Abstract
Background
Phosphatidylcholine Transfer Protein (PCTP) regulates the intermembrane transfer of phosphatidylcholine (PC). Higher platelet PCTP expression is associated with increased platelet responses upon activation of protease-activated receptor 4 (PAR4) thrombin receptors noted in black subjects as compared to white subjects. Little is known regarding regulation of platelet PCTP. Haplodeficiency of RUNX1, a major hematopoietic transcription factor, is associated with thrombocytopenia and impaired platelet responses upon activation. Platelet expression profiling of a patient described by us with a RUNX1 loss-of-function mutation revealed a 10-fold downregulation of PCTP gene compared with healthy controls.
Methods
We pursued the hypothesis that PCTP is regulated by RUNX1 and that PCTP expression is correlated with cardiovascular events. We studied RUNX1 binding to PCTP promoter using DNA-protein binding studies and human erythroleukemia (HEL) cells, and promoter activity using luciferase reporter studies. We assessed the relationship between RUNX1 and PCTP in peripheral blood RNA and PCTP and death or myocardial infarction (MI) in two separate patient cohorts (587 total patients) with cardiovascular disease.
Results
Platelet PCTP protein in the patient was reduced by ~50%. DNA-protein binding studies showed RUNX1 binding to consensus sites in ~1 kB of PCTP promoter. PCTP expression was increased with RUNX1 overexpression and reduced with RUNX1 knockdown in HEL cells, indicating that PCTP is regulated by RUNX1. Studies in two cohorts of patients showed that RUNX1 expression in blood correlated with PCTP gene expression; PCTP expression was higher in black compared to white subjects, and associated with future death/myocardial infarction after adjusting for age, sex, and race (odds ratio 2.05, 95% CI [1.6–2.7], P-value < 0.0001). RUNX1 expression is known to initiate at two alternate promoters, a distal P1 and a proximal P2 promoter. In patient cohorts there were differential effects of RUNX1 isoforms on PCTP expression with a negative correlation in blood between RUNX1 expressed from the P1 promoter and PCTP expression.
Conclusions
PCTP is a direct transcriptional target of RUNX1. PCTP expression is associated with death/myocardial infarction in patients with cardiovascular disease. RUNX1 regulation of PCTP may play a role in the pathogenesis of platelet-mediated cardiovascular events.
Keywords: platelets, RUNX1 haplodeficiency, PCTP, cardiovascular disease
INTRODUCTION
Phosphatidylcholine transfer protein (PCTP) is a member of the START (Steroidogenic Acute Regulatory Protein-Related Transfer) domain superfamily and is responsible for the intermembrane transfer of phosphatidylcholine (PC), a major plasma membrane phospholipid. PCTP has a wide tissue distribution,1 and is abundantly expressed in human platelets, but not in mouse platelets.2 The specific function of PCTP in platelets or other cells is unclear, but several findings suggest that it is important for platelet function. PC constitutes a major fraction of platelet phospholipids and source of arachidonic acid (AA) upon platelet activation for thromboxane production.3,4 PC is also a substrate for phospholipase D, leading to formation of phosphatidic acid and the second messenger diacylglycerol.5 Pharmacological inhibition of PCTP decreased platelet aggregation in response to thrombin protease-activated receptor 4 (PAR4) agonist and knockdown of PCTP in megakaryocytic cells blunted PAR4 induced calcium mobilization.2 Platelet PCTP has been implicated in racial differences in platelet responses; increased PCTP expression has been associated with higher platelet aggregation and calcium mobilization induced by PAR4 in black subjects as compared to white subjects.2 Little is known regarding the regulation of PCTP expression in platelets and megakaryocytes.
RUNX1 is a major hematopoietic transcription factor (TF) and regulates numerous megakaryocytic/platelet genes.6,7 RUNX1 expression is known to initiate at two alternate promoters, a distal P1 and a proximal P2 promoter, which together with alternative splicing results in different isoforms of RUNX1 protein that have distinct roles and tissue expression.8–15 Two isoforms, variant 1 (NM_001754) and variant 2 (NM_001001890.1), expressed from the P1 and P2 promoters encode for RUNX1 proteins of 480 and 453 amino acids, respectively (Supplemental Figure 1). Variants 1 and 2 differ in their exons 1 but otherwise share the remaining RUNX1 exons. Platelets (D. Voora, M.D. and A.K. Rao, M.B.B.S., unpublished data, 2017) and human erythroleukemia (HEL) cells16 express both variants. Human RUNX1 haplodeficiency is associated with thrombocytopenia, platelet function defects, and increased risk of leukemia and myelodysplastic syndromes.17,18 The platelet abnormalities described include diminished aggregation and granule secretion, protein phosphorylation (pleckstrin and myosin), and activation of GPIIb-IIIa complex upon activation.18,19. Expression profiling of platelets from a patient described by us with a heterozygous RUNX1 nonsense mutation20 showed a marked downregulation of PCTP expression (fold change ratio 0.09, p=0.02) compared with normal controls.21 These studies also showed that several other genes were significantly downregulated, including MYL9, PF4, PRCKQ, ALOX12 and PLDN; we have shown that these are transcriptionally regulated by RUNX1.20,22–26
Based on the decreased platelet PCTP expression in our patient with RUNX1 haplodeficiency21 and the association between PCTP and enhanced platelet responses,2 we hypothesized that PCTP is regulated by RUNX1. Our results provide the first evidence that PCTP is a direct transcriptional target of RUNX1, constituting a cogent mechanism for downregulation of platelet PCTP in our patient. To reinforce the hypothesis that RUNX1 regulates PCTP, we evaluated peripheral blood gene expression correlation for RUNX1 and PCTP and report strong correlation between RUNX1 and PCTP. To assess the potential clinical relevance, we assessed the relationship between PCTP in blood and clinical events in two separate cohorts of patients with cardiovascular disease from the Duke Catheterization Genetics (CATHGEN) cohort we have previously studied.27 These studies revealed that PCTP expression is independently associated with death or myocardial infarction (MI) in patients with cardiovascular disease.
METHODS
Patient with RUNX1 Haplodeficiency
We have previously described19,21 the clinical presentation and studies in this 38 year old white male, documenting decreased agonist-stimulated platelet aggregation, secretion, GPIIb-IIIa activation, and phosphorylation of pleckstrin and myosin light chain (MLC); platelet PKC-θ level was also decreased. This patient was initially referred for evaluation of easy bruising and epistaxis. The patient has a single point mutation in RUNX1, in intron 3 at the splice acceptor site for exon 4, leading to a frameshift with premature termination in the conserved Runt homology domain.20
This research in the patient with RUNX1 haplodeficiency and healthy subjects was approved by the institutional human subjects review board of Temple University and all participants gave written informed consent.
Patient cohorts with cardiovascular disease
The Duke Catheterization Genetics (CATHGEN) cohort is a sequential cohort of patients presenting to the Duke University Medical Center cardiac catheterization laboratory who provided informed consent for biologic specimen banking. CATHGEN consists of two cohorts of patients with cardiovascular disease, an observational and a case/control cohort. The baseline clinical, medication, microarray data generated from unfractionated peripheral blood RNA preserved in PAXgene collection tubes at the time of catheterization, and long-term clinical outcomes from the CATHGEN cohorts have been described27. All CATHGEN participants provided informed consent and this analysis was approved by the Duke University Medical Center institutional review board.
