SUMMARY
Sleep inertia is affected by circadian phase, with worse performance upon awakening from sleep during the biological night than biological day. Visual search/selective visual attention performance is known to be sensitive to sleep inertia and circadian phase. Individual differences exist in the circadian timing of habitual wake time, which may contribute to individual differences in sleep inertia. Since later chronotypes awaken at an earlier circadian phase, we hypothesized that later chronotypes would have worse visual search performance during sleep inertia than earlier chronotypes if awakened at habitual waketime. We analyzed performance from eighteen healthy participants [5 females (22.1±3.7 y; mean±SD)] at ~1, 10, 20, 30, 40, and 60 min following electroencephalogram-verified awakening from an 8h in-laboratory sleep opportunity. Cognitive throughput and reaction times of correct responses were impaired by sleep inertia and took ~10–30 min to improve after awakening. Regardless whether chronotype was defined by dim light melatonin onset (DLMO) or mid-sleep clock hour on free days (MSFsc), derived from the Munich ChronoType Questionnaire (MCTQ), the duration of sleep inertia for cognitive throughput and reaction times was longer for later chronotypes (n=7) compared to earlier chronotypes (n=7). Specifically, performance for earlier chronotypes showed significant improvement within ~10–20 min after awakening, whereas performance for later chronotypes took ~30 min or longer to show significant improvement (p<0.05). Findings have implications for decision making immediately upon awakening from sleep and are consistent with circadian theory suggesting that sleep inertia contributes to longer lasting impairments in morning performance in later chronotypes.
Keywords: Circadian clock, morning-eveningness, morning type, evening type
INTRODUCTION
Sleep inertia—the state of cognitive impairment immediately upon awakening from sleep—is characterized by grogginess, disorientation, and confusion. This paradoxical phenomenon of worse performance upon awakening than after a full day of wakefulness has been observed since the 1930’s (Omwake, 1932, Kleitman, 1939). More recently, the duration and magnitude of sleep inertia have been quantified. Impairment from sleep inertia is greatest immediately upon awakening from sleep and performance continues to improve 2–4h after waking (Jewett et al., 1999, Wertz et al., 2006, Burke et al., 2015). Performance immediately upon awakening from an 8h sleep opportunity has been shown to be significantly worse than performance following 26h total sleep deprivation, suggesting that impairments within the first ~10 min after waking are safety significant (Wertz et al., 2006).
Performance impairment caused by sleep inertia is largely affected by prior sleep duration/depth (Dinges et al., 1985) and sleep stage upon awakening (Dinges et al., 1985, Silva and Duffy, 2008). Sleep inertia is also affected by circadian phase, such that impairments are greater when awoken during the biological night, when melatonin levels are high and core body temperature is low, compared to the biological day when melatonin levels are low and core body temperature is high (Scheer et al., 2008, Burke et al., 2015, Dinges et al., 1985, Silva and Duffy, 2008). Lastly, drugs that promote sleep increase sleep inertia upon forced awakening (Frey et al., 2011), whereas drugs that increase arousal reduce sleep inertia when taken prior to a nap (Van Dongen et al., 2001) and upon awakening (Newman et al., 2013).
The phase angle of entrainment—timing of the circadian clock relative to the environmental light-dark cycle—underlies a person’s chronotype (Aschoff, 1965, Aschoff and Wever, 1966). Early chronotypes tend to go to sleep and wake up at an earlier clock hour than late chronotypes. Questionnaires have thus been developed to estimate chronotype based on sleep-wake preferences (Horne and Ostberg, 1976) and sleep behavior (Roenneberg et al., 2003, Kantermann et al., 2015). Phase angle of entrainment is associated with and/or influenced by biological and environmental factors such as the period of the circadian clock (Pittendrigh and Daan, 1976, Aschoff, 1965, Duffy et al., 1999, Lazar et al., 2013), age (LeBourgeois et al., 2013, Crowley et al., 2014, Roenneberg et al., 2004, Duffy et al., 1999), and light exposure history (Wright et al., 2013, Wright et al., 2005). Early chronotypes tend to have shorter circadian periods and advanced circadian phases compared to late chronotypes (Duffy et al., 2001,Carskadon et al., 1999). Further, Duffy and colleagues (1999) showed that when sleeping at their habitual times, late chronotypes have a narrower wake time phase angle of entrainment compared to early chronotypes. Therefore, although late chronotypes tend to wake at a later clock hour than early chronotypes, they wake at an earlier time within their circadian cycle closer to their melatonin onset and core body temperature minimum.
