively, we searched for truncating DNMs in the 15 identified genes with clustering de novo missense mutations. We found only three predicted truncating mutations in two of 15 genes, which is significantly less than expected based on the total number of DNMs found in the total cohort for all HI genes (p<1.00e-05; Permutation test).
We have previously hypothesized that genes with mutations acting through NHI mechanisms might be more intolerant to normal variation than genes with mutations acting though a HI mechanism for ID/DD.8 To test for tolerance to variation, existing scores like pLI15 are not useful because these capture tolerance to mRNA truncating variation rather than tolerance to variation in general. Therefore, we measured tolerance to variation as the ratio of missense over synonymous variation “,” which has been used by us and others previously for predicting disease genes.2, 41, 42 We downloaded all PASS-filtered single nucleotide variants (SNVs) from ExAC (n = 9,035,134) and constructed a “” measure by counting the unique missense SNVs , and the unique synonymous SNVs , while correcting for sequence composition using the total possible unique missense and synonymous SNVs ( and respectively): (Table S16).
Based on calculations of these scores for the sets of 116 NHI, and 183 HI genes, we indeed find that genes with mutations acting through a NHI mechanism are significantly more intolerant to missense variation than genes with mutations acting though a HI mechanism (p = 2.24e-03; permutation test, Figure 2). In line with our hypothesis, also our set of 15 genes with clustered DNMs was significantly less tolerant to missense variation compared to the set of 183 genes with mutations acting through a HI mechanism (p = 8.45e-03; permutation test, Figure 2).
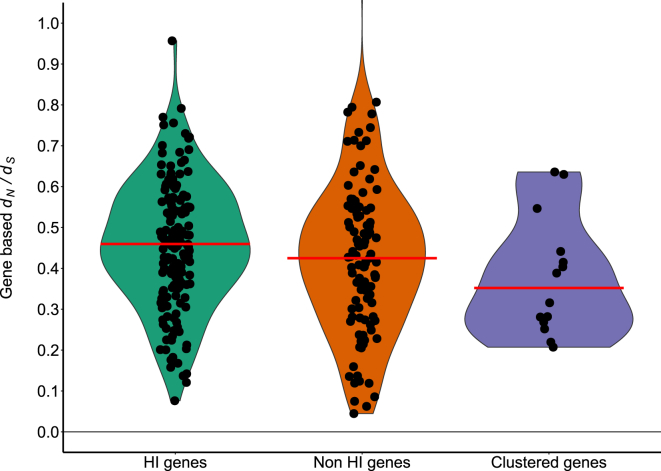
Figure 2.
Intolerance to Missense Variation
Violin plots show the distribution of the gene-based (y axis) per gene set (x axis). The median is indicated by a red horizontal line. The NHI genes are more intolerant to missense variation than HI genes (HI genes median: 0.460; NHI genes median: 0.428; p = 2.24e-03). In addition, the identified genes with clustering mutations are more intolerant to missense variation than HI genes (genes with clustering mutations median: 0.352; p = 8.45e-03).
Modeling of missense mutations in a 3D protein structure is helpful to gain more insight into the possible structural and functional effects.43 Conceptually, mutations in the core of the protein structure are more likely to prevent proper folding than mutations on the protein surface.44 The impact of a surface change, however, depends entirely on the spatial context and is therefore less likely to result in misfolding and subsequent protein degradation.45 Consequently, de novo disease-causing missense mutations preventing proper folding cause protein degradation, and thus indirectly lead to HI, similar to protein truncating mutations in such genes. To test the hypothesis that our clustered de novo missense mutations do not generally result in HI due to protein misfolding, we modeled mutations onto the 3D protein structure using YASARA & WHAT IF Twinset.46, 47 A (partial) protein 3D structure was available or could be created via homology modeling7 for 10 of the 15 identified genes. We assessed 48 missense mutations on the 3D structure (i.e., buried, at the surface, or semi-buried) and whether the mutation was likely to affect protein folding (no effect, local effect, or large effect; Figure 3, Table S17). To compare the results of 3D modeling of clustered mutations, we also modeled 75 de novo disease-causing missense mutations in 25 genes with mutations acting though HI (Table S13) for which a structure was available (Table S17). For the HI genes, 42% of missense mutations were buried and 34% of mutations were located at the protein surface. In the 10 genes for which a mutational NHI effect is proposed, only 11% of mutations was buried whereas 61% was located at the protein surface (p = 1.26E-03, chi-square test; Table S17). Even more strikingly, only 19% of the clustering de novo missense mutations were likely to result in a large structural change that would affect protein function whereas this was observed for 63% of de novo missense mutations in HI genes (p = 8.43E-06, chi-square test). These results support the notion that the majority of clustered de novo disease-causing missense mutations do not result in haploinsufficiency at the protein structure level, but exert their effect through other mechanisms. Possibly this could be through the functional impairment of protein-protein interactions, as we noted that two of the three candidate ID/DD-associated genes require complex formation or joining of protein subunits (e.g., multimerization) to be functional (Table 2).