Materials and Methods
The RUNX1 siRNA, PCTP siRNA, control siRNA and antibodies against RUNX1, PCTP and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phorbol 12-myristate 13-acetate (PMA) was from Enzo life Sciences (Farmingdale, NY). Luciferase Reporter Assay System kit, PCR reagents, PGL3-basic vector, and the Renilla luciferase control vector were purchased from Promega Biotech (Madison, WI). Trizol reagent was from Life Technologies Corporation (Carlsbad, CA). All oligonucleotides and IRDye-labeled probes were synthesized by Integrated DNA Technologies (Coralville, IA). Fluorophore-conjugated secondary antibodies raised in donkey were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Computational Bioinformatics
Potential transcription factor RUNX1 binding sites were predicted by use of TFsearch and TFBIND INPUT (http://www.cbrc.jp/research/db/TFSEARCH.html, http://tfbind.hgc.jp/).
Preparation of platelet RNA and proteins
Whole blood samples were collected from the patient and healthy volunteer donors into 7.5 ml each of acid citrate dextrose (ACD) solution (71.4 mM citric acid, 85 mM sodium citrate-dihydrate, 11.1 mM dextrose) as described previously.19,20 The platelet pellet was washed twice in Hepes buffer (pH 6.5, 20 mM Hepes, 5.5 mM dextrose, 0.376 mM NaH2PO4, 1 mM MgCl2 2.7 mM KCl, 137 mM NaCl and 1 mg/ml bovine serum albumin) and resuspended in TRIzol Reagent (Life Technologies, Carlsbad, CA) for total RNA isolation as recommended by the manufacturer. For preparing platelet protein, pellets were resuspended in M-Per Mammalian Protein Extraction Reagent (ThermoFisher Scientific, Grand Island, NY) with proteinase inhibitors.
Cell Cultures
Human erythroleukemia (HEL) cells obtained from the American Type Culture Collection (Rockville, MD) were cultured in RPMI 1640 medium (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (GE Healthcare, Mississauga) and penicillin/streptomycin (100 U/ml/100 mg/ml, Invitrogen, Carlsbad, CA) at 37°C in a humidified 5% CO2 atmosphere. HEL cells were treated with phorbol myristate acetate (PMA) (10–30 nM) for 24 hours to induce megakaryocytic transformation. Human cervical carcinoma HeLa cells from ATCC, (CCL-2) were cultured in DMEM (Mediatech, Manassas, VA) supplemented with 10% FBS (GE Healthcare, Mississauga), 100 U/mL penicillin, and 100mg/mL streptomycin (Invitrogen), and seeded in 12-well plates (4×105 cells/well).
Chromatin Immunoprecipitation (ChIP) Assays
HEL cells treated with PMA (10 nM) were cross-linked with 1% formadehyde. ChIP analysis was performed using the Active Motif ChIP-IT assay kit (Carlsbad, CA). Chromatin was immunoprecipitated using control IgG from the Active Motif (Carlsbad, CA) and anti-RUNX1 antibodies (Santa Cruz, Cat. No. sc-8563). PCR reactions were performed using primers listed in Supplemental Table 1. PCR products were analyzed by electrophoresis on 3% agarose gels.
Nuclear Extract Preparation and Electrophoretic Mobility Shift Assay
Nuclear protein was extracted from PMA (10 nM)-treated HEL cells using NE-PER Nuclear and Cytoplasmic Extraction Reagents (ThermoFisher Scientific, Grand Island, NY). IRDye-labeled oligonucleotides (IDT) were double stranded. EMSA was done using Odyssey Infrared EMSA Kit (Li-Cor Biosciences, Lincoln, NE). 10 μg of nuclear protein was incubated (20 minutes, room temperature) with 50 nM probe and 1 μg Poly dI-dC in a 20 μl reaction including 10 mM Tris, pH 7.5, 50 mM KCl, 3.5 mM DTT, 0.25% Tween 20, 2.5 % Glycerol, 10 mM EDTA. For super-shift assays, 1 μg of anti-RUNX1 antibodies (Santa Cruz, Cat. No. sc-8563) or control IgG (Santa Cruz) were incubated with the binding reaction (20 minutes, room temperature). Competition assays were performed with 100-fold excess unlabeled probe. Binding complexes were separated by electrophoresis on 5% Tris-borate-EDTA native acrylamide gels (Bio-Rad Laboratories) at 4°C and imaged using the Odyssey Infrared Imaging System (Li-Cor Biosciences). The oligonucleotide sequences used to prepare DNA probes are shown in Supplemental Table 2.
Promoter and Plasmid Construction
Genomic DNA was isolated from human blood using Genomic DNA isolation kit (Qiagen). The promoter region of PCTP was obtained by the PCR amplification of the genomic DNA (primers listed in Supplemental Table 3). Promoter-luciferase constructs were generated with DNA fragments containing human PCTP promoter regions −1015 to −3, and −106 to −3 (from ATG) and cloned into the PGL3-basic vector (Promega, Madison, WI). The constructs with RUNX1 sites mutated were generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene Inc., La Jolla, CA) (Supplemental Table 3).
Luciferase reporter assay
HEL cells were grown in 24-well plates and treated with 30 nM PMA. Transfections were performed using the Lipofectamine™ 2000 Reagent (Invitrogen). Cells (2×105) were co-transfected with 200 ng of PCTP promoter plasmids and either the empty PGL3-basic plasmid (Promega, Madison, WI) or PGL3-basic plasmid containing the PCTP promoter region and 10 ng of pRL-TK vector DNA (Promega) as an internal control transfection. Luciferase activity was analyzed at 24 hours using the Dual Luciferase Reporter Assay (Promega).
Quantitative Real Time PCR
Total RNA was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA). RNA (1 μg) was reverse-transcribed using Superscript III (Applied Biosystems). The cDNA was diluted 1:50 in nuclease-free water for PCR reactions. The primer concentration in each well was 0.1 μM. The real time PCR parameters were: 95°C for 10 min and 40 cycles of 95°C 15 s, 55°C 20 s, and 72°C 20 s, using a Master Cycler Real-Time PCR System (Eppendorf, Hauppauge, NY), and relative abundances were calculated by ΔΔCT method using GAPDH as the reference gene. The primers used are shown in the Supplemental Table 4.
Cell extracts and Immunoblotting
Western blot analysis was performed on cells lysed in M-PER Mammalian Protein Extraction Reagent (ThermoFisher Scientific, Grand Island, NY) with protease inhibitors (Enzo Life Sciences, Farmingdale, NY). Lysates (20 or 30 μg) were subjected to 10% or 12% SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA). Blots were probed with the antibodies against RUNX1, PCTP, and β-actin (Santa Cruz). The secondary antibodies were IRDye-labeled antibodies (Li-Cor Biosciences, Lincoln, NE). Immunoreactive proteins were detected using the Odyssey Infrared Imaging system (Li-Cor Biosciences).
siRNA transfection
PCTP promoter plasmid transfection of PMA-treated HEL cells was performed using Lipofectamine 2000 (Life Technologies). RUNX1 siRNA, a pool of three 20–25 oligonucleotides or non-specific siRNA (50–80 nM) (Santa Cruz Biotechnology) was cotransfected with PCTP −1015/PGL3 reporter plasmid. Cells were harvested at 24 or 48 hours and processed for luciferase reporter activity, immunoblotting and quantitative RT-PCR.
Effect of RUNX1 overexpression on PCTP expression
These studies were performed using the expression plasmid RUNX1-pCMV6-XL4 and empty pCMV6-XL4 vector (OriGene Technologies, Rockville, MD). This plasmid expresses RUNX1 variant 2 (NM__001001890), expressed from RUNX1 P2 promoter. HEL cells were transiently co-transfected using Lipofectamine 2000 (Invitrogen) with 100 ng – 650 ng of RUNX1-pCMV6-XL4 plasmid or empty vector and PCTP promoter luciferase reporter constructs (−1015/PGL3-basic). The cells were harvested 24–48 hours for luciferase activity, immunoblotting or RNA extraction.