Inter-individual differences in the ease of awakening, “easy for some and difficult for others”, was also noted by Kleitman (1939). However, whether performance upon awakening from sleep differs between early and late chronotypes remains unknown and, therefore, was the focus of the current study. Based on circadian theory, we expected later chronotypes to show worse sleep inertia than earlier chronotypes upon awakening from an 8h sleep opportunity at habitual waketime. We tested spatial-configuration visual search performance since cognitive throughput on another version of this task has been shown to be sensitive to sleep inertia and circadian phase (Burke et al., 2015). We also performed exploratory analyses on other performance metrics derived from the visual search task as they have not been previously examined during sleep inertia.
MATERIAL AND METHODS
Participants and protocol
Analyses are of baseline data from a series of larger studies (Burke et al., 2013, McHill et al., 2014). Eighteen healthy adults that performed a spatial-configuration visual search task during sleep inertia (i.e., electroencephalogram [EEG] verified awakening) were included. Written informed consent was obtained and procedures approved by the Colorado Clinical and Translational Science Institute and University of Colorado Institutional Review Board. Participants were healthy as determined by physical, psychological, and sleep disorders screenings at the Clinical and Translational Research Center and the Sleep and Chronobiology Laboratory. Participants were free of medications, nicotine, and recreational drugs, verified by urine toxicology and breath alcohol testing (Lifeloc Technologies Model FC10, Wheat Ridge, Colorado). Shift work within one year prior or travel more than one time zone three weeks prior to the study were exclusionary. Participants maintained regular ~8h sleep schedules at habitual sleep-wake times for one week prior to study, verified via sleep logs, call-ins to a time-stamped recorder, and wrist actigraphy. Caffeine, over-the-counter medications and supplements/vitamins were proscribed two weeks prior, napping one week prior, and alcohol two days prior to study.
Participants practiced the visual search task to remove the steep portion of the learning curve. The visual search task (Wolfe, 1994) used required participants to determine whether a target (the number 5) was present among distractor stimuli (the number 2) of four set sizes with 10, 20, 30, and 40 distractors. Each test consisted of 100 self-paced trials, of which the first 10 were considered practice and not included in analyses; targets were presented on 10% of trials with is considered a rare target prevalence (Wolfe, 2005). Performance was assessed following an 8h in-laboratory sleep opportunity scheduled at participants’ habitual bedtimes, derived from the prior week of ambulatory monitoring. Performance was assessed ~1, 10, 20, 30, 40, and 60 min after EEG-verified awakening. Baseline performance was assessed ~9h prior to awakening; equivalent to ~1h prior to bedtime.
Chronotype of participants was determined in two ways: according to mid-sleep on free days using the Munich ChronoType Questionnaire (chronotypeMSFsc) and according to dim light melatonin onset (chronotypeDLMO). MSFsc was obtained during the consent visit and DLMO was obtained during a constant routine immediately following sleep inertia testing. The 28h modified constant routine included wakefulness in a semirecumbent position except for brief, scheduled bathroom breaks using a commode ~1m from the bed, dim light (~1.9 lux; ~0.6 Watts/m2 in the angle of gaze), and isocaloric hourly snacks. Circadian melatonin phase was estimated via saliva samples collected every 30–60 min (ELISA assay; IBL International, Hamburg, Germany). DLMO was defined as the linearly interpolated time point when melatonin levels exceeded and remained two standard deviations above the stable baseline mean (Burke et al., 2013). Polysomnography (PSG) recordings (Siesta Compumedics USA Ltd, Charlotte, North Carolina) were used to determine participants’ sleep architecture during the sleep opportunity prior to sleep inertia testing. Sleep onset latency (SOL) was defined as time to three continuous epochs of sleep. Recordings were obtained from C3, C4, O1, and F3, referenced to contralateral mastoid electrodes, right and left electrooculogram, chin electromyogram, and electrocardiogram.
Data analysis
Sleep inertia was examined for the top and bottom ~39th percentiles of each chronotype definition: comparisons were made between earlier and later chronotypeMSFsc (n=7 [3 females] and n=7 [2 females], respectively) and between earlier and later chronotypeDLMO (n=7 [1 female] and n=7 [3 females], respectively). Seventeen of the 18 individuals possible contributed to chronotype categorizations and analyses. Categorization of four participants switched when using MSFsc versus DLMO chronotype definitions (two from early to late and two from late to early).