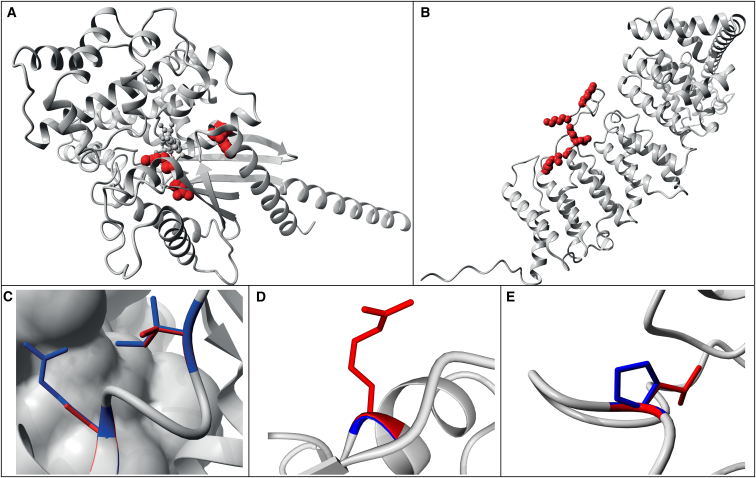
Figure 3.
Examples of Modeling of Missense Mutations on 3D Protein Structures
Wild-type residues are marked in blue; de novo mutations are indicated as red globes or lines (Tables S17).
(A) 3D structure of GNA1, acting through HI, showing that the modeled missense mutations are buried and likely to disrupt protein folding.
(B) Structure of PPP2R5D, acting through NHI, where the modeled missense mutations affect mostly surface residues and are expected to have no or only local structural effects.
(C) Zoom-in of known missense variants p.Arg496Cys and p.Ile500Val in SMAD4 known to act through a gain-of-function mechanism. These variants are located on the surface of the monomer and in contact with another SMAD4 monomer.38
(D) Zoom-in of the missense variant p.Gly343Arg in ACTL6B which is located at the surface. The side-chain points toward the solvent, therefore the larger Arginine will fit.
(E) Zoom-in of the missense variant p.Pro65Leu in PCGF2 close to the interaction site with other molecules.
In conclusion, we developed a method for the identification of disease-associated genes based on the significance of spatial mutation clustering within a gene. We show that our method successfully identifies genes previously implicated in ID/DD. Moreover, we identified three genes with similar clustering patterns that we propose as candidate ID/DD-associated genes. Our findings support the concept that these mutations mostly exert their pathogenic effect through disease mechanisms other than haploinsufficiency. Thus, our findings might indicate a larger contribution of non-haploinsufficient mechanisms to ID/DD than previously thought.
Acknowledgments
This work was in part financially supported by grants from the Netherlands Organization for Scientific Research (916-14-043 to C.G. and 918-15-667 to J.A.V.), the European Research Council (ERC Starting grant DENOVO 281964 to J.A.V.) and from the Radboud Institute for Molecular Life Sciences, Radboud university medical center (R0002793 to G.V.). We thank Stephan Boersma for help with writing the software for analysis of cluster mutations.
Published: August 31, 2017
Footnotes
Supplemental Data include 16 figures and 17 tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2017.08.004.