Immunostaining and microscopy
Glass coverslips were incubated (4°C overnight) with 5 μg/ml plasma fibronectin; after washing, the coverslips or slides were incubated for 30 minutes with heat-inctivated 1% BSA/PBS. Cells were seeded on the coated coverslips for 1 hr at 37°C. Adherent cells were fixed in 3.7% formaldehyde/PBS for 20 min at RT. Fixed cells were washed and permeabilized with 0.1% Triton X-100/PBS for 5 minutes, washed with PBS, and incubated with primary antibodies for 1 hour at RT, washed, then incubated with fluorophore-conjugated secondary antibodies, followed by nuclear staining with 30 nM 4′, 6-diamidino-2-phenylindole (DAPI) for 10 min. Fluorophores used were FITC and Cy3 (Jackson Immunoresearch). Coverslips were washed and mounted on slides with ProLong Gold anti-fade reagent (Invitrogen). Cells were imaged using a Leica DM IRE2 microscope with a TCS SL confocal system, using a 63×/1.40 NA oil immersion objective at room temperature and Leica imaging software, or on a Nikon E800, using a 63×/1.40 NA oil-immersion objective at room temperature and Q-Capture software. Confocal images were captured by line sequential scanning using 4× line averaging and 3× frame averaging at 400 Hz scan speed. Post-acquisition image processing was performed using ImageJ and Adobe Photoshop. Operations included brightness/contrast adjustment to all pixels in the images and grouping of images. Fluorescence intensity analysis was performed using ImageJ and Microsoft Excel. Corrected total cellular fluorescence was calculated as the average of individual cell fluorescence intensities divided by the single cell area, > 100 cells per sample.
Statistical analysis for in vitro studies
Results of the in vitro studies are expressed as the mean ±SEM. Differences were compared using the Student’s t-test.
Statistical analysis of data from CATHGEN study
Microarray gene expression data was normalized using RMA separately for the two cohorts making up the CATHGEN study: observational (N = 190) and case-control (N =397) as previously described.27 To assess for correlation between RUNX1 and PCTP expression we used microarray data from CATHGEN participants. Several potential probe sets available on the Affymetrix microarray map to the RUNX1 locus on chromosome 21. Of the 17 available probe sets, we selected 6 that map to a single locus in the genome and had evidence by PCR for being able to discriminate between RUNX1 P1 vs. P2 expression by PCR (Supplemental Table 5) as previously described16,27. To select microarray probe sets for RUNX1 we used banked, serial peripheral blood RNA from healthy volunteers with matched microarray data27 and performed PCR for RUNX1 isoforms.16 Linear mixed-effects regression with a random effect for subject was used to model the linear relationship between RUNX1 probe set expression and RUNX1 PCR expression. Effect sizes, standard errors, and p-values (calculated using a normal approximation) are reported.
Correlation between PCTP and the selected RUNX1 probe sets was evaluated using linear regression, including age, sex, and race covariates. Race-stratified PCTP-RUNX1 correlations were similarly tested. Both global and within-race correlations were combined across studies using inverse variance weight meta-analysis. PCTP was tested for differential expression due to race using a moderated t-test28 and the log fold changes combined across studies using inverse variance weighted meta-analysis.
To assess for the association between PCTP expression and cardiovascular events we used longitudinal follow up data from CATHGEN participants. In each cohort, the PCTP probe set (218676_s_at) was tested for effect on MI or death events using logistic regression. Two covariate models were evaluated: 1) the reduced covariate model controlling for the effects of age, sex, and race on death or MI outcomes, and 2) the full covariate model controlling for the effects of age, sex, race, smoking status, body mass index, hyperlipidemia, hypertension, diabetes, platelet count, aspirin treatment, and an aspirin response gene expression signature27 previously shown to correlate with death/MI outcomes in these cohorts. Meta-analysis was used to combine evidence of PCTP probe expression on the cumulative incidence of death or MI in each cohort. For each probe, the log odds ratios and their standard errors were pooled as the weighted average of the log odds ratios using inverse variance weights, assuming a fixed effect model The log fold change of PCTP expression associated with death/MI was estimated using linear regression, modeling PCTP expression as response to death or MI outcome, adjusting for age, sex, and race.
All CATHGEN data processing and statistical analyses were conducted in R version 3.2 (http://www.r-project.org/) using packages affy, meta, and limma for normalization, meta-analyses, and moderated t-tests, respectively.
RESULTS
Platelet PCTP expression is decreased in the patient with RUNX1 haplodeficiency
In previously reported expression profiling studies using Affymetrix U133 Gene Chips21 platelet PCTP expression was decreased in the patient (two separate studies) compared to profiles from six healthy control subjects) (fold change 0.1, p=0.028). This was validated using quantitative PCR; the platelet mRNA in the patient was lower than in five healthy subjects matched for race (Figure 1A). Immunofluorescence studies using anti-PCTP antibodies, performed on two separate occasions, showed that the corrected total cellular fluorescence (CTCF) was reduced by ~ 50% in patient platelets compared to platelets from four matched control subjects indicating decreased PCTP protein. Results from the patient and three control subjects studied concurrently are shown (Figure 1B).
Figure 1.
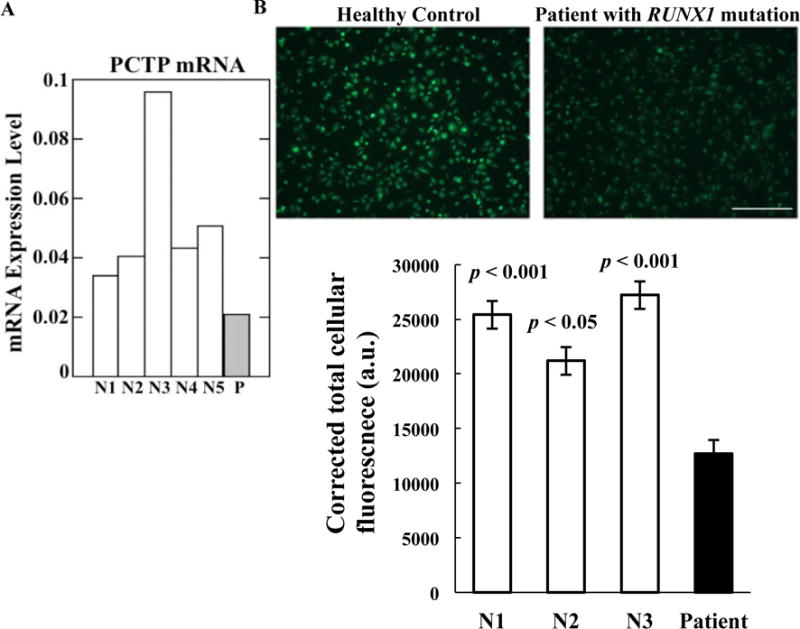
PCTP expression in patient platelets. A. Platelet PCTP mRNA levels in the patient (P) and five race-matched healthy controls (N1-N5) by quantitative PCR. Shown are mRNA levels normalized to GAPDH. B. PCTP immunostaining in platelets from the patient and platelets from three concurrently studied, matched healthy controls and normalized corrected total cellular fluorescence (mean ± SEM) calculated from images. Bar, 50 μm. The p values shown indicate comparison of the patient with each of the control subjects.