Primary outcomes for visual search performance were cognitive throughput—number of correct responses attempted per minute—and overall median reaction time for correct responses (MedRTC). As exploratory outcomes, we also examined MedRTC when a participant responded “yes” when a target was present or “no” when a target was absent overall and across set sizes, percent targets missed, and search slope calculated by the linear fit of MedRTC across set sizes and interpreted as the rate at which attention is shifted from item to item. False alarms and d’ were not examined since false alarms were rare, as known when target prevalence is rare (Wolfe, 2005), and calculation of d’ is problematic as it is derived using false alarms. Eight out of 126 performance tests were missing due to scheduling or technical issues. Performance data were analyzed with mixed-model analysis of variance (ANOVA), with participant as a random factor and time awake, chronotype, and set size as fixed factors using the variance components covariance structure. Independent or dependent parametric t-tests were used for planned comparisons (performance immediately upon awakening compared to other time points within subject; each time point compared between subject) using Statistica version 10.0.
Sleep staging was assessed for 8h sleep episodes, 30 min and 10 min prior to awakening, and sleep stage upon awakening. Sleep data for one participant was missing due to loss of recording (categorized as early for chronotypeMSFsc and late for chronotypeDLMO). Examination of wrist actigraphy showed the participant had low activity consistent with estimated sleep at scheduled waketime.
RESULTS
Chronotype and sleep characteristics
Table 1 shows chronotype and sleep characteristics. On average, habitual bedtime occurred earlier for earlier chronotypes compared to later chronotypes, being significant for chronotypeDLMO groups but non-significant for chronotypeMSFsc groups (p=0.057). MSFsc was significantly earlier for earlier chronotypes compared to later chronotypes in the chronotypeMSFsc groups, but not the chronotypeDLMO groups (p=0.29). In contrast, DLMO occurred significantly earlier for earlier chronotypes compared to later chronotypes in the chronotypeDLMO groups, but not the chronotypeMSFsc groups (p=0.54).
Table 1.
Chronotype and sleep characteristics
| All Subjects (n=18) |
ChronotypeMSFsc | ChronotypeDLMO | |||
|---|---|---|---|---|---|
| Earlier (n=7) |
Later (n=7) |
Earlier (n=7) |
Later (n=7) |
||
| DLMO | 22.1 ± 0.3 | 22.1 ± 0.4 | 22.4 ± 0.4 | 21.2 ± 0.2 | 23.2 ± 0.2*** |
| MSFsc | 4.9 ± 0.2 | 4.2 ± 0.1 | 5.8 ± 0.2*** | 4.6 ± 0.3 | 5.1 ± 0.4 |
| Bedtime | 24.2 ± 0.2 | 23.9 ± 0.4 | 24.9 ± 0.3 | 23.5 ± 0.2 | 24.8 ± 0.3** |
p<0.0001,
p<0.01,
p<0.05 between earlier and later chronotypes within chronotype group; data represent mean±SEM.
Regardless of chronotype definition, no significant differences between earlier and later chronotypes in total sleep time (TST), sleep efficiency (SE), sleep staging, or number of minutes awake within 10 min or 30 min prior to awakening were observed (Table S1; all p>0.11). Further, the majority of participants awakened out of stage 2 sleep (Table S2). Three participants were awake prior to scheduled wake time with an average wake duration of 1.3±0.6 min, ranging 0.5 to 2.5 min.
Impact of sleep inertia on visual search – all 18 participants
Cognitive throughput was significantly worse immediately upon awakening compared to baseline and significantly improved ~10 min after wake time (Fig. 1A), whereas reaction time significantly improved ~30 min after wake time (Fig. 1B). When analyzing target absent and target present trials separately, reaction time for target absent trials significantly improved ~20 min after wake time, and reaction time for target present trials improved ~60 min after wake time (Fig. 1C). Reaction time varied by time and set size when the target was absent such that performance significantly improved at ~20 min after awakening for distractor set sizes 20 and 30, at ~30 min after awakening for distractor set size 40, and at ~60 min after awakening for distractor set size 10 (Fig. 1D). Percent of missed targets was lower immediately upon awakening from sleep and significantly increased at ~20 min after wake time (Fig. 1E). Search slopes for target present trials significantly improved ~10 min after wake time and for target absent trials improved ~60 min after wake time (Fig. 1F). ANOVA output for main effects of time are in Table 2.
Figure 1.