Web Resources
gene2phenotype, http://www.ebi.ac.uk/gene2phenotype/downloads
Genome Diagnostics Gene List: https://www2.radboudumc.nl/Informatievoorverwijzers/Genoomdiagnostiek/en/Pages/Intellectualdisability.aspx
OMIM, http://www.omim.org/
YASARA: http://www.yasara.org/
Supplemental Data
References
- 1.The Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 3.Srour M., Caron V., Pearson T., Nielsen S.B., Lévesque S., Delrue M.A., Becker T.A., Hamdan F.F., Kibar Z., Sattler S.G. Gain-of-Function Mutations in RARB Cause Intellectual Disability with Progressive Motor Impairment. Hum. Mutat. 2016;37:786–793. doi: 10.1002/humu.23004. [DOI] [PubMed] [Google Scholar]
- 4.Hoischen A., van Bon B.W., Gilissen C., Arts P., van Lier B., Steehouwer M., de Vries P., de Reuver R., Wieskamp N., Mortier G. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat. Genet. 2010;42:483–485. doi: 10.1038/ng.581. [DOI] [PubMed] [Google Scholar]
- 5.Schuurs-Hoeijmakers J.H., Oh E.C., Vissers L.E., Swinkels M.E., Gilissen C., Willemsen M.A., Holvoet M., Steehouwer M., Veltman J.A., de Vries B.B. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am. J. Hum. Genet. 2012;91:1122–1127. doi: 10.1016/j.ajhg.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisheker M.R., Heymann G., Wang T., Coe B.P., Turner T.N., Stessman H.A.F., Hoekzema K., Kvarnung M., Shaw M., Friend K. Hotspots of missense mutation identify neurodevelopmental disorder genes and functional domains. Nat. Neurosci. 2017;20:1043–1051. doi: 10.1038/nn.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deciphering Developmental Disorders Study Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lelieveld S.H., Reijnders M.R., Pfundt R., Yntema H.G., Kamsteeg E.J., de Vries P., de Vries B.B., Willemsen M.H., Kleefstra T., Löhner K. Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability. Nat. Neurosci. 2016;19:1194–1196. doi: 10.1038/nn.4352. [DOI] [PubMed] [Google Scholar]
- 9.Huang N., Lee I., Marcotte E.M., Hurles M.E. Characterising and predicting haploinsufficiency in the human genome. PLoS Genet. 2010;6:e1001154. doi: 10.1371/journal.pgen.1001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner T.N., Douville C., Kim D., Stenson P.D., Cooper D.N., Chakravarti A., Karchin R. Proteins linked to autosomal dominant and autosomal recessive disorders harbor characteristic rare missense mutation distribution patterns. Hum. Mol. Genet. 2015;24:5995–6002. doi: 10.1093/hmg/ddv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkie A.O. The molecular basis of genetic dominance. J. Med. Genet. 1994;31:89–98. doi: 10.1136/jmg.31.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehr H., Jang S.H., Duarte J.M., Wierling C., Lehrach H., Lappe M., Lange B.M. The structural impact of cancer-associated missense mutations in oncogenes and tumor suppressors. Mol. Cancer. 2011;10:54. doi: 10.1186/1476-4598-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamburov A., Lawrence M.S., Polak P., Leshchiner I., Lage K., Golub T.R., Lander E.S., Getz G. Comprehensive assessment of cancer missense mutation clustering in protein structures. Proc. Natl. Acad. Sci. USA. 2015;112:E5486–E5495. doi: 10.1073/pnas.1516373112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner T.N., Yi Q., Krumm N., Huddleston J., Hoekzema K., HA F.S., Doebley A.L., Bernier R.A., Nickerson D.A., Eichler E.E. denovo-db: a compendium of human de novo variants. Nucleic Acids Res. 2017;45:D804–D811. doi: 10.1093/nar/gkw865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrow J., Frankish A., Gonzalez J.M., Tapanari E., Diekhans M., Kokocinski F., Aken B.L., Barrell D., Zadissa A., Searle S. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besenbacher S., Sulem P., Helgason A., Helgason H., Kristjansson H., Jonasdottir A., Jonasdottir A., Magnusson O.T., Thorsteinsdottir U., Masson G. Multi-nucleotide de novo Mutations in Humans. PLoS Genet. 2016;12:e1006315. doi: 10.1371/journal.pgen.1006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genome of the Netherlands Consortium Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat. Genet. 2014;46:818–825. doi: 10.1038/ng.3021. [DOI] [PubMed] [Google Scholar]