RUNX1 binds to PCTP promoter region in vivo
Analysis in silico using TFBIND database revealed five RUNX1 consensus binding sites within 1000 base pairs of the PCTP 5′ upstream region from ATG (Figure 2A). ChIP studies using PMA-treated HEL cells showed RUNX1 binding to chromatin regions encompassing RUNX1 binding sites 1 (−345/−340 bp), and 3 (−632/−627 bp), and encompassing sites 4 and 5 (−974/−969; −997/−992 bp) (Figure 2B) in the PCTP promoter. In each case, PCR amplification showed enrichment by anti-RUNX1 antibodies, but not control IgG, of the regions encompassing the RUNX1 consensus sites, indicating RUNX1 binding in vivo to the PCTP promoter.
Figure 2.
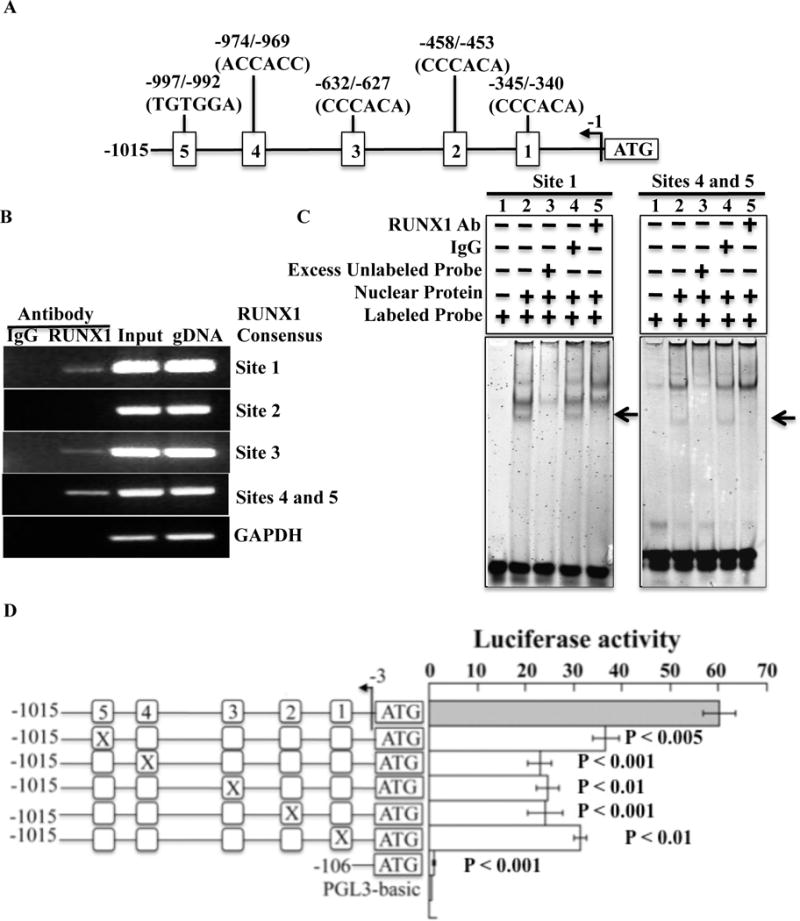
Binding of RUNX1 to PCTP promoter region and functional activity. A. 5′ upstream region of PCTP with the putative RUNX1 consensus bindings sites. B. Binding of RUNX1 to PCTP promoter region in vivo by chromatin immunoprecipitation (ChIP). Column 1 and column 2 show PCR amplification of the chromatin immunoprecipitates with control IgG and RUNX1 antibody, respectively. Column 3 shows PCR amplification of total input DNA and column 4 shows amplification with genomic DNA. Shown are PCR amplification of PCTP promoter regions encompassing RUNX1 binding sites 1–5 and GAPDH. C. Binding of RUNX1 to PCTP promoter region by EMSA. Shown is EMSA using oligonucleotide probe with consensus RUNX1 binding site 1 and probe with sites 4 and 5, and nuclear extracts from PMA-treated HEL cells. For each panel, Lane 1: probe alone; lane 2: probe with nuclear extract; lane 3: competition with 100× molar excess unlabeled probe; lane 4: effect of control IgG; lane 5: effect of RUNX1 antibodies. The arrows indicate areas where the band was super-shifted (probe with site 1) or competed (probe with sites 4 and 5) by the RUNX1 antibodies. Shown are representative of three experiments with each probe. The nucleotide sequences of the probes are shown in Supplemental Table 2. D. Luciferase reporter studies on the PCTP promoter in PMA-treated HEL cells. Shown is luciferase promoter activity at 24 hours of the wild-type construct and the effect of mutating each of the five RUNX1 consensus binding sites in the 5′ upstream region of PCTP. Shown is the ratio of luciferase to Renilla activity of the wild type construct and of constructs with each RUNX1 consensus site mutated. Shown are mean (± SEM) of four experiments. The p values indicate comparisons of the activity of each mutant construct with that of the wild-type contract
RUNX1 binds PCTP promoter sequences
EMSA studies using nuclear extracts from PMA-treated HEL cells showed RUNX1 binding to probe containing site 1 and the probe with both sites 4 and 5 (Figure 2C), but not probes with sites 2 or 3 (data not shown). In studies with the probe containing site 1 there was protein binding (Figure 2C, Panel 1, lane 2) that was eliminated by excess unlabeled probe (lane 3) and super-shifted by anti-RUNX1 antibodies (lane 5), but not by non-specific IgG (lane 4), indicating that RUNX1 was a component of the protein-DNA complex. In studies with probe containing sites 4 and 5 (Figure 2C, Panel 2), there was specific protein binding (lane 2) that was attenuated by excess unlabeled probe (lane 3); the specific binding was inhibited by anti-RUNX1 antibodies (lane 5) but not with non-specific IgG (lane 4). Together, these studies show that RUNX1 binds in the regions encompassing consensus sites 1, 4 and 5 in PCTP promoter.
RUNX1 binding sites have promoter activity
In luciferase reporter studies in HEL cells, mutation of each of RUNX1 binding sites 1–5 in the PCTP promoter resulted in ~50% reduction in promoter activity (Figure 2D), indicating that these sites were functional. Together, the concordant results of the ChIP, EMSA and luciferase reporter studies suggest that RUNX1 interacts with the PCTP 5′-upstream region through consensus sites 1, 4 and 5, and regulates promoter activity.
RUNX1 overexpression and RUNX1 siRNA inhibition modulate PCTP expression
Overexpression of RUNX1 variant 2 in HEL cells showed an increase in RUNX1 protein (Figure 3). There was an increase in PCTP promoter activity, mRNA and protein (Figure 3A). RUNX1 downregulation using non-variant selective siRNA decreased expression of RUNX1 and PCTP mRNA and protein (Figure 3B). Staining with anti-PCTP antibodies showed ~50% reduction in corrected total cellular fluorescence in RUNX1 siRNA-transfected cells (Figure 3C).
Figure 3.

Effect of transient overexpression and siRNA knockdown of RUNX1 on PCTP expression. A. Effect of RUNX1 overexpression. PMA-treated HEL cells were transfected with the empty pCMV6 vector or RUNX1-pCMV6 variant 2 expression vector. Shown (mean ± SEM) are PCTP promoter activity (N=4 experiments; p value shown represents comparison of promoter activities with and without the expression vector), mRNA levels (N=3) and protein expression (shown are immunoblots for RUNX1 and PCTP with β-actin as loading control, representative of 3 experiments). B. Effect of siRNA RUNX1 knockdown in PMA-treated HEL cells transfected with control siRNA or RUNX1 siRNA. Shown are RUNX1and PCTP mRNA (N=4; p values are for comparisons to control siRNA) and protein expression (immunoblots for RUNX1 and PCTP with β-actin as loading control; representative of 4 experiments). C. Immunostaining of PMA-treated HEL cells transfected with the indicated siRNAs, using PCTP antibodies (Bar, 10 μm) and corrected total cellular fluorescence (mean ± SEM) calculated from images.