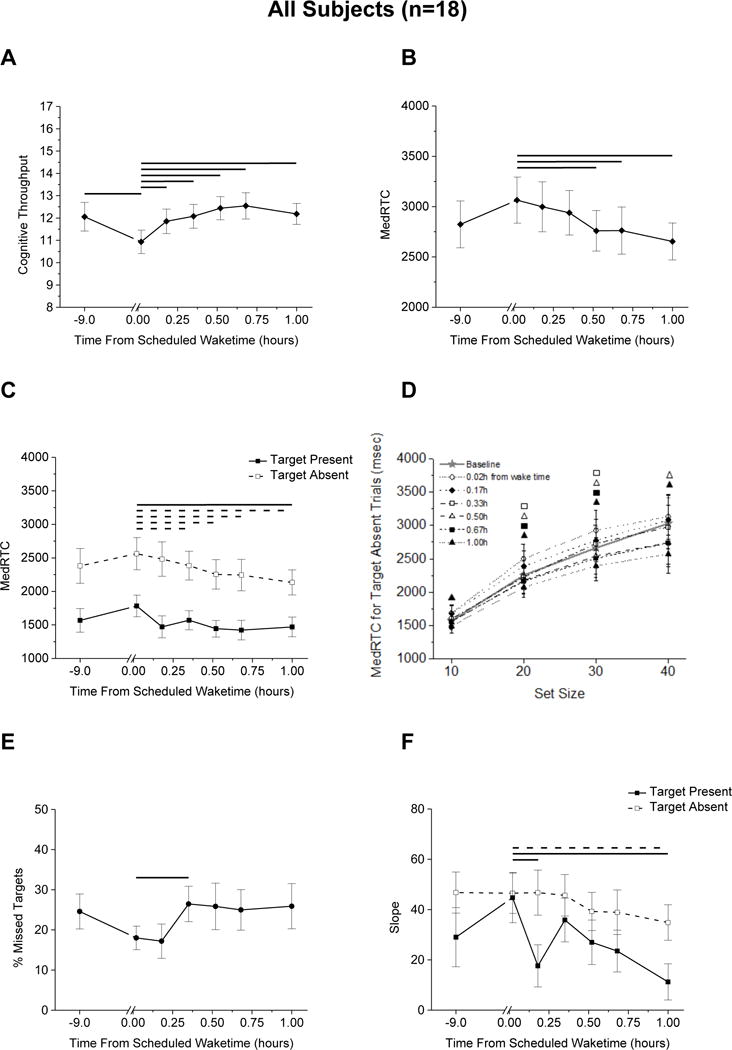
Visual search performance for all subjects (n=18) regardless of chronotype. (A) cognitive throughput; median correct reaction times for correct responses (MedRTC) for (B) all trials and (C) for target absent and target present trials separately and (D) by set size when target absent; (E) percent missed targets; (F) slope. Lines represent significant differences (p<0.05) between time points at the ends of each line (A–C, E and F; solid line overall or target present, dashed line target absent). Symbols in D represent significant differences for that time point from the first sleep inertia test (open circle) 0.02h from wake time (p<0.05).
Table 2.
Mixed model, analysis of cognitive measures
| All Subjects (n=18) | ||
|---|---|---|
| Main effect of time unless noted | ||
| Outcome | F value | p value |
| Cognitive Throughput | 4.54 | 0.0004 |
| Cognitive Throughput for Target Present Trials | 0.29 | 0.9424 |
| Cognitive Throughput for Target Absent Trials | 2.70 | 0.0212 |
| Overall MedRTC | 2.99 | 0.0113 |
| MedRTC on Target Present Trials | 1.25 | 0.2877 |
| set size | 9.15 | 0.0000 |
| Interaction time × set size | 1.28 | 0.1989 |
| MedRTC on Target Absent Trials | 2.69 | 0.0197 |
| set size | 31.82 | 0.0000 |
| Interaction time × set size | 1.80 | 0.0246 |
| % Missed Targets | 1.18 | 0.3217 |
| Slope for Correct Responses on Target Present Trials | 1.62 | 0.1509 |
| Slope for Correct Responses on Target Absent Trials | 2.16 | 0.0544 |
MedRTC, Median reaction time for correct responses.
Influence of chronotype on sleep inertia
In general, sleep inertia induced impairments in cognitive throughput that lasted longer for later chronotypes than earlier chronotypes. Specifically, cognitive throughput significantly improved within 10 min after wake time for both earlier chronotype groups (Fig. 2A and 2B), whereas cognitive throughput did not show significant improvement until ~30 min after wake time for both later chronotype groups. Impairments in MedRTC also lasted longer for later chronotypes than earlier chronotypes. Specifically, reaction time significantly improved within ~20 min after wake time for both earlier chronotype groups (Fig. 2C and 2D). In contrast, reaction time for the later chronotypeMSFsc group became more impaired until ~10 min after wake time. As a result, the magnitude of sleep inertia for MEDRTC was significantly greater for later chronotypes compared to earlier chronotypes 10 min after wake time for the chronotypeMSFsc group (Fig. 2C); there was a non-significant trend for cognitive throughput for the same time point (p=0.08; Fig. 2A). Reaction time for later chronotypes did not show significant improvement until ~60 min after wake time (Fig. 2C). For the later chronotypeDLMO group, reaction time did not show significant improvement until ~40 min after wake time (Fig. 2D). When analyzing target absent trials separately, findings were generally consistent with those seen for overall reaction time with target absent and target present trials combined such that it took longer for later chronotypes to show improvement in performance after awakening as compared to early chronotypes (Fig. S1). ANOVA output for effects of chronotype and time are in Table 3.