- 19.Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krumm N., Turner T.N., Baker C., Vives L., Mohajeri K., Witherspoon K., Raja A., Coe B.P., Stessman H.A., He Z.X. Excess of rare, inherited truncating mutations in autism. Nat. Genet. 2015;47:582–588. doi: 10.1038/ng.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner T.N., Hormozdiari F., Duyzend M.H., McClymont S.A., Hook P.W., Iossifov I., Raja A., Baker C., Hoekzema K., Stessman H.A. Genome Sequencing of Autism-Affected Families Reveals Disruption of Putative Noncoding Regulatory DNA. Am. J. Hum. Genet. 2016;98:58–74. doi: 10.1016/j.ajhg.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conrad D.F., Keebler J.E., DePristo M.A., Lindsay S.J., Zhang Y., Casals F., Idaghdour Y., Hartl C.L., Torroja C., Garimella K.V., 1000 Genomes Project Variation in genome-wide mutation rates within and between human families. Nat. Genet. 2011;43:712–714. doi: 10.1038/ng.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauch A., Wieczorek D., Graf E., Wieland T., Endele S., Schwarzmayr T., Albrecht B., Bartholdi D., Beygo J., Di Donato N. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 24.de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 25.Halvardson J., Zhao J.J., Zaghlool A., Wentzel C., Georgii-Hemming P., Månsson E., Ederth Sävmarker H., Brandberg G., Soussi Zander C., Thuresson A.C., Feuk L. Mutations in HECW2 are associated with intellectual disability and epilepsy. J. Med. Genet. 2016;53:697–704. doi: 10.1136/jmedgenet-2016-103814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finn R.D., Coggill P., Eberhardt R.Y., Eddy S.R., Mistry J., Mitchell A.L., Potter S.C., Punta M., Qureshi M., Sangrador-Vegas A. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44(D1):D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi K.Y., Yoo M., Han J.H. Toward understanding the role of the neuron-specific BAF chromatin remodeling complex in memory formation. Exp. Mol. Med. 2015;47:e155. doi: 10.1038/emm.2014.129. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda Y., Oma Y., Nishimori K., Ohta T., Harata M. Brain-specific expression of the nuclear actin-related protein ArpNalpha and its involvement in mammalian SWI/SNF chromatin remodeling complex. Biochem. Biophys. Res. Commun. 2002;299:328–334. doi: 10.1016/s0006-291x(02)02637-2. [DOI] [PubMed] [Google Scholar]
- 29.Ramírez O.A., Vidal R.L., Tello J.A., Vargas K.J., Kindler S., Härtel S., Couve A. Dendritic assembly of heteromeric gamma-aminobutyric acid type B receptor subunits in hippocampal neurons. J. Biol. Chem. 2009;284:13077–13085. doi: 10.1074/jbc.M900575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins M.J., Calver A.R., Filippov A.K., Hirst W.D., Russell R.B., Wood M.D., Nasir S., Couve A., Brown D.A., Moss S.J., Pangalos M.N. GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J. Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones K.A., Borowsky B., Tamm J.A., Craig D.A., Durkin M.M., Dai M., Yao W.J., Johnson M., Gunwaldsen C., Huang L.Y. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 32.Møller R.S., Wuttke T.V., Helbig I., Marini C., Johannesen K.M., Brilstra E.H., Vaher U., Borggraefe I., Talvik I., Talvik T. Mutations in GABRB3: From febrile seizures to epileptic encephalopathies. Neurology. 2017;88:483–492. doi: 10.1212/WNL.0000000000003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johannesen K., Marini C., Pfeffer S., Møller R.S., Dorn T., Niturad C.E., Gardella E., Weber Y., Søndergård M., Hjalgrim H. Phenotypic spectrum of GABRA1: From generalized epilepsies to severe epileptic encephalopathies. Neurology. 2016;87:1140–1151. doi: 10.1212/WNL.0000000000003087. [DOI] [PubMed] [Google Scholar]