CATHGEN cohorts: PCTP expression in blood correlates with RUNX1 expression and cardiovascular events
To test the validity of using PCTP expression in unfractionated whole blood as a surrogate for platelet PCTP expression, we first confirmed the differential platelet PCTP expression by race in whole blood PCTP gene expression data from patients referred for coronary angiography. We found that in two separate CATHGEN cohorts (Supplemental Table 6), PCTP expression was higher in blacks (log fold-change for PCTP expression in black vs. white subjects in observational cohort: 0.66, 95% confidence interval [CI] [0.42 – 0.91], P-value ≤ 0.0001 and case-control cohort: 0.16, [0.01 – 0.34], P-value = 0.07). In addition, we found a significant correlation between peripheral blood and platelet PCTP (Spearman rho = 0.22, p = 0.002) (D. Voora, M.D., Unpublished, 2017). Based on this, and a consistent higher peripheral blood PCTP expression blacks vs. whites, we used whole blood PCTP expression as a surrogate for platelet PCTP expression.
RUNX1 expression initiates at two alternate promoters, a distal P1 and a proximal P2 promoter, that encode for variants 1 and 2, respectively.8–10 The available probe sets on the Affymetrix microarray that map to the RUNX1 locus on chromosome 21 represent transcripts that originate from both promoters (Figure 4A). To test the hypothesis that RUNX1 expression correlates with PCTP expression in peripheral blood RNA we first selected 6 probe sets that uniquely mapped to the genome and had evidence by PCR for being able to discriminate between RUNX1 P1 vs P2 expression by PCR (See Methods). These probe sets map to the RUNX1 region and also correlate with transcripts from either P1 or P2 by PCR (Table 1) with two probe sets correlating with P1: (correlation p-value provided in brackets, :233690_at [2.46E-06] and 220918_at [4.67E-03]) and four probe sets with P2 (1557527_at [5.23E-03], 210365_at [0.04], 217263_x_at [0.03], and 211181_x_at [0.05]) The relationships between RUNX1 probe sets and PCTP gene expression were analyzed for each patient cohort separately and combining both (Figures 4B and 5). We found that both P1 RUNX1 probe sets were negatively correlated with PCTP gene expression in each cohort and when combined (Figure 5A–B, Table 1) and that effects were similar in blacks vs. whites (Figure 4B). With respect to P2 RUNX1 probe sets, when the cohorts were combined, only two of four probe sets had evidence for a positive correlation (p ≤ 0.05) with PCTP expression (Figures 4B, 5E and 5F). When analyzed separately, the relationship was positive and significant for 3 of the 4 probe sets in the observational cohort and for 2 of 4 probe sets in the case-control cohort. (Figure 5C–F, and Table 1). Overall, while there was a clear negative correlation between RUNX1 P1 and PCTP expression, there was no consistent evidence for a relationship between RUNX1 P2 probe sets and PCTP expression.
Figure 4.

RUNX1 mRNA isoforms and their correlation with PCTP expression in blood. Panel A: Known mRNA from the RUNX1 locus are depicted along with their reference sequence identifiers, intron/exons composition, and genomic coordinates on chromosome 21 based on hg19 human genome assembly. Solid arrows represent RUNX1 isoforms that originate from P1 and P2 promoters. Asterisks indicate approximate location of PCR primers used to distinguish between P1 and P2 derived isoforms. The vertical dashed line represents where the genomic coordinates on the x-axis were interrupted to reduce the width of the overall image. RUNX1 variant 1 isoform (Refseq gene ID: NM_001754) and variant 2 isoform (Refseq gene ID: NM_001001890) employ different exon 1 usage, are expressed form the P1 and P2 promoters, and encode for proteins of 480 and 453 amino acids, respectively. Selected (see Methods for selection criteria) Affymetrix U133 probe sets aligned to the RUNX1 locus are depicted. Panel B, Correlation of PCTP with RUNX1 expression in peripheral blood RNA. The slope of the association between expression of selected RUNX1 probe sets originating from the P1 and P2 promoters and PCTP probe set (218676_s_at) microarray expression stratified by race is shown on the y-axis with the size of the points reflective of the P-value and stratified by race. The data are from two separate cohorts (combined) from the Duke Catheterization Genetic (CATHGEN) biorepository. Slopes greater than zero reflect positive correlation and those less than zero reflect negative correlation.
Table 1.
Correlations between selected RUNX1 probe sets and RUNX1 P1 vs. P2 transcripts by PCR and with PCTP expression by microarray in whole blood RNA.
| Probe Set | Correlation with RUNX1 by PCR* | Correlation with PCTP* | |||||
|---|---|---|---|---|---|---|---|
| P2 | P1 | Case/control cohort | Observational cohort | Combined cohorts | |||
| Effect (SE) | p-value | Effect (SE) | p-value | Effect (SE)/p-value | Effect (SE)/p-value | Effect (SE)/p-value | |
| 1557527_at | 0.18 (0.07) | 5.23E-03 | 0.06 (0.09) | 0.50 | 0.19 (0.09)/0.03 | −0.03 (0.08)/0.65 | 0.07 (0.11)/0.51 |
| 210365_at | 0.2 (0.1) | 0.04 | −0.02 (0.12) | 0.86 | 0.00 (0.06)/0.99 | 0.93 (0.29)/0.001 | 0.42 (0.46)/0.36 |
| 217263_x_at | 0.12 (0.06) | 0.03 | −0.01 (0.08) | 0.94 | 0.30 (0.16)/0.07 | 0.84 (0.26)/0.001 | 0.53 (0.27)/0.05 |
| 211181_x_at | 0.14 (0.07) | 0.05 | −0.08 (0.09) | 0.41 | 0.40 (0.15)/0.008 | 1.19 (0.27)/0.002 | 0.76 (0.39)/0.05 |
| 233690_at | 0.17 (0.09) | 0.07 | 0.51 (0.11) | 2.46E-06 | −0.63 (0.07)/< 1.0E-07 | −0.89 (0.13)/< 1.0E-07 | −0.75 (0.13)/< 1.0E-07 |
| 220918_at | 0.03 (0.08) | 0.72 | 0.28 (0.1) | 4.67E-03 | −0.85 (0.07)/< 1.0E-07 | −1.02 (0.13)/< 1.0E-07 | −0.90 (0.08)/< 1.0E-07 |
Effects refer to slopes from linear regression; SE = Standard error
Figure 5.