Figure 2.
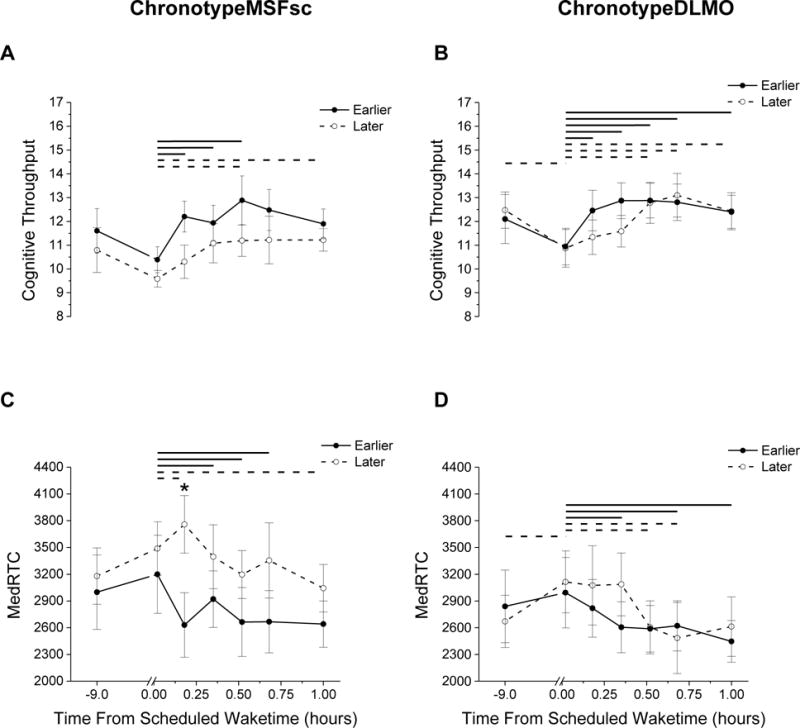
Visual search performance for the ChronotypeMSFsc group (A and C) and ChronotypeDLMO group (B and D). A and B cognitive throughput; C and D median reaction time (MedRTC). Stars above symbols represent significant (p<0.05) differences between earlier and later chronotypes. Solid and dashed lines represent significant differences (p<0.05) for time points at the ends of each line within chronotype group.
Table 3.
Mixed model, analysis of cognitive measures for each chronotype group
| Outcome | ChronotypeMSFsc (n=7 earlier, n=7 later) |
ChronotypeDLMO (n=7 earlier, n=7 later) |
|||
|---|---|---|---|---|---|
| F value | p value | F value | p value | ||
| Cognitive Throughput | Time | 5.34 | 0.0001 | 6.54 | 0.0000 |
| Chronotype | 1.59 | 0.2311 | 0.06 | 0.8123 | |
| T × C | 0.46 | 0.8338 | 1.60 | 0.1603 | |
| Cognitive Throughput for Target Present Trials | Time | 0.27 | 0.9472 | 0.56 | 0.7621 |
| Chronotype | 0.00 | 0.9632 | 0.23 | 0.6433 | |
| T × C | 0.73 | 0.6288 | 0.71 | 0.6436 | |
| Cognitive Throughput for Target Absent Trials | Time | 2.81 | 0.0214 | 4.75 | 0.0004 |
| Chronotype | 1.35 | 0.2674 | 0.08 | 0.7830 | |
| T × C | 1.83 | 0.1076 | 1.04 | 0.4082 | |
| Overall MedRTC | Time | 3.01 | 0.0128 | 5.21 | 0.0002 |
| Chronotype | 1.46 | 0.2501 | 0.05 | 0.8258 | |
| T × C | 2.32 | 0.0431 | 1.49 | 0.1943 | |
| MedRTC on Target Present Trials | Time | 1.27 | 0.2828 | 1.17 | 0.3309 |
| Chronotype | 0.22 | 0.6503 | 0.01 | 0.9298 | |
| T × C | 0.15 | 0.9875 | 1.19 | 0.3205 | |
| MedRTC on Target Absent Trials | Time | 3.04 | 0.0117 | 4.92 | 0.0003 |
| Chronotype | 1.34 | 0.2697 | 0.04 | 0.8449 | |
| T × C | 1.91 | 0.0917 | 1.06 | 0.3925 | |
| % Missed Targets | Time | 0.90 | 0.5034 | 0.72 | 0.6351 |
| Chronotype | 2.56 | 0.1361 | 0.00 | 0.9460 | |
| T × C | 0.96 | 0.4616 | 0.49 | 0.8126 | |
| Slope for Correct Responses on Target Present Trials | Time | 1.75 | 0.1222 | 1.64 | 0.1496 |
| Chronotype | 1.23 | 0.2891 | 3.42 | 0.0898 | |
| T × C | 0.46 | 0.8365 | 0.34 | 0.9146 | |
| Slope for Correct Responses on Target Absent Trials | Time | 2.35 | 0.0409 | 5.01 | 0.0003 |
| Chronotype | 1.01 | 0.3355 | 0.03 | 0.8595 | |
| T × C | 1.67 | 0.1427 | 0.73 | 0.6282 | |
MedRTC, Median reaction time for correct responses; T × C, interaction for time by chronotype; ChronotypeMSFsc, chronotype derived using the mid sleep phase corrected from the Munich chronotype questionnaire; ChronotypeDLMO, chronotype derived using the timing of the dim light melatonin onset.
DISCUSSION