- 34.Acuna-Hidalgo R., Bo T., Kwint M.P., van de Vorst M., Pinelli M., Veltman J.A., Hoischen A., Vissers L.E., Gilissen C. Post-zygotic Point Mutations Are an Underrecognized Source of De Novo Genomic Variation. Am. J. Hum. Genet. 2015;97:67–74. doi: 10.1016/j.ajhg.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goriely A., Wilkie A.O. Missing heritability: paternal age effect mutations and selfish spermatogonia. Nat. Rev. Genet. 2010;11:589. doi: 10.1038/nrg2809-c1. [DOI] [PubMed] [Google Scholar]
- 36.Endele S., Rosenberger G., Geider K., Popp B., Tamer C., Stefanova I., Milh M., Kortüm F., Fritsch A., Pientka F.K. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 2010;42:1021–1026. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- 37.Lemke J.R., Hendrickx R., Geider K., Laube B., Schwake M., Harvey R.J., James V.M., Pepler A., Steiner I., Hörtnagel K. GRIN2B mutations in West syndrome and intellectual disability with focal epilepsy. Ann. Neurol. 2014;75:147–154. doi: 10.1002/ana.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Goff C., Mahaut C., Abhyankar A., Le Goff W., Serre V., Afenjar A., Destrée A., di Rocco M., Héron D., Jacquemont S. Mutations at a single codon in Mad homology 2 domain of SMAD4 cause Myhre syndrome. Nat. Genet. 2011;44:85–88. doi: 10.1038/ng.1016. [DOI] [PubMed] [Google Scholar]
- 39.Gallione C., Aylsworth A.S., Beis J., Berk T., Bernhardt B., Clark R.D., Clericuzio C., Danesino C., Drautz J., Fahl J. Overlapping spectra of SMAD4 mutations in juvenile polyposis (JP) and JP-HHT syndrome. Am. J. Med. Genet. A. 2010;152A:333–339. doi: 10.1002/ajmg.a.33206. [DOI] [PubMed] [Google Scholar]
- 40.Wuttke T.V., Jurkat-Rott K., Paulus W., Garncarek M., Lehmann-Horn F., Lerche H. Peripheral nerve hyperexcitability due to dominant-negative KCNQ2 mutations. Neurology. 2007;69:2045–2053. doi: 10.1212/01.wnl.0000275523.95103.36. [DOI] [PubMed] [Google Scholar]
- 41.Ge X., Kwok P.Y., Shieh J.T. Prioritizing genes for X-linked diseases using population exome data. Hum. Mol. Genet. 2015;24:599–608. doi: 10.1093/hmg/ddu473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge X., Gong H., Dumas K., Litwin J., Phillips J.J., Waisfisz Q., Weiss M.M., Hendriks Y., Stuurman K.E., Nelson S.F. Missense-depleted regions in population exomes implicate ras superfamily nucleotide-binding protein alteration in patients with brain malformation. Npj Genomic Medicine. 2016;1:16036. doi: 10.1038/npjgenmed.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C., Levitt M. Accurate prediction of the stability and activity effects of site-directed mutagenesis on a protein core. Nature. 1991;352:448–451. doi: 10.1038/352448a0. [DOI] [PubMed] [Google Scholar]
- 44.Yue P., Li Z., Moult J. Loss of protein structure stability as a major causative factor in monogenic disease. J. Mol. Biol. 2005;353:459–473. doi: 10.1016/j.jmb.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 45.Venselaar H., Camilli F., Gholizadeh S., Snelleman M., Brunner H.G., Vriend G. Status quo of annotation of human disease variants. BMC Bioinformatics. 2013;14:352. doi: 10.1186/1471-2105-14-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krieger E., Koraimann G., Vriend G. Increasing the precision of comparative models with YASARA NOVA--a self-parameterizing force field. Proteins. 2002;47:393–402. doi: 10.1002/prot.10104. [DOI] [PubMed] [Google Scholar]
- 47.Vriend G. WHAT IF: a molecular modeling and drug design program. J Mol Graph. 1990;8:52–56. doi: 10.1016/0263-7855(90)80070-v. 29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.