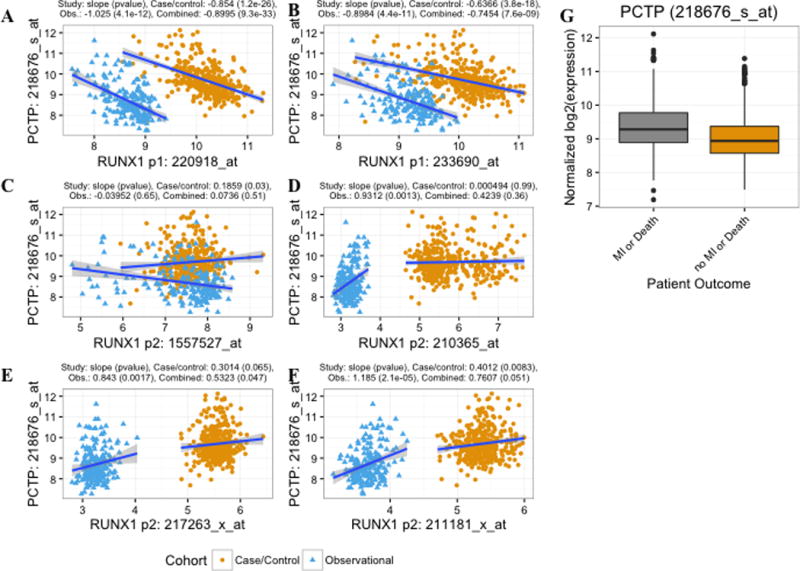
Correlation of PCTP with RUNX1 expression in peripheral blood RNA and with future cardiovascular events. Panels A-F Normalized PCTP expression plotted as a function of normalized RUNX1 P1 (A and B) and P2 (C – D) probe set expression. Points are color coded by cohort and trend lines represent the unadjusted linear relationships between probe set expression within each of the cohorts. Above each graph, the covariate (see Methods) adjusted slope from the regression of RUNX1 vs. PCTP expression and p-value is listed for each cohort separately and combined through meta-analysis. Panel G shows PCTP probe set expression (adjusted for cohort effects) in CATHGEN participants with subsequent death or myocardial infarction (MI) versus those who did not develop death or MI.
Platelet hyperreactivity is associated with a higher risk of cardiovascular events.29 Higher platelet PCTP expression is associated with an enhanced platelet response to PAR4 agonists.2 Our previous studies have revealed that gene expression in peripheral blood correlates with platelet functionality.27 To test the hypothesis that peripheral blood PCTP expression was correlated with future cardiovascular events we used follow-up death and MI data from the two CATHGEN cohorts previously studied by us.27 We found that higher PCTP expression was associated with future death/MI after adjusting for age, sex, and race across cohort (Figure 5G, meta-analysis odds ratio [OR] 2.10, 95% CI [1.61–2.10], P-value < 0.0001 and meta analysis log2 fold change 0.35 [0.23–0.47], P-value < 0.0001) and was consistent within each cohort: observational cohort OR, 2.37, [1.45 – 3.89], p = 0.00067, and case-control OR 2.00 [1.46–2.73], p-value = 1.51e-05. The association between PCTP expression and death/MI persisted (meta-analysis odds ratio = 2.03, [1.50 – 2.75], P-value = 5e-06) after adjustment for cardiovascular risk factors, patient reported aspirin use, genes we have previously identified as associated with death/MI,27 and platelet count and was consistent within each cohort separately: observational and case-control cohort ORs, 95% CIs, and P-values were: 2.16, [1.4 – 3.4], p = 0.0007937 and 2.01, [1.5–2.8], p-value = 1.07e-05, respectively. Thus, higher PCTP expression is associated with a higher risk of cardiovascular events. In a separate CATHGEN analysis,16 we previously showed that RUNX1 P1 probe sets (233690_at and 220918_at) are significantly lower in CATHGEN patients who develop death or MI. Of the RUNX1 P2 probe sets described above, one 211181_x_at was associated with higher risk of death/MI in CATHGEN (OR = 3.3 [1.5–7.3], p-value = 0.003. Together, these findings demonstrate a consistent directionality of associations and that RUNX1 regulation of PCTP expression may be clinically relevant for the development of cardiovascular events.
DISCUSSION
Our studies provide the first evidence that PCTP is a direct transcriptional target and is regulated in platelets/megakaryocytic cells by transcription factor RUNX1 in an isoform dependent manner, and that blood PCTP expression is associated with future death or MI in patients with cardiovascular disease. Platelet PCTP expression was decreased in our patient with RUNX1 haplodeficiency (Figure 1). Our studies provide evidence that RUNX1 binds to the PCTP promoter, and that the sites have promoter activity (Figure 2). Overexpression of RUNX1 variant 2 increased PCTP expression (Figure 3). RUNX1 downregulation elicited the opposite effects (Figure 3). These findings provide a mechanism for the decreased platelet PCTP expression in our patient with RUNX1 haplodeficiency. Previous studies showing that inhibiting PCTP blunts PAR4 agonist stimulated platelet responses2 suggest that decreased platelet PCTP expression may contribute to the platelet dysfunction in RUNX1 haplodeficiency. We now show also that higher PCTP expression in blood is associated with a higher risk of platelet-mediated events in patients with cardiovascular disease. Together, our findings implicate RUNX1 regulation of PCTP as a novel mechanism underlying myocardial infarction.
Although PCTP is recognized for many years, the pathways directly regulated by PCTP in various cells remains unclear. In mouse models, PCTP has been shown to play a role in lipid and glucose metabolism.1 Humans genetic polymorphisms in PCTP may modulate cardiovascular risk through influencing low-density lipoprotein (LDL) size.30 In platelets, PC is a major source of arachidonic acid mobilized on platelet activation and modulates thromboxane A2 synthesis.3,4 However, knockdown of PCTP in HEL cells had no effect on thromboxane production in response to agonists, including a PAR4 agonist (F. Del-Carpio Cano, B.S. and A.K. Rao, M.B.B.S., 2017). In addition, thrombin induced free arachidonic acid release was unaffected in two patients with a RUNX1 mutation identical to that of the present patient.31 Thus, the reported effects of inhibiting PCTP on platelet responses2 are unlikely due to decreased thromboxane production.
Edelstein et al2 reported an association between the increased PAR4 agonist-induced platelet aggregation among healthy black subjects and increased expression of platelet PCTP. Our studies in the CATHGEN cohorts revealed higher PCTP expression in black subjects (Figure 4). These studies provide the first evidence that RUNX1 expression correlates with PCTP expression (Figure 5). We found a strong negative relationship of blood PCTP expression to RUNX1 P1 probe sets and evidence for differential regulation by P1 and P2-driven RUNX1 (Figures 4 and 5). This divergence may be related to the biology of RUNX1. RUNX1 expression is driven by two promoters – the proximal P2 and distal P1 promoter, which have distinct roles and tissue expression,9–15 influenced also by different binding partners.11,32 For example, during hematopoietic development from human embryonic stem cells RUNX1 isoforms expressed from P1 and P2 promoters were differentially expressed; while total RUNX1 and variant 2 expression were constant throughout the differentiation process, variant 1 expression was dynamic, peaking at day 12 with a pattern similar to that of hematopoietic stem cell markers.13 The RUNX1 variant 1 expression coincided with the emergence of definitive hematopoietic stem cells.13 Platelets and HEL cells express both variants at mRNA and protein levels16 (D. Voora, M.D., and A.K. Rao, M.B.B.S. unpublished, 2017). Moreover, in our unpublished studies in HEL treated with PMA to induce megakaryocytic transformation there was decrease in RUNX1 expression from P1 promoter with an increase in P2 driven expression. These reflect the dynamic nature of regulation from the two promoters. Little is known regarding the relative abundance and differential regulation of variants 1 or 2 in cells, or the impact on target gene expression.