Our findings demonstrate that cognitive throughput, cognitive speed, and search rate components of visual selective attention are impaired by sleep inertia and take between ~10–30 min after wake time to improve. Improvements during sleep inertia were first observed for set sizes with intermediate attentional load and took the longest to improve for the set size with the lowest attentional load. Missed targets were fewer immediately upon awakening from sleep and worsened at ~20 min after awakening, consistent with prior findings (Burke et al., 2015). During sleep inertia, participants took especially longer to correctly identify that a target was absent. Slower, less efficient, visual search during sleep inertia may have safety significant consequences immediately upon awakening from sleep, such as when on-call medical personnel need to quickly search for multiple injuries in a patient brought into the emergency room or when military or security personnel need to quickly make decisions on how to respond to an emerging event.
Regardless of whether chronotype is defined by self-report on the Munich ChronoType questionnaire (MSFsc) or by the timing of the internal circadian clock (DLMO), the duration of sleep inertia for cognitive throughput and cognitive speed on a visual search task was longer for later chronotypes than earlier chronotypes. Specifically, cognitive performance for earlier chronotypes improved within ~10–20 min following wake time, whereas performance for later chronotypes took ~30 min or longer to show improvement. There were no main effects of chronotype nor interaction effects between chronotype and time, and planned comparisons showed no significant differences between chronotypes with respect to the magnitude of impairment aside from one time point in the chronotypeMSFsc group. Therefore, these results suggest that chronotype has a larger impact on the time course of improvement in cognition after awakening rather than the magnitude of sleep inertia, at least for the visual search task examined. Cognitive throughput and overall reaction time performance were the primary outcome variables and thus further research is needed to replicate findings for exploratory outcomes.
The time to improvement during sleep inertia was longer for later than earlier chronotypes when participants maintained self-selected 8h sleep opportunities for one-week prior to study. Consistent with prior findings, sleep architecture was similar among chronotypes (Mongrain et al., 2005). Prior sleep has been shown to influence sleep inertia such that sleep restriction/deprivation increases sleep pressure and sleep depth, and consequently worsens cognitive impairment upon awakening (Dinges et al., 1985). Although no differences in homeostatic sleep drive (i.e., slow wave activity) is seen between chronotypes when sleeping at their habitual circadian phase, early chronotypes show more slow wave activity after sleep fragmentation suggesting that sleep homoestasis may interact with chronotype to influence sleep inertia (Mongrain et al., 2007).