RUNX1 is a positive regulator for many genes and a repressor for some.18,33 RUNX1 regulates the thrombopoietin receptor gene MPL promoter both positively or negatively according to cell types.32 Moreover, RUNX1 autoregulates its gene transcription through the P1 promoter: in recent studies9 two conserved RUNX1 binding sites in the P1 promoter were positive regulators while three sites in the 5′UTR were repressors. Our studies show that P1 (variant 1) and P2 (variant 2) derived RUNX1 isoforms may have differential effects in regulating PCTP (Figures 4 and 5) and on clinical events.16
In HEL cells, RUNX1-P2 driven variant 2 was a positive regulator of PCTP (Figure 3), as also shown by us for other platelet/megakaryocyte genes.18,22–25 Of note, we have previously shown16 that in HEL cells aspirin inhibits RUNX1 P2 transcript expression, increases RUNX1-P1 expression with a decrease in P2 to P1 ratio; this was associated with a decrease in expression of MYL9 (myosin light chain), a known RUNX1 target.16,22 Overall, our studies support the premise that RUNX1 regulates expression of PCTP and other genes through mechanisms that involve also autoregulation of its own variants, in addition to differential effects of the variants on gene expression. Other evidence indicates that regulation of expression of P1 and P2 RUNX1 isoforms may be controlled by different signaling or upstream mechanisms.34,35 For example, Wnt/β-catenin signaling induces RUNX1 transcription by binding to a conserved region within P1 promoter.34 Further studies are needed to elucidate these complex mechanisms and relative roles of RUX1 isoforms in gene expression in megakaryocytes/platelets.
We found an association between PCTP expression and future death/MI in patients with cardiovascular disease, which persisted after adjustment for age, sex, race, cardiovascular risk factors, aspirin use, and platelet count. In studies in healthy human volunteers administered aspirin, Voora et al27 have identified a set of 62 genes in blood (aspirin-responsive signature genes, ARS) that is associated with platelet responses in in vitro platelet function testing. This ARS was associated with death/MI27 in the CATHGEN cohort; PCTP was not one of the ARS genes. In present studies the association of PCTP with cardiac events persisted even after adjusting for the ARS genes. Overall, these studies implicate PCTP in outcomes of cardiovascular disease.
Our studies demonstrate that PCTP is a transcriptional target of RUNX1 in megakaryocytes/platelets with direct evidence from the patient with RUNX1 haplodeficiency and the studies in platelets and megakaryocytic cells. The studies in the CATHGEN cohort were performed in unfractionated blood samples containing other cells (e.g. leukocytes), which also express PCTP. In our previous studies in the CATHGEN cohorts and healthy subjects,27 gene expression in whole blood correlated with platelet function, and the aspirin-responsive gene signature (ARS) identified in whole blood included many genes that are platelet derived27 and have RUNX1 binding sites.16 These indicate that gene expression in whole blood may reflect expression of certain platelet genes. Future studies on regulation of PCTP in leukocytes, outside the scope of the present project, may provide important insights.
In summary, these studies provide evidence for platelet PCTP deficiency in RUNX1 haplodeficiency and that PCTP is a direct transcriptional target of RUNX1 with a differential regulation by RUNX1 isoforms arising from the proximal and distal RUNX1 promoters. They advance an association between blood PCTP expression and major clinical events in patients with cardiovascular disease and suggest a potentially important mechanism between RUNX1, PCTP, and outcomes in cardiovascular disease.
Supplementary Material
Clinical Perspective.
1. What is new?
Phosphatidylcholine transfer protein (PCTP) regulates inter-membrane transfer of phosphatidylcholine (PC). Platelet PCTP expression is associated with increased platelet responses upon activation of protease-activated receptor 4 (PAR4) thrombin receptors noted in black subjects. Little is known regarding regulation of platelet PCTP.
Our studies showed that PCTP is a direct transcriptional target of RUNX1, a major hematopoietic transcription factor that regulates platelet production and function.
Our studies in patients with cardiovascular disease showed that PCTP expression in blood correlates with RUNX1 expression and was independently associated with future death/myocardial infarction.
2. What are the clinical implications?
Platelet PCTP has been shown to regulate platelet responses upon activation with PAR4 thrombin receptors.
Our studies identify a novel association between PCTP expression in blood and death/myocardial infarction (MI) in patients with cardiovascular disease and suggest that regulation of PCTP by transcription factor RUNX1 may play a role in the pathogenesis of platelet-mediated cardiovascular events.
Our findings implicate PCTP as a novel gene in the pathogenesis of MI and motivate novel biomarkers and therapeutics to prevent MI.
Acknowledgments
The assistance of Denise Tierney in manuscript preparation is gratefully acknowledged.
SOURCES OF FUNDING
This study was supported by research funding from NIH (NHLBI) R01HL109568 and R01HL085422 to AKR, R01HL118049 to DV, and American Heart Association 16GRNT27260319 to LEG. Clinical data and peripheral blood RNA were collected by the Duke Catheterization Genetics (CATHGEN) biorepository, and microarray data from CATHGEN participants were generated with funding provided for the Measurement to Understand the Reclassification of Disease of Cabarrus/Kannapolis (MURDOCK) Horizon 1 Cardiovascular Disease Study through a gift to Duke University from the David H. Murdock Institute for Business and Culture.
Footnotes
DISCLOSURES
None. The authors have no conflicts to declare.
References
- 1.Kang HW, Wei J, Cohen DE. PC-TP/StARD2: Of membranes and metabolism. Trends Endocrinol Metab. 2010;21:449–456. doi: 10.1016/j.tem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelstein LC, Simon LM, Montoya RT, Holinstat M, Chen ES, Bergeron A, Kong X, Nagalla S, Mohandas N, Cohen DE, Dong JF, Shaw C, Bray PF. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med. 2013;19:1609–1616. doi: 10.1038/nm.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holinstat M, Boutaud O, Apopa PL, Vesci J, Bala M, Oates JA, Hamm HE. Protease-activated receptor signaling in platelets activates cytosolic phospholipase A2alpha differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler Thromb Vasc Biol. 2011;31:435–442. doi: 10.1161/ATVBAHA.110.219527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler DH, Cogan JD, Phillips JA, 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, Oates JA. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Exton JH. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990;265:1–4. [PubMed] [Google Scholar]
- 6.Ichikawa M, Asai T, Chiba S, Kurokawa M, Ogawa S. Runx1/AML-1 ranks as a master regulator of adult hematopoiesis. Cell Cycle. 2004;3:722–724. [PubMed] [Google Scholar]