Due to having a delayed circadian phase, late chronotypes typically have difficulty falling asleep early on work/school days when social constraints require them to awaken early. As a result, late chronotypes may be chronically sleep restricted during the work/school week–a phenomenon known as social jetlag (Wittmann et al., 2006). Roenneberg, Wirz-Justice, and Merrow (2003) showed that self-reported sleep inertia, or the time to feel fully awake in the morning, is longer for late chronotypes than early chronotypes during the workweek, but that this difference is not apparent on free days. Our findings, however, demonstrate that the duration of sleep inertia for a visual search task is longer for late chronotypes compared to early chronotypes when prior sleep duration is similar and when awakening at their habitual times. Although sleep inertia was observed to last longer for late chronotypes than early chronotypes when sleep history was similar, it is possible that sleep inertia would be exacerbated during the work/school week when late chronotypes may get less sleep and awaken at an even earlier circadian time due to social jetlag. Future research examining differences in sleep inertia between chronotypes on Monday morning after a weekend, when late chronotypes are expected to delay further, is necessary to determine effects of sleep inertia.
Current findings are consistent with prior findings regarding the relationship between circadian phase and cognitive performance. The circadian phase at which alertness and cognitive performance are most impaired is near the end of the biological night (Dijk et al., 1992, Wright et al., 2002, Burke et al., 2015). As noted, late chronotypes awaken closer to their biological night compared to early chronotypes (Duffy et al., 1999). Furthermore, it has been shown that performance level across the day varies between chronotypes, such that when analyzing the same clock hour, early chronotypes’ performance is better than late chronotypes’ performance during the morning hours (Horne et al., 1980, Monk, 1986).
Findings from prior studies indicate that DLMO and MSFsc are significantly correlated (Wright et al., 2013, Kitamura et al., 2014, Kantermann et al., 2015). In the current study, circadian DLMO phase was not significantly different for chronotypes derived from MSFsc, and MSFsc was not significantly different for chronotypes derived from DLMO. Such findings may reflect that MSFsc is influenced by behavioral choices, whereas DLMO is more strongly determined by circadian period and light exposure history (Wright et al., 2005, Duffy et al., 2011). Alternatively, participants were not selected based on chronotype extremes and this may contribute to this finding. Later chronotypes were however consistent with the “late” chronotype definition, as defined by Roenneberg (2003). Nonetheless, performance outcomes were similar with later chronotypes derived from both MSFsc and DLMO showing longer time to improvement during sleep inertia. Our findings may underestimate the influence of chronotype on sleep inertia as examining extreme chronotypes may result in larger effects. The absence of extreme chronotypes makes our findings applicable to more individuals in society, as extreme types are rare in the general population compared to intermediate types (Roenneberg et al., 2003). Research examining extreme chronotypes and how other cognitive functions are affected by interactions between sleep inertia and chronotype are needed (Santhi et al., 2013).
In summary, visual selective attention was impaired during sleep inertia with lower cognitive throughput and slower reaction time and search rates. The slower termination of searching may be due to participants revisiting or dwelling on distractors and examination of other search tasks is needed to explore contributing mechanisms. Impairments resulting from sleep inertia appear to last longer for later chronotypes compared to earlier chronotypes, even when sleep duration and history were similar. It appears that sleep inertia is influenced by individual circadian characteristics, such as the circadian timing of wake time, yet it is possible that other factors, such as social jetlag, could make sleep inertia even worse. Late chronotypes are likely to awaken later and therefore have less time to commute to school or work. If commuting within 30–40 min following awakening, it is likely that they will be driving under the influence of sleep inertia, putting themselves and others at risk. In conclusion, this study provides evidence for chronotype being another factor that influences the duration of sleep inertia.
Supplementary Material
Acknowledgments
Funding: NIH HL081761, HL109706 and TR001082, Office of Naval Research N00014-15-1-2809, Howard Hughes Medical Institute in collaboration with the Biological Sciences Initiative and Undergraduate Research Opportunities Program at the University of Colorado Boulder.
Footnotes
Author Contributions: H.K.R. and J.A. analyzed data and wrote the paper; T.M.B. and T.B.D. collected data and wrote the paper; A.W.M. collected and analyzed data, and wrote the paper; K.P.W. secured funding, collected and analyzed data, and wrote the paper.
Conflicts of Interest: H.K.R., T.M.B., T.B.D., A.W.M. and J.A. report no conflicts; K.P.W.: Funding: NIH, Office of Naval Research, Philips Inc., Torvec Inc.; Consulting fees or served as a paid member of scientific advisory boards for NIH, Torvec Inc.; Speaker honorarium from American College of Chest Physicians, The Obesity Society, Obesity Medicine Association.
References
- Aschoff J. Circadian rhythms in man: a self-sustained oscillator with an inherent frequency underlies human 24-hour periodicity. Science. 1965;148:1427–32. doi: 10.1126/science.148.3676.1427. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Wever R. Circadian period and phase-angle difference in chaffinches (Fringilla coelebs L) Comp Biochem Physiol. 1966;18:397–404. doi: 10.1016/0010-406x(66)90197-6. [DOI] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, Chinoy ED, et al. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep. 2013;36:1617–24. doi: 10.5665/sleep.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke TM, Scheer FA, Ronda JM, Czeisler CA, Wright KP., Jr Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res. 2015;24:364–71. doi: 10.1111/jsr.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–32. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Van Reen E, Lebourgeois MK, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PloS one. 2014;9:e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Orne MT, Orne EC. Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Beh Res Meth, Instr Comp. 1985;17:37–45. [Google Scholar]
- Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 2011;108:15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–50. [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–9. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Ortega JD, Wiseman C, Farley CT, Wright KP., Jr Influence of zolpidem and sleep inertia on balance and cognition during nighttime awakening: a randomized placebo-controlled trial. J Am Geriat Soc. 2011;59:73–81. doi: 10.1111/j.1532-5415.2010.03229.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Brass CG, Pettitt AN. Circadian performance differences between morning and evening types. Ergon. 1980;23:29–36. doi: 10.1080/00140138008924715. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Sung H, Burgess HJ. Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. J Biol Rhythms. 2015;30:449–53. doi: 10.1177/0748730415597520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Hida A, Aritake S, et al. Validity of the Japanese version of the Munich ChronoType Questionnaire. Chronobiol Int. 2014;31:845–50. doi: 10.3109/07420528.2014.914035. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Sleep and Wakefulness. The University of Chicago Press; Chicago, Illinois: p. 1939. [Google Scholar]
- Lazar AS, Santhi N, Hasan S, et al. Circadian period and the timing of melatonin onset in men and women: predictors of sleep during the weekend and in the laboratory. J Sleep Res. 2013;22:155–9. doi: 10.1111/jsr.12001. [DOI] [PubMed] [Google Scholar]
- Lebourgeois MK, Carskadon MA, Akacem LD, et al. Circadian phase and its relationship to nighttime sleep in toddlers. J Biol Rhythms. 2013;28:322–31. doi: 10.1177/0748730413506543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchill AW, Smith BJ, Wright KP., Jr Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythms. 2014;29:131–43. doi: 10.1177/0748730414523078. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Chronotype and sex effects on sleep architecture and quantitative sleep EEG in healthy young adults. Sleep. 2005;28:819–27. doi: 10.1093/sleep/28.7.819. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Dumont M. Increased homeostatic response to behavioral sleep fragmentation in morning types compared to evening types. Sleep. 2007;30:773–80. doi: 10.1093/sleep/30.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Leng VC. Interactions between inter-individual and inter-task differences in the diurnal variation of human performance. Chronobiol Int. 1986;3:171–77. doi: 10.3109/07420528609066364. [DOI] [PubMed] [Google Scholar]
- Newman RA, Kamimori GH, Wesensten NJ, Picchioni D, Balkin TJ. Caffeine gum minimizes sleep inertia. Percept Mot Skills. 2013;116:280–93. doi: 10.2466/29.22.25.PMS.116.1.280-293. [DOI] [PubMed] [Google Scholar]
- Omwake KT. Effect of varying periods of sleep on nervous stability. J Appl Physiol. 1932;16:623–32. [Google Scholar]
- Pittendrigh CS, Daan S. A Functional Analysis of Circadian Pacemakers in Nocturnal Rodents: IV. Entrainment: Pacemakers as Clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–9. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Santhi N, Groeger JA, Archer SN, Gimenez M, Schlangen LJ, Dijk DJ. Morning sleep inertia in alertness and performance: effect of cognitive domain and white light conditions. PloS one. 2013;8:e79688. doi: 10.1371/journal.pone.0079688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23:353–61. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EJ, Duffy JF. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav Neurosci. 2008;122:928–35. doi: 10.1037/0735-7044.122.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen HP, Price NJ, Mullington JM, Szuba MP, Kapoor SC, Dinges DF. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep. 2001;24:813–9. doi: 10.1093/sleep/24.7.813. [DOI] [PubMed] [Google Scholar]
- Wertz AT, Ronda JM, Czeisler CA, Wright KP., Jr Effects of sleep inertia on cognition. JAMA. 2006;295:163–64. doi: 10.1001/jama.295.2.163. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided Search 2.0 - a Revised Model of Visual-Search. Psychon B Rev. 1994;1:202–38. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Horowitz TS, Kenner NM. Cognitive psychology: rare items often missed in visual searches. Nature. 2005;435:439–40. doi: 10.1038/435439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–77. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol. 2002;283:R1370–7. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Mchill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–8. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