- 7.Tijssen MR, Cvejic A, Joshi A, Hannah RL, Ferreira R, Forrai A, Bellissimo DC, Oram SH, Smethurst PA, Wilson NK, Wang X, Ottersbach K, Stemple DL, Green AR, Ouwehand WH, Gottgens B. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev Cell. 2011;20:597–609. doi: 10.1016/j.devcel.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyoshi H, Ohira M, Shimizu K, Mitani K, Hirai H, Imai T, Yokoyama K, Soeda E, Ohki M. Alternative splicing and genomic structure of the AML1 gene involved in acute myeloid leukemia. Nucleic Acids Res. 1995;23:2762–2769. doi: 10.1093/nar/23.14.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez M, Hinojosa M, Trombly D, Morin V, Stein J, Stein G, Javed A, Gutierrez SE. Transcriptional Auto-Regulation of RUNX1 P1 Promoter. PLoS One. 2016;11:e0149119. doi: 10.1371/journal.pone.0149119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghozi MC, Bernstein Y, Negreanu V, Levanon D, Groner Y. Expression of the human acute myeloid leukemia gene AML1 is regulated by two promoter regions. Proc Natl Acad Sci U S A. 1996;93:1935–1940. doi: 10.1073/pnas.93.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telfer JC, Rothenberg EV. Expression and function of a stem cell promoter for the murine CBFalpha2 gene: distinct roles and regulation in natural killer and T cell development. Dev Biol. 2001;229:363–382. doi: 10.1006/dbio.2000.9991. [DOI] [PubMed] [Google Scholar]
- 12.Bee T, Liddiard K, Swiers G, Bickley SR, Vink CS, Jarratt A, Hughes JR, Medvinsky A, de Bruijn MF. Alternative Runx1 promoter usage in mouse developmental hematopoiesis. Blood Cells Mol Dis. 2009;43:35–42. doi: 10.1016/j.bcmd.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Challen GA, Goodell MA. Runx1 isoforms show differential expression patterns during hematopoietic development but have similar functional effects in adult hematopoietic stem cells. Exp Hematol. 2010;38:403–416. doi: 10.1016/j.exphem.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ran D, Shia WJ, Lo MC, Fan JB, Knorr DA, Ferrell PI, Ye Z, Yan M, Cheng L, Kaufman DS, Zhang DE. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121:2882–2890. doi: 10.1182/blood-2012-08-451641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draper JE, Sroczynska P, Tsoulaki O, Leong HS, Fadlullah MZ, Miller C, Kouskoff V, Lacaud G. RUNX1B Expression Is Highly Heterogeneous and Distinguishes Megakaryocytic and Erythroid Lineage Fate in Adult Mouse Hematopoiesis. PLoS Genet. 2016;12:e1005814. doi: 10.1371/journal.pgen.1005814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voora D, Rao AK, Jalagadugula GS, Myers R, Harris E, Ortel TL, Ginsburg GS. Systems Pharmacogenomics Finds RUNX1 Is an Aspirin-Responsive Transcription Factor Linked to Cardiovascular Disease and Colon Cancer. EBioMedicine. 2016;11:157–164. doi: 10.1016/j.ebiom.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song WJ, Sullivan MG, Legare RD, Hutchings S, Tan X, Kufrin D, Ratajczak J, Resende IC, Haworth C, Hock R, Loh M, Felix C, Roy DC, Busque L, Kurnit D, Willman C, Gewirtz AM, Speck NA, Bushweller JH, Li FP, Gardiner K, Poncz M, Maris JM, Gilliland DG. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 18.Songdej N, Rao AK. Hematopoietic Transcription Factors Mutations – Important Players in Inherited Platelet Defects. Blood. 2017;129:2873–2881. doi: 10.1182/blood-2016-11-709881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabbeta J, Yang X, Sun L, McLane MA, Niewiarowski S, Rao AK. Abnormal inside-out signal transduction-dependent activation of glycoprotein IIb-IIIa in a patient with impaired pleckstrin phosphorylation. Blood. 1996;87:1368–1376. [PubMed] [Google Scholar]
- 20.Sun L, Mao G, Rao AK. Association of CBFA2 mutation with decreased platelet PKC-θ and impaired receptor-mediated activation of GPIIb-IIIa and pleckstrin phosphorylation: proteins regulated by CBFA2 play a role in GPIIb-IIIa activation. Blood. 2004;103:948–954. doi: 10.1182/blood-2003-07-2299. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Gorospe JR, Hoffman EP, Rao AK. Decreased platelet expression of myosin regulatory light chain polypeptide (MYL9) and other genes with platelet dysfunction and CBFA2/RUNX1 mutation: insights from platelet expression profiling. J Thromb Haemost. 2007;5:146–154. doi: 10.1111/j.1538-7836.2006.02271.x. [DOI] [PubMed] [Google Scholar]
- 22.Jalagadugula G, Mao G, Kaur G, Goldfinger LE, Dhanasekaran DN, Rao AK. Regulation of platelet myosin light chain (MYL9) by RUNX1: implications for thrombocytopenia and platelet dysfunction in RUNX1 haplodeficiency. Blood. 2010;116:6037–6045. doi: 10.1182/blood-2010-06-289850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aneja K, Jalagadugula G, Mao G, Singh A, Rao AK. Mechanism of platelet factor 4 (PF4) deficiency with RUNX1 haplodeficiency: RUNX1 is a transcriptional regulator of PF4. J Thromb Haemost. 2011;9:383–391. doi: 10.1111/j.1538-7836.2010.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalagadugula G, Mao G, Kaur G, Dhanasekaran DN, Rao AK. Platelet PKC-θ deficiency with human RUNX1 mutation: PRKCQ is a transcriptional target of RUNX1. Arterioscler Thromb Vasc Biol. 2011:921–927. doi: 10.1161/ATVBAHA.110.221879. BS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur G, Jalagadugula G, Mao G, Rao AK. RUNX1/core binding factor A2 regulates platelet 12-lipoxygenase gene (ALOX12): studies in human RUNX1 haplodeficiency. Blood. 2010;115:3128–3135. doi: 10.1182/blood-2009-04-214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao GF, Goldfinger LE, Fan DC, Lambert MP, Jalagadugula G, Freishtat R, Rao AK. Dysregulation of PLDN (Pallidin) is a mechanism for platelet dense granule deficiency in RUNX1 haplodeficiency. J Thromb Haemost. 2017;15:792–801. doi: 10.1111/jth.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voora D, Cyr D, Lucas J, Chi JT, Dungan J, McCaffrey TA, Katz R, Newby LK, Kraus WE, Becker RC, Ortel TL, Ginsburg GS. Aspirin exposure reveals novel genes associated with platelet function and cardiovascular events. J Am Coll Cardiol. 2013;62:1267–1276. doi: 10.1016/j.jacc.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 29.Frelinger AL, 3rd, Li Y, Linden MD, Barnard MR, Fox ML, Christie DJ, Furman MI, Michelson AD. Association of cyclooxygenase-1-dependent and -independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization. Circulation. 2009;120:2586–2596. doi: 10.1161/CIRCULATIONAHA.109.900589. [DOI] [PubMed] [Google Scholar]
- 30.Dolley G, Berthier MT, Lamarche B, Despres JP, Bouchard C, Perusse L, Vohl MC. Influences of the phosphatidylcholine transfer protein gene variants on the LDL peak particle size. Atherosclerosis. 2007;195:297–302. doi: 10.1016/j.atherosclerosis.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Rao AK, Poncz M. Defective Acid Hydrolase Secretion in RUNX1 Haplodeficiency: Evidence for a global Platelet Secretory Defect. Haemophilia. 2017 doi: 10.1111/hae.13280. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh Y, Matsumura I, Tanaka H, Ezoe S, Fukushima K, Tokunaga M, Yasumi M, Shibayama H, Mizuki M, Era T, Okuda T, Kanakura Y. AML1/RUNX1 works as a negative regulator of c-Mpl in hematopoietic stem cells. J Biol Chem. 2008;283:30045–30056. doi: 10.1074/jbc.M804768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michaud J, Simpson KM, Escher R, Buchet-Poyau K, Beissbarth T, Carmichael C, Ritchie ME, Schutz F, Cannon P, Liu M, Shen X, Ito Y, Raskind WH, Horwitz MS, Osato M, Turner DR, Speed TP, Kavallaris M, Smyth GK, Scott HS. Integrative analysis of RUNX1 downstream pathways and target genes. BMC Genomics. 2008;9:363. doi: 10.1186/1471-2164-9-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina MA, Ugarte GD, Vargas MF, Avila ME, Necunir D, Elorza AA, Gutierrez SE, De Ferrari GV. Alternative RUNX1 Promoter Regulation by Wnt/beta-Catenin Signaling in Leukemia Cells and Human Hematopoietic Progenitors. J Cell Physiol. 2016;231:1460–1467. doi: 10.1002/jcp.25258. [DOI] [PubMed] [Google Scholar]
- 35.Wu JQ, Seay M, Schulz VP, Hariharan M, Tuck D, Lian J, Du J, Shi M, Ye Z, Gerstein M, Snyder MP, Weissman S. Tcf7 is an important regulator of the switch of self-renewal and differentiation in a multipotential hematopoietic cell line. PLoS Genet. 2012;8:e1002565. doi: 10.1371/journal.pgen.1002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


